Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.575
Revised: March 18, 2024
Accepted: April 9, 2024
Published online: May 26, 2024
Processing time: 127 Days and 7.6 Hours
Atherosclerosis (AS), a chronic inflammatory disease of blood vessels, is a major contributor to cardiovascular disease. Dental pulp stem cells (DPSCs) are capable of exerting immunomodulatory and anti-inflammatory effects by secreting cytokines and exosomes and are widely used to treat autoimmune and inflammation-related diseases. Hepatocyte growth factor (HGF) is a pleiotropic cytokine that plays a key role in many inflammatory and autoimmune diseases.
To modify DPSCs with HGF (DPSC-HGF) and evaluate the therapeutic effect of DPSC-HGF on AS using an apolipoprotein E-knockout (ApoE-/-) mouse model and an in vitro cellular model.
ApoE-/- mice were fed with a high-fat diet (HFD) for 12 wk and injected with DPSC-HGF or Ad-Null modified DPSCs (DPSC-Null) through tail vein at weeks 4, 7, and 11, respectively, and the therapeutic efficacy and mechanisms were analyzed by histopathology, flow cytometry, lipid and glucose measurements, real-time reverse transcription polymerase chain reaction (RT-PCR), and enzyme-linked immunosorbent assay at the different time points of the experiment. An in vitro inflammatory cell model was established by using RAW264.7 cells and human aortic endothelial cells (HAOECs), and indirect co-cultured with supernatant of DPSC-Null (DPSC-Null-CM) or DPSC-HGF-CM, and the effect and mechanisms were analyzed by flow cytometry, RT-PCR and western blot. Nuclear factor-κB (NF-κB) activators and inhibitors were also used to validate the related signaling pathways.
DPSC-Null and DPSC-HGF treatments decreased the area of atherosclerotic plaques and reduced the expression of inflammatory factors, and the percentage of macrophages in the aorta, and DPSC-HGF treatment had more pronounced effects. DPSCs treatment had no effect on serum lipoprotein levels. The FACS results showed that DPSCs treatment reduced the percentages of monocytes, neutrophils, and M1 macrophages in the peripheral blood and spleen. DPSC-Null-CM and DPSC-HGF-CM reduced adhesion molecule expression in tumor necrosis factor-α stimulated HAOECs and regulated M1 polarization and inflammatory factor expression in lipopolysaccharide-induced RAW264.7 cells by inhibiting the NF-κB signaling pathway.
This study suggested that DPSC-HGF could more effectively ameliorate AS in ApoE-/- mice on a HFD, and could be of greater value in stem cell-based treatments for AS.
Core Tip: In this study, we found that dental pulp stem cells (DPSCs) treatment reduced atherosclerotic plaque formation in apolipoprotein E-knockout (ApoE-/-) mice fed a high-fat diet and that Ad-hepatocyte growth factor (HGF) modified DPSCs (DPSC-HGF) treatment was more effective than Ad-Null modified DPSCs (DPSC-Null) treatment, which depended on the reduced expression of aortic endothelial cell adhesion molecule and inflammatory macrophages. In addition, DPSC-HGF had a greater inhibitory effect on the nuclear factor-κB signaling pathway in RAW264.7 cells and HAOECs cells under inflammatory stimulation than DPSC-Null. Taken together, these data suggest for the first time that DPSCs treatment can ameliorate AS in ApoE-/- mice and that DPSC-HGF has more impressive therapeutic potential than DPSC-Null and might be a new therapy for AS patients in the future.
- Citation: Duan H, Tao N, Lv L, Yan KX, You YG, Mao Z, Wang CY, Li X, Jin JY, Wu CT, Wang H. Hepatocyte growth factor enhances the ability of dental pulp stem cells to ameliorate atherosclerosis in apolipoprotein E-knockout mice. World J Stem Cells 2024; 16(5): 575-590
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/575.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.575
Cardiovascular disease due to atherosclerosis (AS) is a major health problem in modern life[1]. A large body of clinical data suggests that patients who use statins have a significant residual cardiovascular risk of not achieving the desired therapeutic level[2]. Therefore, new therapeutic strategies are needed.
In recent years, numerous experimental studies have shown that inflammation plays a crucial role in the development of AS[3,4], and endothelial cells and monocyte-macrophages may act as major intervention target cells for treating AS[5-7]. Mesenchymal stem cells (MSCs), which can be isolated from different tissues, including bone marrow, umbilical cord, placenta, adipose tissue, and dental pulp, have self-renewal and multiple differentiation potential[8]. MSCs have received extensive attention because of their ability to repair damaged tissues, modulate immune responses, and promote angiogenesis and anti-inflammatory reactions[9,10]. Human dental pulp stem cells (DPSCs) are MSCs that have the advantages of high anti-inflammatory capacity, low immunogenicity, easy isolation and in vitro expansion, and have been shown in previous studies to have powerful anti-inflammatory and immunomodulatory functions[11].
Hepatocyte growth factor (HGF) is a polypeptide growth factor belonging to the fibrinogen family that is secreted mainly by mesenchymal cells[12]. HGF is involved in a variety of pathophysiological processes, including cell migration, maturation, cytokine production, anti-apoptosis, and T-cell effector functions that regulate acute and chronic inflammation in various disease models[13-15]. However, recombinant HGF protein is highly unstable in serum and has a short half-life and weak targeting effect, so continuous administration of recombinant HGF protein is required to obtain an effective treatment effect.
In this study, we modified DPSCs with an adenovirus carrying the HGF gene (DPSC-HGF), which enabled DPSCs to highly express HGF to provide more powerful anti-inflammatory functions and play more long-lasting and accurate therapeutic roles at the same time. Using in vivo experiments, we evaluated the therapeutic effect of DPSC-HGF treatment on atherosclerotic plaque development in apolipoprotein E-knockout (ApoE-/-) mice fed a high-fat diet (HFD). Furthermore, we examined the effects and underlying mechanism of DPSC-HGF supernatant (DPSC-HGF-CM) on human aortic endothelial cells (HAOECs) and mouse mononuclear macrophages (RAW264.7) in vitro by establishing an inflammatory cellular model.
Clinical-grade DPSCs were obtained from Beijing SH Biotechnology (http://www.bjshbio.com/) and were isolated and cultured as previously described[16]. The DPSCs were cultured in α-MEM (Thermo Fisher Scientific, Gaithersburg, MD, United States) supplemented with 10% fetal bovine serum (ExCell Bio, Uruguay), and cells at passages 4-5 were used in the experiments to achieve stable and reliable results. HAOECs and RAW264.7 cells were purchased from ATCC and cultured in DMEM (Thermo Fisher Scientific, Gaithersburg, MD, United States) supplemented with 10% fetal bovine serum.
Ad-HGF, a replication-defective adenovirus expressing human HGF, and Ad-Null, a replication-defective adenovirus not carrying exogenous genes, were used in this study. Both vectors were constructed as previously described[17]. 8 × 106 per petri dish of DPSCs were infected at a multiplicity of infection of 150 with Ad-HGF or Ad-Null. Four hours after infection, the culture media were discarded, the cells were washed twice with phosphate-buffered saline (PBS), and fresh culture media were added. Forty-eight hours postinfection, the cell culture medium and cells were collected. The cell culture medium was centrifuged at 1500 rpm for 10 min at 4 °C, and the supernatant was collected as conditioned media [e.g., DPSC-HGF-CM and Ad-Null modified DPSCs (DPSC-Null-CM), respectively] and used for indirect co-culture experiments. The collected cells were used for AS treatment.
