INTRODUCTION
In this article, we comment on an article written by Li et al[1] published in the recent issue of World Journal of Stem Cells. High hopes have been given to mesenchymal stem cells (MSCs) as an optimal cell source for stem cell therapies because of their prominent availability, validated regeneration ability and safety[2]. However, after injection, MSCs face hypoxic and inflammatory environments within targeted tissues, which negatively affect MSC vitality and diminish their therapeutic efficacy[3,4]. This negative impact could be attributed to the impairment of mitochondrial function, immunomodulatory activity, and paracrine communication[5]. Moreover, the in vitro treatment of MSCs by physical and biochemical factors has been proven to enhance survival[6], guide differentiation[7] and promote regeneration[8]. Researchers have therefore focused on finding the optimal preconditioning procedure to maximize MSC therapeutic ability. Previous studies mainly focused on bone marrow stem cells and adipose-derived stem cells, while umbilical cord MSC (UC-MSC) were also a potential cell source. It has been reported that pretreatment by antioxidants[9], cytokines[10] and a hypoxia environment[11] could enhance UC-MSC survival, proliferation, oxidative resistance, tumor suppression and angiogenic ability. However, our knowledge of UC-MSC preconditioning is still insufficient to determine the optimal procedure.
PRECONDITIONING PROCEDURE OF UC-MSCS
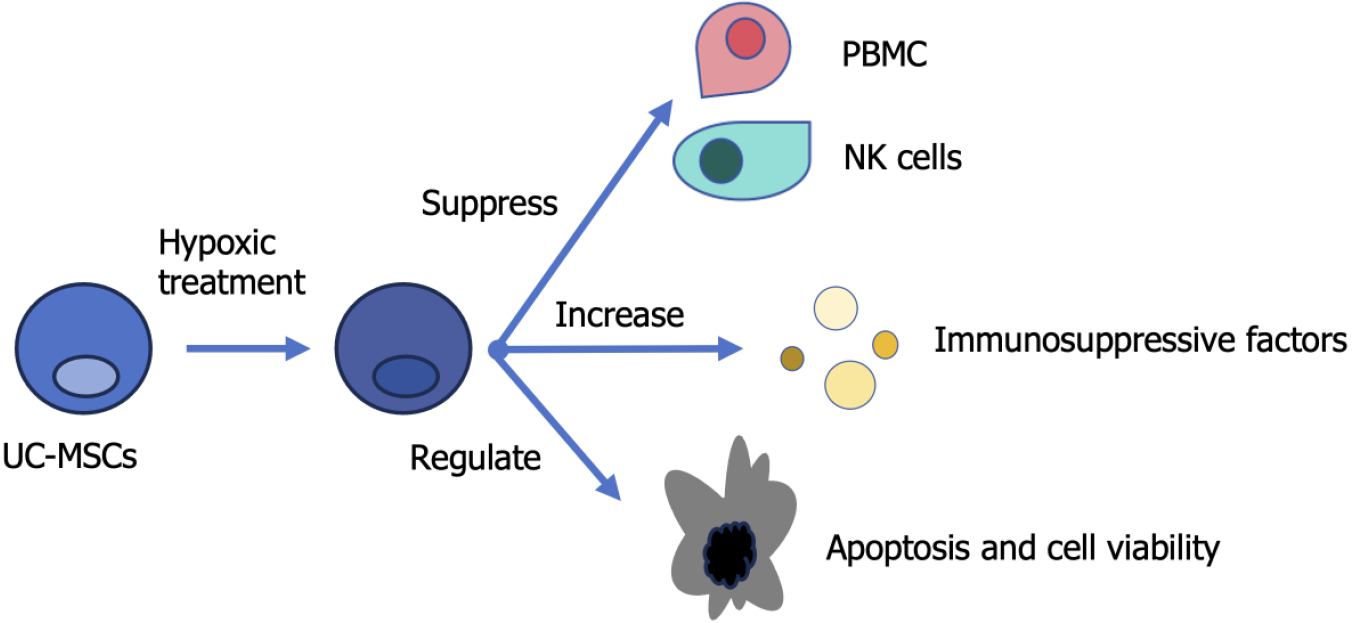
In their recent study entitled “Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells” published in this journal, Li et al[1] pretreated UC-MSCs with a novel combination of hypoxia (2% O2) and inflammatory factors for 24 hours (Figure 1). The authors discovered that the immunosuppressive ability of UC-MSCs was enhanced after pretreatment. They cocultured UC-MSCs with natural killer cells and peripheral blood mononuclear cells (PBMCs) to test their immunosuppressive ability. The results showed that after treatment, the UC-MSCs displayed increased inhibition of PBMC proliferation and cytotoxic activity of natural killer cells. An increased level of paracrine immunosuppressive soluble factors, such as inducible indoleamine 2,3-dioxygenase, prostaglandin E2, transforming growth factor-β1, tumor necrosis factor-stimulated gene-6 and interleukin-10, was also found, suggesting a higher immunosuppressive ability after pretreatment. In addition, UC-MSC proliferation rate and viability did not change before or after pretreatment. However, the study found enhanced apoptotic and senescent induction by pretreatment, which could be attributed to the increased reactive oxygen species level within UC-MSCs, while mitochondrial function was not damaged, as shown by mitochondrial membrane potential and antioxidant level. In addition, an CD142 overexpression was induced after preconditioning, raising potential safety concerns about unwanted clotting.
Figure 1 Graphic abstract representing the main points of the commented article.
NK: Natural killer; PBMC: Peripheral blood mononuclear cell; UC-MSC: Umbilical cord mesenchymal stem cell.
This work provided us with important insights into the preconditioning procedure of UC-MSCs and left us with one question: What is the optimal combination of hypoxia and inflammatory stimulation for generating the best therapeutic capacity of UC-MSCs, or all kinds of MSCs, and minimizing the side effects such as oxidative injuries and other safety issues? UC-MSCs have been proven to possess therapeutic potential in a wide range of diseases including autoimmune thyroiditis[12], myeloid leukemia[10], stroke[13], bronchopulmonary dysplasia[14], bone tumors[15] and several degenerative diseases[16]. Some of them were undergoing clinical trials[17]. A consensus view has been reached that hypoxic and inflammatory pretreatment might benefit cell function after injection. Hao et al[14] suggested a 48 hour pretreatment with deferoxamine and Aslam et al[18] proposed 24 hour treatment with isorhamnetin. Zhang et al[19] suggested that culturing UC-MSCs in 3% oxygen for 72 hours allowed for better proliferation and differentiation ability than 1% or 5% oxygen, and Kang et al[20] suggested there was even heterogeneity in the response to hypoxic treatment between allogeneic MSC donors. Exposing UC-MSCs to an excessively hypoxic environment may over-activate the reactive oxygen species system and harm cell viability, while insufficient hypoxic levels could not generate such an effect. Furthermore, different MSCs and differentiation directions might require different preconditioning procedures; the number of studies involving the variation between cells and directions was insufficient. In this case, we still could not determine the optimal treatment time, oxygen level and biochemical factors. Additionally, this article prompted meaningful safety concerns because preconditioning increased CD142, also called tissue factor (TF). TF plays an important role in the natural clotting process by promoting coagulation. Previous studies have suggested that UC-MSCs expressed higher CD142 levels than other MSCs and long-lasting clotting was observed in the same study[21]. The mechanisms underlying whether this phenomenon would exist in vivo and would cause coagulation disorder still lacked clarity.
CONCLUSION
Generally, the study by Li et al[1] was important not only because they made great progress in discovering the optimal preconditioning procedure for UC-MSCs but also because they raised an essential safety concern for further discussion and investigation. Furthermore, detailed horizontal comparisons of the optimal time, oxygen level and cytokine supplementation is necessary to reach a unanimous conclusion. Considering the almost infinite combinations of physical environment, biochemical factors (including hypoxia and inflammation stimulation) and the diseases we are dedicated to curing, standardized protocols for further research is urgently needed to make the results more comparable and facilitate researchers’ work. Apart from hypoxic pretreatment of MSCs, studies focused on reversing the hypoxic microenvironment within the transplanted area by encapsulating MSCs in certain materials. Oxygen releasing materials, such as sodium percarbonate, and biomechanical scaffolds were used to create a long-lasting oxygen generating system to support cell survival, viability, and regeneration[22,23]. While the applications of MSCs are far from abundant[24], researchers have noticed that paracrine function was also donor-dependent, and therefore, it was necessary to pay more attention to the donor side of MSC transplantation[25]. In conclusion, although MSC therapy remains to be promising, it might face safety challenges. More work, including standardized procedures, should be done to offer comparable results.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade A, Grade C
P-Reviewer: Phinney DG, United States; Rotondo JC, Italy S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhang XD