INTRODUCTION
Numerous pathological settings such as acute coronary syndrome/acute myocardial infarction, stroke, hemorrhagic shock, and solid organ transplant are particularly prone to cause ischemia-reperfusion injury[1]. Local inflammatory responses, which can spread to more advanced systemic inflammation, are a major cause of damage due to ischemia-reperfusion syndrome[2]. In this connection, a management of ischemia-reperfusion remains to be a crucial element of individualizing strategy of improving clinical settings and prognosis.
Extracellular vesicles (EVs), which are enriched in numerous active molecules, such as microRNAs (miRNAs), regulatory peptides, growth factors, have been identified as key mediators of myocardial structure and functions paying a pivotal role in regulation of several cardiac protective mechanisms: Inhibition of cell death, attenuation of oxidative stress and synthesis of inflammatory cytokines, and enhancement of mitochondrial function.
REPARATIVE POTENCY OF ADIPOSE-DERIVED MESENCHYMAL STEM CELL-DERIVED EVS
Cell-free therapy, which regulates cell-to-cell communication and multiple physiological processes through modulating the synthesis and secretion of adipose-derived mesenchymal stem cell (ADMSC)-derived secretom such as EVs, is attracting the most attention in the management of cardiac ischemia/reperfusion[3,4]. Indeed, ADMSC-derived exosomes represent a promising cell-free tool for therapeutic applications, not only due to their high physical stability and functional properties during GMP manufacturing, but also due to their higher safety approach to achieve a sufficient reparative effect[5].
ADMSC-derived EVs, which are loaded with biologically active components, exert a sufficient paracrine modality on several target cells including resident progenitors, antigen-presenting cells, fibroblasts, cardiac myocytes and subsequently ensure immunomodulatory, anti-inflammatory, angiopoietic, reparative, protective and metabolic activities[6,7]. It has been established that EVs derived from ADMSC contain a wide range of biological active molecules. They include mRNAs, miRNAs, long non coding RNAs, double-stranded and single-stranded DNAs along with dsDNA-binding histone proteins and mitochondrial DNA, growth factors, lipids, cytokines, chemokines, immunomodulatory factors, which may regulate differentiation, proliferation, and migration, activate autophagy and/or inhibited apoptosis, ferroptosis, necrosis and oxidative stress in injured cardiac myocytes[8]. In addition, EV-encapsulated cell organelles such as mitochondria play a crucial role in mediating the structure and energy metabolism of target cells[9]. Moreover, ADMSC-derived EVs can transfer the stem cell-derived mitochondria components to target cells in a dose-dependent manner[10]. It has been found that ADMSC-derived EVs demonstrated their ability to markedly increase levels of mitochondrial DNA, mitochondrial membrane potential, OXPHOS activity and ATP generation, probably by complementing damaged mitochondria[10]. Perhaps ADMSC-derived EVs could down-regulate the synthesis of pro-inflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor-alpha and inducible NO-synthase secretion, and mediate an increase in the production of anti-inflammatory cytokines, including IL-10 and arginase-1, through unrecognised mechanisms. Overall, the exact inner molecular mechanisms of the reparative potential of ADMSC-derived EVs have not yet been fully understood. In this context, it has been suggested that the beneficial effects of ADMSC-derived EVs may be enhanced by the influence of γ-aminobutyric acid (GABA)[11].
GABA’S ROLE IN ENHANCING EV THERAPY
Indeed, GABA pre-treatment of ADMSC improved systemic insulin sensitivity and insulin-dependent glucose uptake, reduced macrophage accumulation in subcutaneous inguinal adipose tissue and suppressed systemic and adipose tissue inflammation in obese animals[12,13]. Moreover, GABA is likely to be a modulator of energy homeostasis in ischemia/reperfusion-damaged myocardium acting in connection with Kbhb-proteins, which are modifiers of mitochondrial energy homeostasis through β-hydroxybutyrylation - a lysine-based post-translational modification[14]. Although the underlying molecular mechanisms of the EV secretome derived from ADMSCs previously treated with GABA on cardiac myocytes damaged by ischemia/reperfusion remain elusive, it has been suggested that the cardioprotective effects of ADMSCs may be regulated through non-coding miRNAs such as miR-21-5p.
MECHANISM OF MIR-21-5P
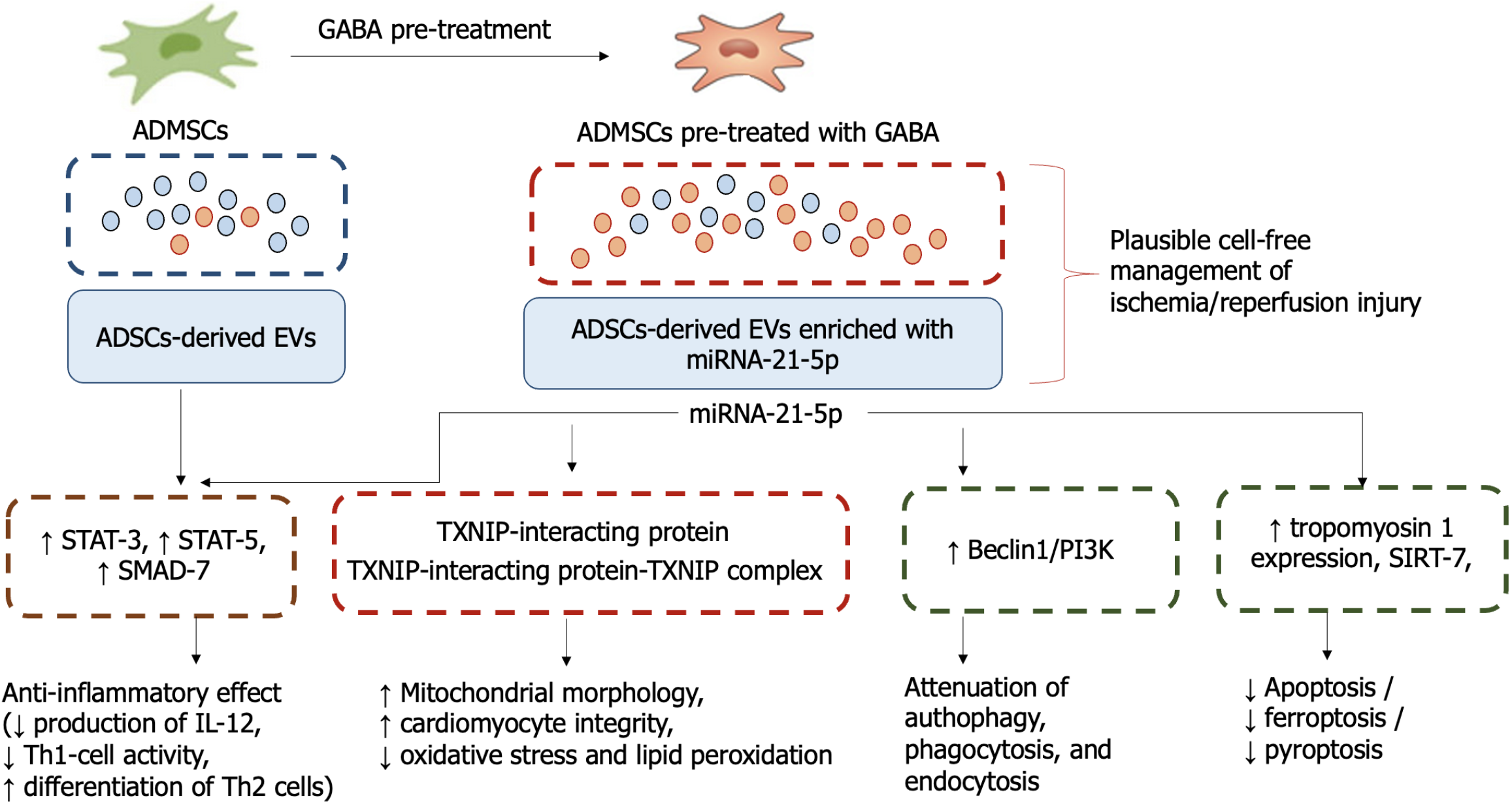
In previous studies, overexpression of miR-21-5p was found to regulate mitochondrial lipid content, lipid peroxidation and mitochondrial respiration, and to attenuate hypoxia-induced inflammation and apoptosis/ferroptosis/pyroptosis in target cells through the overexpression of tropomyosin 1, resulting in cell protection[15-17]. In addition, miR-21-5p acts through SMAD-7, activators of signal transducer and activator of transcription 5 and signal transducer and activator of transcription 3/nuclear factor-kappaB pathway to reduce the production of IL-12, thereby inhibiting T helper type 1 (Th1) cells and promoting the differentiation of Th2, Th17 and regulatory T cells, which are involved in suppressing the inflammatory response (Figure 1). It has been found that miR-21-5p downregulated the ubiquitination level of sirtuin-7 and an expression of protein Beclin1/phosphatidylinositol 3-phosphate complex known as a regulator of autophagy, membrane trafficking, phagocytosis, and endocytosis[16,17].
Figure 1 The mechanisms of action of γ-aminobutyric acid and miR-21-5p.
GABA: γ-aminobutyric acid; ADMSCs: Adipose-derived mesenchymal stem cell; ADSCs: Adipose-derived stem cells; EVs: Extracellular vesicles; STAT: Signal transducer and activator of transcription; TXNIP: Thioredoxin interacting protein; PI3K: Phosphatidylinositol 3-phosphate; SIRT: Sirtuin; IL: Interleukin; Th1: T helper type 1.
GABA ENHANCEMENT OF MIRNA-21-5P IN ADMSC-DERIVED EV
The study by Wang et al[17], which has been published in this issue of the World Journal of Stem Cells, reported that the protective effects of EVs secreted by subcutaneous inguinal adipose tissue after GABA induction are mainly due to miRNA-21-5p, which suppresses mitochondrial oxidative stress and exerts anti-inflammatory activity. In addition, the researchers found that thioredoxin interacting protein (TXNIP), which can be used as a target for miRNA-21-5p, upregulates the formation of the TXNIP-interacting protein-TXNIP complex and subsequently enhances the antioxidant function of TXNIP. It also restores cardiomyocyte integrity and improves mitochondrial morphology.
These findings suggest a deeper understanding of the mechanisms involved in the suppression of mitochondrial oxidative stress and the improvement of mitochondrial structure, as well as opening up new perspectives in the treatment of both ischaemic and early reperfusion myocardial injury. This is particularly important for the development of a new strategy to reduce sensitivity to ischaemia and reperfusion, which can be achieved by modulating the expression of structural components of oxidative phosphorylation in mitochondria. These, as this study by Wang et al[17] has shown, are under the control of miRNA-21-5p, which plays a pivotal role in the pathogenesis of ischaemia/reperfusion through binding with TXNIP-interacting protein. Thus, pre-treatment with GABA shows promise in terms of establishing the efficacy potential of miRNA-21-5p incorporated into ADMSC-derived EV and seems to be a certain breakthrough in individualizing molecular targets for patients with ischemia/reperfusion injury, which represents an attractive area of future work with therapeutic implications. Future studies are require to clearly elucidate the therapeutic potency of the approach in clinical settings.
CONCLUSION
The adipose-derived stem cells-derived EVs treated with GABA effectively ameliorate mitochondrial oxidative stress and mitigate ischemia/reperfusion damage of cardiomyocyte. Cardiac protective effects of ADMSC-derived EVs may be attributed through their ability to deliver miR-21-5p acting via TXNIP. Administration of stem cell-based cell-free therapy based on ADMSC-derived EVs is considered a promising approach to individualized management of ischemia-induced cardiomyopathy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country of origin: Austria
Peer-review report’s classification
Scientific Quality: Grade A, Grade B, Grade B, Grade C, Grade C, Grade D
Novelty: Grade A, Grade A, Grade B, Grade B, Grade B, Grade C
Creativity or Innovation: Grade A, Grade A, Grade A, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade B, Grade B, Grade B, Grade B, Grade C
P-Reviewer: Wang KY; Wang R; Wang ZS S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD