Published online Feb 26, 2024. doi: 10.4252/wjsc.v16.i2.163
Peer-review started: October 18, 2023
First decision: December 2, 2023
Revised: December 14, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: February 26, 2024
Processing time: 130 Days and 18.6 Hours
In vitro expansion to increase numbers of hematopoietic stem cells (HSCs) in cord blood could improve clinical efficacy of this vital resource. Nicotinamide (NAM) can promote HSC expansion ex vivo, but its effect on hematopoietic stem and progenitor cells (HSPCs, CD34+CD38) and functional subtypes of HSCs – short-term repopulating HSCs (ST-HSCs, CD34+CD38CD45RACD49f+) and long-term repopulating HSCs (LT-HSCs, CD34+CD38CD45RACD49f+CD90+) is not yet known. As a sirtuin 1 (SIRT1) inhibitor, NAM participates in regulating cell ad
To evaluate the effects and underlying mechanisms of action of different concentrations of NAM on HSC proliferation and differentiation.
CD34+ cells were purified from umbilical cord blood using MacsCD34 beads, and cultured for 10–12 d in a serum-free medium supplemented with cytokines, with different concentrations of NAM added according to experimental requirements. Flow cytometry was used to detect phenotype, cell cycle distribution, and apop
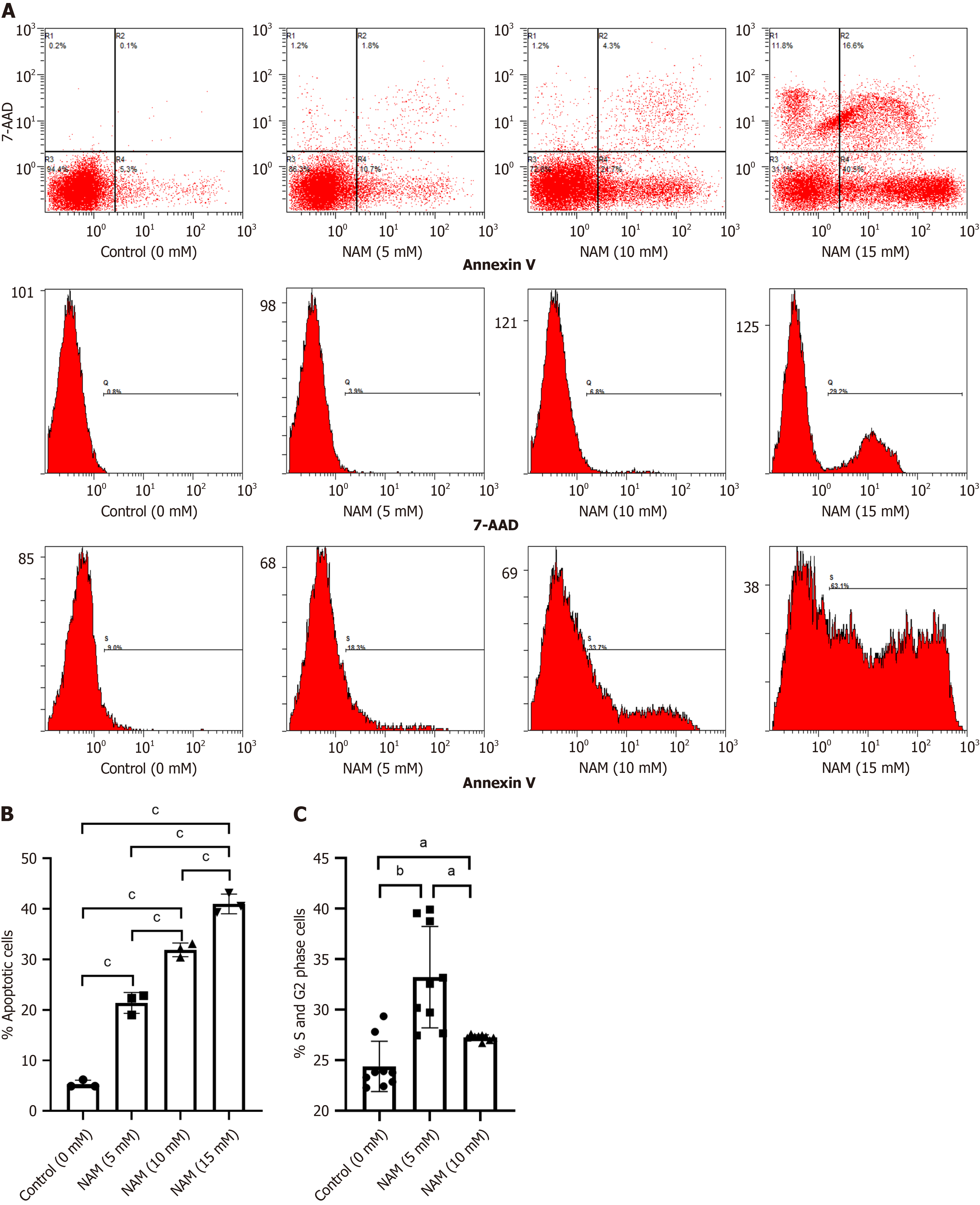
Compared with the control group, the proportion and expansion folds of HSPCs (CD34+CD38) incubated with 5 mmol/L or 10 mmol/L NAM were significantly increased (all P < 0.05). The ST-HSCs ratio and fold expansion of the 5 mmol/L NAM group were significantly higher than those of the control and 10 mmol/L NAM groups (all P < 0.001), whereas the LT-HSCs ratio and fold expansion of the 10 mmol/L NAM group were significantly higher than those of the other two groups (all P < 0.05). When the NAM concentration was > 10 mmol/L, cell viability significantly decreased. In addition, compared with the 5 mmol/L NAM group, the proportion of apoptotic cells in the 10 mmol/L NAM group increased and the proportion of cells in S and G2 phase decreased. Compared with the 5 mmol/L NAM group, the HSCs incubated with 10 mmol/L NAM exhibited significantly inhibited SIRT1 ex
Low concentrations (5 mmol/L) of NAM can better regulate the balance between proliferation and differentiation, thereby promoting expansion of HSCs. These findings allow adjustment of NAM concentrations according to ex
Core tip: This study reveals the dominant subgroups of hematopoietic stem cells (HSCs) and molecular mechanisms underlying the effects of different nicotinamide (NAM) concentrations. Activation or inhibition of sirtuin 1 (SIRT1) is determined by the concentration of NAM. High concentrations inhibit SIRT1 but are not conducive to self-renewal of HSCs, whereas low concentrations balance HSC proliferation and differentiation by regulating the SIRT1–HIF1A pathway and reactive oxygen species production, effectively promoting in vitro expansion of the stem cells. These findings could allow adjustment of NAM concentrations according to expansion needs and may help predict small molecules that synergistically promote expansion with NAM.
- Citation: Ren Y, Cui YN, Wang HW. Effects of different concentrations of nicotinamide on hematopoietic stem cells cultured in vitro. World J Stem Cells 2024; 16(2): 163-175
- URL: https://www.wjgnet.com/1948-0210/full/v16/i2/163.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i2.163
Hematopoietic stem cells (HSCs) can self-renew and give rise to mature cells of all hematopoietic lineages following extensive proliferation and differentiation[1,2]. Umbilical cord blood (UCB) is an important source of HSCs because it can be obtained through noninvasive means, has low requirements for the matching of human leukocyte antigens, and has a low incidence of chronic graft-versus-host disease. However, the limited cell dose in a single UCB sample can lead to de
Small molecules represent novel modalities for ex vivo expansion of HSCs[4-6], and the roles of different molecules in HSC transplantation are diverse. As a form of vitamin B-3, nicotinamide (NAM) can delay differentiation and maintain stemness, as well as promote neutrophil recovery in patients after transplantation[7-9]. In humans, CD34+CD38 cells are classic hematopoietic stem and progenitor cells (HSPCs), and HSCs can be functionally divided into short-term repopulating HSCs (ST-HSCs, CD34+CD38CD45RACD49f+) and long-term repopulating HSCs (LT-HSCs, CD34+CD38CD
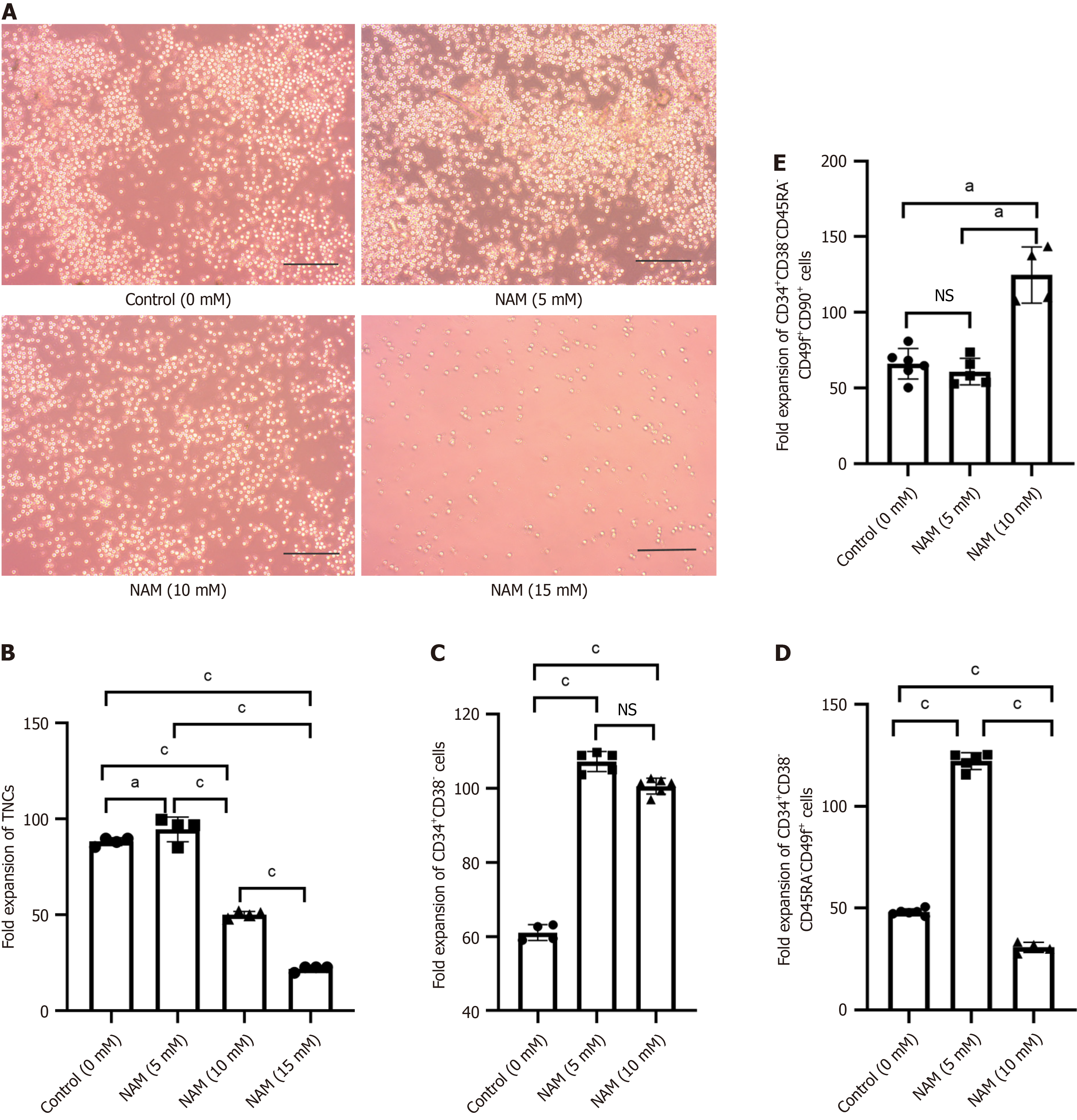
In this study, we evaluated the effects of different concentrations of NAM on the proliferation and differentiation of HSCs during in vitro culture. Proliferation is reflected by fold expansion of total nucleated cells (TNCs) and differentiation is reflected by phenotype analysis of HSPC, ST-HSC and LT-HSC populations. Apoptosis ratio and cell cycle distribution were used to reveal the influencing factors of cell quantity differences; reactive oxygen species (ROS) production and cytokine levels were evaluated to reveal