Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.560
Revised: February 26, 2024
Accepted: April 12, 2024
Published online: May 26, 2024
Processing time: 146 Days and 22.9 Hours
Alveolar bone defects caused by inflammation are an urgent issue in oral implant surgery that must be solved. Regulating the various phenotypes of macrophages to enhance the inflammatory environment can significantly affect the progression of diseases and tissue engineering repair process.
To assess the influence of interleukin-10 (IL-10) on the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) following their interaction with macrophages in an inflammatory environment.
IL-10 modulates the differentiation of peritoneal macrophages in Wistar rats in an inflammatory environment. In this study, we investigated its impact on the proliferation, migration, and osteogenesis of BMSCs. The expression levels of signal transducer and activator of transcription 3 (STAT3) and its activated form, phos
IL-10-stimulated macrophages underwent polarization to the M2 type through substitution, and these M2 macrophages actively facilitated the osteogenic differentiation of BMSCs. Mechanistically, STAT3 signaling plays a crucial role in the process by which IL-10 influences macrophages. Specifically, IL-10 stimulated the activation of the STAT3 signaling pathway and reduced the macrophage inflammatory response, as evidenced by its diminished impact on the osteogenic differentiation of BMSCs.
Stimulating macrophages with IL-10 proved effective in improving the inflammatory environment and promoting the osteogenic differentiation of BMSCs. The IL-10/STAT3 signaling pathway has emerged as a key regulator in the macrophage-mediated control of BMSCs’ osteogenic differentiation.
Core Tip: This study investigated the mechanism of interleukin-10 (IL-10) affecting macrophages in inflammatory environments, observed the effects of different macrophages on the biological behavior and osteogenic differentiation of bone marrow mesenchymal stem cells, and found that IL-10/signal transducer and activator of transcription 3 signaling plays a crucial role in promoting bone formation by affecting macrophages. This study provides a new strategy for solving the problem of poor osteogenesis in bone defect repair caused by an excessive inflammatory response in clinical work.
- Citation: Lyu MH, Bian C, Dou YP, Gao K, Xu JJ, Ma P. Effects of interleukin-10 treated macrophages on bone marrow mesenchymal stem cells via signal transducer and activator of transcription 3 pathway. World J Stem Cells 2024; 16(5): 560-574
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/560.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.560
Long-term tooth defects or loss is frequently accompanied by bone loss in the edentulous area. Substantial bone aug
Previous studies on osteogenesis primarily focused on the biological behavior of osteoblasts, osteoclasts, or fibroblasts, explaining osteogenesis by investigating the behavioral phenotypes of these cells[3]. The concept of “osteoimmunology” was introduced in 2000, which prompted scholars to explore osseointegration and bone loss from an immunological per
Macrophages are vital components of the intrinsic immune system and play a crucial role in various immune respon
The effect of IL-4 on the biological behavior and function of BMSCs following the induction of macrophage M2 polarization has been previously studied[12]. However, the effects of the induced macrophages on the biological behavior and function of BMSCs in an inflammatory environment remain unknown. IL-10, recognized as a potent anti-inflammatory cytokine that plays a crucial role in the immune response to external substances[13,14]. IL-10 is the founding member of a family of cytokines that includes IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B, and IL-29[15]. The cytokine IL-10 is a key mediator that ensures the protection of a host from over-exuberant responses to pathogens and microbiota while playing important roles in other settings, such as sterile wound healing, autoimmunity, cancer, and homeostasis[16]. Type 2 helper T cells were the first identified cellular source of IL-10[13], more and more cells were subsequently confirmed to produce IL-10, such as CD4/CD8 T cells, B cells, macrophages, monocytes, dendritic cells, neutrophils, mast cells, eosinophils, and natural killer (NK) cells[17]. In addition to being produced by different cell types, IL-10 targets different cells and exerts a wide range of anti-inflammatory activities. Macrophages express high levels of the IL-10R on their surface, IL-10 have been shown to trigger strong immunosuppressive responses, primarily through transcriptional inhibition of cytokines and chemokines, as well as major histocompatibility complex class II[18]. IL-10 exerts regulatory effects on various cells via the IL-10R. After stimulation with IL-10, the intracellular phosphorylation of IL-10R related Janus kinase 1 and tyrosine kinase 2 occurs[19]. These kinases further phosphorylate tyrosine residues within IL-10R cells, thereby recruiting the signal transducer and activator of transcription 3 (STAT3). After phosphorylation of STAT3, it enters the nucleus and initiates a specific transcriptional program that largely defines the IL-10-mediated anti-inflammatory response[20,21].
This study aimed to investigate the mechanism by which IL-10 influences macrophages in an inflammatory envi
Primary peritoneal macrophages were obtained from Wistar rats by intraperitoneal lavage, followed by cultivation
Total RNA was extracted from macrophages using TRIzol reagent, and RNA purity and concentration were assessed using a spectrophotometer. Real-time fluorescence quantitative polymerase chain reaction (RT-qPCR) was conducted according to the SYBR Green Real-time PCR reagent instructions (Kangwei Century Biotechnology Co., Ltd., China). The reaction time and temperature were determined through preliminary experiments. The RT-qPCR primer sequences (Shanghai Biotech, China) are shown (Table 1).
| Primer | Sequences |
| Actb | Forward: 5’-GGAGATTACTGCCCTGGCTCCTAGC-3’ |
| Reverse: 5’-GGCCGGACTCATCGTACTCCTGCTT-3’ | |
| Gapdh | Forward: 5’-TATGACTCTACCCACGGCAA-3’ |
| Reverse: 5’-ATACTCAGCACCAGCATCACC-3’ | |
| Arg1 | Forward: 5’-AGTGTGGTGCTGGGTGGAGAC-3’ |
| Reverse: 5’-GCGGAGTGTTGATGTCAGTGTGAG-3’ | |
| Il10 | Forward: 5’-CGCATCCAGACACACACAGACTAG-3’ |
| Reverse: 5’-GCCCAGAGACAGACAAGCAAGAG-3’ | |
| Inos | Forward: 5’-GAGACGCACAGGCAGAGGTTG-3’ |
| Reverse: 5’-AGCAGGCACACGCAATGATG G-3’ | |
| Tnfa | Forward: 5’-ATGGGCTCCCTCTCATCAGT-3’ |
| Reverse: 5’-GCTTGGTGGTTTGCTACGAC-3’ |
Femoral and tibial BMSCs from Wistar rats were extracted using the whole bone marrow apposition method and cultured (37 °C, 5% CO2, saturated humidity) in MSCM (ScienCell, United States) containing 10% fetal bovine serum (Gibco, United States) and 1% cyanostatin (Gibco, United States) by volume. The 3rd generation BMSCs were obtained by digestion with 0.25% trypsin (Gibco, United States) when cell growth reached 75%-85% confluence. After culturing each group of macrophages for 24 h using the method described above, the culture medium was changed to normal DMEM, and the cells were incubated for another 24 h. The supernatants from each group of macrophage cultures were collected for non-contact co-culture with BMSCs. Non-contact co-culture persisted throughout the osteogenic differentiation of BMSCs. Differences in the biological behavior of BMSCs were observed, and the osteogenic differentiation and mineralization of BMSCs were examined after switching to an osteogenic induction medium (Cyagen, China).
Proliferation of BMSCs: The 3rd generation BMSCs were uniformly seeded into 24-well plates at a density of 1 × 104/well. The culture supernatant of the macrophages after different treatments was added for non-contact co-culture. The proliferation of BMSCs was assessed on days 0, 1st, 3rd, 5th, and 7th d using the Cell Counting Kit-8 (CCK-8, DOJINDO, Japan). A CCK-8 reaction solution (CCK-8 solution: Culture medium = 1:10) was prepared and added to each well containing BMSCs. After incubation for 3 h in the dark, the absorbance (OD) was measured at 495 nm using an enzyme marker (Molecular Devices, China).
Migration of BMSCs: The 3rd generation BMSCs were uniformly seeded in 6-well plates at a density of 3 × 105/well. Once the cells adhered to the well surface, a straight-line scratch was created along the midline of the well bottom using a 1 mL pipette tip. Subsequently, the culture medium was aspirated and serum-free medium along with the macrophage culture supernatant from each group was added. The migration ability of the BMSCs was then observed.
Differentiation of BMSCs: The 3rd generation BMSCs were uniformly seeded in 6-well plates at a density of 3 × 105/well. Upon cell adhesion, the medium was switched to osteogenic induction medium (Cyagen, China) and macrophage culture supernatants from different groups were added for non-contact co-culture. Total RNA from BMSCs after 3 d of osteogenic induction was extracted using a previously described method and subjected to RT-qPCR. The PCR primer sequences (Shanghai Biotech, China) are listed (Table 2).
| Primer | Sequences |
| Gapdh | Forward: 5’-TATGACTCTACCCACGGCAA-3’ |
| Reverse: 5’-ATACTCAGCACCAGCATCACC-3’ | |
| Alp | Forward: 5’-AACGTGGCCAAGAACATCATCA-3’ |
| Reverse: 5’-TGTCCATCTCCAGCCGTGTC-3’ | |
| Ocn | Forward: 5’-AAAGCCCAGCGACTCT-3’ |
| Reverse: 5’-CTAAACGGTGGTGCCATAGAT-3’ | |
| Runx2 | Forward: 5’-GCTTCTCCAACCCACGAATG-3’ |
| Reverse: 5’-GAACTGATAGGACGCTGACGA-3’ |
Mineralization of BMSCs: Alizarin red staining was conducted for each group of BMSCs following a 14-d osteogenic induction culture to observe cell mineralization. The cells were fixed with 4% paraformaldehyde for 15 min. Subse
Macrophages were extracted and cultured using the same method as previously described. They were then treated differently and grouped as follows: IL-10 (100 ng/mL) was added to macrophages in the experimental group, while Tyrphostin AG490 (10 μM), a specific inhibitor of STAT3 phosphorylation (MCE, United States), was added to macro
Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto a polyvinylidene fluoride membrane. The membranes were blocked with 5% skim milk at room temperature for 1 h. Pri
Cell migration images were processed using the ImageJ software. One-way analysis of variance and paired sample T-test was used for statistical analyses between different groups. Data were statistically analyzed using SPSS 26.0, with the significance level set at P < 0.05. GraphPad Prism software (version 8.0) was used to generate graphs.
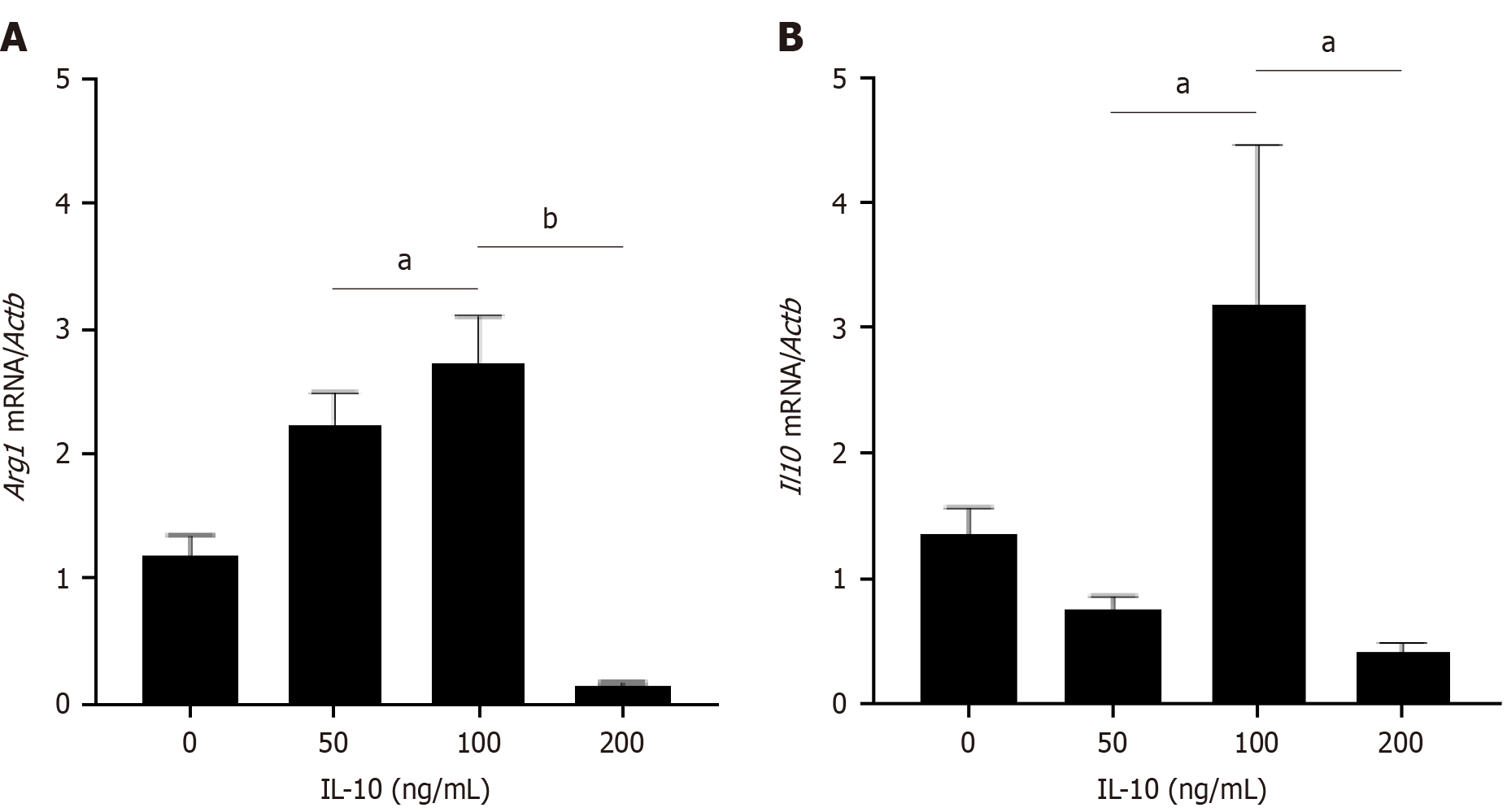
In this study, the optimal concentration of IL-10 was determined using a gradient of 0, 50, 100, and 200 ng/mL. The expression of Arg1 and Il10 in each group of macrophages was assessed via RT-PCR, revealing the highest expression of Arg1 and Il10 at an active concentration of 100 ng/mL (Figure 1). Therefore, 100 ng/mL was selected as the optimal concentration of IL-10 for subsequent experiments.
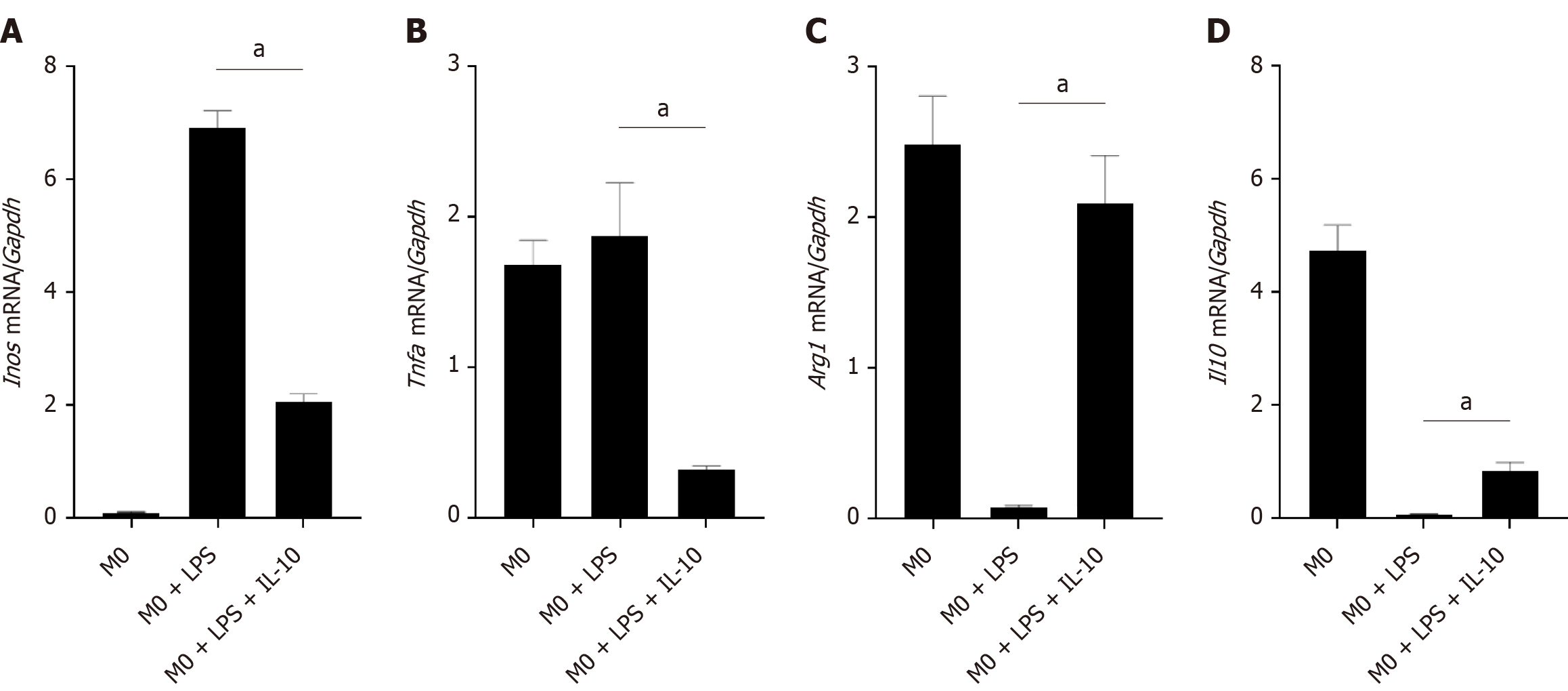
To investigate the impact of IL-10 on macrophages in an inflammatory environment, we induced a macrophage inflammatory response model using LPS (1 μg/mL) for 12 h. Subsequently, IL-10 (100 ng/mL) was added, and the macrophages were cultured for an additional 12 h before collection. The expression of the M1 markers inducible isoform of nitric oxide synthase (Inos) and Tnfa, as well as the M2 markers Arg1 and Il10, in macrophages was simultaneously assessed by RT-PCR. Under LPS-stimulated conditions, an elevation was noted in the expression of the inflammatory factors Inos and Tnfa, indicating polarization toward the M1 type. Conversely, after the addition of IL-10, there was a decrease in the expression of the inflammatory factors Inos and Tnfa and an increase in the expression of the anti-inflammatory factors Arg1 and Il10 (Figure 2).
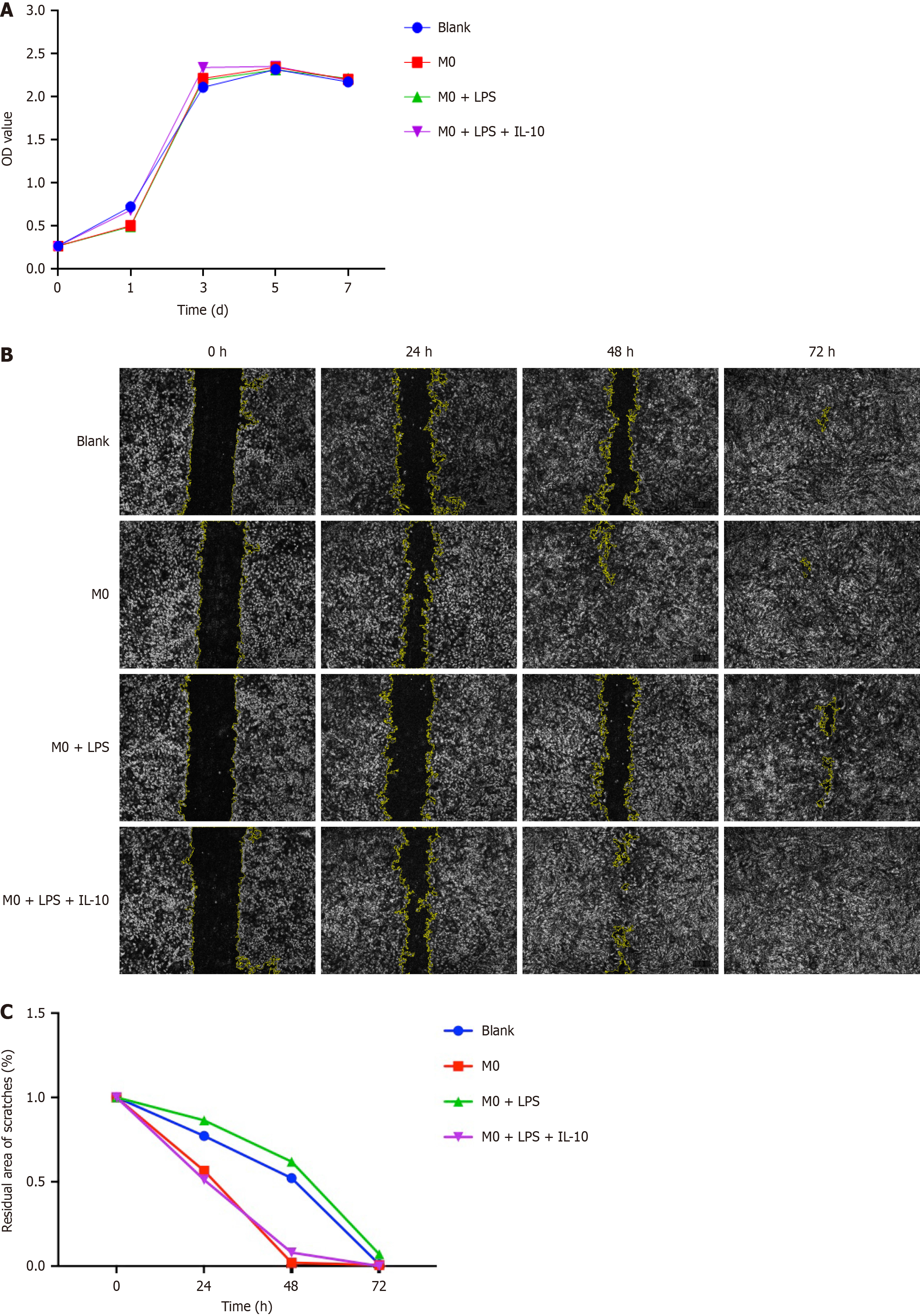
To explore the effects of different types of macrophages on BMSCs’ proliferation, we co-cultured macrophages and BMSCs. The OD values of the 3rd generation BMSCs were measured and recorded at days 1, 3, 5, and 7 using the CCK-8 method, and proliferation curves were plotted. The results indicated that the proliferation curves of BMSCs in each group exhibited exponential growth in the first 3 d and leveled off by the 5th d, with no significant differences among the groups. Additionally, no significant variation was observed in the proliferation rate of BMSCs in each group. Hence, macrophages in each group had no discernible effect on the proliferation of BMSCs (Figure 3A).
To assess the impact of different macrophages on BMSC migration, we created a straight-line scratch along the midline of the bottom of the well and recorded the scratch width after 24 h, 48 h, and 72 h. Among the various BMSC groups, the BMSCs in the M0 + LPS + IL-10 group exhibited the fastest migration rate after exposure to the supernatant, with scratches healing the earliest. In contrast, the scratches in the M0 + LPS group healed the latest, and the cells at the scratches were sparsely arranged. These results indicated that the migration rate of BMSCs was slowed in the inflammatory environment, and IL-10 treatment promoted BMSC migration, accelerating scratch healing (Figure 3B). The residual area of the scratches was quantitatively analyzed using ImageJ software (Figure 3C).
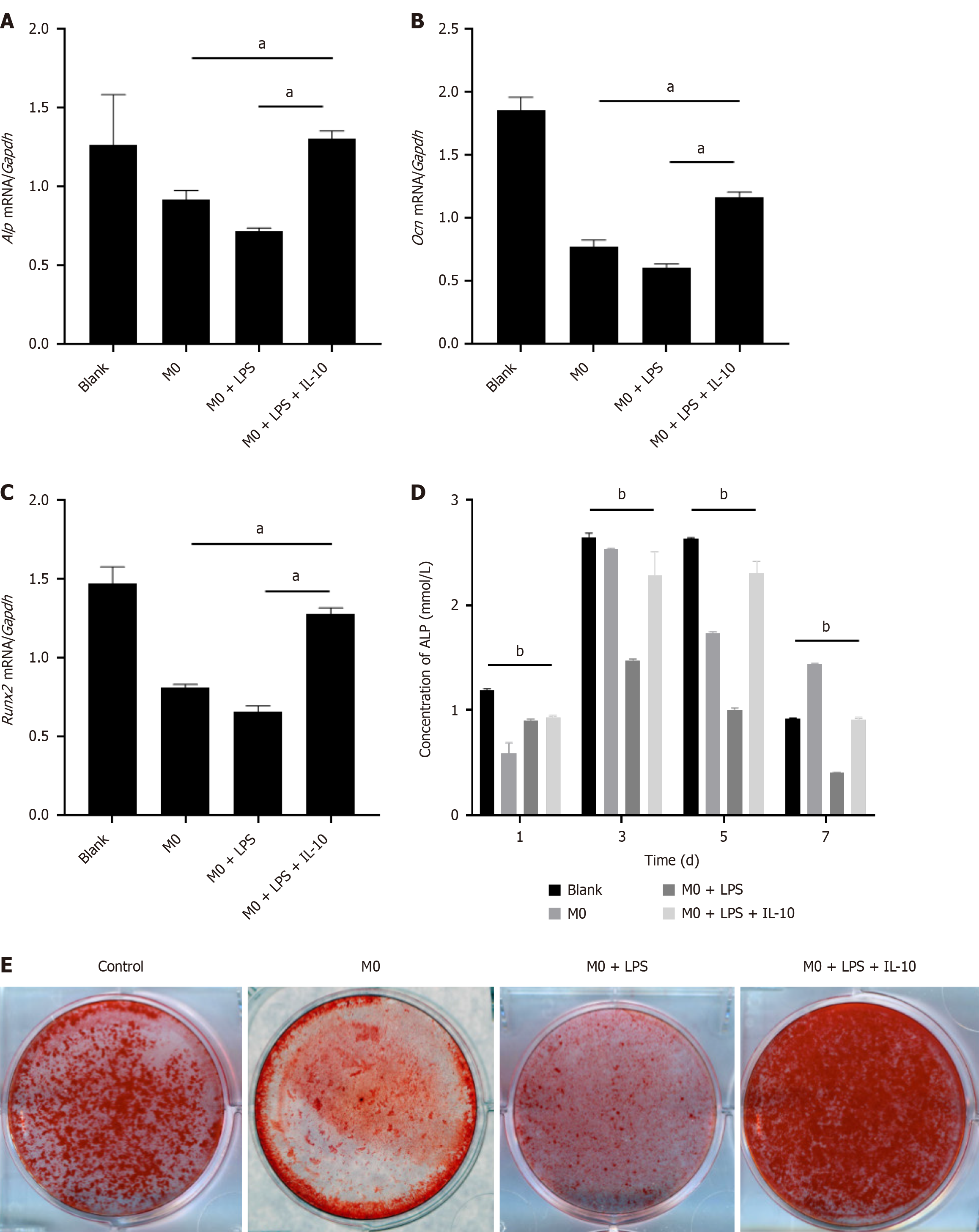
In the RT-PCR analysis of osteogenic markers [alkaline phosphatase (Alp), osteocalcin (Ocn), and related transcription factor 2 (Runx2)] in each BMSC group on the 3rd d after post-osteogenic induction, we observed a reduction in the inhibitory effect of the inflammatory state of macrophages on LPS-induced BMSC osteogenesis due to LPS. Additionally, the expression of osteoblast-related genes increased after the addition of IL-10 (Figure 4A-C).
ALP served as an early marker in osteoblast differentiation. To assess BMSC differentiation, BMSCs were cocultured with macrophages and treated with osteogenic liquids. ALP expression was measured on the 1st, 3rd, 5th, and 7th after the post-osteogenic induction. ALP expression in all BMSC groups peaked on the 3rd d. The M0 + LPS coculture group exhibited the lowest ALP expression, whereas BMSCs in the M0 + LPS + IL-10 group showed elevated ALP expression, indicating improved osteogenic differentiation in the M0 + LPS + IL-10 group (Figure 4D).
Subsequently, BMSCs were subjected to osteoblast induction for 14 d. Each group was stained with alizarin red to observe cell mineralization. Red-stained Ca2+ nodules were observed in all the groups. Photographs revealed that The M0 + LPS + IL-10 group exhibited the deepest staining, whereas the M0 + LPS group showed poor osteogenic effect. These results indicate that under inflammatory conditions, macrophages weaken the osteogenic effects of BMSCs. However, this phenomenon was ameliorated by IL-10 treatment, which restored osteogenic effects (Figure 4E).
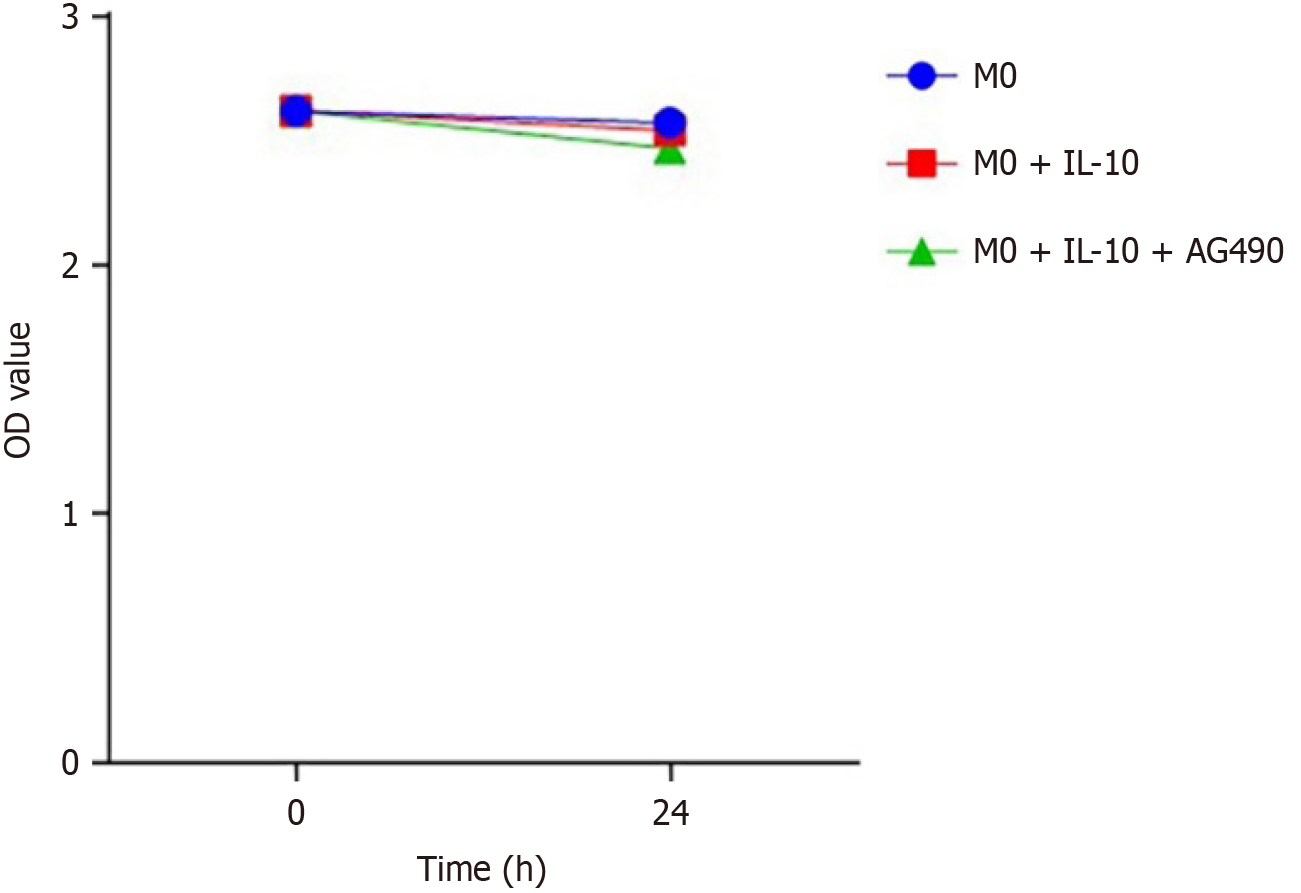
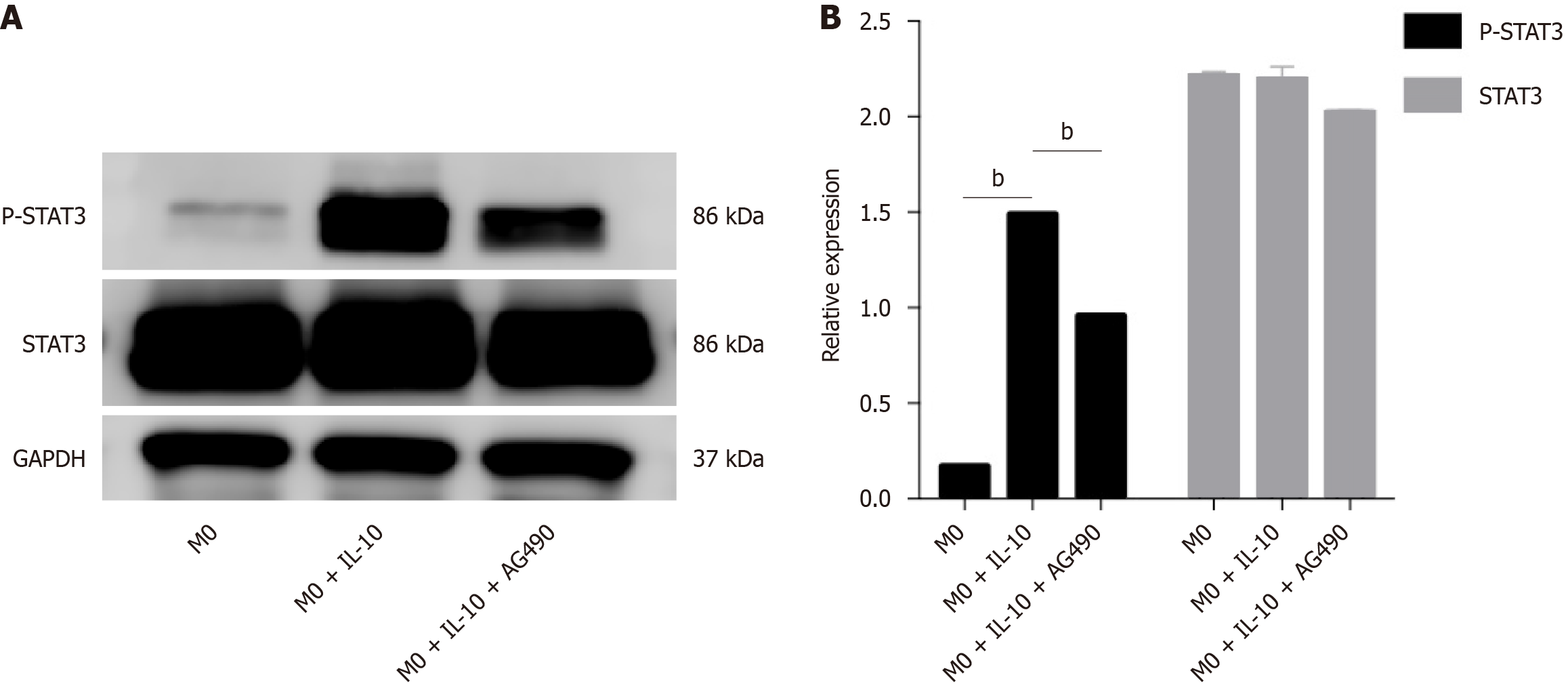
To further explore the signaling pathway of IL-10 in macrophages, we used a STAT3 phosphorylation-specific inhibitor (AG490) to elucidate its function. The effect of the STAT3 inhibitor on macrophage activity was assessed using a CCK-8 assay. Macrophage activity was not significantly affected in the STAT3 inhibitor group compared to the other groups, and the cytotoxicity of the inhibitor AG490 was negligible (Figure 5). Inhibition of STAT3 phosphorylation was evaluated by western blotting, which revealed a significant increase in p-STAT3 expression levels in macrophages after IL-10 stimulation. However, AG490 effectively inhibited STAT3 phosphorylation (Figure 6).
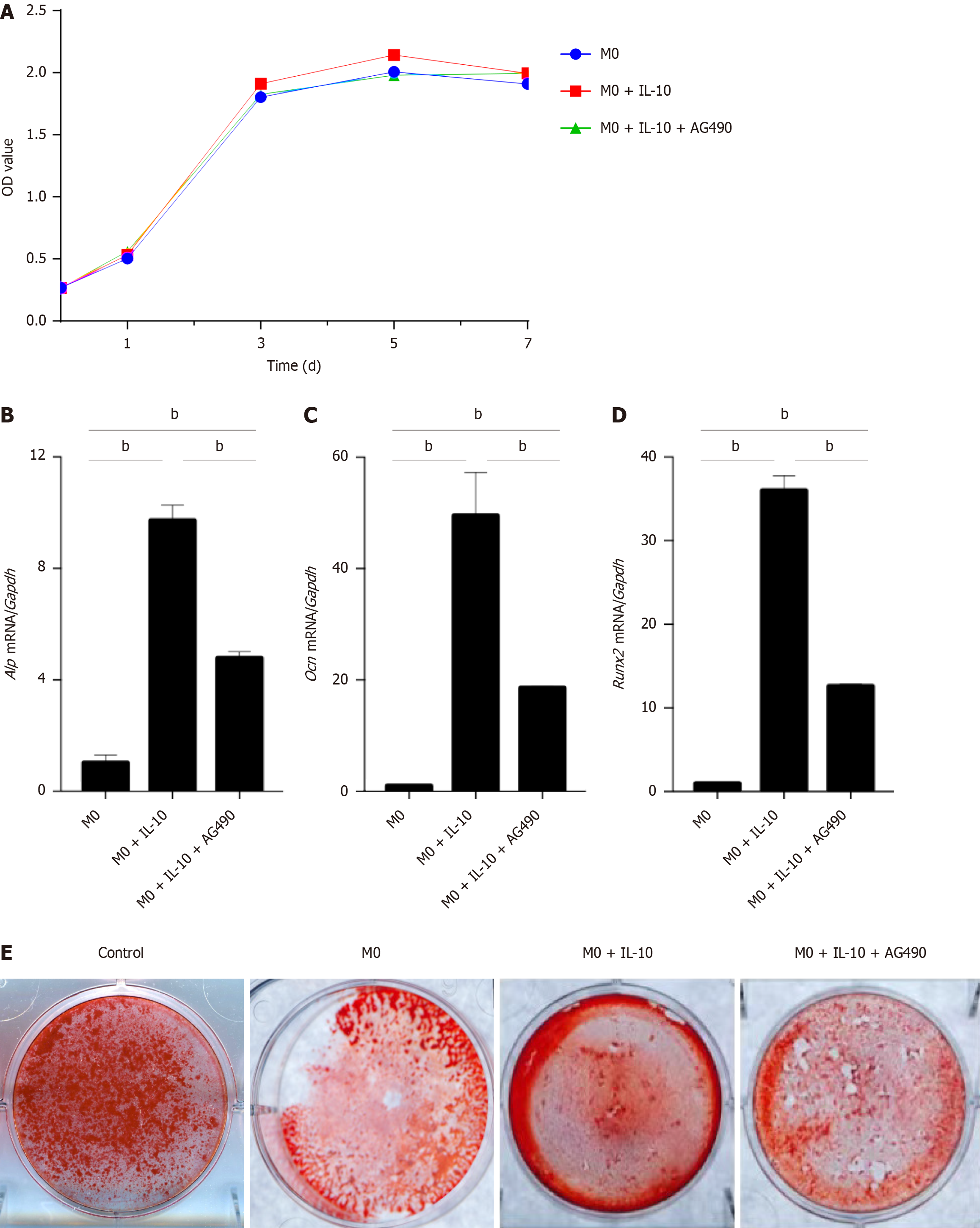
To confirm the role of STAT3 in IL-10-treated macrophages, BMSCs were co-cultured with macrophages in which p-STAT3 expression was inhibited. The proliferation of 3rd-generation BMSCs was assessed using the CCK-8 assay, and proliferation curves were plotted. The results indicated no significant difference in the proliferation rate of BMSCs between the groups. Furthermore, macrophages did not have any discernible effects on the proliferation of BMSCs in any group (Figure 7A).
RT-PCR was conducted on osteogenic markers (Alp, Ocn, and Runx2) in each group of BMSCs at 3 d post-osteogenic induction. The expression of osteogenic markers in the co-cultured BMSCs decreased after p-STAT3 inhibition in macrophages, thereby diminishing the osteogenesis-promoting effects of macrophages (Figure 7B-D).
After 14 d of osteogenic induction culture for alizarin red staining, the degree of cell mineralization was reduced after the addition of AG490 to inhibit p-STAT3 compared to the M0 + IL-10 group. These results suggested that the inhibition of STAT3 phosphorylation in macrophages weakened their ability to promote osteogenesis in BMSCs (Figure 7E).
The discovery of receptor activator of nuclear factor-κ B ligand (RANKL) has drawn attention to the relationship between the immune system and bone, leading to the emergence of a field known as “osteoimmunology”. The concept of “osteoimmunology” has significantly expanded our understanding of the role of immune cells in bone repair process[22]. BMSCs were crucial engineered cells in bone tissue engineering and represented the primary cells influenced by relevant immune cells within the framework of “osteoimmunology”[23]. During the induction of new bone formation in areas with bone defects, BMSCs initially migrated, divided, and proliferated towards the bone defect area. They differentiate into osteoblasts in response to environmental stimuli. Osteoblasts secrete collagen fibers that envelop the cells, and with the gradual deposition of calcium on these collagen fibers, osteoblasts undergo further transformation into bone cells.
Throughout this process, various types of immune cells accumulate in the defect area alongside the BMSCs. Macro
Numerous studies have indicated a close relationship between osteogenic differentiation of BMSCs and the inflammatory state of macrophages. When macrophages exhibit an imbalance in polarization, they secrete more inflammatory factors, thereby maintaining the body in a constant inflammatory state. Influenced by inflammatory factors, MSCs can alter their differentiation direction, resulting in poor bone regeneration and repair or even failure[26]. Yuan et al[27] discovered that the expression levels of osteogenic-related genes, such as Ocn, Alp, and Runx2 decreased during osteogenesis under the influence of inflammatory factors, such as TNF-α[27,28]. Inhibition of the proliferation and differentiation ability of BMSCs led to a decrease in the number of osteoblasts and a poor osteogenic effect. Besides the effects of immune cells on bone cells, there are also reciprocal effects of osteoclasts and osteoblasts on the cells of the immune system. During inflammatory conditions, the so-called “inflammatory osteoclasts” originating from dendritic cells influenced CD4+ T lymphocytes in an antigen-dependent manner and altered their TNFα production[29]. However, osteoblasts are an important source of activated complement proteins under inflammatory conditions[30], thereby activating immune system[31]. With increasing in-depth research, this field of osteoimmunology has convinced the scientific community that a two-way communication exists between the bone and immune system.
In the present study, an inflammatory environment was created by the LPS-induced differentiation of macrophages into the M1 type, followed by non-contact co-culture of the induced macrophages with BMSCs. The results also demonstrated a decrease in the expression of genes, such as Ocn, Alp, and Runx2 during osteogenesis in BMSCs, indicating that macrophages in an inflammatory environment exert an inhibitory effect on osteogenesis. Moreover, implantation of a material containing the cytokine bone morphogenetic protein-4 regulates the polarization of the mouse macrophage line RAW264.7 towards an anti-inflammatory type, ultimately promoting BMSC osteogenesis[25]. These results suggested that improving the inflammatory microenvironment can accelerate bone repair.
Previous studies have confirmed that cytokines in the osteogenic environment are crucial for bone regeneration. However, in an in vivo environment, the bone is a very specific microenvironment, and bone remodeling is a continuous dynamic equilibrium process, while repair and regeneration are ongoing. The innate immune system is the main source of various cytokines, providing an immediate response to infection and injury and initiating tissue repair[32]. Immune cells, such as macrophages utilize pattern recognition receptors (PRR) to recognize local “danger signals” such as damage-associated molecular patterns released by tissue damage caused by infectious organisms. The activation of PRRs can also lead these cells to produce inflammatory cytokines such as TNFα, IL-1, IL-6, and IFN-γ. In the bone microenvironment, these inflammatory cytokines have direct effects on both osteoclasts and osteoblasts as well as indirect effects on osteoclasts via osteoblast upregulation of RANKL[33,34]. Therefore, studying the molecular mechanisms underlying the cross-relationship between immune cells and MSCs is important to clarify the role of the immune system in recruiting MSCs during bone regeneration and regulating their differentiation. This paves the way for exploring new therapeutic methods to enhance bone regeneration by leveraging interactions between immune cells and MSCs[35].
IL-10 is a potent anti-inflammatory cytokine that participates in various cellular activities and regulates cell growth and differentiation. Although IL-10 is primarily produced by mononuclear macrophages and T cells, it can also be synthesized by immune cells, such as dendritic cells, B cells, NK cells, and neutrophils in vivo[36,37]. In one study, IL-10 was found to reduce NO production in LPS-stimulated mouse RAW 264.7, leading to a significant decrease in the expression of iNOS and cyclooxygenase 2, as well as other related proteins, resulting in a reduced inflammatory response[38]. However, although IL-10 has a strong inhibitory effect on the inflammatory response, its effect on BMSCs is two-sided. IL-10 can promote the osteogenic differentiation of BMSCs at low concentrations, while high concentrations demonstrated an inhibitory effect on the osteogenic differentiation of BMSCs. Another study discovered that a certain concentration of IL-10 enhanced the osteogenic differentiation of dental pulp stem cells (DPSCs) by regulating DPSC metabolism[27].
In this study, we focused on the role of IL-10 in the inflammatory response. Therefore, we created an inflammatory en
Most anti-inflammatory factors regulate cell expression by binding to cell surface receptors and subsequently activating various signaling pathways[39]. This is also true of the anti-inflammatory mechanisms of IL-10. STAT3 sig
Returning to the inception of our research, it holds significance not only within the realm of oral alveolar bone re
IL-10 can improve the inflammatory response of macrophages. However, macrophages in different states did not induce changes in the proliferation ability of BMSCs. Microenvironments constructed by macrophages in different states can affect osteogenic differentiation of BMSCs. Macrophages treated with IL-10 enhanced the osteogenic differentiation of BMSCs. The IL-10/STAT3 signal played a crucial role in promoting bone formation by influencing macrophages.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Cao B, China S-Editor: Wang JJ L-Editor: A P-Editor: Zheng XM
| 1. | Al-Harthi S, Barbagallo G, Psaila A, d'Urso U, Nibali L. Tooth loss and radiographic bone loss in patients without regular supportive care: A retrospective study. J Periodontol. 2022;93:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Ji H, Wang Y, Liu H, Liu Y, Zhang X, Xu J, Li Z, Luo E. Programmed core-shell electrospun nanofibers to sequentially regulate osteogenesis-osteoclastogenesis balance for promoting immediate implant osseointegration. Acta Biomater. 2021;135:274-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Zemtsova EG, Yudintceva NM, Morozov PE, Valiev RZ, Smirnov VM, Shevtsov MA. Improved osseointegration properties of hierarchical microtopographic/nanotopographic coatings fabricated on titanium implants. Int J Nanomedicine. 2018;13:2175-2188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Yang D, Xiao J, Wang B, Li L, Kong X, Liao J. The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;104:109927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Walsh MC, Takegahara N, Kim H, Choi Y. Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity. Nat Rev Rheumatol. 2018;14:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 6. | Trindade R, Albrektsson T, Tengvall P, Wennerberg A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin Implant Dent Relat Res. 2016;18:192-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 7. | Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1178] [Cited by in RCA: 1560] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 8. | He J, Chen G, Liu M, Xu Z, Chen H, Yang L, Lv Y. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;108:110411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Li X, Huang Q, Elkhooly TA, Liu Y, Wu H, Feng Q, Liu L, Fang Y, Zhu W, Hu T. Effects of titanium surface roughness on the mediation of osteogenesis via modulating the immune response of macrophages. Biomed Mater. 2018;13:045013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Lin H, Sohn J, Shen H, Langhans MT, Tuan RS. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 11. | Löffler J, Sass FA, Filter S, Rose A, Ellinghaus A, Duda GN, Dienelt A. Compromised Bone Healing in Aged Rats Is Associated With Impaired M2 Macrophage Function. Front Immunol. 2019;10:2443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Kazimierczak P, Koziol M, Przekora A. The Chitosan/Agarose/NanoHA Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation In Vitro. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081-2095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2007] [Cited by in RCA: 2093] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 14. | Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 829] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Ouyang W, O'Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 2019;50:871-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 715] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 16. | Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 617] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 17. | Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4725] [Cited by in RCA: 4953] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 18. | D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1026] [Cited by in RCA: 1077] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 19. | Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513-16521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 345] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 21. | Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013;12:489-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 22. | Yang W, Liu H, Xu L, Yu T, Zhao X, Yao S, Zhao Q, Barnes S, Cohn SM, Dann SM, Zhang H, Zuo X, Li Y, Cong Y. GPR120 Inhibits Colitis Through Regulation of CD4(+) T Cell Interleukin 10 Production. Gastroenterology. 2022;162:150-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 23. | Xiao F, Wang C, Gao Y, Zhang X, Chen X. BMPER Enhances Bone Formation by Promoting the Osteogenesis-Angiogenesis Coupling Process in Mesenchymal Stem Cells. Cell Physiol Biochem. 2018;45:1927-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Trifunović J, Miller L, Debeljak Ž, Horvat V. Pathologic patterns of interleukin 10 expression--a review. Biochem Med (Zagreb). 2015;25:36-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 25. | Sun X, Ma Z, Zhao X, Jin W, Zhang C, Ma J, Qiang L, Wang W, Deng Q, Yang H, Zhao J, Liang Q, Zhou X, Li T, Wang J. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact Mater. 2021;6:757-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Duan R, Zhang Y, van Dijk L, Barbieri D, van den Beucken J, Yuan H, de Bruijn J. Coupling between macrophage phenotype, angiogenesis and bone formation by calcium phosphates. Mater Sci Eng C Mater Biol Appl. 2021;122:111948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Yuan L, You H, Qin N, Zuo W. Interleukin-10 Modulates the Metabolism and Osteogenesis of Human Dental Pulp Stem Cells. Cell Reprogram. 2021;23:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Solís-Martínez R, Cancino-Marentes M, Hernández-Flores G, Ortiz-Lazareno P, Mandujano-Álvarez G, Cruz-Gálvez C, Sierra-Díaz E, Rodríguez-Padilla C, Jave-Suárez LF, Aguilar-Lemarroy A, Bravo-Cuellar A. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol Lett. 2018;196:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Madel MB, Ibáñez L, Ciucci T, Halper J, Rouleau M, Boutin A, Hue C, Duroux-Richard I, Apparailly F, Garchon HJ, Wakkach A, Blin-Wakkach C. Dissecting the phenotypic and functional heterogeneity of mouse inflammatory osteoclasts by the expression of Cx3cr1. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Mödinger Y, Löffler B, Huber-Lang M, Ignatius A. Complement involvement in bone homeostasis and bone disorders. Semin Immunol. 2018;37:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Halbgebauer R, Schmidt CQ, Karsten CM, Ignatius A, Huber-Lang M. Janus face of complement-driven neutrophil activation during sepsis. Semin Immunol. 2018;37:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Charles JF, Nakamura MC. Bone and the innate immune system. Curr Osteoporos Rep. 2014;12:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Baker-LePain JC, Nakamura MC, Lane NE. Effects of inflammation on bone: an update. Curr Opin Rheumatol. 2011;23:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Zhao B, Ivashkiv LB. Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res Ther. 2011;13:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Zhu Y, Liang H, Liu X, Wu J, Yang C, Wong TM, Kwan KYH, Cheung KMC, Wu S, Yeung KWK. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 36. | Li D, Wang C, Chi C, Wang Y, Zhao J, Fang J, Pan J. Bone Marrow Mesenchymal Stem Cells Inhibit Lipopolysaccharide-Induced Inflammatory Reactions in Macrophages and Endothelial Cells. Mediators Inflamm. 2016;2016:2631439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Bouvet-Gerbettaz S, Boukhechba F, Balaguer T, Schmid-Antomarchi H, Michiels JF, Scimeca JC, Rochet N. Adaptive immune response inhibits ectopic mature bone formation induced by BMSCs/BCP/plasma composite in immune-competent mice. Tissue Eng Part A. 2014;20:2950-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Neranjan Tharuka MD, Bathige SDNK, Oh M, Lee S, Kim MJ, Priyathilaka TT, Lee J. Molecular characterization and expression analysis of big-belly seahorse (Hippocampus abdominalis) interleukin-10 and analysis of its potent anti-inflammatory properties in LPS-induced murine macrophage RAW 264.7 cells. Gene. 2019;685:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Czimmerer Z, Daniel B, Horvath A, Rückerl D, Nagy G, Kiss M, Peloquin M, Budai MM, Cuaranta-Monroy I, Simandi Z, Steiner L, Nagy B Jr, Poliska S, Banko C, Bacso Z, Schulman IG, Sauer S, Deleuze JF, Allen JE, Benko S, Nagy L. The Transcription Factor STAT6 Mediates Direct Repression of Inflammatory Enhancers and Limits Activation of Alternatively Polarized Macrophages. Immunity. 2018;48:75-90.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 40. | Degboé Y, Rauwel B, Baron M, Boyer JF, Ruyssen-Witrand A, Constantin A, Davignon JL. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front Immunol. 2019;10:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 41. | Chu C, Deng J, Sun X, Qu Y, Man Y. Collagen Membrane and Immune Response in Guided Bone Regeneration: Recent Progress and Perspectives. Tissue Eng Part B Rev. 2017;23:421-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 42. | Chu C, Deng J, Xiang L, Wu Y, Wei X, Qu Y, Man Y. Evaluation of epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes and concerns on osteoblasts. Mater Sci Eng C Mater Biol Appl. 2016;67:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 43. | Chu C, Liu L, Wang Y, Wei S, Man Y, Qu Y. Macrophage phenotype in the epigallocatechin-3-gallate (EGCG)-modified collagen determines foreign body reaction. J Tissue Eng Regen Med. 2018;12:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |