TO THE EDITOR
We have read a recent article entitled “Yes-associated protein-mediated melatonin regulates the function of periodontal ligament stem cells under oxidative stress conditions” by Gu et al[1] from Nankai University, which we find particularly intriguing. Using human periodontal ligament stem cells (hPLSCs) as a cellular model, the study demonstrates that treatment with melatonin, a biomolecule with potent free radical scavenging properties, delays their senescence, supports cell proliferation, and enhances differentiation under oxidative stress in vitro[1]. The other highlighting feature of the study is elucidating the underlying molecular mechanism, which the authors attribute to the Yes-associated protein (YAP) activity after melatonin priming of hPLSCs (Figure 1). YAP is a crucial regulator of cell proliferation and apoptosis, and its activation secures the stemness characteristics of hPLSCs under oxidative stress conditions. This role of YAP in stem cell priming is significant as it provides a potential target for future research and therapeutic interventions. Incidentally, first reported for their presence in the third molar tooth, hPLSCs are identified by the surface expression of mesenchymal stem cell-specific markers, i.e., CD13, CD44, CD73, CD90, and CD105, and absence of hematopoietic specific markers, i.e., CD14, CD19, CD40, CD45, CD80 and CD86, besides showing multilineage differentiation potential including bone, periodontal ligament, peripheral nerves, blood vessels, etc[2]. In one of the recently published studies regarding the fate determination of Gli-1+ PLSCs using the Gli1-CreERT2 mouse model for lineage tracing, the cells displayed a significant number change in response to periodontal inflammation due to extensive apoptosis in the diseased teeth[3]. Also, they showed significantly reduced osteogenic differentiation in the periodontitis-induced teeth. While elucidating the molecular mechanism involved in the osteogenic differentiation of the cells, Mazziotta et al[4] have provided an excellent review of the role of circular RNAs in abrogating the inhibitory activity of microRNAs in the downstream signaling pathways.
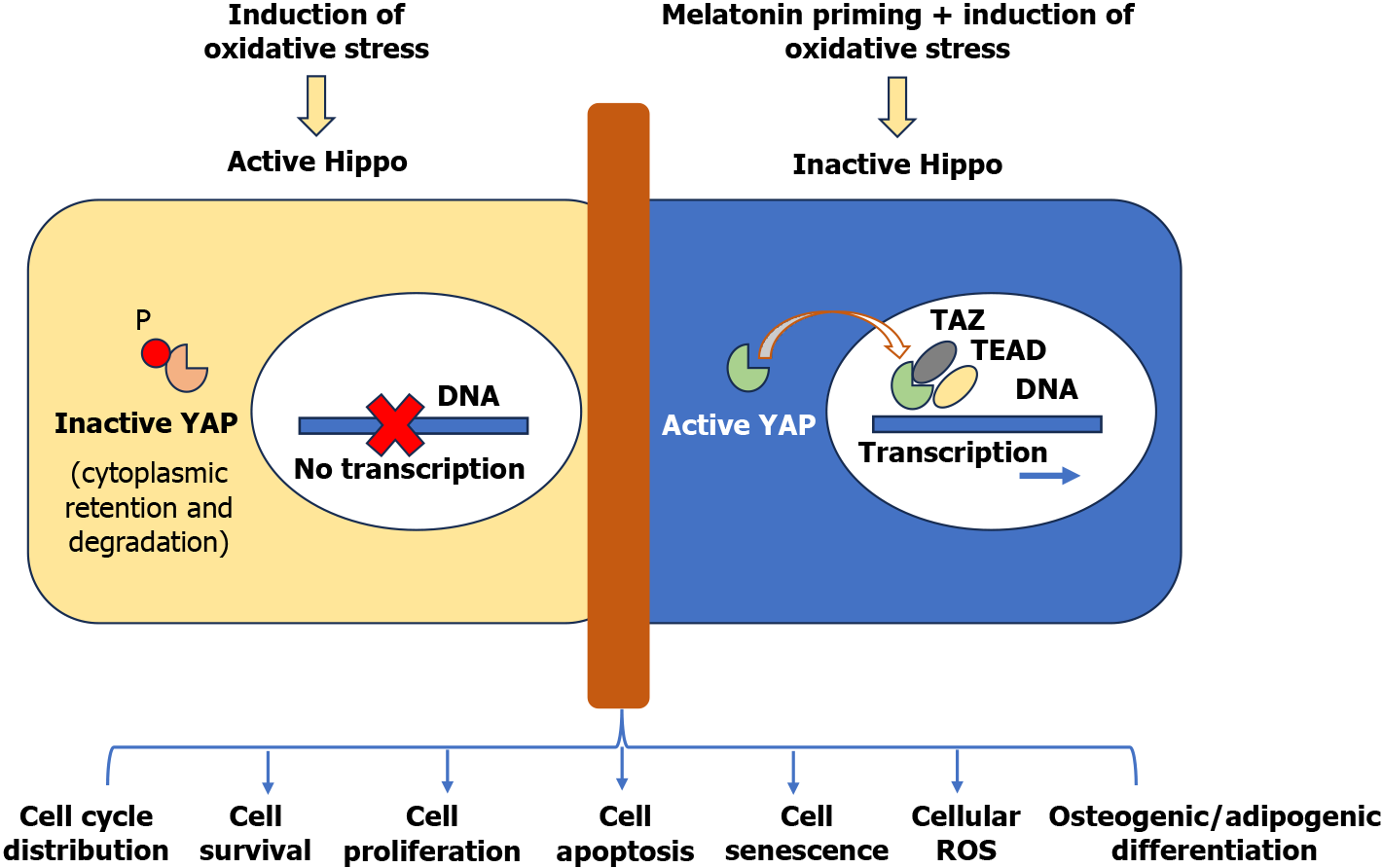
Figure 1 Summary result from Gu et al[1].
YAP: Yes-associated protein; TAZ: Transcriptional co-activator with PDZ-binding motif; TEAD: TEA domain DNA-binding family of transcription factor; ROS: Reactive oxygen species.
Stem cells primarily reside in niches with hypoxic microenvironments to sustain their stemness and undifferentiated self-renewal. Therefore, they have an exaggerated response to oxidative stress changes, culminating in cell senescence and apoptosis[5]. Even when expanded in vitro under normoxic culture conditions, they experience multiple stresses, including oxidative stress, that initiate diverse signaling pathways as a protective response to sustain their stemness and biology[6]. Stem cell priming is a clinically relevant strategy to counter oxidative stress-related molecular changes and enhance stem cell survival and functionality during their in vitro expansion in culture and post-engraftment in the harsh microenvironment of the ischemically injured tissue, which is replete with infiltrating pro-inflammatory cells and bioactive molecules, especially during the acute phase of injury, when the tissue is most vulnerable to oxidative stress and inflammation[7]. These molecular and cellular level changes result in massive cell death, and those cells that survive the harsh microenvironment onslaught undergo senescence besides the loss of stemness and repairability. Although different priming strategies have been reported in the published literature, encompassing physical, chemical, pharmacological, or genetic manipulation, using biomolecules for stem cell priming is a safer approach from the clinical perspective[7].
Melatonin, generally known for its role in sleep and circadian rhythm[8], has also been extensively studied for its antioxidant properties via multifactorial mechanisms. These mechanisms encompass direct antioxidant activity, which involves melatonin directly scavenging free radicals, and receptor-dependent mechanisms, which involve melatonin binding to its specific G-protein coupled receptors and enhancing the expression of genes encoding for enzymes with antioxidant activity and their activation. These receptors have a wide distribution in different central and peripheral tissues and organs, including the brain, heart, small intestine, gallbladder, and even B lymphocytes and T lymphocytes, and hence have broad therapeutic potential, including Alzheimer’s and other neurodegenerative disorders[9]. Even the metabolites of melatonin, i.e., hydroxy melatonin and hydroxy melatonin, show antioxidant activity, providing a safe and effective option for stem cell priming[10]. An overview of melatonin mechanisms of action has been elegantly tabulated by Chitimus et al[11].
H2O2 treatment for oxidative stress induction and its biological relevance
During exposure to oxidative stress, the antioxidant capacity of a cell to maintain physiological homeostasis is exhausted by the rise of reactive oxygen species, i.e., H2O2, superoxide anion radicals, etc[12]. These molecular changes result in the oxidation of macromolecular structures in the cells, with cellular damage ranging from mild damage to complete loss of cellular functions and cell death. For example, at the genomic level, oxidative stress causes DNA and protein damage; at the organelle level, it causes mitochondrial iron overload, leading to mitochondrial dysfunction that may lead to ferroptosis[13,14]. During in vitro experimentation in cellular models, the use of H2O2 in high molar concentrations is the most common practice to study the effect of generating oxidative stress on cell biology and functionality[15] and using natural compounds and bioactive molecules to alleviate the damaging effects of oxidative stress[16]. Gu et al[1] used the same experimental design to successfully alleviate the harmful effects of incremental oxidative stress using melatonin. While their reported data are significant, it is pertinent to mention that the dynamics of the chemically generated external oxidative stress in vitro using H2O2 differs from that in vivo, especially regarding the release of oxygen, which is much more than the oxygen levels in the niche microenvironment. Using proximal tubular kidney cells as a model, Van de Water et al[17] have shown that treatment with nephrotoxin 1,2-dichlorovinyl-L-cysteine elevated the levels of hydroperoxide that could be prevented by diphenylphenylenediamine and deferoxamine. While elucidating the molecular mechanism of the 1,2-dichlorovinyl-L-cysteine-induced oxidative stress, the authors reported increased intracellular and mitochondrial free Ca2+ with concomitant loss of mitochondrial membrane potential. These data evidenced the role of mitochondrial-free Ca2+ in oxidative stress induction. It would be interesting to see if melatonin can interfere with mechanistically diverse oxidative stress inducers and prevent cell damage during future studies.
On the contrary, in vivo oxidative stress, in general, and in the niche microenvironment in particular, is a controlled process regulated by the interplay of cytokines and growth factors in the presence of more than 40 enzymes, i.e., superoxide dismutase, catalase, thiorexidine, etc. It helps the cells to adapt to the subtly changing microenvironment[18]. Therefore, it would be interesting to study in the future whether the same data can be generated after subjecting the stem cells to hypoxia or, for that matter, post-transplantation in ischemic tissues in an experimental animal model to enhance our understanding of the study results in clinical perspective[19].
Hippo signaling and oxidative stress
Another salient feature of Gu et al’s reported data is elucidating the underlying molecular signaling during melatonin priming of the cells that restored the YAP gene and protein expression, which was otherwise abrogated under oxidative stress[1]. YAP is a critical transcription factor acting downstream of Hippo. Given its crucial role as a negative regulator of YAP/transcriptional co-activator with PDZ-binding motif (TAZ) activity required for controlling gene activity for cell proliferation, apoptosis, differentiation, growth, etc., the Hippo signaling pathway is one of the evolutionarily conserved mechanisms regulating physiological processes such as embryogenesis, development, regeneration, and wound healing. Activation of the Hippo cascade causes LATS1/2 kinases to phosphorylate YAP and TAZ, resulting in their nuclear export, cytoplasmic retention, and degradation by the proteasome[20]. On the contrary, abrogation of Hippo cascade dephosphorylates YAP/TAZ, allowing them to wield their nuclear function to promote transcription of oncogenes in association with oncogenic transcription factors such as TEA domain DNA-binding family of transcription factors, suppressor of mother against decapentaplegics, and others. Given the significant role of Hippo signaling in cell survival and growth, dysregulation of Hippo signaling has been implicated in the loss of cellular homeostasis in terms of development and homeostasis of the organ, thus leading to various diseases, including cancer[21,22]. Elegantly discussed by Zhong et al[23] in their recently published review of Hippo signaling, the signaling pathway provides a potential target for using small molecules and macromolecules to interfere with the dysregulated Hippo for implications in disease and regenerative medicine[23]. The significance of the Hippo pathway is even more critical because it is a hub where multiple signaling pathways converge, including the melatonin-induced G-protein coupled receptor signaling, thus rendering melatonin a choice molecule for priming the cells via manipulation of Hippo[24,25]. This renders the potential of the Hippo signaling pathway in stem cell research not just promising; it is intriguing and warrants further exploration, offering new avenues for understanding and enhancing stem cells’ stemness, biology, and functionality[26]. Therefore, intrinsic and extrinsic stress regulation is crucial for maintaining functional stem cell populations both in vitro and in vivo. For example, initiated by the overloading of reactive oxygen species in the cells, its activation is a part of the stem cell’s characteristic response to oxidative stress involving the MST/YAP/FoxO pathway, leading to cell apoptosis. Gu et al[1] also showed that melatonin treatment helped the cells express stemness factors like OCT4, cMyc, and Nanog, which was abolished by H2O2-induced stress besides delaying senescence during the culture.
In conclusion, oxidative stress has emerged as a standard feature that limits stem cell maintenance and disrupts its functions[27]. The data provided by Gu et al[1] is a good foundation for establishing melatonin-induced priming protocol for stem cells and understanding the underlying molecular mechanism involved therein. As stem cell biology advances into the clinic, managing oxidative stress becomes increasingly important in producing viable and functional stem cells as living bio-drugs to promote their differentiation and produce morphofunctionally competent desired cell types for treating diseases and aging[22].