the relevant factors of differentiation differences; and real-time polymerase chain reaction (RT-PCR) detection of genes related to stemness, antioxidant enzymes, and homing were used to explore the molecular mechanisms underlying the actions of NAM. NAM at 5 mmol/L and 10 mmol/L could inhibit differentiation and maintain HSPCs, with the higher concentration being more conducive to the maintenance of LT-HSCs. However, owing to significant proapoptotic effects, this high concentration of 10 mmol/L NAM reduced cell survival and self-re
A gynecologist collected UCB samples from consenting donors according to ethical procedures approved by the Second Hospital of Shanxi Medical University (Shanxi, China). UCB mononuclear cells (MNCs) were isolated by Ficoll (Tianjin Haoyang Biological Products Technology) and density-gradient centrifugation. Specifically, UCB depleted of erythrocytes (by adding hydroxyethyl starch) was slowly pipetted on Ficoll at a ratio of 2:1 and centrifuged (2000 rpm, 20 min), fo
Before being seeded onto 24-well plates (Corning), CD34+ cells were resuspended in serum-free medium (5 × 104/mL), which was composed of Iscove Modified Dulbecco Medium (IMDM; Gibco), supplemented with 10 ng/mL human stem cell factor (Miltenyi Biotec), 100 ng/mL thrombopoietin (Miltenyi Biotec), 1% penicillin–streptomycin–glutamine (Gibco), and NAM at different concentrations. According to relevant research data[25,26] and preliminary experimental results (Supplementary Figure 1), we set the concentration of NAM to 5 mmol/L, 10 mmol/L,or 15 mmol/L. Based on the di
Trypan Blue cell viability counting was performed. Trypan Blue (Thermo Fisher Scientific) can only pass through an incomplete cell membrane, and dead cells were dyed clear blue. After mixing 10 μL cell suspension with 10 μL Trypan Blue, the viability and size of the cells in suspension were measured by a Countess 3 Automated Cell Counter (Thermo Fisher Scientific).
After incubation with NAM for 10–12 d, the cells were collected and washed with PBS. Next, the cells were stained in PBS supplemented with the following antibody and fluorophore combinations for 30 min at 4°C: ECD-labeled anti-human CD34 (IM2709U; Beckman), FITC-labeled anti-human CD38 (A07778; Beckman), Pacific Blue-labeled anti-human CD45RA (A82946; Beckman), APC-CY7-labeled anti-human CD49f (313628; Biolegend), and PerCP-CY5-labeled anti-human CD90 (IM3703; Beckman). After a washing step, the stained cells were analyzed by Navios flow cytometer (Be
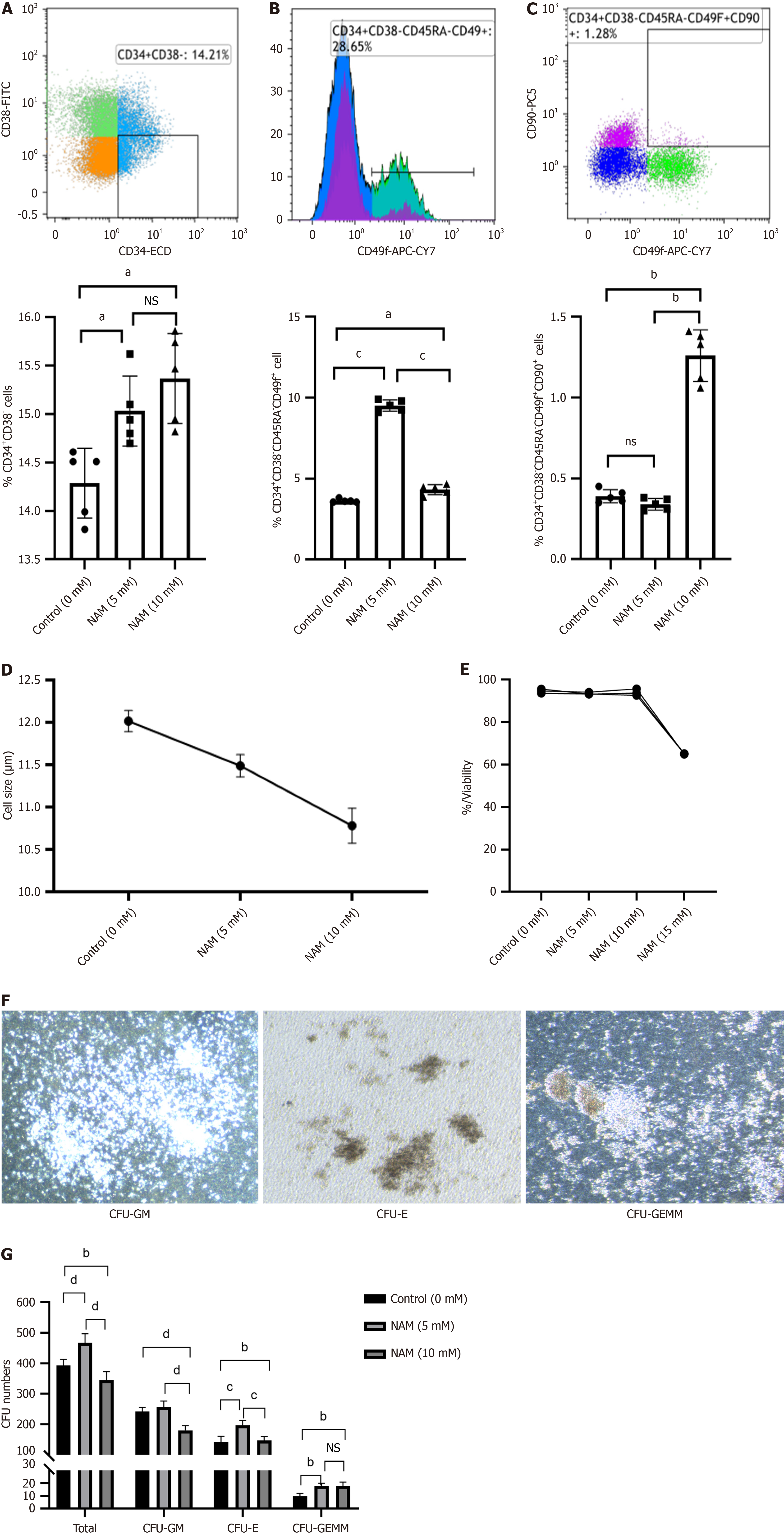
According to the cell count, we adjusted the concentration of CD34+ cells of each group to 105/mL, add 50 μL cells to 450 μL IMDM (Gibco) for mixing, and added 150 μL cells to 1.5 mL MethodCultTM medium (Stem Cell Technologies). We connected a 1.6-mm needle to a 2-mL disposable sterile syringe and distributed the MethodCultTM mixture containing cells into a 35-mm culture dish (Corning), so that the medium was evenly distributed on the surface of each dish. The cells were cultured in 5% CO2 at 37 °C for 14 d, and culture dishes were visually scored for colony-forming unit (CFU)-granulocyte/macrophage, CFU-erythrocyte (CFU-E) and CFU-granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM).
The cultured cells were collected and washed twice with precooled PBS. Cells were stained for 15 min at room tem
The cultured cells were washed with precooled PBS, resuspended in 70% ethanol at 4°C for 2–6 h, and washed with PBS and incubated with 0.4 mL propidium iodide staining solution (Biosciences) for 30 min at 37°C in the dark. The 0.4 mL of propidium iodide staining solution was composed of 384 μL staining buffer, 15 μL 25× propidium solution, and 1 μL RNase (10 mg/mL). A minimum of 40 000 cells were collected at a low speed for each sample.
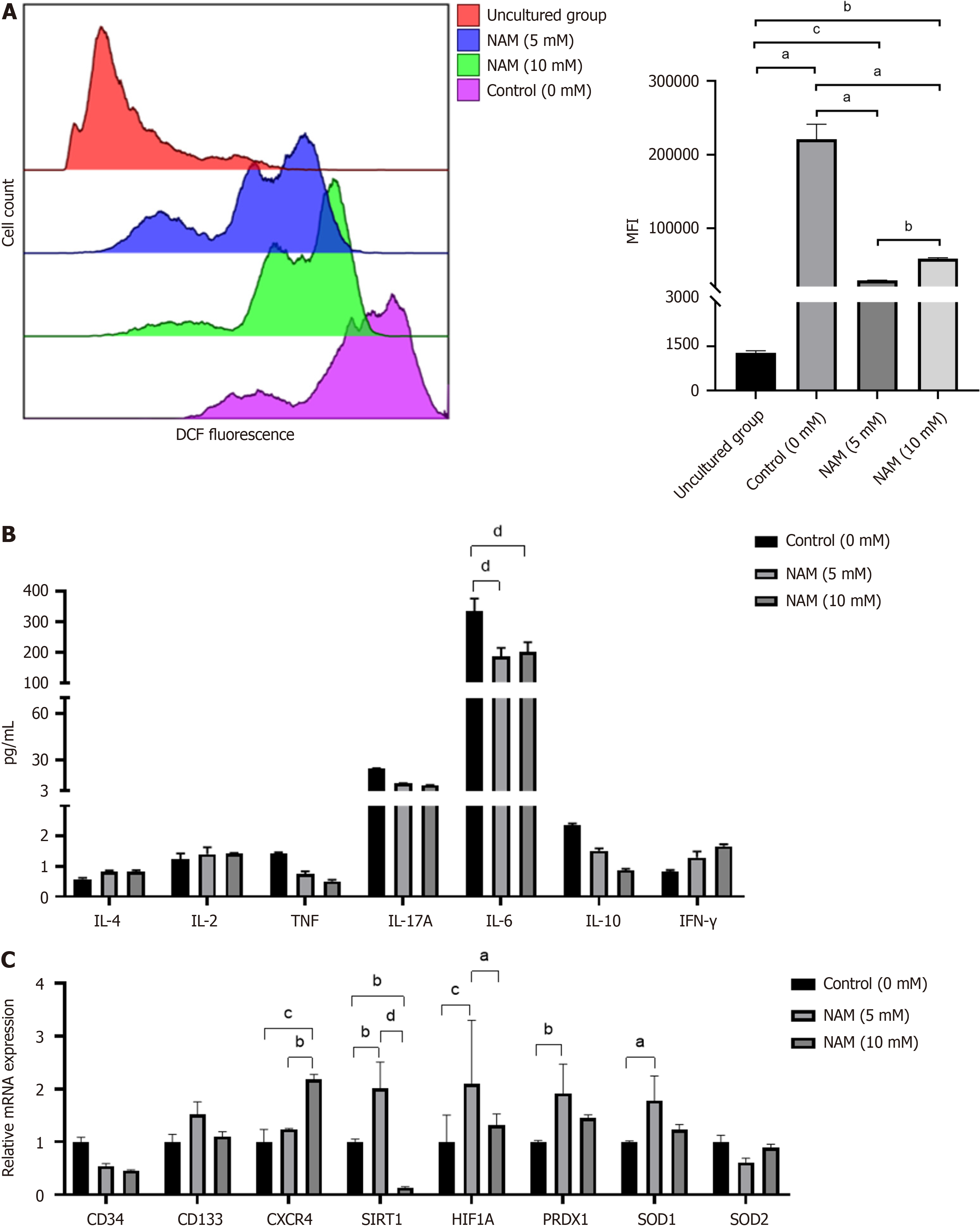
The cultured cells were resuspended at a density of 5 × 105 to 1 × 106 cells/mL, and the dichloro-dihydro-fluorescein diacetate (DCFH-DA) probe was diluted with serum-free medium (Beyotime Biotechnology). The cells were resuspended in diluted DCFH-DA and incubated for 20 min in a 37°C incubator. The cells were mixed upside down every 3–5 min to ensure full contact between the probe and the cell. The cells were washed with serum-free medium three times to fully remove DCFH-DA that had not entered the cells. The fluorescence intensity of ROS was measured within 2 h by Navios flow cytometer. The median level of mean fluorescence intensity of ROS was obtained in Kaluza software (Beckman) for each sample after adjusting them to the same measuring cell number.
Total RNA was extracted from the cells cultured with or without NAM by the Trizol method. For cDNA synthesis, total RNA was reverse transcribed with a cDNA synthesis kit (Bio-Rad). PCR was performed using a SYBR Premix Ex TaqTMII (Tli RNaseH Plus; TaKaRa) and the CFX96 RT-PCR detection system (Bio-Rad). Each reaction was repeated at least three times to demonstrate reproducibility, and data were analyzed using the CFX96 Real-Time System. Normalized values were obtained by subtracting the threshold cycle (Ct) of GAPDH from the Ct values of the target genes, yielding
This assay was conducted according to the Instruction Manual of BD™ Cytometric Bead Array Human Th1/Th2/Th17 Cytokine Kit (Catalog No. 560484). The fresh cytokine standards were prepared to run with each experiment. We added 50 μL sample and 50 μL Human Th1/Th2/Th17 PE Detection Reagent to the sample tubes and incubate the assay tubes for 3 h at room temperature, protected from light. Data were analyzed using FCAP Array software.
The significant differences between each group were analyzed using SPSS 22.0 for all experimental data. The comparison was analyzed between two groups with an independent sample t test and among three groups with single-factor analysis of variance. The values were plotted as the mean ± SD. Probability values P < 0.05 were considered significant.
We measured the proportion of CD34+CD38 (HSPCs), CD34+CD38CD45RACD49f+ (ST-HSCs), and CD34+CD38CD
Fold expansion of TNCs and the degree of cell differentiation were reliable indicators for evaluating the ex vivo expansion efficiency of HSCs. Under the microscope, small and round HSCs were visible, and the number of cells in the 15 mmol/L NAM group was significantly lower than in the other groups (Figure 2A). The 5 mmol/L NAM group had the highest fold expansion of TNCs, followed by the control group; both of which were significantly higher than the 10 mmol/L and 15 mmol/L NAM groups, with the 15 mmol/L NAM group having the lowest fold expansion of TNCs (Figure 2B). The fold expansion of HSPCs in the 5 mmol/L (107.25 ± 2.71) and 10 mmol/L (100.65 ± 2.13) NAM groups was sig
To investigate whether NAM affected other biological behaviors of HSCs, we measured the proportion of apoptotic cells and cell cycle distribution in each group. The proportion of apoptotic cells in the 10 mmol/L NAM group was signi
In vivo, primitive HSCs can be distinguished by their low mitochondrial activity, as they primarily rely on glycolytic metabolism as their energy source to maintain a dormant state. Ex vivo culture conditions cannot completely mimic the niche of HSCs, but do drive metabolic transitions toward oxidative phosphorylation and activation of inflammatory pa
The expression of stemness, chemotaxis, hypoxia pathway and antioxidant enzyme genes are common indicators for exploring the mechanisms of action of different small molecule compounds in HSC amplification systems[34-39]. To further explore the molecular mechanisms by which NAM affects the proliferation and differentiation of HSCs, we com
The basic HSCs in vitro culture consists of a serum-free medium supplemented with cytokines, and the addition of small molecule substances can significantly enhance amplification efficiency[15,40]. For example, NAM can significantly in
In this study, we evaluated the amplification efficiency of different concentrations of NAM on HSPCs, ST-HSCs, and LT-HSCs, and found that 5 mmol/L and 10 mmol/L NAM are beneficial for maintaining HSPCs. The lower concentration (5 mmol/L) of NAM was more conducive to expansion of ST-HSCs, whereas the higher concentration (10 mmol/L) had a more significant effect on promoting expansion of LT-HSCs. Based on these findings, the working concentration of NAM can be adjusted according to the expansion requirements.
The key to promoting self-renewal of HSCs lies in regulating the balance between proliferation and differentiation, which requires activating dormant stem cells to enter the proliferative cell cycle while also preventing excessive oxidative phosphorylation and ROS production. For example, the HSC self-renewal agonist UM171 stimulates ex vivo HSC ex
In our research, low concentrations of NAM caused HSCs to exit G0 phase and enter the proliferative cell cycle, leading to an increase in cell numbers. In addition, the low concentrations of NAM inhibited differentiation by reducing ROS production. This indicates that low concentrations of NAM can better regulate the balance between proliferation and differentiation, thereby promoting effective expansion of HSCs. When the concentration of NAM exceeds 10 mmol/L, apoptosis and cell necrosis increase, which is not conducive to the survival and self-renewal of HSCs. The high in
Activating SIRT1 can protect cells from oxidative stress damage and regulate cell proliferation by upregulating the SIRT1–HIF1A pathway[46,47], whereas inhibiting SIRT1 may be related to delaying differentiation[48]. NAM is con
Different concentrations of NAM have distinct effects on proliferation and differentiation of HSCs. Although a high concentration of NAM is more conducive to the expansion of LT-HSCs, the cells are affected by oxidative stress and apoptosis, which may negatively impact hematopoietic reconstitution after transplantation. Low concentrations of NAM can better regulate the balance between proliferation and differentiation, thereby promoting effective expansion of HSCs. In addition, the transcription level of SIRT1 is correlated with the concentration of NAM. Upregulation of SIRT1–HIF1A can promote proliferation, whereas inhibition of SIRT1 may achieve a delayed differentiation effect.
The in vitro expansion strategy of increasing the number of hematopoietic stem cells (HSCs) in cord blood is expected to improve its clinical efficacy. Nicotinamide (NAM) is one of the small molecules that can promote the expansion of he
The effects of different concentrations of NAM on proliferation and differentiation of HSCs, as well as whether it affects sirtuin 1 (SIRT1) transcription levels, have not been reported. There are various small molecule substances used for in vitro culture of HSCs (including UM171, SR1, VPA, NAM and ID8), which may affect the maintenance, proliferation, differentiation and homing of HSCs by regulating different pathways, and different molecular pathways may have synergistic effects. This study aimed to provide a theoretical basis for the future joint application of multiple small mo
To evaluate the effects and mechanisms of different concentrations of NAM on HSC proliferation and differentiation.
In this study, we added different concentrations of NAM to serum-free culture medium inoculated with CD34+ cells. We then measure the number, molecular phenotype, cycle distribution, and apoptosis of each group of cells and explore the mechanism by detecting the levels of reactive oxygen species (ROS), inflammatory factors and related gene transcription.
We evaluated the expansion efficiency of different concentrations of NAM on HSPCs, ST-HSCs as well as LT-HSCs, and the results showed that 5 mmol/L and 10 mmol/L NAM were beneficial for maintaining HSPCs, with low concentrations (5 mmol/L) of NAM being more conducive to ST-HSCs expansion, while high concentrations (10 mmol/L) of NAM had a more significant effect on promoting LT-HSCs expansion. Low concentrations of NAM can better regulate the balance between proliferation and differentiation, thereby promoting effective expansion of HSCs.
Low concentration of NAM did not inhibit but upregulated the transcription of SIRT1, promoting cell proliferation by activating the SIRT1–HIF1A pathway, delaying stem cell differentiation by increasing ROS clearance, and ultimately promoting effective expansion of HSCs.
Drugs that specifically target LT-HSCs or ST-HSCs may help in the development of tailored HSC grafts that either fa
We thank Dr Yong-Hong Wang (Department of Maternity, The Second Hospital of Shanxi Medical University) for pro
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SC, United States; Rezus E, Romania; Roomi AB, Iraq; Salceda R, Mexico S-Editor: Wang JJ L-Editor: Kerr C P-Editor: Xu ZH
| 1. | Tajer P, Pike-Overzet K, Arias S, Havenga M, Staal FJT. Ex Vivo Expansion of Hematopoietic Stem Cells for Therapeutic Purposes: Lessons from Development and the Niche. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Zimran E, Papa L, Hoffman R. Ex vivo expansion of hematopoietic stem cells: Finally transitioning from the lab to the clinic. Blood Rev. 2021;50:100853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Ren Y, Cui Y, Tan Y, Xu Z, Wang H. Expansion strategies for umbilical cord blood haematopoietic stem cells in vitro. Vox Sang. 2023;118:913-920. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Sakurai M, Ishitsuka K, Ito R, Wilkinson AC, Kimura T, Mizutani E, Nishikii H, Sudo K, Becker HJ, Takemoto H, Sano T, Kataoka K, Takahashi S, Nakamura Y, Kent DG, Iwama A, Chiba S, Okamoto S, Nakauchi H, Yamazaki S. Chemically defined cytokine-free expansion of human haematopoietic stem cells. Nature. 2023;615:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 5. | Ding S, Gray NS, Wu X, Ding Q, Schultz PG. A combinatorial scaffold approach toward kinase-directed heterocycle libraries. J Am Chem Soc. 2002;124:1594-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, Csaszar E, Knapp DJ, Miller P, Ngom M, Imren S, Roy DC, Watts KL, Kiem HP, Herrington R, Iscove NN, Humphries RK, Eaves CJ, Cohen S, Marinier A, Zandstra PW, Sauvageau G. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 447] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 7. | Ghafouri-Fard S, Niazi V, Taheri M, Basiri A. Effect of Small Molecule on ex vivo Expansion of Cord Blood Hematopoietic Stem Cells: A Concise Review. Front Cell Dev Biol. 2021;9:649115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Horwitz ME, Stiff PJ, Cutler C, Brunstein C, Hanna R, Maziarz RT, Rezvani AR, Karris NA, McGuirk J, Valcarcel D, Schiller GJ, Lindemans CA, Hwang WYK, Koh LP, Keating A, Khaled Y, Hamerschlak N, Frankfurt O, Peled T, Segalovich I, Blackwell B, Wease S, Freedman LS, Galamidi-Cohen E, Sanz G. Omidubicel vs standard myeloablative umbilical cord blood transplantation: results of a phase 3 randomized study. Blood. 2021;138:1429-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Horwitz ME, Wease S, Blackwell B, Valcarcel D, Frassoni F, Boelens JJ, Nierkens S, Jagasia M, Wagner JE, Kuball J, Koh LP, Majhail NS, Stiff PJ, Hanna R, Hwang WYK, Kurtzberg J, Cilloni D, Freedman LS, Montesinos P, Sanz G. Phase I/II Study of Stem-Cell Transplantation Using a Single Cord Blood Unit Expanded Ex Vivo With Nicotinamide. J Clin Oncol. 2019;37:367-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 620] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 11. | Kent D, Dykstra B, Eaves C. Isolation and assessment of long-term reconstituting hematopoietic stem cells from adult mouse bone marrow. Curr Protoc Stem Cell Biol. 2007;Chapter 2:Unit 2A.4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Kent DG, Dykstra BJ, Eaves CJ. Isolation and Assessment of Single Long-Term Reconstituting Hematopoietic Stem Cells from Adult Mouse Bone Marrow. Curr Protoc Stem Cell Biol. 2016;38:2A.4.1-2A.4.24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 850] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 14. | Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 644] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 15. | Cheung AM, Nguyen LV, Carles A, Beer P, Miller PH, Knapp DJ, Dhillon K, Hirst M, Eaves CJ. Analysis of the clonal growth and differentiation dynamics of primitive barcoded human cord blood cells in NSG mice. Blood. 2013;122:3129-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegué E. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 664] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 17. | Akunuru S, Geiger H. Aging, Clonality, and Rejuvenation of Hematopoietic Stem Cells. Trends Mol Med. 2016;22:701-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2046] [Cited by in RCA: 1828] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 20. | Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, Ragusa S, Stefanidis E, Ryu D, Rainer PY, Nikitin G, Giger S, Li TY, Semilietof A, Oggier A, Yersin Y, Tauzin L, Pirinen E, Cheng WC, Ratajczak J, Canto C, Ehrbar M, Sizzano F, Petrova TV, Vanhecke D, Zhang L, Romero P, Nahimana A, Cherix S, Duchosal MA, Ho PC, Deplancke B, Coukos G, Auwerx J, Lutolf MP, Naveiras O. The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance. Cell Stem Cell. 2019;24:405-418.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 21. | Jung JJ, Buisman SC, de Haan G. Do hematopoietic stem cells get old? Leukemia. 2017;31:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Van Zant G, Liang Y. Measuring the aging process in stem cells. Methods Mol Biol. 2015;1235:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Sun X, Cao B, Naval-Sanchez M, Pham T, Sun YBY, Williams B, Heazlewood SY, Deshpande N, Li J, Kraus F, Rae J, Nguyen Q, Yari H, Schröder J, Heazlewood CK, Fulton M, Hatwell-Humble J, Das Gupta K, Kapetanovic R, Chen X, Sweet MJ, Parton RG, Ryan MT, Polo JM, Nefzger CM, Nilsson SK. Nicotinamide riboside attenuates age-associated metabolic and functional changes in hematopoietic stem cells. Nat Commun. 2021;12:2665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 24. | Vaca P, Berná G, Martín F, Soria B. Nicotinamide induces both proliferation and differentiation of embryonic stem cells into insulin-producing cells. Transplant Proc. 2003;35:2021-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Son MJ, Son MY, Seol B, Kim MJ, Yoo CH, Han MK, Cho YS. Nicotinamide overcomes pluripotency deficits and reprogramming barriers. Stem Cells. 2013;31:1121-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Horwitz ME, Chao NJ, Rizzieri DA, Long GD, Sullivan KM, Gasparetto C, Chute JP, Morris A, McDonald C, Waters-Pick B, Stiff P, Wease S, Peled A, Snyder D, Cohen EG, Shoham H, Landau E, Friend E, Peleg I, Aschengrau D, Yackoubov D, Kurtzberg J, Peled T. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124:3121-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 27. | Li X, Zeng X, Xu Y, Wang B, Zhao Y, Lai X, Qian P, Huang H. Mechanisms and rejuvenation strategies for aged hematopoietic stem cells. J Hematol Oncol. 2020;13:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 28. | Mantel CR, O'Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, Brustovetsky N, Srour EF, Lee MR, Messina-Graham S, Haas DM, Falah N, Kapur R, Pelus LM, Bardeesy N, Fitamant J, Ivan M, Kim KS, Broxmeyer HE. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell. 2015;161:1553-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 29. | Filippi MD, Ghaffari S. Mitochondria in the maintenance of hematopoietic stem cells: new perspectives and opportunities. Blood. 2019;133:1943-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 30. | Vannini N, Girotra M, Naveiras O, Nikitin G, Campos V, Giger S, Roch A, Auwerx J, Lutolf MP. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat Commun. 2016;7:13125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 31. | Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 919] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 32. | Carroll D, St Clair DK. Hematopoietic Stem Cells: Normal Versus Malignant. Antioxid Redox Signal. 2018;29:1612-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Wilkinson AC, Ishida R, Kikuchi M, Sudo K, Morita M, Crisostomo RV, Yamamoto R, Loh KM, Nakamura Y, Watanabe M, Nakauchi H, Yamazaki S. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019;571:117-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 296] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 34. | Felker S, Shrestha A, Bailey J, Pillis DM, Siniard D, Malik P. Differential CXCR4 expression on hematopoietic progenitor cells versus stem cells directs homing and engraftment. JCI Insight. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 35. | Calloni R, Cordero EA, Henriques JA, Bonatto D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013;22:1455-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Zhang QS, Deater M, Schubert K, Marquez-Loza L, Pelz C, Sinclair DA, Grompe M. The Sirt1 activator SRT3025 expands hematopoietic stem and progenitor cells and improves hematopoiesis in Fanconi anemia mice. Stem Cell Res. 2015;15:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Henry E, Picou F, Barroca V, Dechamps N, Sobrino S, Six E, Gobeaux C, Auberger P, Hérault O, Pflumio F, Arcangeli ML. The Antioxidant TEMPOL Protects Human Hematopoietic Stem Cells From Culture-Mediated Loss of Functions. Stem Cells Transl Med. 2023;12:676-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Wielockx B, Grinenko T, Mirtschink P, Chavakis T. Hypoxia Pathway Proteins in Normal and Malignant Hematopoiesis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Ratajczak MZ, Suszynska M. Emerging Strategies to Enhance Homing and Engraftment of Hematopoietic Stem Cells. Stem Cell Rev Rep. 2016;12:121-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Branco A, Bucar S, Moura-Sampaio J, Lilaia C, Cabral JMS, Fernandes-Platzgummer A, Lobato da Silva C. Tailored Cytokine Optimization for ex vivo Culture Platforms Targeting the Expansion of Human Hematopoietic Stem/Progenitor Cells. Front Bioeng Biotechnol. 2020;8:573282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Danet GH, Lee HW, Luongo JL, Simon MC, Bonnet DA. Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34(+) cells after ex vivo expansion. Exp Hematol. 2001;29:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95:102-110. [PubMed] |
| 43. | Ratajczak MZ. Phenotypic and functional characterization of hematopoietic stem cells. Curr Opin Hematol. 2008;15:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Grahn THM, Niroula A, Végvári Á, Oburoglu L, Pertesi M, Warsi S, Safi F, Miharada N, Garcia SC, Siva K, Liu Y, Rörby E, Nilsson B, Zubarev RA, Karlsson S. S100A6 is a critical regulator of hematopoietic stem cells. Leukemia. 2020;34:3323-3337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Chagraoui J, Lehnertz B, Girard S, Spinella JF, Fares I, Tomellini E, Mayotte N, Corneau S, MacRae T, Simon L, Sauvageau G. UM171 induces a homeostatic inflammatory-detoxification response supporting human HSC self-renewal. PLoS One. 2019;14:e0224900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Tan P, Wang M, Zhong A, Wang Y, Du J, Wang J, Qi L, Bi Z, Zhang P, Lin T, Zhang J, Yang L, Chen J, Han P, Gong Q, Liu Y, Chen C, Wei Q. SRT1720 inhibits the growth of bladder cancer in organoids and murine models through the SIRT1-HIF axis. Oncogene. 2021;40:6081-6092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 47. | Ren Y, Cui Y, Feng J, Tan Y, Ren F, Zhang Y, Wang H. Synergistic effect and molecular mechanism of PVA and UM171 in ex vivo expansion of primitive hematopoietic stem cells. J Cell Biochem. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer G N, Lerrer B, Cohen HY, Nagler A, Fibach E, Peled A. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40:342-55.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |