Published online Feb 26, 2024. doi: 10.4252/wjsc.v16.i2.176
Peer-review started: October 20, 2023
First decision: December 5, 2023
Revised: December 15, 2023
Accepted: January 19, 2024
Article in press: January 19, 2024
Published online: February 26, 2024
Processing time: 128 Days and 14.7 Hours
Cartilage defects are some of the most common causes of arthritis. Cartilage lesions caused by inflammation, trauma or degenerative disease normally result in osteochondral defects. Previous studies have shown that decellularized extracellular matrix (ECM) derived from autologous, allogenic, or xenogeneic mesenchymal stromal cells (MSCs) can effectively restore osteochondral integrity.
To determine whether the decellularized ECM of antler reserve mesenchymal cells (RMCs), a xenogeneic material from antler stem cells, is superior to the currently available treatments for osteochondral defects.
We isolated the RMCs from a 60-d-old sika deer antler and cultured them in vitro to 70% confluence; 50 mg/mL L-ascorbic acid was then added to the medium to stimulate ECM deposition. Decellularized sheets of adipocyte-derived MSCs (aMSCs) and antlerogenic periosteal cells (another type of antler stem cells) were used as the controls. Three weeks after ascorbic acid stimulation, the ECM sheets were harvested and applied to the osteochondral defects in rat knee joints.
The defects were successfully repaired by applying the ECM-sheets. The highest quality of repair was achieved in the RMC-ECM group both in vitro (including cell attachment and proliferation), and in vivo (including the simultaneous regeneration of well-vascularized subchondral bone and avascular articular hyaline cartilage integrated with surrounding native tissues). Notably, the antler-stem-cell-derived ECM (xenogeneic) performed better than the aMSC-ECM (allogenic), while the ECM of the active antler stem cells was superior to that of the quiescent antler stem cells.
Decellularized xenogeneic ECM derived from the antler stem cell, particularly the active form (RMC-ECM), can achieve high quality repair/reconstruction of osteochondral defects, suggesting that selection of decellularized ECM for such repair should be focused more on bioactivity rather than kinship.
Core Tip: Our study has shown that a decellularized xenogeneic extracellular matrix (ECM) derived from antler reserve mesenchymal cells, provided high quality repair of osteochondral defects in rat knee joints. This was achieved without detectable immune reaction in the recipient animals. This finding provides insights for the development of an effective therapeutic material for osteochondral defect repair and highlights the opportunity for use of this novel stem cell-derived ECM.
- Citation: Wang YS, Chu WH, Zhai JJ, Wang WY, He ZM, Zhao QM, Li CY. High quality repair of osteochondral defects in rats using the extracellular matrix of antler stem cells. World J Stem Cells 2024; 16(2): 176-190
- URL: https://www.wjgnet.com/1948-0210/full/v16/i2/176.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i2.176
Because of their avascular nature and the resultant limitations on nutrient supply, cartilage lesions caused by inflammation, trauma or degenerative disease of joints normally result in osteochondral defects, which eventually lead to osteoarthritis[1-3]. Currently, it is difficult to fully repair this type of damage because three types of tissue are involved in the process, namely articular cartilage (avascular), the osteochondral interface and the subchondral bone (richly vascularized), all of which need to be regenerated simultaneously[4].
Cell-based approaches with or without biomaterial scaffolds have been developed for the treatment of osteochondral defects[5-7]. Mesenchymal stromal cells (MSCs) not only self-renew, but also differentiate into many other distinct cell types[1,2,5]. Hence, they are an ideal candidate for cell-based therapies. However, because of the scarcity of MSCs in adult bone marrow (BM) (approximately 0.001%) and in some other types of MSCs that may be more readily available, such as adipose tissue-derived MSCs[6,8], the MSCs must be expanded in vitro to provide sufficient numbers for implantation. The expansion of MSCs requires a medium containing fetal bovine serum (FBS), which imposes biosafety concerns, such as xeno-immunization and the risk of disease transmission[9-11]. Although specially designed serum-free media (SFM) containing various growth factors has been developed to overcome the xeno-serum problem for propagating MSCs in vitro[12,13], the SFM still lacks a critical component found in the native MSC environment, namely the extracellular matrix (ECM). In vivo, MSCs are surrounded by a rich ECM, including collagens, adhesion proteins, proteoglycans, and growth factors which together, provide a unique microenvironment or “niche”[14,15].
Certain type of engineered cell-free scaffolds capable of recruiting host endogenous MSCs can undoubtedly circumvent the problems that arise from a cell-based approach[16]. Unfortunately, there are few synthetic materials that can fill this role for osteochondral regeneration. Notably, Chen et al[17] fabricated an ECM-sheet by mimicking the natural approach using BM-derived MSCs (bMSCs). However, the bMSC-ECM comprises at least 70 different components although the main constituents are collagens (types I and III), fibronectin, small leucine-rich proteoglycans (biglycan and decorin), and basement membrane constituents (perlecan and laminin). It is believed that these matrix proteins play key roles in regulating cell adhesion, migration, proliferation, differentiation, and survival. MSCs can be readily expanded on a bMSC-ECM-sheet in vitro, and when implanted into immunocompromised mice, this approach generated five times more bone and eight times more hematopoietic marrow tissue than MSCs expanded on a plastic surface[18-20].
Jin et al[21] constructed ECM-sheets derived from cartilage cells, and after freeze-drying to remove the residential cells, the sheets, including cultured allogenous chondrocytes, were implanted into the articular cartilage defects. The outcome was that the defects were repaired by filling with well-formed hyaline cartilage tissue. Wei et al[22] reported that ECM-sheets, made by adding vitamin C (VC) to cultured periodontal ligament stem cells, while keeping these cells intact, effectively treated periodontal defects in a swine model. Yoshida et al[23] established a method in a rat femoral meta
Consequently, it seems that ECM-sheets, without retained/attached cells, can still effectively repair osteochondral defects/damage by forming hyaline cartilage-like tissue. In fact, ECM-sheets with allogenic/xenogeneic cells can cause unwanted side-effects. These include host inflammatory reactions, limited capacity to generate microscale vascularization, different rates of cell proliferation compared with scaffold degradation, and the inability to generate functional tissues with the architectural complexity of native tissues[22]. Above all, the cells that are to be used with ECM-sheets must be autologous to avoid causing immune rejection; thus, the clinical application of cell-containing MSC-ECM is restricted due to limited source of autologous cells and inevitable donor morbidity. In contrast, should cell-free MSC-ECM prove equally effective, an almost unlimited source can become available. In such cases, even xenogeneic decellularized ECMs can be used (e.g., from adipose tissue and skin) and these xenogeneic ECMs have shown excellent biocompatibility and biological safety[25-27].
Deer antlers are the only mammalian organ that can fully regenerate and are known for their very rapid elongation during their growth phase[28]. Antlers are organs of bone and formed through endochondral ossification[29].The growth centre of the antler is located in the tip and the antler reserve mesenchymal cells (RMCs) reside in the outermost layer of the centre with sequential differentiation towards cartilage cells through precartilage cells[30]. Hence, the cells of the antler growth centre could offer an abundant source of MSCs, precartilage and cartilage cells. Most importantly the ECM produced by these cells can sustain a very high rate of expansion of cartilage tissue as evident in regenerating antlers (2 cm/d). The aim of the present study was to evaluate whether RMC-ECM from regenerating antlers could reproduce its potent effects in the repair of osteochondral defects in rats through implantation of cell-free RMC-ECM sheets. Positive results would provide a new, almost unlimited, source of effective cell-free MSC-ECM, compared with those currently available, that could be evaluated for applications in human health.
All experiments were performed in accordance with the guidelines and study protocols of the Animal Ethics Committee of the Institute of Antler Science and Product Technology, Changchun Sci-Tech University (AEC No: CKARI202309). Thirty-six healthy, 8-wk-old, male Sprague-Dawley rats were used in this study. They were randomly divided into four groups (9/group) with three treatment groups [aMSC, antlerogenic periosteal cell (APC) and RMC] and a control [BM stimulation (BMS)].
aMSCs: Adipose tissue was obtained from the inguinal region of a 4-wk-old rat and rinsed immediately with phosphate buffered saline (PBS). The tissue was cut into small pieces using scissors. Collagenase (Wako Pure Chemical Corp.) was dissolved in PBS (150 U/mL collagenase; Invitrogen, United States) and used for digestion of adipose tissue at 37 °C to release aMSCs. After completion of the reaction, 20 mL standard medium containing 10% FBS (and 1% Penicillin-Streptomycin solution) was added to quench the collagenase activity prior to filtering the resultant solution. The filtrates were centrifuged at 170 × g for 5 min, and the resultant cell fraction was cultured in the standard medium in an incubator at 37 °C. The aMSCs were cultured up to the second passage before being frozen down.
bMSCs: bMSCs were derived from 4-wk-old male rats as per a previous report[31]. Briefly, after euthanization, femurs were collected under sterile conditions and thoroughly rinsed in PBS. Both ends of each femur were cut and BM was flushed out of the cavity using 5 mL complete culture medium. The collected BM was cut into fine pieces and the suspension was transferred to a 100 mm cell culture dish, which was then placed in an incubator (37 °C and 5% CO2) for culture. After 24-h cultivation, the dish was washed using PBS, non-adherent cells were removed and replaced by fresh medium. When the culture reached around 85%-90% confluence, the cells were detached enzymatically and split 1:2 for the continuous culture. At the time of testing, the bMSCs were at the third passage.
APCs and RMCs: Antlerogenic periosteum (AP, tissue that overlies a frontal crest in the prepubertal male deer), was obtained from a 6-month-old male sika deer calf following the procedure reported by Li and Suttie[32]. Reserve mesenchyme (RM) in the growth centre of a 60-d-growing antler was harvested from a 4-year-old sika deer following the procedure reported by Li et al[30]. Both AP and RM tissues were cut into small pieces (about 0.7 mm in thickness) using our custom-made tissue cutter (Patent No: ZL201420335401.8). These small tissue pieces were digested in the digestion medium (DMEM containing 150 U/mL collagenase, Invitrogen, United States) at 37 °C for around 1 h to release APCs or RMCs and then centrifuged at 170 × g for 20 min. Each precipitate was resuspended and washed in 10 mL culture medium (DMEM containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin). The washed precipitate was transferred to T75 cell culture flasks (Nunc, Danmark) containing 20 mL culture medium, and the flasks were cultured in an incubator (37 °C and 5% CO2). The APCs and RMCs were cultured to the second passage before being frozen down.
Each cell type (aMSCs, APCs and RMCs, at the third passage) was seeded in 100 mm petri dishes containing 12 mL culture medium; when the cells had reached 70% confluence, 50 μg/mL L-ascorbic acid (Sigma-Aldrich, United States) was added to the medium to stimulate ECM deposition[33]. Three weeks after ascorbic acid stimulation, the formed aMSC-ECM, APC-ECM and RMC-ECM sheets were peeled gently from the petri dishes, using a pair of fine tweezers, washed gently but thoroughly in PBS at 37 °C, and subsequently treated with 0.5% Triton-X100 containing 20 mmol/L NH4OH for 30 min to remove residential cells. To further clear residual nuclear debris from these ECM-sheets, a nucleic acid scavenging solution containing DNA enzyme (50 U/mL) and RNase (1 U/mL) was used. The resultant ECM sheets were washed in PBS three times, in sterile distilled water once, and then stored at 4 °C for future use.
Each type of ECM-sheets (aMSC, APC and RMC) was divided into two groups (3 per group) for the purpose of quality assessment pre- and post-decellularization. Each ECM-sheet was fixed in 4% paraformaldehyde, dehydrated in a series of ethanol ‘washes’, embedded in paraffin wax, and sectioned at 6 μm thickness to assess whether the ECM sheets were cell-free under a microscope. The comparison between pre- and post-decellularization in each type of ECM-sheet (aMSC, APC and RMC) proceeded as follows: DNA contents were measured using a dsDNA extraction kit (Takara Bio, Japan); the content of glycosaminoglycan (GAG) was measured using a dimethylmethylene blue colorimetric assay (GAG test kit, Yuduo Shanghai); the content of collagen was measured using a test kit for the main indicator of collagen, hydro
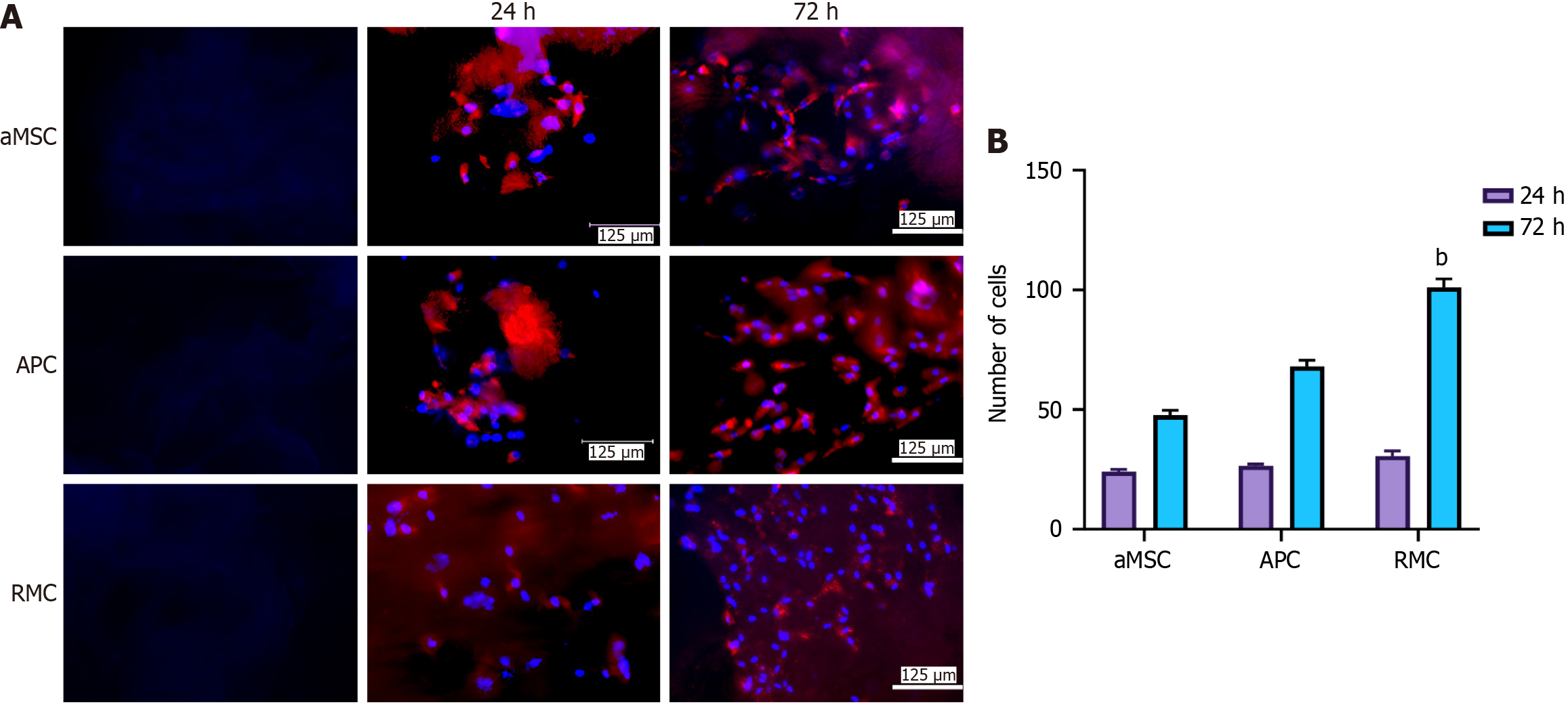
The bMSCs were labeled with PKH26 according to the manufacturer’s instructions (Sigma-Aldrich, United States) and then cultured on each ECM-sheet to assess the effects of different ECMs on the attachment and proliferation of bMSCs. The procedures were as follows: The third passage of each bMSC was digested with 0.25% trypsin, and then suspended in 1 mL DMEM complete medium after a PBS wash. The cells (in total, 4.7 × 106) were mixed with 1 μL dye and 0.5 mL reagent C evenly and incubated for 3 min at 37 °C. The incubation was stopped by adding the same volume of FBS. The solution was centrifuged at 400 × g for 5 min and the supernatant decanted before adding 1 mL culture medium to re-suspend the cells. The PKH26 labeling status was assessed under a fluorescent microscope after 24 h culture.