Eight-week-old ApoE-/- male mice on the C57BL/6J background were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The HFD containing 20% fat and 1.254% cholesterol (CHO) was purchased from HFK Bioscience Co., Ltd. (Beijing, China). Thirty-six ApoE-/- mice were randomly divided into 4 groups (9 mice per group): The normal formula diet group (NFD), which was fed normal chow for 12 wk; the HFD group, which was fed high-fat chow for 12 wk; the DPSC-Null-treated group (DPSC-Null) and DPSC-HGF-treated group (DPSC-HGF), which were fed high-fat chow for 12 wk; and, on the first day of weeks 4, 7 and 11, which were administered DPSC-Null or DPSC-HGF (1 × 106 cells/150 μL), respectively, by intravenous injection. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Beijing Institute of Radiation Medicine (IACUC-DWZX-2021-714).
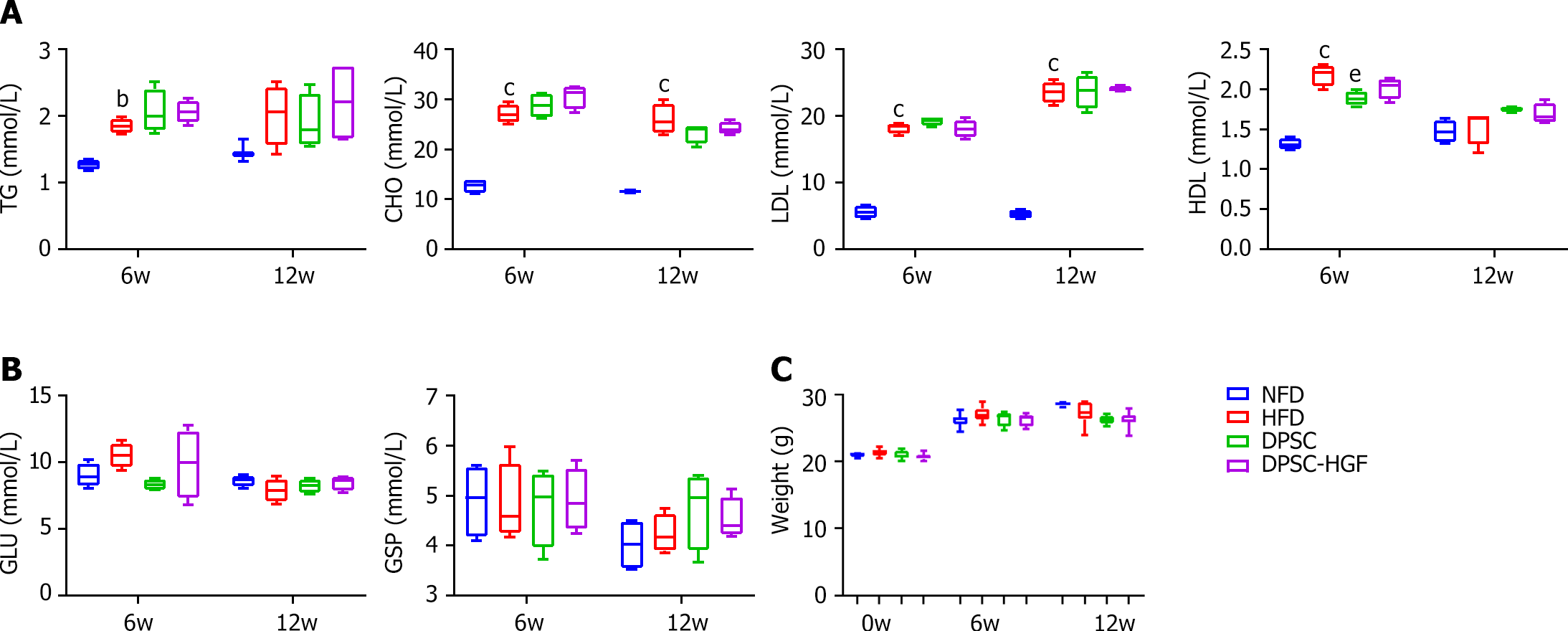
Peripheral blood samples were collected from the inner canthus vein at weeks 6 and 12, and serum was sampled to measure total CHO (S03042), triglyceride (TG, S03027), high-density lipoprotein (HDL, S03025), low-density lipoprotein (LDL, S03029) [kits were purchased from Rayto Life Sciences (Shenzhen, China)], glucose (GLU) concentration (S03039) (kit was purchased from Rayto Life Science Co.) and glycosylated serum protein (GSP, C024) [kit was purchased from Changchun Huili Biotechnology Co Ltd (Jilin, China)]. The operation was processed according to the manufacturer’s instructions and then analyzed using a fully automated biochemical analyzer (Chemray 800, Rayto Life Sciences Co. Shenzhen, China).
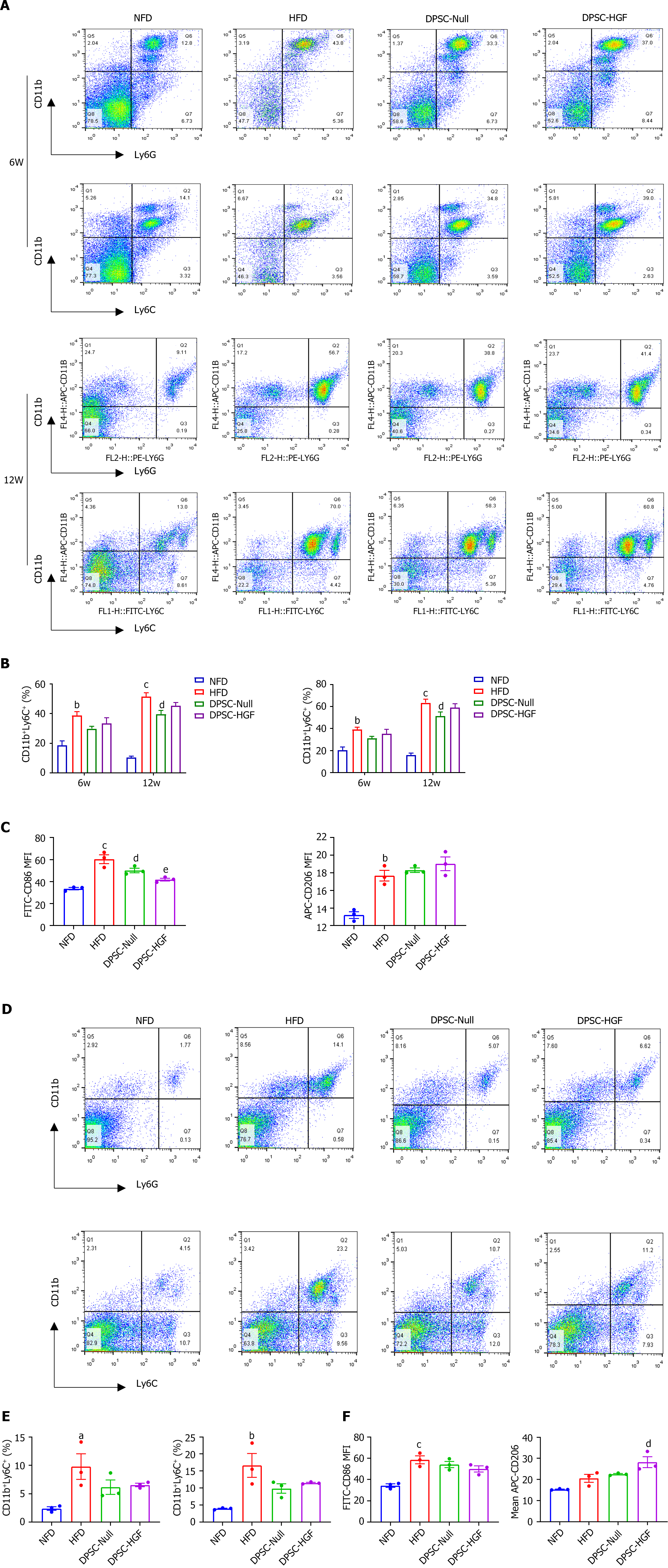
Peripheral blood was collected at weeks 6 and 12, the spleen was sampled, and splenocytes were isolated at week 12. Peripheral blood cells and splenocytes were lysed with RBC lysis buffer (BD Pharmingen, San Diego, CA, United States) and then 1 × 106 cells per tube were labeled with FITC-conjugated rat anti-mouse CD86 antibody (350862, TONBO biosciences, San Diego, CA, United States), APC-conjugated rat anti-mouse CD206 antibody (2140730, eBioscience, CA, United States), PE-conjugated rat anti-mouse F4/80 (565410), FITC-conjugated rat anti-mouse Ly6C (553104), PE-conjugated rat anti-mouse Ly6G (551461), and APC-conjugated rat anti-mouse CD11b (553312, BD Pharmingen, San Diego, CA, United States). The immune phenotypes were analyzed by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, United States).
We assessed the effects of DPSC-Null and DPSC-HGF on the polarization of RAW264.7 cells. For M1 macrophages in vitro, 150 ng/mL lipopolysaccharide (LPS) (Sigma) was used to induce M1 polarization of RAW264.7 cells, and then DPSC-Null-CM or DPSC-HGF-CM was incubated with RAW264.7 cells for 24 h. Then, the cells were stained with a FITC-conjugated rat anti-mouse CD86 antibody (350862; TONBO biosciences, San Diego, CA, United States), and the mean fluorescence intensity (MFI) of CD86 was analyzed by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, United States).
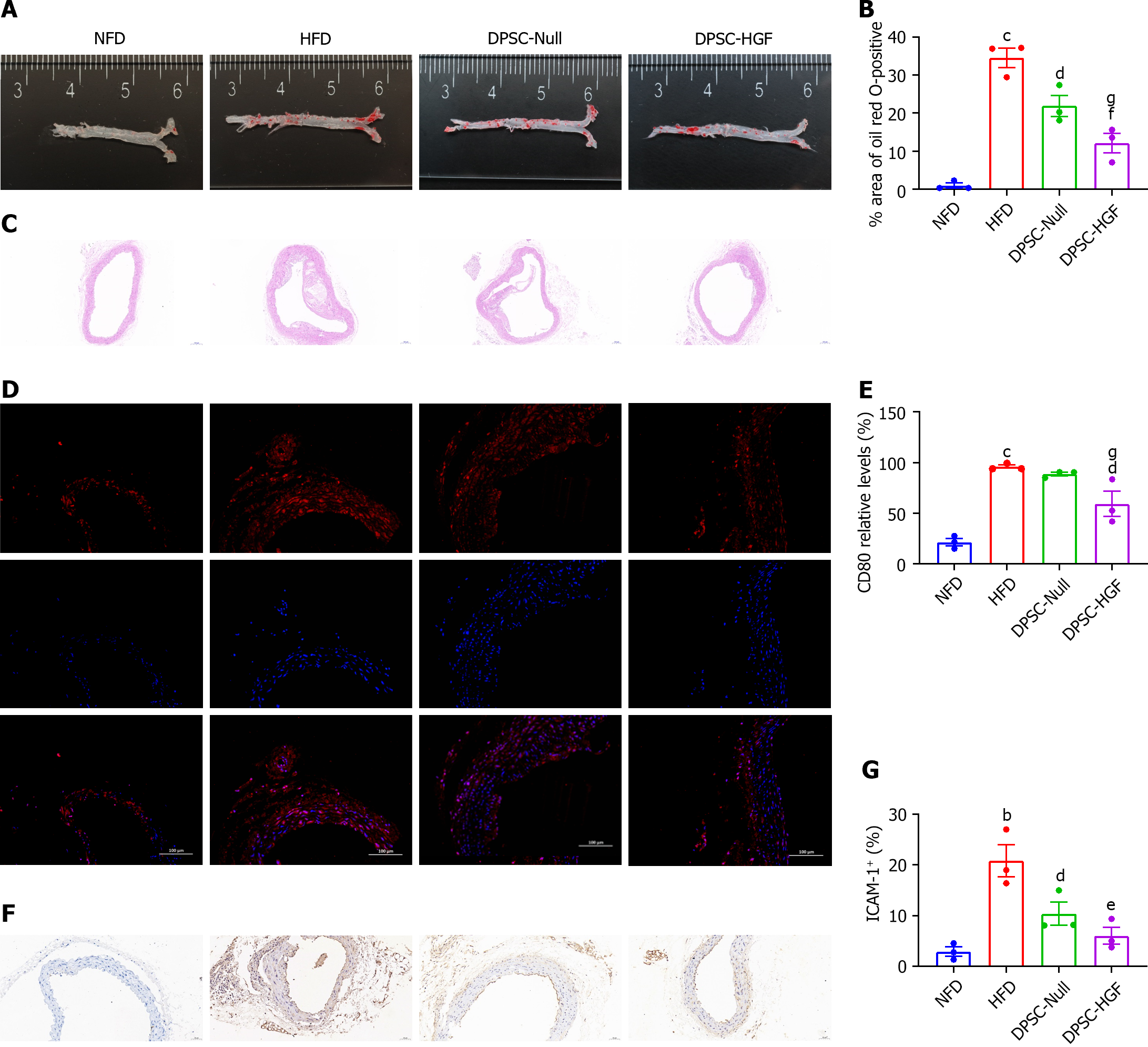
To evaluate plaque sizes, the left ventricles (3 mice per group) were perfused with 10 mL of PBS followed by 5 mL of buffered formalin, and the aortic roots, aortic arches, and abdominal aorta were collected. Excess fat on the tissues was removed, and the tissues were then fixed with 4% neutral buffered formalin (Servicebio Technology, Wuhan, Hubei) at room temperature (RT). Oil red O staining was performed to determine the locations and sizes of the aortic plaques, and the oil red O-positive areas were statistically analyzed by ImageJ software.
The left ventricles of another 3 mice per group were treated as described above and then paraffin-embedded and sectioned (5 μm). Hematoxylin and eosin (HE) staining was used to assess the size of the plaques in the lumen. Immunohistochemical staining was performed to evaluate the local inflammatory status. The samples were blocked with 5% (w/v) BSA, and incubated overnight with primary antibodies against intercellular adhesion molecule-1 (ICAM-1) (GB11106; Servicebio Technology, Wuhan, Hubei) and CD80 (bs-221R; Beijing BoaoSen Biotechnology, Beijing) and incubated with the corresponding secondary antibodies.
Total RNA was extracted from mouse aortic tissues (3 mice per group), HAOECs and RAW264.7 cells by using an RNA-quick purification kit (Yishan Biotechnology, Shanghai, China) according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of total RNA using a cDNA reverse transcription kit (fast all-in-one RT kit, ES Science, Shanghai, China). The mRNA expression of inflammation-related cytokines was quantified by real-time reverse transcription polymerase chain reaction (RT-PCR) using RealStar Green Fast Mixture with ROX II (GenStar, Beijing, China) on an ABI 7500 fast system (Applied Biosystems, Thermo Fisher Scientific, CA, United States). The expression of the target genes was normalized to mouse β-actin/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression or human GAPDH. The detailed primer sequences are shown in Table 1.
| Target gene | Forward sequences (5’-3’) | Reverse sequences (5’-3’) |
| Mouse INF-γ | ACTGGCAAAAGGATGGTG | GTTGCTGATGGCCTGATT |
| Mouse IL-10 | AGTGGTATAGACAGGTCTGTTGG | GCAGCTCTAGGAGCATGTGG |
| Mouse MMP-2 | CACCACCGAGGACTATGACC | TGTTGCCCAGGAAAGTGAAG |
| Mouse MMP-9 | ACAAGACCCTCAGGCCGTAA | TAGCGGTACAAGTATGCCTGG |
| Mouse IL-6 | ACAGAAGGAGTGGCTAAGGA | AGGCATAACGCACTAGGTTT |
| Mouse IL-1β | AGTTGACGGACCCCAAA | TCTTGTTGATGTGCTGCTG |
| Mouse COX-2 | AATGCTGACTATGGCTACAAAA | AAAACTGATGCGTGAAGTGCTG |
| Mouse TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| Mouse Arg1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| Mouse Arg2 | CTGAAGTGGTTAGTAGAGCTGTG | GGGCGTGACCGATAATGGT |
| Mouse β-Actin | AGGCCAACCGTGAAAAGATG | TGGCGTGAGGGAGAGCATAG |
| Mouse GAPDH | GGCAAAGTGGAGATTGTTGC | AATTTGCCGTGAGTGGAGTC |
| Human ICAM-1 | AAAGGAGTTGCTCCTGCCTG | AGTCCAGTACACGGTGAGGA |
| Human MCP-1 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT |
| Human VCAM-1 | CAGCACCACAGGCTCTTTTC | TTGACTGTGATCGGCTTCCC |
| Human E-selectin | AAGGCTTCATGTTGCAGGGA | ATTCATGTAGCCTCGCTCGG |
| Human GAPDH | GTTGCAACCGGGAAGGAAATG | GGAGAAATCGGGCCAGCTA |
Peripheral blood was collected at week 12, and the serum was isolated. Serum samples were diluted 1:2 with sample diluents. Fifty microliters per well of diluted standards or samples were added to a precoated 96-well plate (MEK1016, BOSTER, Wuhan, China) within ten minutes and incubated for 1 h at RT. After the plate was washed three times, 50 μL of detection mix was added to each well, and the plate was incubated for 1 h at RT. The plate was washed three times, 50 μL of streptavidin-HRP was added to each well, and the plate was then incubated for 15 min at RT. The plate was washed six times, and 50 μL per well of substrate was added and then analyzed by using a Q-View Imager LS system (QuansysBio, Shanghai, China) within 15 min.
5 × 104 RAW264.7 cells/well were added to a six-well plate, and when the cell confluence reached 70%, 150 ng/mL LPS was added to induce M1 polarization and indirect co-cultured with DPSC-Null-CM or DPSC-HGF-CM for 24 h, after which FACS and RT-PCR were performed. RAW264.7 cells were first treated with 5 mM nuclear factor-κB (NF-κB) inhibitor (SC75741, Selleck Chemicals, Houston, TX, United States) for 24 h, after which they were pretreated with DPSC-Null-CM or DPSC-HGF-CM for 4 h. Then, 150 ng/mL LPS was added to the cells for 30 min, and western blotting was used to detect the expression of phosphorylated NF-κB-p65.
1 × 105 HAOECs cells/well were added to a six-well plate, and when the cell confluence reached 70%, 5 mM NF-κB inhibitor (SC75741, Selleck Chemicals, Houston, TX, United States) was added to treat HAOECs for 24 h, after which HAOECs were pretreated with DPSC-Null-CM or DPSC-HGF-CM for 4 h, and then 100 ng/mL tumor necrosis factor (TNF)-α was added to the cells for 2 or 8 h. RT-PCR was subsequently performed to detect CAM expression. HAOECs were pretreated with DPSC-Null-CM or DPSC-HGF-CM for 4 h, 100 ng/mL TNF-α was added for 5 min, and western blotting was used to detect the expression of phosphorylated NF-κB-p65.
To determine the role of the NF-κB signaling pathway in the inflammatory responses induced by TNF-α and LPS in HAOECs and RAW264.7 cells, HAOECs and RAW264.7 cells were first pretreated with DPSC-Null-CM or DPSC-HGF-CM for 4 h. Then, 5 mM of the NF-κB activator ALS-357 (S3603, Selleck Chemicals, Houston, TX, United States) was added and incubated for 1 h (western blotting) or 12 h (FACS).
The abovementioned cells were lysed with RIPA buffer supplemented with premixed protease and phosphatase inhibitors (Beyotime, Shanghai, China). The supernatant was collected, and the total proteins were quantified by a BCA Protein Assay Kit (Thermo Scientific, Rockford, United States). Proteins (10 μg) were resolved on 10% polyacrylamide gels before being transferred to polyvinylidene fluoride membranes. Then, the membranes were blocked with 5% nonfat milk and incubated with primary antibodies, including anti-phospho-p65 (diluted 1:1000, catalog number: 3033S), anti-p65 (diluted 1:1000, catalog number: 8242S, Cell Signaling Technology), and anti-GAPDH (1:10000, catalog number: ab181602, Abcam), overnight at 4 °C. After the membranes were washed, they were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5000, catalog number: ZB-2301, ZSGB-BIO, Beijing, China) for 1 h at RT. Signals were detected by an enhanced chemiluminescence western blotting substrate (Solarbio Life Science, Beijing, China) and a Tanon imaging system (Tanon, Shanghai, China). Each experiment was carried out at least three times.
The values are expressed as the mean ± standard error of the mean (SEM) and were tested for normal distributions. Data from three or more samples were subjected to one-way ANOVA, and data from groups with more than one of the two variables were subjected to multiway ANOVA, followed by Bonferroni post hoc tests using GraphPad Prism software version 8.0. Probability values of P < 0.05 were considered significant.
Atherosclerotic plaques were assessed by staining aortic sections with oil red O (Figure 1A), and the stained areas were quantified (Figure 1B). Compared with those in the HFD group, the stained aortic areas in the DPSC-Null group (21% vs. 32%, P < 0.05) and DPSC-HGF group (12% vs. 32%, P < 0.001) were significantly smaller, and these differences were more obvious in the DPSC-HGF group than in the DPSC-Null group (P < 0.05). HE staining further verified the ability of DPSC-HGF to inhibit plaque formation (Figure 1C).
Aortic arch tissue sections were stained with an anti-mCD80 antibody to detect M1 macrophages. CD80-positive plaques were smaller in the DPSC-Null and DPSC-HGF groups (P < 0.05) than in the HFD group (Figure 1D and E). ICAM-1 is the main CAM involved in the development of AS during monocyte adhesion to endothelial cells. Therefore, we performed immunohistochemical staining and quantification of aortic arch tissue sections with an anti-mICAM-1 antibody to evaluate the effects of DPSC-Null and DPSC-HGF on the recruitment of monocytes to endothelial cells (Figure 1F and G). The quantification results showed that ICAM-1 expression in the DPSC-Null and DPSC-HGF groups decreased compared with that in the HFD group (P < 0.05, P < 0.001). DPSC-HGF treatment was more efficient than DPSC-Null treatment in reducing CD80+ macrophages and ICAM-1+ endothelial cells.
The TG, CHO, LDL, and HDL levels increased in the HFD group compared to those in the NFD group, but there were no significant differences between the DPSC-Null or DPSC-HGF group and the HFD group due to the HFD (Figure 2A). We also determined the GLU and GSP levels in serum and body weights, and the results showed that there were nearly no differences among the groups (Figure 2B and C).
Local macrophages in lesions are mainly differentiated from circulating monocytes, which are stimulated by inflammation and polarize toward the M1 phenotype, promoting the development of AS. All of these circulating monocytes are produced by hematopoietic progenitor cells in the bone marrow and spleen. To verify the effects of DPSC-Null and DPSC-HGF on atherosclerotic macrophage formation, flow cytometry was used to examine neutrophils (CD11b+Ly6G+) and monocytes (CD11b+Ly6C+) in the peripheral blood at weeks 6 and 12 and in the spleen at week 12. The percentages of CD11b+Ly6G+ and CD11b+ Ly6C+ cells in the peripheral blood (Figure 3A and B) and spleen (Figure 3D and E) were significantly greater after HFD feeding and decreased after treatment with DPSC-Null or DPSC-HGF.
M1 and M2 macrophages (CD86 and CD206, respectively) in the peripheral blood and spleen were also detected at week 12. Compared to HFD feeding, DPSC-Null treatment had little effect on macrophages, while DPSC-HGF treatment reduced the MFI of CD86 in the peripheral blood and elevated the MFI of CD206 in the spleen (P < 0.001, P < 0.05) (Figure 3C and F).
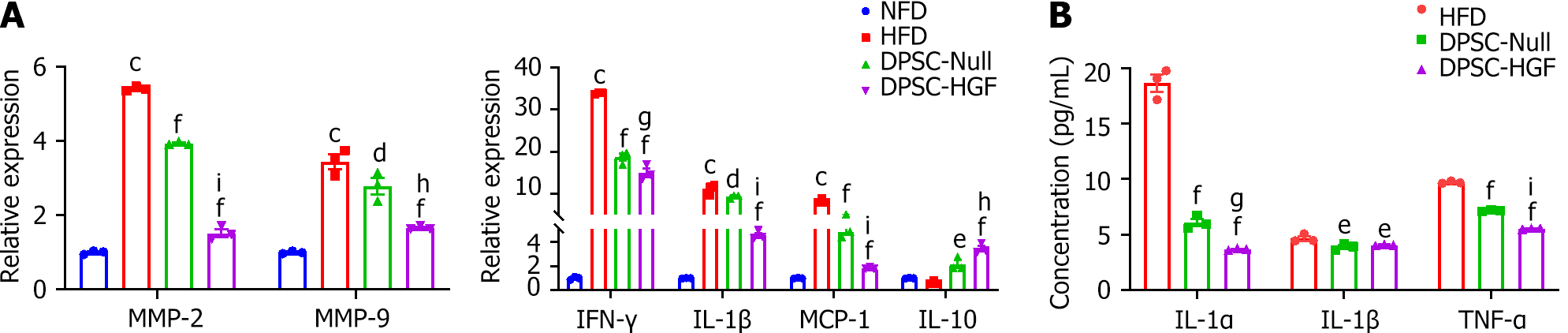
The expression of AS markers and inflammatory and anti-inflammatory cytokines in aortic tissues was detected by RT-PCR, and the expression levels of AS markers [metallopeptidase-2 (MMP-2) and MMP-9] and inflammatory cytokines [monocyte chemotactic protein-1 (MCP-1), interleukin-1β (IL-1β) and interferon-γ] were reduced. The expression levels of the anti-inflammatory cytokine IL-10 were elevated after DPSC-Null and DPSC-HGF treatment (P < 0.01, P < 0.001), and DPSC-HGF performed better than DPSC-Null (P < 0.01) (Figure 4A).
At the end of the experiment, we also determined the expression levels of inflammatory cytokines in the serum by using enzyme-linked immunosorbent assay. Compared with those in the HFD group, the expression levels of IL-1, IL-1β, and TNF-α were lower in the DPSC-Null and DPSC-HGF groups. The concentrations of IL-1α and TNF-α in the DPSC-HGF group were significantly lower than those in the DPSC-Null group (Figure 4B). These results suggested that DPSC-Null and DPSC-HGF can reduce AS formation partly by modulating the expression of local and serum inflammatory cytokines and that DPSC-HGF exerts much stronger anti-inflammatory effects.
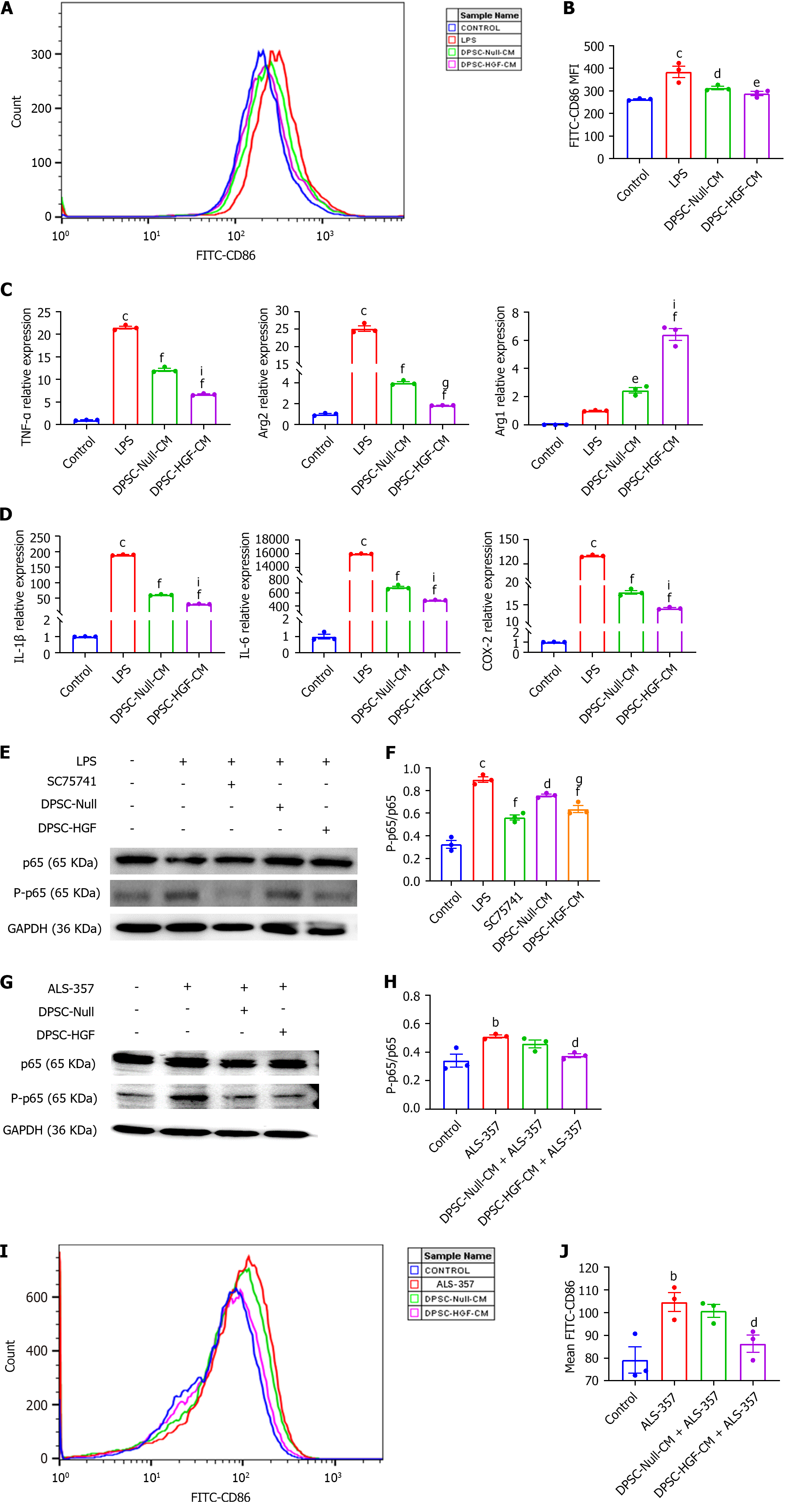
An in vitro cellular inflammation model of RAW264.7 cells was established to verify the effects of DPSC-Null-CM and DPSC-HGF-CM on macrophage polarization and inflammatory factor expression. A comparison of the MFI of the M1 macrophage marker CD86 by flow cytometry revealed that the MFI of CD86 was significantly greater in each group after LPS stimulation (P < 0.001), decreased in both the DPSC-Null-CM and DPSC-HGF-CM treatment groups compared with the LPS group (P < 0.01, P < 0.001), and increased significantly in the DPSC-HGF-treated group (Figure 5A and B). The RT-PCR results showed that the expression levels of the M1 macrophage markers TNF-α and arginase 2 (Arg2) were reduced and that the expression levels of the M2 macrophage marker Arg1 were increased after DPSC-Null-CM and DPSC-HGF-CM treatment, especially in the DPSC-HGF-CM group (Figure 5C). In addition, DPSC-Null-CM and DPSC-HGF-CM significantly decreased the expression of the inflammatory factors IL-1β, IL-6, and cyclooxygenase-2, and the effect of DPSC-HGF-CM was more pronounced than that of DPSC-Null-CM (Figure 5D).
NF-κB can regulate the polarization of macrophages. To investigate the mechanism by which DPSC-Null-CM and DPSC-HGF-CM regulate RAW264.7 polarization, RAW264.7 cells were treated with an NF-κB inhibitor for 24 h and then indirect co-cultured with DPSC-Null-CM or DPSC-HGF-CM for 4 h. Then, 150 ng/mL LPS was added for 30 min, and the expression of NF-κB-p65 was detected by western blotting. The results showed that the expression of phosphorylated NF-κB-p65 significantly increased after LPS treatment (P < 0.001) and significantly decreased in the NF-κB inhibitor-treated group (P < 0.001) and the DPSC-Null-CM treated group (P < 0.05) and DPSC-HGF-CM treated groups (P < 0.001). The effect of DPSC-HGF-CM was greater than that of DPSC-Null-CM (Figure 5E and F). To further confirm the relationships between DPSC-Null-CM and DPSC-HGF-CM and between DPSC-CM and NF-κB, RAW264.7 cells were pretreated with DPSC-Null-CM or DPSC-HGF-CM for 4 h and then treated with the NF-κB activator betulic acid (ALS-357) for 1 h. NF-κB-p65 expression was detected by western blotting, and the expression of CD86 was determined by flow cytometry. The results showed that phosphorylated NF-κB-p65 was upregulated after NF-κB activator treatment (P < 0.01), whereas the expression of phosphorylated NF-κB-p65 was inhibited in the DPSC-Null-CM treatment group and DPSC-HGF-CM treatment group (P < 0.05) compared with the NF-κB activator group (Figure 5G and H). Furthermore, the CD86 MFI was obviously increased after NF-κB activator treatment. Compared to that in the NF-κB activator group, the CD86 MFI was decreased in the DPSC-Null-CM and DPSC-HGF-CM-treated groups (Figure 5I and J). This finding suggested that DPSC-Null-CM and DPSC-HGF-CM may regulate the polarization of RAW264.7 cells by suppressing the NF-κB pathway to reduce inflammation, and this effect was more pronounced after HGF modification.
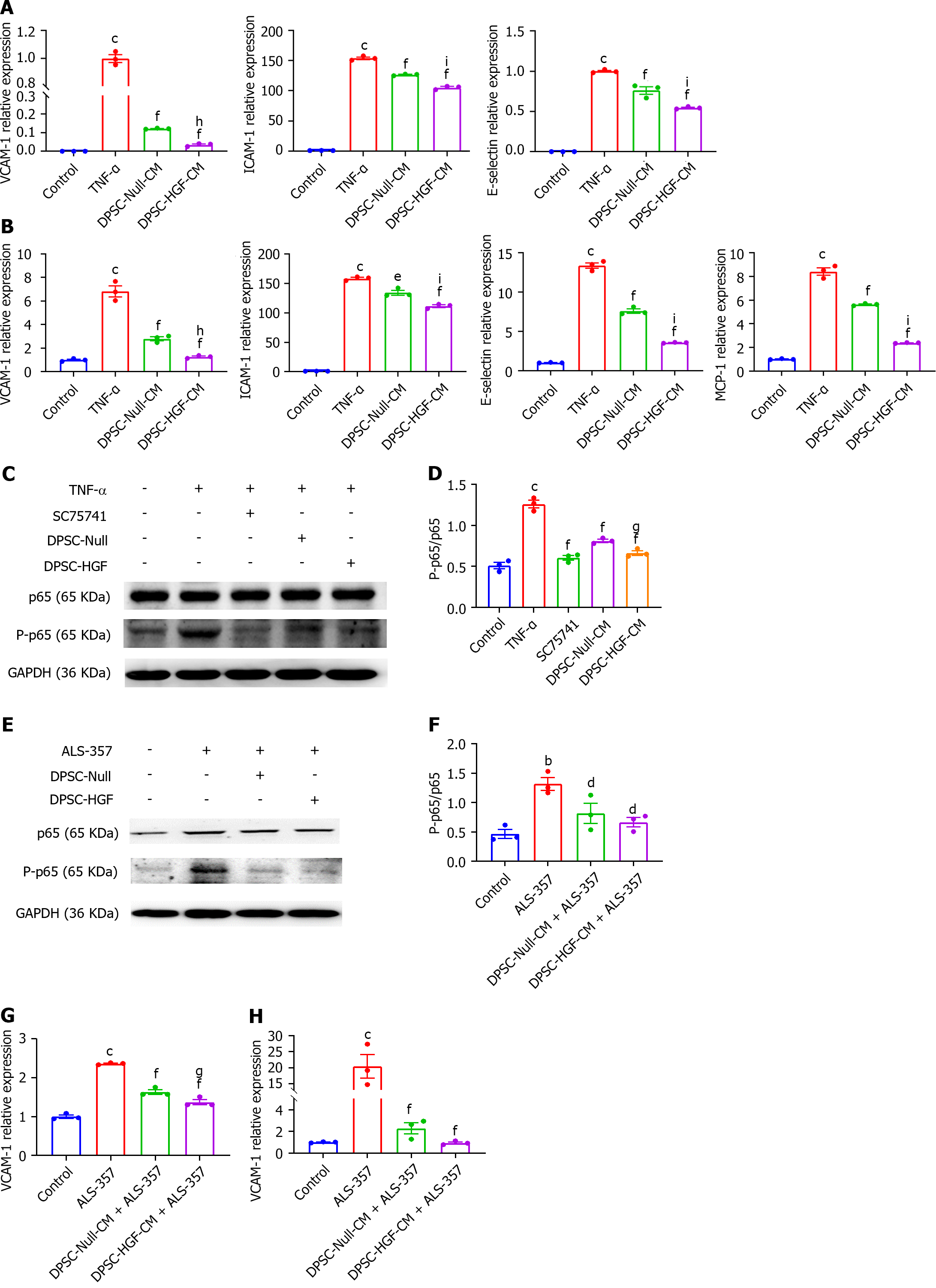
We indirectly co-cultured HAOECs with DPSC-Null-CM and DPSC-HGF-CM in the presence of 100 ng/mL TNF-α for 2 or 8 h to investigate the effects of DPSC-Null-CM and DPSC-HGF-CM on CAM and MCP-1 expression in HAOECs activated by inflammatory stimulation. The RT-PCR results revealed that the DPSC-Null-CM and DPSC-HGF-CM treatments downregulated the expression levels of vascular cell adhesion molecule-1 (VCAM-1), ICAM-1, and E-selectin in HAOECs at 2 h and of VCAM-1, ICAM-1, E-selectin and MCP-1 at 8 h of indirect co-culture, and the effect was more pronounced after DPSC-HGF-CM treatment (Figure 6A and B).
We further investigated the mechanism of the anti-inflammatory effects of DPSC-Null-CM and DPSC-HGF-CM on TNF-α-stimulated HAOECs. We found that DPSC-Null-CM and DPSC-HGF-CM inhibited the phosphorylation of NF-κB-p65 compared to that in the TNF-α group (P < 0.001, P < 0.001), and DPSC-HGF-CM decreased this phosphorylation better than DPSC-Null-CM did (Figure 6C and D). The addition of the NF-κB activator significantly increased phosphorylated NF-κB-p65; similarly, DPSC-Null-CM and DPSC-HGF-CM downregulated phosphorylated NF-κB-p65 expression after NF-κB activator treatment (P < 0.05, P < 0.05) (Figure 6E and F). We further investigated the effect of ALS-357 on CAM expression in HAOECs. RT-PCR results showed that VCAM-1 expression increased after 2 h and 8 h of NF-κB activator treatment (P < 0.001, P < 0.001). Furthermore, VCAM-1 expression was decreased in the DPSC-Null-CM and DPSC-HGF-CM groups (P < 0.001, P < 0.001), and the effect of DPSC-HGF-CM was more significant (Figure 6G and H).
The development of atherosclerotic plaques is a multistep inflammatory process[18]. Inflammation is common in all stages of AS and has an important effect on the development of AS[19]. MSCs have outstanding self-renewal, pluripotency, and immunomodulatory functions and are currently widely used to treat various diseases. However, due to their therapeutic limitations, they often do not consistently exert effective therapeutic effects[20]. With the rapid development of genetic engineering, adenoviruses can be used as cytokine carriers to further play a mutually synergistic role by expressing related factors in many cells. HGF is a multipotent factor that has been actively used to treat chronic inflammatory and autoimmune diseases. It has been reported that modification of the HGF gene favors the efficacy of MSCs. MSC-HGF facilitates the survival of grafts in the host and inhibits the expression of inflammatory factors such as ICAM-1 and myeloperoxidase more potently than MSCs. In addition, MSC-HGF promotes cell migration and engraftment, cell-cell junction restoration and soft tissue re-epithelialization, angiogenesis and neurogenesis, antifibrotic effects, anti-inflammatory effects, antiapoptotic effects and antioxidant effects[21,22]. Thus, HGF gene-modified stem cells could partly overcome the limitations of stem cell therapy and will have the dual advantages of stem cell therapy and cytokine therapy. This study is the first to apply HGF-modified DPSCs in the treatment of AS and provides a new therapeutic strategy for the treatment of AS.
In the present study, we determined the specific content of HFD based on relevant studies that successfully established an animal model of AS[23,24]. Previous studies have shown that in vivo injections of MSCs can reduce atherosclerotic lesions by activating anti-inflammatory responses, including reductions in the number of circulating monocytes and the numbers of macrophages and T cells in the aortic roots of LDLR-/- mice fed a HFD. However, the body weights and serum TG and LDL levels in mice did not change after the administration of MSCs[25-27]. Similarly, in the present study, we found that DPSC-Null and DPSC-HGF treatments reduced the plaque area in ApoE-/- mice fed a HFD, as observed by oil red O staining, while the treatments did not alter body weight or serum lipid levels in the mice.
Macrophages play a key role in all stages of AS development, with local proliferation of macrophages at lesions dominating macrophage aggregation and monocytes and neutrophils being the main sources of lesion macrophages[28-30]. Our results suggested that DPSC-Null and DPSC-HGF could reduce the aggregation of M1 macrophages in arterial tissue and alleviate AS. This may be related to the reductions in circulating monocytes and neutrophils and the inhibition of M1 macrophage polarization in peripheral blood. DPSC-HGF inhibited M1 polarization better than did DPSC-Null. As the spleen is a major immune organ and an important site for macrophage, monocyte, and neutrophil production, DPSC-Null and DPSC-HGF treatments reduced monocyte and neutrophil levels in the spleen. Although the MFI of M1 macrophages in the spleen did not decrease, the MFI of M2 macrophages increased after DPSC-HGF treatment. Inflammation is an important prerequisite for eliciting an immune response, so we examined the expression of inflammatory factors in local aortic tissues and systemic circulation. The significant therapeutic effect of DPSC-HGF may be achieved in part by reducing the levels of inflammatory factors in local tissues and serum. It is unclear why HGF modification could not reduce the percentages of monocytes and neutrophils in the peripheral blood or spleen further when compared with DPSC-Null, which remains to be elucidated by further studies. We also verified the anti-inflammatory effect of DPSC-HGF on macrophages in cell models constructed in vitro, in which DPSC-Null-CM and DPSC-HGF-CM treatments reduced the MFI of CD86 and inflammatory factor expression in RAW264.7 cells under inflammatory conditions. DPSC-HGF-CM had a stronger inhibitory effect than DPSC-Null-CM. The NF-κB signaling pathway is involved in regulating AS[31]. To further investigate the underlying mechanisms of these effects, we first confirmed the effects of DPSC-Null-CM and DPSC-HGF-CM treatments on the phosphorylation of NF-κB-p65 in RAW264.7 cells under inflammatory stimulation. Subsequently, to verify the relationship between DPSC-Null-CM and DPSC-HGF-CM treatments and NF-κB-p65, we treated RAW264.7 cells with DPSC-Null-CM or DPSC-HGF-CM and an NF-κB activator at the same time. Our results suggested that DPSC-Null and DPSC-HGF could inhibit the phosphorylation of NF-κB-p65, which might be partly associated with reducing inflammation and the percentages of monocytes and neutrophils, inhibiting the migration and polarization of invasive inflammatory macrophages, and thus suppressing plaque formation.
An increasing number of studies have shown a close link between inflammation, endothelial cell damage, and macrophages in AS[32]. When stimulated by inflammation, endothelial cells are damaged and release many adhesion molecules, which are the targets for monocyte and inflammatory macrophage clustering[33-35]. Inflammation-stimulated endothelial cell secretion of MCP-1 directs free monocytes to migrate and infiltrate the subendothelium while inducing them to polarize toward the M1 phenotype, further activating inflammatory mechanisms[36]. We previously verified that DPSC-Null and DPSC-HGF treatments can reduce the percentages of monocytes and macrophages in ApoE-/- mice fed a HFD. To investigate the relationship between monocytes/macrophages and the release of adhesion molecules from endothelial cells, we examined the expression of ICAM-1 in arterial tissues by immunohistochemistry and found that DPSC-Null and DPSC-HGF treatments reduced ICAM-1 expression. To further investigate the relationship between endothelial cell damage and inflammation, we established a cell model in vitro by using HAOECs and examined CAM expression. We found that DPSC-Null and DPSC-HGF reduced the expression of CAMs in endothelial cells cultured under inflammatory conditions, and similar to the abovementioned results, DPSC-HGF performed better than DPSC-Null. Inhibition of NF-κB-p65 activation was also reported to reduce macrophage polarization toward M1 and the expression of ICAM and VCAM[37-39]. In the present study, we also observed that the NF-κB signaling pathway was activated in HAOECs after TNF-α stimulation, whereas DPSC-Null and DPSC-HGF inhibited activation of the NF-κB pathway and thus reduced CAM expression in inflamed HAOECs. Furthermore, we applied NF-κB activators to demonstrate the relationship between the NF-κB signaling pathway and CAM production by endothelial cells. Our results suggest that DPSC-Null and DPSC-HGF can inhibit the expression of the NF-κB pathway, which may be associated with reduced inflammation and downregulated expression of adhesion molecules, thereby inhibiting plaque formation.
We found that DPSC-Null and DPSC-HGF treatments reduced atherosclerotic plaque formation in ApoE-/- mice fed a HFD and that DPSC-HGF treatment was more effective than DPSC-Null treatment because of the reduced expression of aortic endothelial cell adhesion molecules and inflammatory macrophages. In vitro, we found that DPSC-Null-CM and DPSC-HGF-CM inhibited the NF-κB signaling pathway in RAW264.7 and HAOECs cells under inflammatory stimulation. Taken together, these data suggest for the first time that DPSC-Null and DPSC-HGF could ameliorate AS in ApoE-/- mice and that DPSC-HGF has greater therapeutic potential than DPSC-Null. However, there are still some shortcomings in the experiments. The mechanism by which DPSC-HGF regulates NF-κB activation is not fully understood and needs further study for verification.
| 1. | Khambhati J, Engels M, Allard-Ratick M, Sandesara PB, Quyyumi AA, Sperling L. Immunotherapy for the prevention of atherosclerotic cardiovascular disease: Promise and possibilities. Atherosclerosis. 2018;276:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Ruscica M, Ferri N, Banach M, Sirtori CR, Corsini A. Side effects of statins: from pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc Res. 2023;118:3288-3304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 3. | Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, Luo S, Li Z, Liu P, Han J, Harding IC, Ebong EE, Cameron SJ, Stewart AG, Weng J. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol Rev. 2021;73:924-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 595] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 4. | Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 1162] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 5. | Björkegren JLM, Lusis AJ. Atherosclerosis: Recent developments. Cell. 2022;185:1630-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 602] [Article Influence: 200.7] [Reference Citation Analysis (0)] |
| 6. | Gimbrone MA Jr, García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016;118:620-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1448] [Cited by in RCA: 2346] [Article Influence: 260.7] [Reference Citation Analysis (0)] |
| 7. | Ruparelia N, Choudhury R. Inflammation and atherosclerosis: what is on the horizon? Heart. 2020;106:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Caplan AI. MSCs: The Sentinel and Safe-Guards of Injury. J Cell Physiol. 2016;231:1413-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Zheng G, Huang R, Qiu G, Ge M, Wang J, Shu Q, Xu J. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 11. | Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, Lin Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 12. | Yamaji D, Soliman MM, Kamikawa A, Ito T, Ahmed MM, Okamatsu-Ogura Y, Saito M, Kimura K. Species-specific control of hepatocyte growth factor expression and production in adipocytes in a differentiation-dependent manner. Domest Anim Endocrinol. 2018;62:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Kitamura K, Nagoshi N, Tsuji O, Matsumoto M, Okano H, Nakamura M. Application of Hepatocyte Growth Factor for Acute Spinal Cord Injury: The Road from Basic Studies to Human Treatment. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Wang LS, Wang H, Zhang QL, Yang ZJ, Kong FX, Wu CT. Hepatocyte Growth Factor Gene Therapy for Ischemic Diseases. Hum Gene Ther. 2018;29:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Molnarfi N, Benkhoucha M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor: A regulator of inflammation and autoimmunity. Autoimmun Rev. 2015;14:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Hu J, Cao Y, Xie Y, Wang H, Fan Z, Wang J, Zhang C, Wu CT, Wang S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 2016;7:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Wang H, Yang YF, Zhao L, Xiao FJ, Zhang QW, Wen ML, Wu CT, Peng RY, Wang LS. Hepatocyte growth factor gene-modified mesenchymal stem cells reduce radiation-induced lung injury. Hum Gene Ther. 2013;24:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 583] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 19. | Bäck M, Yurdagul A Jr, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16:389-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 658] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 20. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 21. | Meng H, Wei F, Zhou Y, Hu L, Ge Z, Jin J, Wang H, Wu CT. Overexpression of Hepatocyte Growth Factor in Dental Pulp Stem Cells Ameliorates the Severity of Psoriasis by Reducing Inflammatory Responses. Stem Cells Dev. 2021;30:876-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Meng HF, Jin J, Wang H, Wang LS, Wu CT. Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings. J Cell Mol Med. 2022;26:4745-4755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 23. | Shi H, Liang M, Chen W, Sun X, Wang X, Li C, Yang Y, Yang Z, Zeng W. Human induced pluripotent stem cellderived mesenchymal stem cells alleviate atherosclerosis by modulating inflammatory responses. Mol Med Rep. 2018;17:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 461] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 25. | Frodermann V, van Duijn J, van Pel M, van Santbrink PJ, Bot I, Kuiper J, de Jager SC. Mesenchymal Stem Cells Reduce Murine Atherosclerosis Development. Sci Rep. 2015;5:15559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Li Q, Sun W, Wang X, Zhang K, Xi W, Gao P. Skin-Derived Mesenchymal Stem Cells Alleviate Atherosclerosis via Modulating Macrophage Function. Stem Cells Transl Med. 2015;4:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Takafuji Y, Hori M, Mizuno T, Harada-Shiba M. Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr-/- mice. Cardiovasc Res. 2019;115:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Bartlett B, Ludewick HP, Misra A, Lee S, Dwivedi G. Macrophages and T cells in atherosclerosis: a translational perspective. Am J Physiol Heart Circ Physiol. 2019;317:H375-H386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Liberale L, Dallegri F, Montecucco F, Carbone F. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb Haemost. 2017;117:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 31. | Karunakaran D, Nguyen MA, Geoffrion M, Vreeken D, Lister Z, Cheng HS, Otte N, Essebier P, Wyatt H, Kandiah JW, Jung R, Alenghat FJ, Mompeon A, Lee R, Pan C, Gordon E, Rasheed A, Lusis AJ, Liu P, Matic LP, Hedin U, Fish JE, Guo L, Kolodgie F, Virmani R, van Gils JM, Rayner KJ. RIPK1 Expression Associates With Inflammation in Early Atherosclerosis in Humans and Can Be Therapeutically Silenced to Reduce NF-κB Activation and Atherogenesis in Mice. Circulation. 2021;143:163-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 32. | Meng B, Li Y, Ding Y, Xu X, Wang L, Guo B, Zhu B, Zhang J, Xiang L, Dong J, Liu M, Xiang G. Myeloid-derived growth factor inhibits inflammation and alleviates endothelial injury and atherosclerosis in mice. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 508] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 34. | Hashem RM, Rashed LA, Abdelkader RM, Hashem KS. Stem cell therapy targets the neointimal smooth muscle cells in experimentally induced atherosclerosis: involvement of intracellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM). Braz J Med Biol Res. 2021;54:e10807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Motawi T, Shaker O, Taha N, Abdel Raheem M. Genetic variations in E-selectin and ICAM-1: relation to atherosclerosis. Med Sci Monit. 2012;18:CR381-CR389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101:107598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 482] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 37. | Lee K, Yim JH, Lee HK, Pyo S. Inhibition of VCAM-1 expression on mouse vascular smooth muscle cells by lobastin via downregulation of p38, ERK 1/2 and NF-κB signaling pathways. Arch Pharm Res. 2016;39:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Somensi N, Rabelo TK, Guimarães AG, Quintans-Junior LJ, de Souza Araújo AA, Moreira JCF, Gelain DP. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int Immunopharmacol. 2019;75:105743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Ding S, Liu J, Han X, Ding W, Liu Z, Zhu Y, Zhan W, Wan Y, Gai S, Hou J, Wang X, Wu Y, Wu A, Li CY, Zheng Z, Tian XL, Cao H. ICAM-1-related noncoding RNA accelerates atherosclerosis by amplifying NF-κB signaling. J Mol Cell Cardiol. 2022;170:75-86. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |