Published online Feb 26, 2025. doi: 10.4252/wjsc.v17.i2.101030
Revised: December 9, 2024
Accepted: February 7, 2025
Published online: February 26, 2025
Processing time: 174 Days and 17.5 Hours
Mesenchymal stem cells, found in various tissues, possess significant healing and immunomodulatory properties, influencing macrophage polarization, which is essential for wound repair. However, chronic wounds present significant the
To investigate the potential of fetal dermal mesenchymal stem cells (FDMSCs) in enhancing wound healing through modulation of macrophage polarization, specifically by promoting the M2 phenotype to address inflammatory responses in chronic wounds.
FDMSCs were isolated from BalB/C mice and co-cultured with RAW264.7 ma
FDMSCs induced macrophage polarization from the M1 to M2 phenotype, as demonstrated by a reduction in pro-inflammatory markers (inducible nitric oxide synthase, interleukin-6) and an increase in anti-inflammatory markers [mannose receptor (CD206), arginase-1] in co-cultured RAW264.7 macrophages. These shifts were confirmed by flow cytometry. In an acute skin wound model, FDMSC-treated mice exhibited faster wound healing, enhanced collagen deposition, and improved vascular regeneration compared to controls. Significantly higher expression of arginase-1 further indicated an enriched M2 macrophage environment.
FDMSCs effectively modulate macrophage polarization from M1 to M2, reduce inflammation, and enhance tissue repair, demonstrating their potential as an immunomodulatory strategy in wound healing. These findings highlight the promising therapeutic application of FDMSCs in managing chronic wounds.
Core Tip: This study investigates the role of fetal dermal mesenchymal stem cells in wound healing by modulating macrophage polarization towards a pro-healing M2 phenotype. Using flow cytometry, reverse transcriptase polymerase chain reaction, and histology, we demonstrate that fetal dermal mesenchymal stem cells promote a reparative immune environment, accelerating wound closure, enhancing collagen deposition, and supporting vascular regeneration in a mouse model.
- Citation: Xia ZY, Wang Y, Shi N, Lu MQ, Deng YX, Qi YJ, Wang XL, Zhao J, Jiang DY. Fetal mice dermal mesenchymal stem cells promote wound healing by inducing M2 type macrophage polarization. World J Stem Cells 2025; 17(2): 101030
- URL: https://www.wjgnet.com/1948-0210/full/v17/i2/101030.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i2.101030
Mesenchymal stem cells (MSCs) are multipotent precursor cells with self-renewal capacity, found in various tissues such as bone marrow, adipose tissue, umbilical cord blood, dental pulp, dermis, and the endometrial lining of the uterus[1]. MSCs are known for their robust regenerative properties, including the ability to migrate to injury sites, differentiate into various cell types, and release cytokines, growth factors, and chemokines that promote tissue repair[2]. In addition to their direct regenerative effects, MSCs modulate the immune response during healing, particularly by influencing macrophage behavior, which plays a critical role in tissue repair and inflammation resolution[3]. This immunomodulatory function enhances the healing process, positioning MSCs as promising candidates for treating chronic wounds, autoimmune disorders, and inflammation-driven diseases[4,5].
Macrophages are critical immune cells involved in the inflammatory, proliferative, and remodeling phases of wound healing[6]. Initially, granulocytes and monocytes are recruited to the injury site, with monocytes differentiating into pro-inflammatory M1 macrophages characterized by high CD86 and programmed death-ligand 1 expression[7]. These M1 macrophages secrete inflammatory mediators such as inducible nitric oxide synthase (iNOS), tumor necrosis factor-alpha, interleukin-1 beta (IL-1β), and IL-6, and play a key role in phagocytosis and debris clearance[8]. As healing progresses, macrophages transition to an anti-inflammatory M2 phenotype, marked by high mannose receptor (CD206), arginase-1 (Arg-1), and FIZZ1 expression, along with the secretion of transforming growth factor-beta and IL-10[9]. M2 macrophages support tissue repair by promoting angiogenesis and collagen deposition[10]. They also produce cytokines like vascular endothelial growth factor and fibroblast growth factor to stimulate fibroblast and endothelial cell activity[11]. An overabundance of pro-inflammatory macrophages in chronic wounds can impair healing by hindering tissue remodeling[12]. Recent research has revealed that macrophage polarization is more complex than the classical M1/M2 dichotomy, with various intermediate phenotypes influencing the wound healing process. These phenotypes, shaped by factors such as the tissue microenvironment, activation timing, and the presence of other immune cells, demonstrate that macrophage polarization exists on a spectrum. This dynamic understanding is essential for developing more targeted immunomodulatory therapies for chronic wounds and inflammation[13].
Fetal dermal MSCs (FDMSCs) are a distinct category of MSCs extracted from the dermal tissue of BalB/C mice on the fifteenth day of gestation. In comparison to adult MSCs, including those sourced from bone marrow and adipose tissue, FDMSCs exhibit enhanced multidirectional differentiation potential, decreased immunogenicity, and a significant correlation with the scarless healing properties inherent to fetal skin. Previous studies have demonstrated that FDMSCs play a crucial role in promoting wound healing and angiogenesis, with evidence showing that they facilitate tissue repair by inducing macrophage polarization toward the M2 phenotype[14,15]. This study aims to investigate the role of FDMSCs in modulating macrophage polarization, specifically promoting the transition from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, and to evaluate their impact on wound healing.
BalB/C mice, aged 6 to 8 weeks, were procured from Jinan Pengyue Laboratory Animal Breeding Co., Ltd. and were maintained in groups at the Animal Facility of our hospital. All animal care and experimental techniques were conducted in accordance with the protocols sanctioned by the Institutional Animal Care and Use Committee at our hospital. Furthermore, all procedures were conducted in compliance with applicable rules and legislation to guarantee the ethical treatment of animals. This study adhered to ARRIVE principles, and all techniques are documented to guarantee transparency and reproducibility of the experimental processes. Upon the completion of the experiments, euthanasia was performed to alleviate suffering. Mice were executed via CO2 inhalation, followed by cervical dislocation as a supplementary method to confirm death, in compliance with the American Veterinary Medical Association euthanasia re
FDMSC was isolated from the dorsal dermis of BalB/C mice on gestational day 15. The epidermis and dermis were dissociated using neutral protease, followed by digestion of the dermis with type I collagenase. The FDMSCs were subjected to filtration using a 70-micrometer filter and subsequently cultivated in DMEM low glucose medium (HyClone, UT, United States) supplemented with 10% fetal bovine serum (Gibco, CA, United States) and 1% Penicillin-Streptomycin at a concentration of 100 U/mL (Gibco, CA, United States).
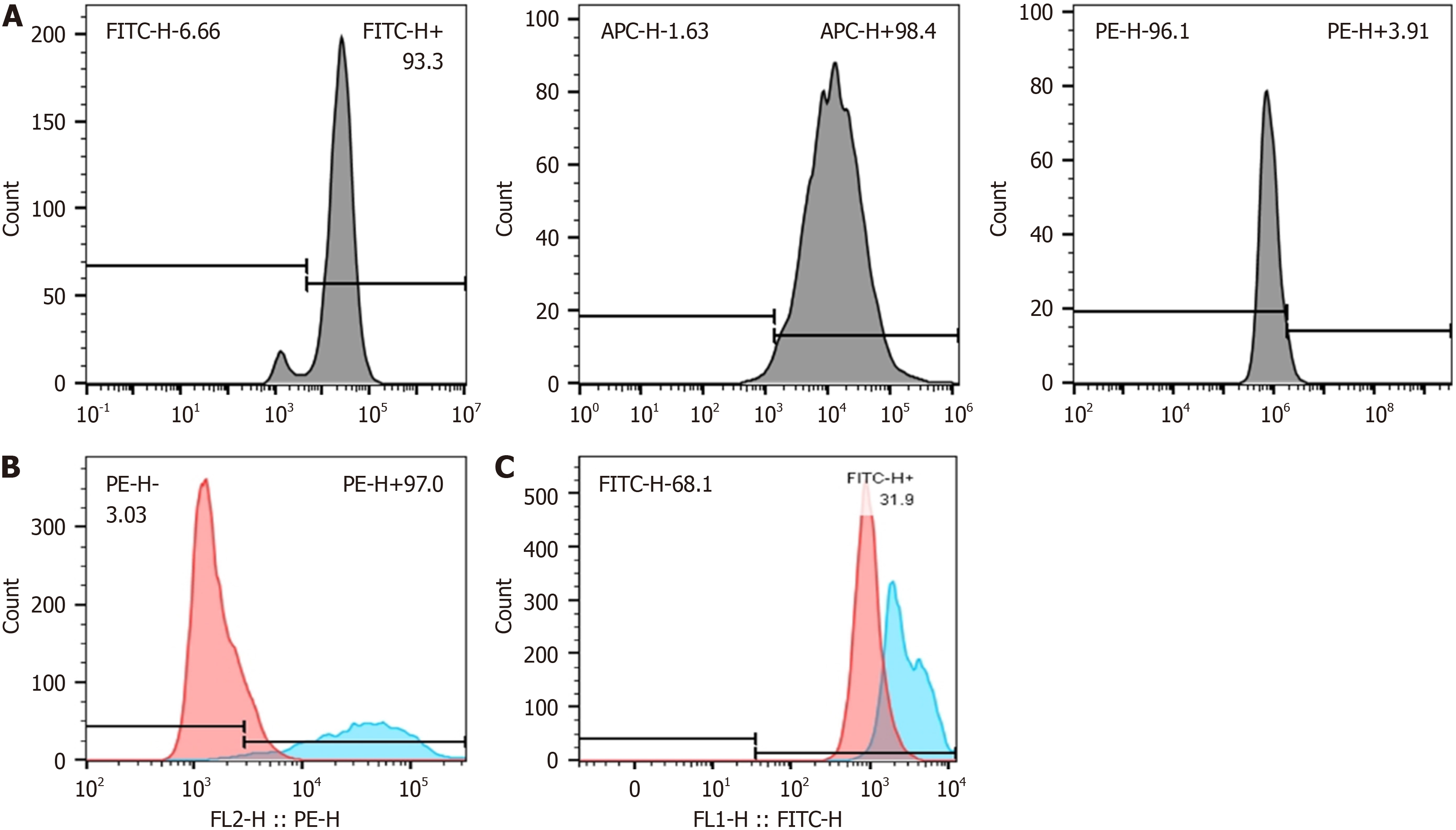
The surface markers of FDMSCs and M1/M2 type RAW264.7 were evaluated by flow cytometry. Macrophages were stimulated to the M1 phenotype using 100 ng/mL lipopolysaccharide (LPS) and 20 ng/mL interferon (IFN)-β, and to the M2 phenotype using 20 ng/mL IL-4 for intracellular cytokine detection. In summary, 5 × 105 cells were harvested and resuspended in phosphate buffered saline buffer, followed by a 15-minute incubation with particular antibodies in the dark. The cells were subsequently rinsed with phosphate buffered saline solution and evaluated by flow cytometry. Results were evaluated using FlowJo Software (Ashland, OR, United States).
Co-culture investigations were conducted utilizing 0.4 μm pore transwell chambers (Corning, CA, United States). RAW264.7 cells were cultured in the lower compartment and polarized to the M1 phenotype using 100 ng/mL LPS and 20 ng/mL IFN-β; 20 ng/mL IL-4 was employed to produce the M2 macrophage phenotype. The FDMSCs were cultured in the upper chamber. Co-culture for a duration of 24 hours prior to evaluation.
Mice were randomly assigned to control and FDMSCs-treated groups. A full-thickness skin splinting model in mice with a diameter of 1 cm was established. In the control group, 100 μL of normal saline was administered at the wound’s periphery. In the FDMSCs group, 100 μL of normal saline containing approximately 106 FDMSCs was administered. The injury was dressed with sterile gauze.
Total cellular RNA was extracted using a TRIzol reagent (Invitrogen, Carlsbad, CA, United States), according to the manufacturer’s instructions. Isolated total RNA was then subjected to reverse transcription using Oligo dT primer and PrimeScript® RTase (Takara, Dalian, China), according to the manufacturer’s instructions. Quantitative reverse tran
The excised tissues from wound sites were preserved in 4% paraformaldehyde for 24 hours and subsequently dehydrated using graded ethanol. Following the embedding of collected tissues in paraffin, the samples were sectioned into 4 μm thick slices. Hematoxylin and eosin (HE) staining was performed to examine the histological alterations in the wound healing process. The Masson’s trichrome staining assay was conducted to assess collagen maturity in accordance with the manufacturer’s guidelines. Microscopic images were obtained and analyzed using Image J software.
Statistical analysis was conducted on GraphPad Prism 8. Three or more separate experiments were conducted for each outcome, and the mean and standard deviation were computed. One-way ANOVA or Student’s t-test were employed to identify statistically significant differences. A P value of less than 0.05 was deemed statistically significant.
In accordance with the MSC identification criterion, we discovered three biomarkers: CD29, CD44, and CD45. Among these, CD29 and CD44 were highly expressed, while CD45 was little expressed. RAW264.7 was stimulated with conditioned media for 24 hours, and the expression levels of CD86 and CD206 were assessed using flow cytometry. In comparison to uninduced RAW264.7 cells, M1-type macrophages exhibited elevated expression of CD86, while M2-type macrophages demonstrated increased expression of CD206. The induction with 100 ng/mL LPS, 20 ng/mL IFN-γ, and
We investigated the influence of FDMSCs on macrophage polarization through a coculture method. Prior to co-culture, RAW264.7 was polarized to the M1 phenotype using LPS and IFN-γ, followed by co-culturing with FDMSCs. We identified the gene expression of iNOS and IL-6, which are hallmark pro-inflammatory components of M1 macrophages. In comparison to the control group, the expressions of iNOS and IL-6 in M1 macrophages co-cultured with FDMSC were decreased. Furthermore, we identified the expression of the anti-inflammatory cytokine IL-10. The expression of the IL-10 gene was elevated in the MSC group relative to the control group. Ultimately, we identified two cell markers, CD86 and CD206, by flow cytometry. The results indicated a decrease in CD86 expression, indicative of M1 polarization, and an increase in CD206 expression, indicative of M2 polarization. Consequently, we have demonstrated that FDMSC can diminish the polarization of M1 macrophages, decrease the expression levels of pro-inflammatory factors IL-6 and iNOS; concurrently, it can facilitate the transformation of M1 macrophages to the M2 phenotype, resulting in an elevated expression level of IL-10 (Figure 2).
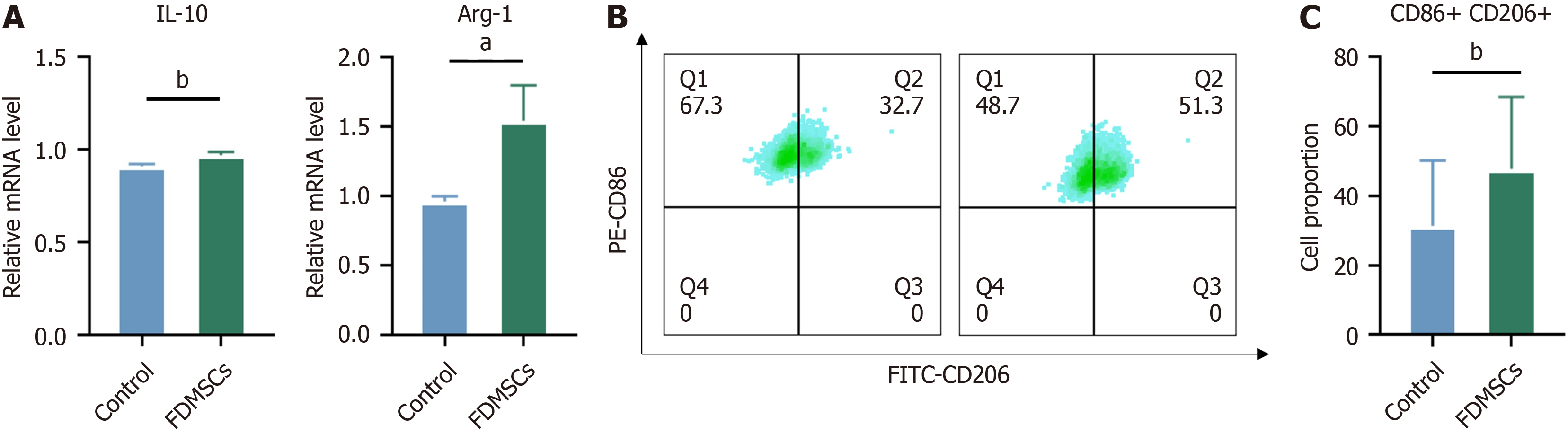
We employed the identical methodology to investigate the influence of FDMSCs on M2-polarized macrophages, which were induced to the M2 phenotype using IL-4 prior to co-culture. We subsequently analyze the distinctive cellularity that indicates the degree of polarization of M2 macrophages.
Following FDMSC injection, the gene expression of IL-10 in M2 macrophages was elevated in comparison to the control group. The flow cytometry data indicated a reduction in CD86 expression and an elevation in CD206 expression relative to the control group. Furthermore, we observed that the expression of the Arg-1 gene in M2 macrophages increased following the administration of FDMSC, in comparison to the control group. It may be inferred that FDMCS can enhance macrophage polarization towards the M2 phenotype and elevate the gene expression of key anti-inflammatory proteins IL-10 and Arg-1 (Figure 3).
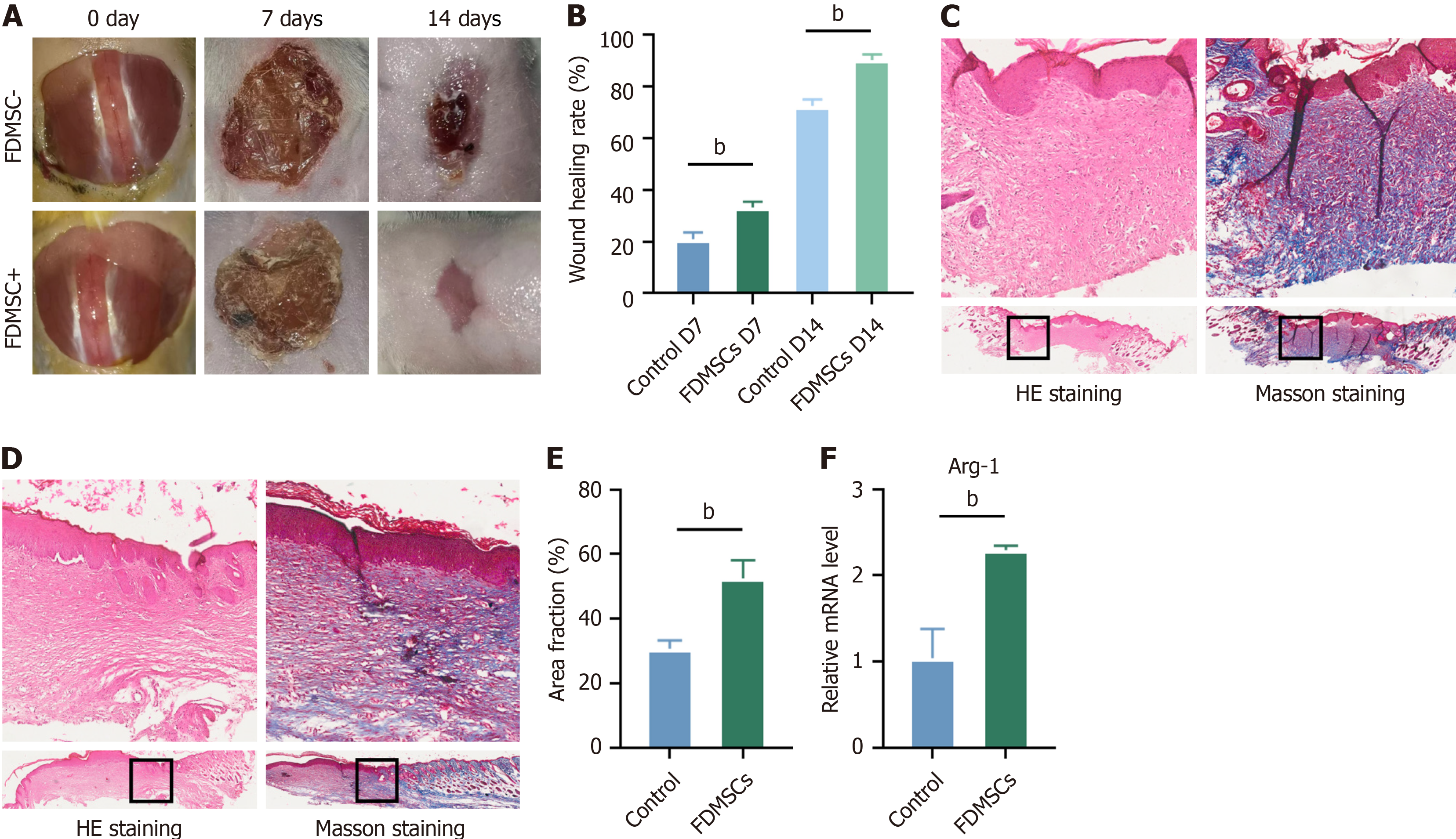
We developed a model of acute total trauma in mice and treated the trauma with FDMSCs. The healing process de
To further substantiate the role of FDMSCs in the wound healing process, we collected wound specimens on day 14 and assessed the expression of iNOS and Arg-1 via quantitative reverse transcriptase polymerase chain reaction. The results indicated that Arg-1 expression was elevated in the FDMSCs-intervention group relative to the control group (Figure 4F), whereas no statistically significant difference was noted for iNOS. This indicates a potentially elevated proportion of M2-type macrophages in the FDMSCs group during the wound healing process, hence facilitating wound repair.
Our study reveals the innovative potential of FDMSCs for wound healing, highlighting their capability to shift macrophage polarization from M1 to M2, thereby fostering an anti-inflammatory milieu conducive to tissue repair. This effect was validated through co-culture experiments showing decreased pro-inflammatory markers and increased anti-inflammatory markers. Further, FDMSC treatment markedly improved wound closure, collagen deposition, and vascular regeneration in a mouse model, supported by increased expression of the M2 marker Arg-1. These findings underscore the therapeutic promise of FDMSCs for chronic wound management and tissue regeneration, leveraging their low immunogenicity and high biocompatibility. This research advances the field of regenerative medicine, offering new strategies for treating non-healing wounds and enhancing tissue repair outcomes. Current therapies for wound healing primarily involve the use of growth factors, stem cell therapies, and skin grafts. However, these approaches often face limitations such as prolonged healing times, immune rejection, and risk of scarring. Our study introduces FDMSCs as a promising alternative. FDMSCs promote macrophage polarization toward the M2 phenotype, enhancing tissue re
Numerous studies have documented the beneficial effects of MSCs on various diseases, particularly through their influence on macrophage polarization, which impacts the progression of certain inflammatory disorders, myocardial infarction, spinal cord injury, and wound healing[16,17]. MSCs operate by multiple mechanisms, including the secretion of diverse cytokines and exosomes, facilitation of angiogenesis, tissue healing, immunological modulation, and cell recruitment. Our prior research has established that FDMSCs contribute positively to angiogenesis, wound healing, and keloid management. We concentrated on the impact of FDMSC on macrophage polarization. During tissue regeneration, MSCs can facilitate the change of macrophages from the inflammatory M1 phenotype to the anti-inflammatory M2 phenotype[18]. Bone marrow MSCs and human placenta MSCs have been shown to suppress M1 marker expression and facilitate polarization towards the M2 phenotype, characterized by decreased levels of tumor necrosis factor-alpha and iNOS, alongside increased levels of IL-10, CD206, and Arg1.
Macrophages engage in tissue repair via phenotypic conversion, facilitate the phagocytosis of pathogenic microbes and cellular debris during the inflammatory phase, release various essential cytokines during this period, and aid in cell recruitment[19]. During the tissue repair phase, it demonstrates an anti-inflammatory activity, releases various cytokines, and activates fibroblasts and endothelial cells. Research has established that MSCs are pivotal in cardiac healing and the reduction of fibrosis following acute kidney damage via macrophage polarization[20].
FDMSC, a type of MSC isolated from fetal mouse dermis, has low immunogenicity and excellent biocompatibility. Its dermal origin suggests a superior capacity for in situ skin wound repair and it is considered a crucial cell in scarless healing. The interaction between FDMSCs and macrophages, essential immune cells in tissue regeneration, remains unexplored. This study demonstrates that coculturing FDMSC with macrophages facilitates the polarization of ma
To further validate the characteristics of FDMSCs, we conducted a morphological examination and confirmed their typical fibroblast-like spindle-shaped appearance under a microscope, which is consistent with MSC properties. Ad
While our study demonstrates the promising therapeutic potential of FDMSCs in promoting wound healing and ma
To address these limitations, future studies should employ clinically relevant models, such as 3D bioprinted human skin constructs or diabetic wound models, to better simulate human wound healing. We also plan to investigate the po
Our research demonstrates that FDMSC can induce a phenotypic transition of RAW264.7 from M1 to M2, hence inhibiting inflammation during the initial phase of wound healing and enhancing tissue regeneration in the subsequent phase. This establishes a basis for more research on FDMSCs in relation to wound healing via the immunomodulation of macro
| 1. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2700] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 2. | Zheng G, Ge M, Qiu G, Shu Q, Xu J. Mesenchymal Stromal Cells Affect Disease Outcomes via Macrophage Polarization. Stem Cells Int. 2015;2015:989473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 197] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 4. | Yu Y, Yue Z, Xu M, Zhang M, Shen X, Ma Z, Li J, Xie X. Macrophages play a key role in tissue repair and regeneration. PeerJ. 2022;10:e14053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 5. | Chen C, Liu T, Tang Y, Luo G, Liang G, He W. Epigenetic regulation of macrophage polarization in wound healing. Burns Trauma. 2023;11:tkac057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7233] [Cited by in RCA: 6864] [Article Influence: 403.8] [Reference Citation Analysis (0)] |
| 7. | DiPietro LA, Wilgus TA, Koh TJ. Macrophages in Healing Wounds: Paradoxes and Paradigms. Int J Mol Sci. 2021;22:950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Rauchenwald T, Handle F, Connolly CE, Degen A, Seifarth C, Hermann M, Tripp CH, Wilflingseder D, Lobenwein S, Savic D, Pölzl L, Morandi EM, Wolfram D, Skvortsova II, Stoitzner P, Haybaeck J, Konschake M, Pierer G, Ploner C. Preadipocytes in human granulation tissue: role in wound healing and response to macrophage polarization. Inflamm Regen. 2023;43:53. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 2079] [Article Influence: 231.0] [Reference Citation Analysis (0)] |
| 10. | Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 945] [Cited by in RCA: 861] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 11. | Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2865] [Cited by in RCA: 2959] [Article Influence: 328.8] [Reference Citation Analysis (0)] |
| 12. | Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in Chronic Wounds. Int J Mol Sci. 2016;17:2085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 672] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 13. | Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW, Knawy BA. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep. 2013;9:620-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 14. | Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3128] [Cited by in RCA: 3363] [Article Influence: 305.7] [Reference Citation Analysis (0)] |
| 15. | Luque-Campos N, Bustamante-Barrientos FA, Pradenas C, García C, Araya MJ, Bohaud C, Contreras-López R, Elizondo-Vega R, Djouad F, Luz-Crawford P, Vega-Letter AM. The Macrophage Response Is Driven by Mesenchymal Stem Cell-Mediated Metabolic Reprogramming. Front Immunol. 2021;12:624746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Wang M, Zhang G, Wang Y, Liu T, Zhang Y, An Y, Li Y. Crosstalk of mesenchymal stem cells and macrophages promotes cardiac muscle repair. Int J Biochem Cell Biol. 2015;58:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Wise AF, Williams TM, Kiewiet MB, Payne NL, Siatskas C, Samuel CS, Ricardo SD. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306:F1222-F1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 460] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 19. | Ni R, Jiang L, Zhang C, Liu M, Luo Y, Hu Z, Mou X, Zhu Y. Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation. Int J Mol Sci. 2023;24:8358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, Huang X, Yan H, He J, Cai Z. Metabolic reprogramming in macrophage responses. Biomark Res. 2021;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 339] [Article Influence: 84.8] [Reference Citation Analysis (0)] |