For each type of ECM-sheet, PKH26-labeled bMSCs (2 × 104 cells/type/sq.cm) were inoculated onto the sheet and then cultured in an incubator (37 °C and 5% CO2). At 24 h and 72 h respectively, a sheet was removed from the medium and fixed in 4% paraformaldehyde for 15 min. After washing with PBS, 1 mL 4’,6-diamidino-2-phenylindole (DAPI) (Product, Beyotime, China) staining solution was added to the sheets for 5 min, and the status of cell attachment and proliferation was assessed under fluorescent microscope (EVOS M5000, United States). The number of cells in the selected view field of was counted using ImageJ.
Both knee joints of the hind legs of each rat were operated on. Arthrotomy was performed using the medial parapatellar approach. The patella was dislocated laterally to expose the articular capsule. A full-thickness cylindrical osteochondral defect (1.5 mm in diameter and 2 mm in depth) was created in the trochlear groove using a 1.5-mm drill. After removing cartilage and bone debris, the boundary surrounding each defect was trimmed using a pair of surgical scissors. Treatments were carried out immediately after surgery with four groups of rats (9 rats/group) namely, BMS (the control group) plus the three treatment groups (aMSC, APC and RMC). In the control group, the defects on each knee were left untreated after BMS; in the treatment groups, the defects on each knee were filled with the particular type of ECM-sheet (1/4 of each sheet for each defect hole) via fitting under pressure. After the operation, tramadol analgesia was administered (50 mg/kg; intramuscular injection; once a day for 3 d). In each group, three rats were sacrificed at each of 4, 8 and 12 wk after surgery and treatment, and the tissues from the treated areas were collected for further analysis.
Femurs of both knees from each rat were collected after removing surrounding periarticular soft tissues. The defect areas of the articular cartilage samples were photographed using a high-resolution camera (Canon) to reveal the status of repair. Macroscopic assessment based on the International Cartilage Repair Society score (ICRS) was performed by three raters for each experimental group and the mean was calculated. For histological evaluation, the samples were fixed in 10% formalin for 48 h, decalcified with formic acid for 10 d, embedded in paraffin wax, and sectioned at 6 μm. The sections were then stained with Alcian blue or Safranin O, respectively.
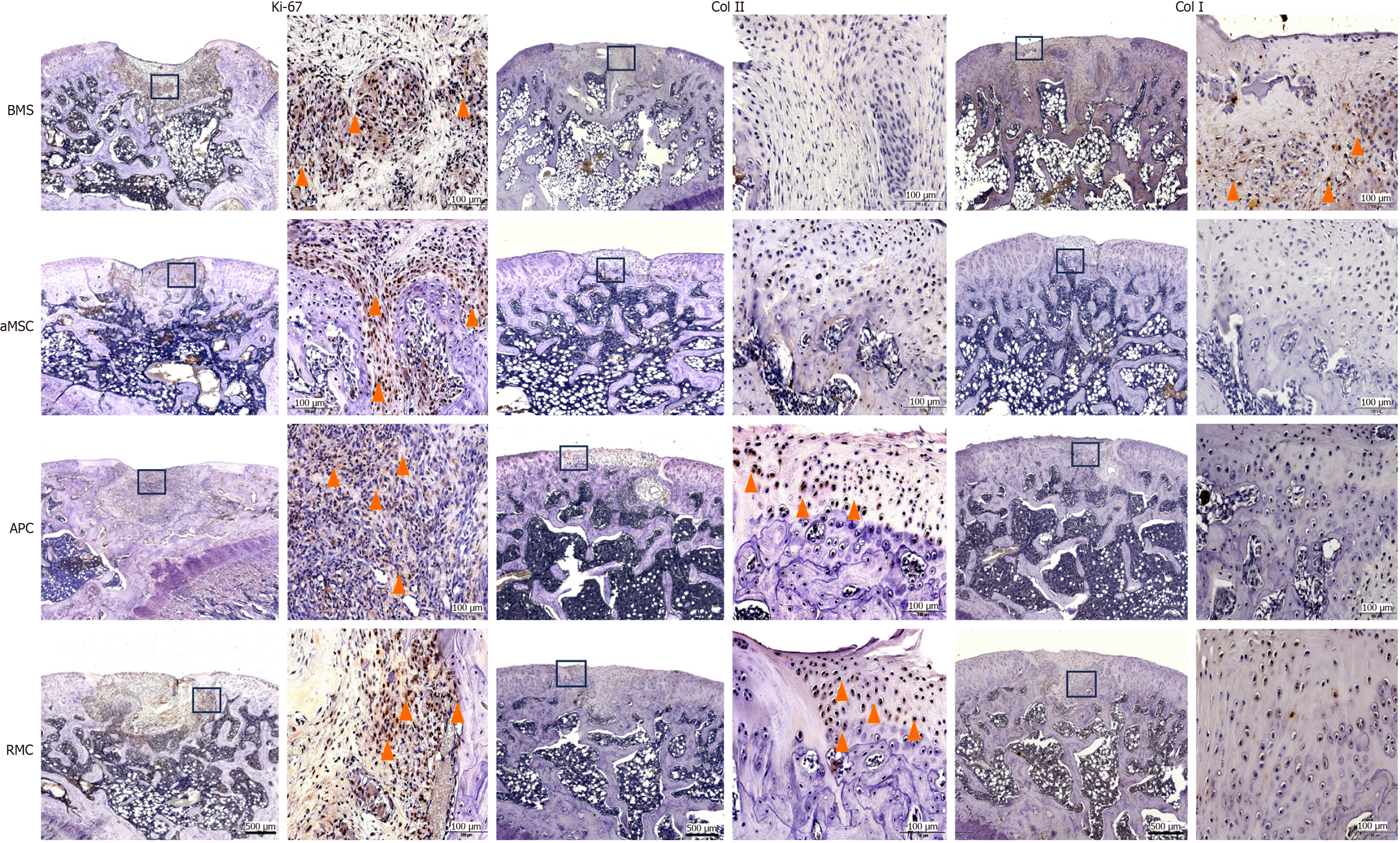
Ki-67, type I collagen (Col I) and type II collagen (Col II) were detected via immunohistochemistry (IHC) using a 9710 kit (Maixin Biotechnologies, China). Mouse monoclonal antibodies of anti-rabbit ki-67, Col I and Col II were used (Abcam, Cambridge, United Kingdom). The procedure for IHC was: Following dewaxing and rehydration, antigen retrieval was performed in the boiled sodium citrate buffer for 15 min, endogenous peroxidase was quenched through incubation with H2O2, and non-specific reaction sites were blocked by use of goat serum at room temperature for 10 min. The first antibodies of Col I and Col II (1:200) and ki-67 (1:1000) were added to the tissue sections and incubated at 4 °C overnight. Next, slides were incubated with biotinylated goat-anti-rabbit immunoglobulin G antibody. Diaminobenzidine was used as the chromogenic agent (15 min at 37 °C) and incubated with avidin peroxidase reagent and hematoxylin for counter-staining. Finally, slides were photographed under a microscope (EVOS M5000, United States). We selected six random fields per section and six sections in total (2 sections/rat from 3 rats) for quantification of IHC results. The IHC results were calculated via ImageJ.
The results are presented as mean ± SD. Statistical significance was evaluated using GraphPad Prism 9.0.0 software. Statistical analysis for the comparisons of multiple variables was performed using a two-way ANOVA, and Student’s t-test was used to compare two variables. All experiments were performed in triplicate. The values were set at P < 0.05 for statistical significance.
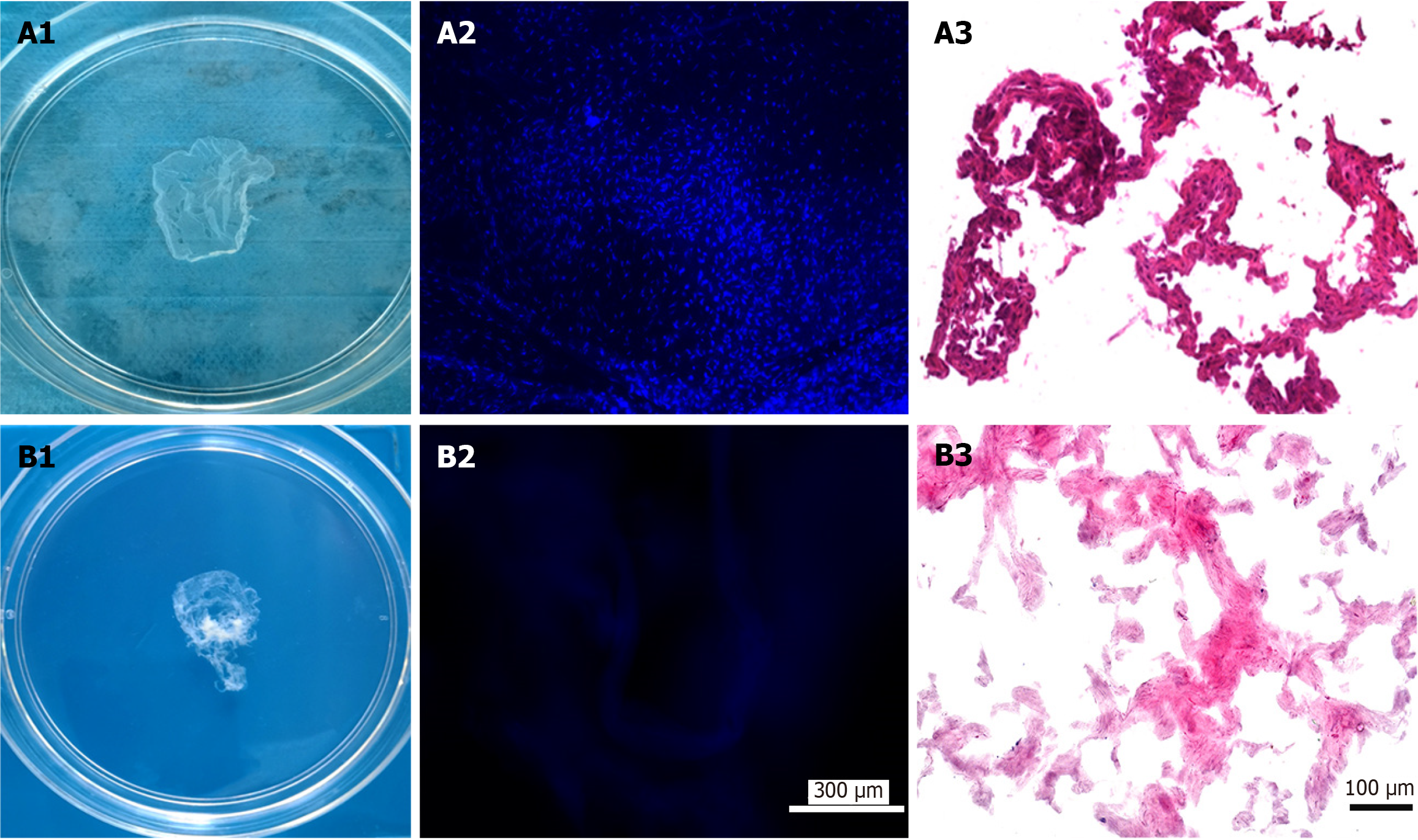
Before decellularization, the fabricated ECM-sheets appeared intact with a smooth surface (Figure 1A-A1; Supple
The quantitative results (Table 1) show that decellularization almost completely eliminated cellular DNA contents from all types of ECM-sheets: aMSC, APC and RMC were reduced to 1.9%, 2.2% and 2.8% of their original levels, respectively, and the residual DNA was detected at 23.22 ng/mg in the RMC group. After decellularization, the contents of GAG and collagen retained in the ECM-sheets were: 79.8% and 66.2% (aMSC), 81.8% and 72.7% (APC) and 90.0% and 80.8% (RMC). Therefore, the decellularization process, besides removing the DNA, essentially preserved other extracellular compo
| ECM sheet | DNA | GAG | Collagen | ||||||
| Before (ng/mg) | After (ng/mg) | Retention rate (%) | Before (ng/mg) | After (ng/mg) | Retention rate (%) | Before (ng/mg) | After (ng/mg) | Retention rate (%) | |
| aMSC | 1287.36 ± 51.28 | 25.73 ± 3.61 | 1.9 | 472.73 ± 21.54 | 376.93 ± 11.83 | 79.83 | 756.93 ± 17.37 | 500.49 ± 9.13 | 66.18 |
| APC | 1017.78 ± 35.92 | 26.27 ± 1.6 | 2.2 | 559.21 ± 10.93 | 457.31 ± 5.13 | 81.79 | 874.71 ± 23.53 | 635.62 ± 29.61 | 72.67 |
| RMC | 1194.36 ± 64.37 | 23.22 ± 2.94 | 2.8 | 611.83 ± 14.29 | 550.78 ± 6.27 | 90.00 | 915.61 ± 27.37 | 740.08 ± 15.52 | 80.84 |
To assess the suitability of these MSC-sheets for cell attachment and proliferation, we seeded bMSCs on each type of sheet in vitro. The results showed that bMSCs were readily attached and actively proliferated at 24 h after seeding (Figure 2A), although no significant difference was detected amongst the three types of ECM-sheets (Figure 2B). At 72 h, cell numbers in each group were significantly increased compared to those at 24 h (P < 0.001). There were also significant differences in cell numbers amongst the three groups: The RMC (P < 0.01) and the APC (P < 0.05) groups were significantly higher than the aMSC group (Figure 2B). Our conclusion was supported by the evidence that PKH26 color (red) was more diluted on the cells of the RMC group than those of the aMSC group and APC group due to more cycles of division (Figure 2A).
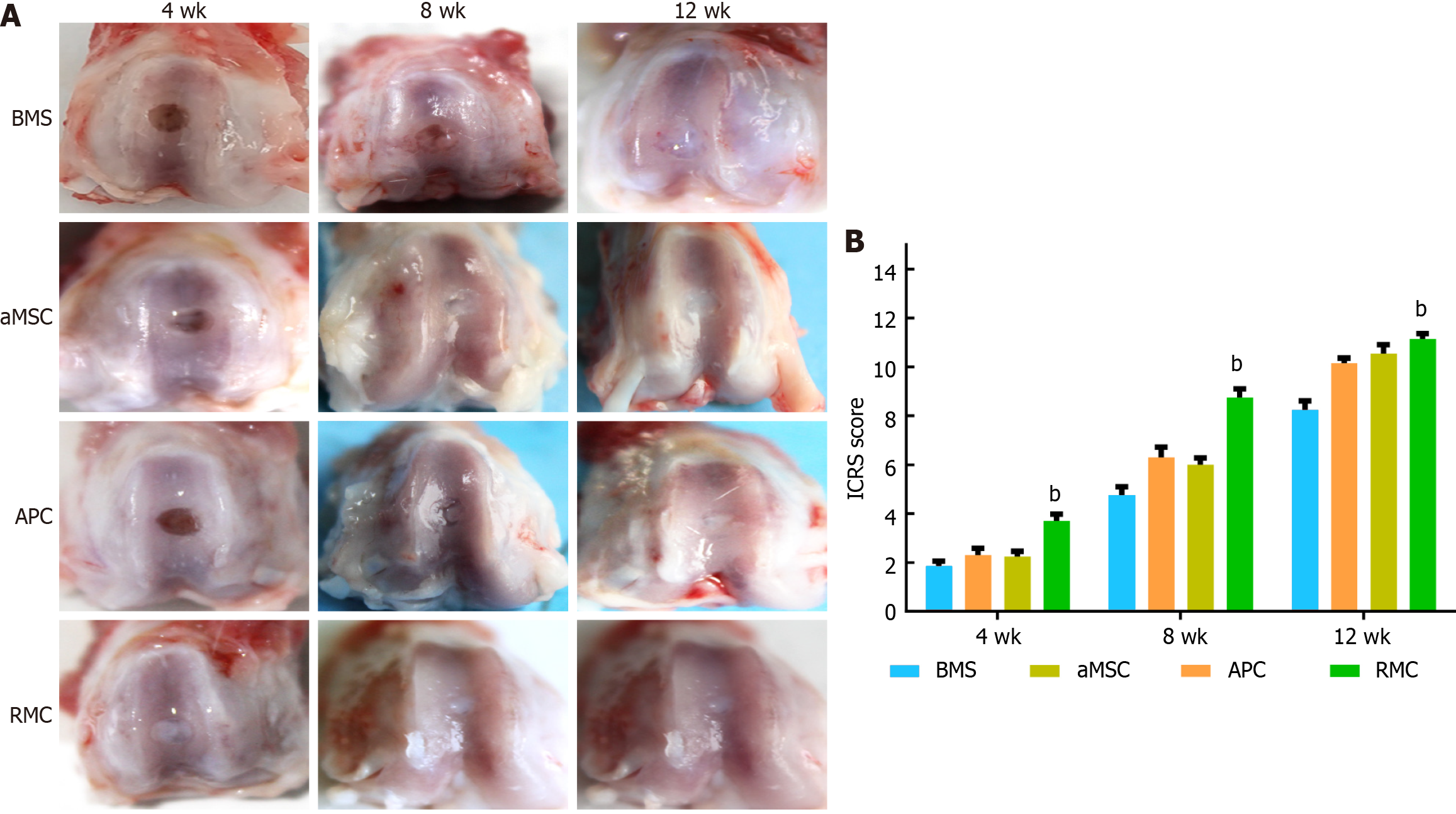
At the morphological level: There was a general trend in the degree of repair over the time points (4, 8 and 12 wk) and with the different types of ECM treatment (BMS, aMSC, APC and RMC; Figure 3A).
At week 4, the appearance of the defects in the respective groups were: BMS - appeared empty with a reddish color and without any visible regenerated tissue; aMSC - showed some white-colored tissue but defects were still deeply concave; APC - showed markedly more tissue than the aMSC group but the reddish-colored tissue appeared watery; RMC - defects appeared fully filled with firm ivory white-colored tissue.
At week 8, the sizes of the defects in the four groups were substantially reduced although to various degrees. The defects in the aMSC, APC and RMC groups were filled with a white-colored tissue, but the defects in the BMS group were still partly filled and contained a fibrous-like tissue.
At week 12, the sizes of the defects in all four groups were further reduced although still to varying degrees among the different groups: The defects in the RMC and APC groups were the smallest and BMS and aMSC groups were the largest. All the defects appeared to be fully filled with a firm white-colored tissue (cartilage-like), although the demarcation between the preexisting cartilage and newly regenerated cartilage-like tissue was still clearly discernible. The ICRS scores (Figure 3B) were consistent overall with the results of the morphological observation: RMC group was the highest (best) and BMS group was the lowest (worst) in terms of the quality score of repair.
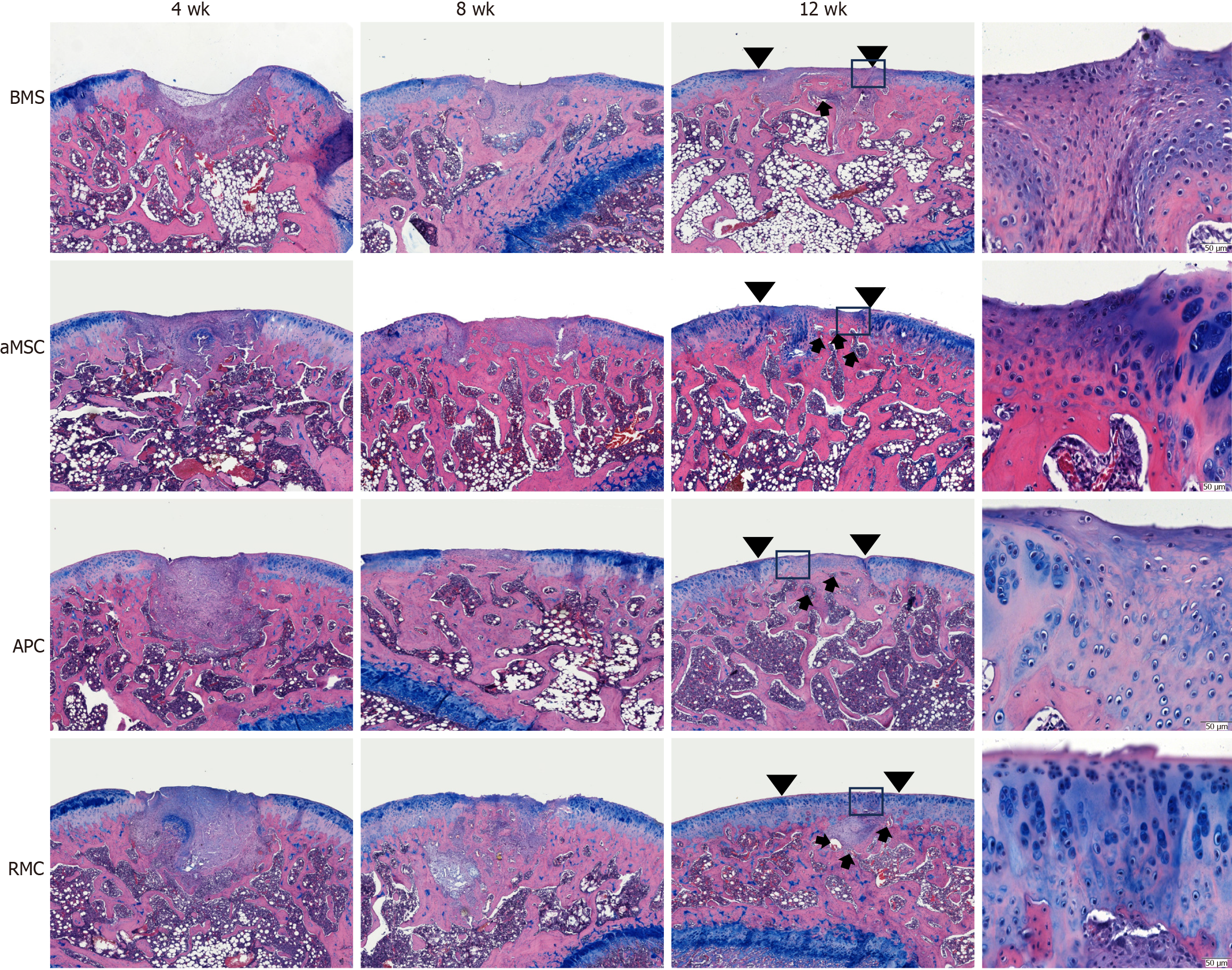
At the histological level: Similar trends were observed at the histological [HE and alcian blue (AB) counter-staining] level as at the morphological level (Figure 4; Supplementary Figure 3).
At week 4, the defects in the BMS group were at a very early stage of repair with fibrous tissue; in the other three groups, the defects were almost fully filled with regenerated tissue; however, only the RMC group had regenerated substantial cartilage tissue, while the aMSC and APC groups were filled with either osseous or fibrous tissue (lack of AB staining) and were still slightly concave on the surfaces of the defects.
At week 8, the difference between the newly formed (replacement) and the native tissues was still discernible. The defect surface of each rat in the BMS group was still slightly concave and the tissue was mainly fibrous in nature. The defects in both the aMSC and APC groups had almost been repaired fully, albeit solely with osseous tissue. Hyaline cartilage was detected only on the defect surface in the RMC group and merged with the native hyaline cartilage of the joints, although the surface was not as smooth as the original tissue.
At week 12, the repair process of defects in all groups was essentially complete although the replacement tissue varied in nature and arrangement in the different groups. The replacement tissue in the BMS group was a mixture of osseous and fibrous tissues; in the aMSC group, it was mainly osseous tissue with a very thin layer of fibrous (inset) cartilage surface (around 1/5-1/3 thickness of existing cartilage layer); in the APC group, it was osseous tissue of a similar level of thickness to the existing cartilage surface, but fibrous in nature (inset); in the RMC group, it was osseous tissue of a similar level of thickness to the hyaline cartilage surface (inset) while the regenerated cartilage surface appeared to merge seamlessly with the existing surrounding cartilage (inset).
At the molecular level: Expression levels of critical factors (Ki-67, Col I and Col II) in the different groups and at different time points of the repair process varied greatly based on the results of IHC (Figure 5).
At week 4, proliferating cells (Ki-67 positive) were densely populated in the regenerating tissue in the defects in all groups. In the BMS and aMSC groups, most dividing cells were located either in the perivascular area or intertrabecular area; in the APC group, dividing cells were more evenly distributed in the ‘repairing’ trabecular bone area of the defects; in the RMC group, dividing cells were mainly located in the vicinity of trabecular bone and seemed to be actively participating in bone tissue formation (Supplementary Figure 4).
By week 12, the repair process of defects in all groups was essentially complete. Col II was detected only in the top layers covering the defects in the APC and RMC groups (Supplementary Figure 5), indicating that these layers covering the defects in the APC and RMC groups were hyaline cartilage-like, and those in the BMS and aMSC groups were either fibrous- (BMS) or fibrocartilage- (aMSC) like. In contrast, Col I was detected only in the top layer covering the defects in the BMS group, whereas it was barely detectable in the rats in the other three groups (Supplementary Figure 6), which further confirms that the top layer of regenerated tissue in the defects of the BMS group was fibrous in nature.
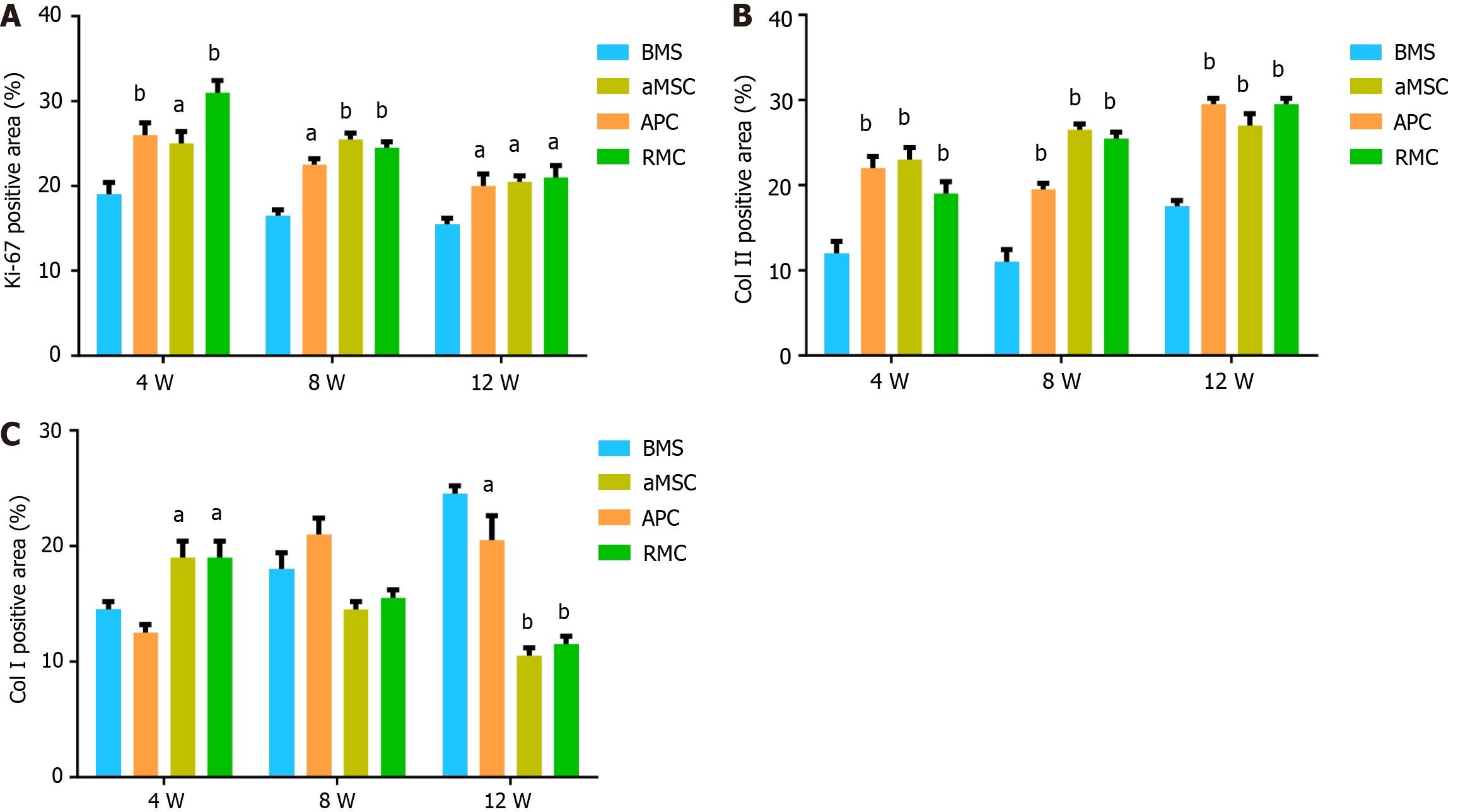
Quantification of the IHC results confirmed the staining findings. Overall, the number of dividing cells (Figure 6A) and the Col II (Figure 6B) content in the BMS group were significantly lower than those of the other three groups over the 12-wk period. The Col II contents in the APC and RMC groups were significantly higher than that of the aMSC group and the latter was significantly higher than that of the BMS group (Figure 6B). The changes in Col I content were more complex than those of Col II (Figure 6C): Initially (week 4), the APC and RMC groups were significantly higher than the BMS and aMSC groups; but from week 8 onwards, the Col I contents of the rats in the BMS and aMSC groups became significantly higher than APC and RMC groups, indicating that hyalin cartilage came to dominate in the later stage of repair in the antler stem cell group.
To the best of our knowledge, this is the first investigation where decellularized antler ECM-sheets [a type of xenogeneic ECM derived from either quiescent (APC) or active (RMC) stem cells] have been used to treat full osteochondral defects. The results have shown that the defects were successfully repaired by applying these ECMs without adding living cells.
The best results of repair were achieved in the RMC group; this included both simultaneous regeneration of well-vascularized subchondral bone and avascular articular hyaline cartilage that integrated with the surrounding native tissues. The defects in the APC group were less well repaired but were superior to the aMSC group; the poorest results were found in the BMS group, where the defects were filled mainly with fibrous tissue/fibrocartilage. Therefore, our study demonstrates that decellularized xenogeneic ECM (such as RMC), without attached living cells, can repair osteochondral defects to a very high level of tissue integrity. This finding underscores the great promise of decellularized xenogeneic ECM for osteochondral repair in the clinical setting.
BMS techniques, such as drilling, abrasion, and microfracture, have been developed to stimulate the migration of endogenous bMSCs into the damaged area of articular cartilage, thereby enhancing defect repair[31]. However, the repaired tissue resulting from applying BMS techniques is mainly fibrous tissue or fibrocartilage, which are inferior to the original hyaline cartilage[8]. To address this problem, Gille et al[34] combined the BMS technique with autologous ECM to fill the defects and found that the ECM significantly improved the outcomes of BMS by forming hyaline cartilage. However, there are many drawbacks associated with using autologous ECM, such as donor morbidity and the limited source of autologous cells[1,7,35].
Notably in this respect, Wang et al[25] found that ECM derived from allogeneic bMSCs can successfully substitute for autologous ECM to facilitate repair of osteochondral defects, and surprisingly without causing any obvious immune reaction when the allogenic ECM had been decellularized prior to implantation. Hoganson et al[36] and Benders et al[27] took this finding further and found that decellularized xenogeneic ECM scaffolds from several tissues, including the dermis, cornea, and small intestine, could be used successfully for treating osteochondral defects, because of their superior bioactivity and biocompatibility[27,37]. Along this line, in the present study we used an alternative type of xenogeneic ECM, namely an ECM derived from antler stem cells, that successfully repaired osteochondral defects; the ECM from the active antler cells (RMCs) achieved what appears to be, perfect repair, without causing detectable immune reaction and inflammation.
The reasons that ECM from deer antler stem cells (xenogeneic) performed better than that from rat aMSC (allogenic) in the repair of osteochondral defects, and why antler ECM from active cells (RMCs) was better than that from quiescent cells (APCs) can only be subject to speculation. We know that antlers are the only mammalian bony organs that can fully regenerate and exhibit very rapid growth over their growth phase[28]. The antler growth centre is located in the antler tip and consists of RM, precartilage and cartilage layers[29]. Given that antlers can grow at an exceptionally high rate (up to 2 cm or more daily), the cells of the antler growth must be sustained by a unique niche, within which the ECM is the indispensable main player. Therefore, it may not be surprising that the antler ECM generated the highest quality of repair, compared with the other types of ECMs in the present study.
With regard to the results showing that the repair using antler RMC-ECM were superior to the APC-ECM, we propose that this is related to the developmental stage of the cells at the time of tissue sampling. APCs are the initial antler stem cells responsible for the formation of the deer pedicle and the first antler[38,39]. During pedicle development, activated APCs differentiate initially into osteoblasts to build up bone, and then slowly and gradually switch to differentiate to chondroblasts to form cartilage[40,41]. In contrast, RMCs are cells from the antler growth centre that are directly responsible for extremely rapid chondrogenesis[30]. Therefore, it is not surprising that ECM from RMCs performed better than that from APCs in the present study.
The general view from the literature is that the underlying mechanism by which implanted ECM is capable of facilitating repair of osteochondral defects is because ECM can mix quickly with the blood clots formed in the defect via BMS to form a complex, whereby this complex serves as a favorable niche for endogenous cell recruitment and tissue regeneration[37]. In addition, decellularized ECM, irrespective of its source, would be expected to have an advantage over the cell-impregnated ECM, because: (1) The pores and empty lacuna of decellularized ECM match those of recruited bMSCs/prechondroblasts very well, thus favoring the migration and adhesion of the recruited cells; and (2) The cell-free approach provides the opportunity for selective use of more effective autologous, allogenic, or even xenogeneic ECMs, without causing significant immune reaction. Therefore, we conclude that the selection of decellularized ECM for repair of osteochondral defects in the future should be more focused on bioactivity rather than kinship. In this respect, the successful use of allogenic or autologous ECM in the clinic would be expected to provide a way to reduce patient morbidity.
The bioactivity of different ECMs must be dependent on their composition. It is reported that natural ECM bioma
One may argue that, although we removed virtually all of the cellular DNA from the ECM-sheets, residual DNA debris may still exist, and the debris could elicit an immune reaction which may be detrimental to the tissue repair. The immune response to foreign cellular materials (allogenic or xenogeneic), if it occurs, would be partly macrophage-mediated[52]. However, a macrophage response to the implantation of an ECM-sheet is a necessary event, because macrophages are involved in scaffold degradation[52]. It is known that products and bioactive molecules originating from ECM degra
The use of decellularized ECM including xenogeneic ECM is gaining ground within the field of cartilage tissue engineering/regeneration and may prove to be of great potential because it allows for multifactorial mimicry that has not yet been achieved by man-made biomaterials. The avascular nature of cartilage is one of the major challenges in initiating intrinsic repair but may also be advantageous, because the tissue is immune-privileged to a large extent, which opens up many more options in choosing the ECM source, including allogeneic and xenogeneic sources, with less potential for rejection issues[57]. In addition, the dense nature of cartilage ECM (say antler cartilage ECM) may further contribute to the weakly immunogenic, or even non-immunogenic status, because it physically protects chondrocytes from T and natural killer cells that are released in graft rejection[57]. The application of xenogeneic products for cartilage repair in clinics is still in its infancy but should be explored further because it has great potential to overcome the limited availability of human tissue or cells.
In the present study, we successfully identified a novel type of xenogeneic ECM for the repair of osteochondral defects. The RMC-ECM is a product of the reserve mesenchymal cells in a regenerating antler. The decellularized RMC-ECM is found to have the ability to quality repair the osteochondral defects in a rat model and cause barely detectable immune response, thus has the potential to be used in the clinical setting.
To properly repair articular cartilage defects is still a big challenge in the clinical field. Notably, cell-free xenogeneic extracellular matrix (ECM) of mesenchymal stromal cells (MSCs) has been found to be effective on the restoration of articular defects. Identification of more potent xenogeneic ECM for cartilage defect repair has been being conducted. Deer antlers are the only mammalian organ that once lost can fully regenerate. During the rapid growing period, antlers can elongate at unprecedented rate (2 cm/d). Research finds that the ability of this full regeneration and extremely rapid growth of antlers is underpinned by its ECM and soluble factors, besides the presence of antler stem cells. Consequently, we decided to apply ECM from the antler mesenchymal cells located at its growth centre [antler reserve mesenchymal cells (RMCs)] to the rat articular cartilage defects to evaluate the effects of RMC-ECM.
After 4 decades of research on deer antler biology in our research group, we found that deer antlers, fastest growing bony organ (2 cm/d), would be superior for the reparation of bone defects if being applied to the clinical situation.
To identify potent cell-free xenogeneic ECM for high quality repair of articular cartilage defects.
RMCs were isolated from a 60-d-growth (most rapid growth period) antler, and RMCs were stimulated to produce ECM (RMC-ECM) using ascorbic acid. Holes (1.5 mm in diameter and 2.0 mm in depth) were drilled on the rat articular cartilage and filled with RMC-ECM sheet before closure. The repaired tissue was collected at three different times: 4, 8 and 12 wk after surgery and treatment for histological and immunohistochemistry analyses.
In vitro trials demonstrated that RMC-ECM was superior for attracting mesenchymal cells to attach and proliferate. In vivo, RMC-ECM was used to fill the drilled holes on the rat articular cartilage surface and successfully repaired these defects. The repaired quality (hyaline cartilage-like) of RMC-ECM was superior to the controls of both adipocyte-derived MSCs-CM and antlerogenic periosteal cell-ECM.
Decellularized RMC-ECM, a novel type of xenogeneic ECM that derived from the active type of antler stem cell, achieved high quality repair/reconstruction of rat articular osteochondral defects.
Eventual solve the problem of articular cartilage defects, thus arthritis, would be cell-free allogenic/xenogeneic ECM. Based on its attributes, RMC-ECM is considered as one of the most potent natural ECM for the repair of cartilage defects.
We are grateful to Dr. Peter Fennessy for his critical reading and polishing of the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Elder SH, United States; Jeyaraman M, India; Li SC, United States S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Deng C, Chang J, Wu C. Bioactive scaffolds for osteochondral regeneration. J Orthop Translat. 2019;17:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Xue J, Feng B, Zheng R, Lu Y, Zhou G, Liu W, Cao Y, Zhang Y, Zhang WJ. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials. 2013;34:2624-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Prakash D, Learmonth D. Natural progression of osteo-chondral defect in the femoral condyle. Knee. 2002;9:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 849] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 5. | Taghiyar L, Jahangir S, Khozaei Ravari M, Shamekhi MA, Eslaminejad MB. Cartilage Repair by Mesenchymal Stem Cell-Derived Exosomes: Preclinical and Clinical Trial Update and Perspectives. Adv Exp Med Biol. 2021;1326:73-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4207] [Cited by in RCA: 3643] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 7. | Mellor LF, Nordberg RC, Huebner P, Mohiti-Asli M, Taylor MA, Efird W, Oxford JT, Spang JT, Shirwaiker RA, Loboa EG. Investigation of multiphasic 3D-bioplotted scaffolds for site-specific chondrogenic and osteogenic differentiation of human adipose-derived stem cells for osteochondral tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2020;108:2017-2030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170-176. [PubMed] |
| 9. | Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 10. | Schallmoser K, Rohde E, Reinisch A, Bartmann C, Thaler D, Drexler C, Obenauf AC, Lanzer G, Linkesch W, Strunk D. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Tonti GA, Mannello F. From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera? Int J Dev Biol. 2008;52:1023-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Chase LG, Lakshmipathy U, Solchaga LA, Rao MS, Vemuri MC. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther. 2010;1:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Swistowski A, Peng J, Han Y, Swistowska AM, Rao MS, Zeng X. Xeno-free defined conditions for culture of human embryonic stem cells, neural stem cells and dopaminergic neurons derived from them. PLoS One. 2009;4:e6233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1223] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 15. | Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1060] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 16. | Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 517] [Cited by in RCA: 455] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 17. | Chen CH, Tsai CC, Chen W, Mi FL, Liang HF, Chen SC, Sung HW. Novel living cell sheet harvest system composed of thermoreversible methylcellulose hydrogels. Biomacromolecules. 2006;7:736-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Gospodarowicz DJ. Extracellular matrices and the control of cell proliferation and differentiation in vitro. Prog Clin Biol Res. 1984;145. |
| 19. | Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 364] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 20. | Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2194] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 21. | Jin CZ, Choi BH, Park SR, Min BH. Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A. 2010;92:1567-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Wei F, Qu C, Song T, Ding G, Fan Z, Liu D, Liu Y, Zhang C, Shi S, Wang S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227:3216-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Yoshida Y, Matsubara H, Fang X, Hayashi K, Nomura I, Ugaji S, Hamada T, Tsuchiya H. Adipose-derived stem cell sheets accelerate bone healing in rat femoral defects. PLoS One. 2019;14:e0214488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Tang C, Jin C, Du X, Yan C, Min BH, Xu Y, Wang L. An autologous bone marrow mesenchymal stem cell-derived extracellular matrix scaffold applied with bone marrow stimulation for cartilage repair. Tissue Eng Part A. 2014;20:2455-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Wang Z, Han L, Sun T, Ma J, Sun S, Ma L, Wu B. Extracellular matrix derived from allogenic decellularized bone marrow mesenchymal stem cell sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomater. 2020;118:54-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Agrawal V, Johnson SA, Reing J, Zhang L, Tottey S, Wang G, Hirschi KK, Braunhut S, Gudas LJ, Badylak SF. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci U S A. 2010;107:3351-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 374] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 28. | Li C, Fennessy P. The periosteum: a simple tissue with many faces, with special reference to the antler-lineage periostea. Biol Direct. 2021;16:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Ba H, Wang D, Yau TO, Shang Y, Li C. Transcriptomic analysis of different tissue layers in antler growth Center in Sika Deer (Cervus nippon). BMC Genomics. 2019;20:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Li C, Clark DE, Lord EA, Stanton JA, Suttie JM. Sampling technique to discriminate the different tissue layers of growing antler tips for gene discovery. Anat Rec. 2002;268:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Chen Y, Tang X, Li P, Zhou Y, Xue T, Liu J, Yu C. Bone Marrow Derived Mesenchymal Stromal Cells Ameliorate Ischemia/Reperfusion Injury-Induced Acute Kidney Injury in Rats via Secreting Tumor Necrosis Factor-Inducible Gene 6 Protein. Biomed Res Int. 2019;2019:9845709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Li C, Suttie JM. Tissue collection methods for antler research. Eur J Morphol. 2003;41:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Zhong D, Xu GZ, Wu JZ, Liu H, Tang JY, Wang CG. Circ-ITCH sponges miR-214 to promote the osteogenic differentiation in osteoporosis via upregulating YAP1. Cell Death Dis. 2021;12:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, Anders S. Outcome of Autologous Matrix Induced Chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133:87-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 35. | Filová E, Rampichová M, Litvinec A, Držík M, Míčková A, Buzgo M, Košťáková E, Martinová L, Usvald D, Prosecká E, Uhlík J, Motlík J, Vajner L, Amler E. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int J Pharm. 2013;447:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Hoganson DM, O'Doherty EM, Owens GE, Harilal DO, Goldman SM, Bowley CM, Neville CM, Kronengold RT, Vacanti JP. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials. 2010;31:6730-6737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Erggelet C, Neumann K, Endres M, Haberstroh K, Sittinger M, Kaps C. Regeneration of ovine articular cartilage defects by cell-free polymer-based implants. Biomaterials. 2007;28:5570-5580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Li C, Harris AJ, Suttie JM. Tissue interactions and antlerogenesis: new findings revealed by a xenograft approach. J Exp Zool. 2001;290:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Yang F, Wang W, Li J, Haines S, Asher G, Li C. Antler development was inhibited or stimulated by cryosurgery to periosteum or skin in a central antlerogenic region respectively. J Exp Zool B Mol Dev Evol. 2011;316:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Li C, Suttie JM. Light microscopic studies of pedicle and early first antler development in red deer (Cervus elaphus). Anat Rec. 1994;239:198-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Li C. Histogenetic aspects of deer antler development. Front Biosci (Elite Ed). 2013;5:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367-377. [PubMed] [DOI] [Full Text] |
| 43. | Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 932] [Cited by in RCA: 818] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 44. | Pei M, Zhang Y, Li J, Chen D. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev. 2013;22:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Stone N, Meister A. Function of ascorbic acid in the conversion of proline to collagen hydroxyproline. Nature. 1962;194:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Nandi D, Patra RC, Swarup D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology. 2005;211:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Korkmaz A, Kolankaya D. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Ren Fail. 2009;31:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78:2879-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 302] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Prockop DJ, Kivirikko KI. Heritable diseases of collagen. N Engl J Med. 1984;311:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 402] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Han EH, Chen SS, Klisch SM, Sah RL. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J. 2011;101:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Chun SY, Lim GJ, Kwon TG, Kwak EK, Kim BW, Atala A, Yoo JJ. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials. 2007;28:4251-4256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 447] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 53. | D'Onofrio A, Cresce GD, Bolgan I, Magagna P, Piccin C, Auriemma S, Fabbri A. Clinical and hemodynamic outcomes after aortic valve replacement with stented and stentless pericardial xenografts: a propensity-matched analysis. J Heart Valve Dis. 2011;20:319-25; discussion 326. [PubMed] |
| 54. | Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E, Badylak SF, Braunhut SJ. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233-3243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2839] [Cited by in RCA: 2430] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 56. | Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 688] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 57. | Revell CM, Athanasiou KA. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B Rev. 2009;15:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |