Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.421
Peer-review started: January 29, 2023
First decision: February 13, 2023
Revised: February 27, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: May 26, 2023
Processing time: 116 Days and 16.7 Hours
Multiple myeloma (MM) is a hematological malignancy characterized by the accumulation of immunoglobulin-secreting clonal plasma cells at the bone marrow (BM). The interaction between MM cells and the BM microenvironment, and specifically BM mesenchymal stem cells (BM-MSCs), has a key role in the pathophysiology of this disease. Multiple data support the idea that BM-MSCs not only enhance the proliferation and survival of MM cells but are also involved in the resistance of MM cells to certain drugs, aiding the progression of this hematological tumor. The relation of MM cells with the resident BM-MSCs is a two-way interaction. MM modulate the behavior of BM-MSCs altering their expression profile, proliferation rate, osteogenic potential, and expression of senescence markers. In turn, modified BM-MSCs can produce a set of cytokines that would modulate the BM microenvironment to favor disease progression. The interaction between MM cells and BM-MSCs can be mediated by the secretion of a variety of soluble factors and extracellular vesicles carrying microRNAs, long non-coding RNAs or other molecules. However, the communication between these two types of cells could also involve a direct physical interaction through adhesion mole
Core Tip: Mesenchymal stem cells (MSCs), the main cell population of the bone marrow (BM) stroma, can influence BM microenvironment through their paracrine activity, involving both soluble factors and extracellular vesicles, but also through direct communication. Being the BM the predominant localization of multiple myeloma cells (MM), finding the appropriate conditions at this niche, is key for the survival and expansion of tumour cells and thus, for the progression of the disease. Since the activity of BM-MSCs could determine the fate of MM cells at BM, these cells could be interesting targets for the design of new antitumor drugs.
- Citation: García-Sánchez D, González-González A, Alfonso-Fernández A, Del Dujo-Gutiérrez M, Pérez-Campo FM. Communication between bone marrow mesenchymal stem cells and multiple myeloma cells: Impact on disease progression. World J Stem Cells 2023; 15(5): 421-437
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/421.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.421
Multiple myeloma (MM) is one of the most common hematological diseases, only second to non-Hodgkin lymphoma[1]. MM affects mainly older adults, with the median age of diagnosis being around 69 years. Only in 2020, 32270 new cases and 12830 deaths in the United States were estimated by the American Cancer Society Statistics Centre. In global terms, the cases would reach 160000, accounting for 0.9% of all cancer diagnosis. Importantly, incidence of MM has risen 126% globally, and hence, there is an increasing need to find new effective treatments for this incurable disease[2,3].
Besides the initial treatments for MM, consisting in alkylating agents often combined with corticosteroids, the last couple of decades have seen an important advance in the available treatments for this disease. We first saw the introduction of proteasome inhibitors (Bortezomib), histone deacetylase inhibitors (Panobinostat) and drugs such as Selinexor, with a nuclear export inhibition activity. In recent years monoclonal antibodies such as Daratumumab (anti-CD38) or Elotuzumab (anti-SLAMF7), and more recently the use of chimeric antibody receptor (CAR) T-cell products, has introduced immunotherapy as a viable approach to MM treatment[4]. According to data from the National Cancer Institute (Bethesa, MD, United States), all these treatments have had a deep impact on patients' survival, substantially raising the survival rate to 55% in the period between 2011 and 2017. More recently, the use of small molecules, with a molecular weight smaller than 1kDa, has also improved treatments, since it offers important advantages compared to the former therapies, as the easy cell entry, the simplicity of the molecules, and a much lower production cost than other drugs[5]. However, despite these advancements, there are still limitations to existing treatment options. Some patients may not respond to or may develop resistance to certain medications, many patients can become refractory to treatment and thus, there is a high risk of relapse. This promotes the search for new treatments to handle relapsed or refractory MM.
MM is caused by aberrant plasma cells (PC) proliferation in the bone marrow (BM). The premalignant states, known as monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM, transition under specific conditions to the malignant state of PC leukemia or extramedullary myeloma[6]. A key characteristic of MM is the infiltration into and the colonization of the BM, one of the two primary lymphoid organs[7]. This colonization produces typical lytic bone lesions that would be present in approximately 80% of patients with newly diagnosed MM and are the major source of morbidity[8]. The bone lesions, resulting from the stimulation of bone resorption by B-cell plasmacytomas, are associated with hypercalcemia and often, severe bone pain and bone fractures[8,9].
While the initiation of a tumor mainly depends on the accumulation of genetic defects, the transition from a premalignant to a malignant state highly relies on the interaction of the tumor cell with a permissive microenvironment that would support the malignant transformation and the proliferation of the tumor cells, aiding them to evade apoptosis. The relevance of tumor microenvironment in disease progression was first discussed in the “seed and soil hypothesis” formulated by Stephen Paget in 1889, where the establishment of tumor metastatic sites is influenced by the cross-interaction between the seeds (cancer cells) and the soil (a particular microenvironment)[10]. This is not different in MM[11,12]. The progression to MM, which would occur in approximately 50% of patients diagnosed with MGUS[6], requires multiple genomic events, but also a permissive BM microenvironment[13]. MM cells proliferate almost exclusively within the BM niche, highlighting the role of this microenvironment in supporting cancer growth. In fact, there is also mounting evidence indicating this BM microenvironment is not only key for PCs survival, but also has a crucial role in resistance to treatment and disease recurrence[14,15].
The MM cells infiltrating the BM will encounter a complex microenvironment formed by cellular and non-cellular components. Amongst the non-cellular components influencing the BM microenvironment, it is important to consider the extracellular matrix (ECM) proteins as well as a milieu of cytokines, chemokines, and growth factors. Many of these factors can have a positive effect on MM cells, boosting their proliferation and survival and the resistance to different types of drugs. A good example of these cytokines supporting MM progression are interleukin (IL)-6 and ligands of the B-cell maturation antigen, such as a proliferation-inducing ligand and B-cell activating factor (BAFF)[16,17]. Regarding the cellular components of the BM niche, many different types of bone cells (osteoblasts, or bone forming cells, and osteoclasts, or bone resorbing cells, and osteocytes) and cells from the immune system (macrophages, natural killer cells and regulatory T-cells) share this niche. Other cells present here are fat cells (adipocytes), fibroblasts, endothelial cells and two multipotent stem cells, BM mesenchymal stem cells (BM-MSCs), which differentiate into different mesodermal cell lineages, and hematopoietic stem cells (HSCs), that would differentiate into hematological lineages, including the myeloid lineage that would give rise to osteoclasts. MM cells are likely to interact with all the cells in the BM niche and elicit mutual influence[18]. In fact, it is known that communication between MM cells and BM-MSCs is essential in the progression of MM[19]. Once MM cells infiltrate the BM, their presence in the BM niche alters the activity of many of the cells found there, including those involved in bone homeostasis such as osteoclasts[20,21] and osteoblasts[22-24]. While in normal bone homeostasis, the activities of osteoblast and osteoclasts are carefully balanced to ensure a correct bone regeneration, the influence of MM cells disrupts this balance increasing both the resorptive activity of osteoclast and their numbers, and decreasing osteoblasts numbers as well as their osteogenic capacity[25], overall leading to an increase in bone destruction and the appearance of the aforementioned osteolytic lesions typical of this disease. Other cells at the BM niche which activity is highly influenced by MM cells are BM-MSCs. The presence of MM cells at the BM niche alters the MSCs behavior in different ways. In fact, changes in the expression of certain microRNAs (miRNAs) in BM-MSCs leading to important alterations of their secretory profile and osteogenic differentiation potential have been observed after co-cultivation of BM-MSCs and MM cells[26,27]. These changes at the BM niche upon MM invasion produce a microenvironment that would support disease progression. Indeed, there is strong evidence indicating that is precisely this interaction what leads to the formation of the lytic bone lesions[28]. One of the characteristics of this permissive microenvironment is the high presence of pro-inflammatory cytokines that would favor the progression of neoplasia[29]. The crosstalk between MM cells and the BM-MSCs at the BM niche is key to sustain this pro-inflammatory microenvironment and thus, to allow MM cell persistence and growth[30]. It is important to clarify, that this pro-inflammatory microenvironment would be the result of the action not only of the infiltrated MM cells but also of other cells residing at the BM niche, including BM-MSCs.
MSCs, have a key role in regulating the BM microenvironment through their paracrine activity, but also through direct cell-to-cell interaction. Regarding their paracrine activity, these cells produce a plethora of soluble biomolecules and vesicular components, known altogether as “secretome”, that exert multiple actions on other cells at the BM microenvironment[31]. BM-MSCs role in MM disease development and progression has been reported as having both inhibitory[32] and supportive roles[33,34]. Sadly, the latter is the most frequent. Once at the BM niche, MM will exert their influence on resident MSCs, altering their signaling and gene expression pattern and thus, also their secretion pattern. After interaction with MM cells, MSCs will produce a secretome rich in pro-inflammatory cytokines. In fact, it has been previously described how MSCs react to IL-1 produced by the myeloma PCs by producing large quantities of IL-6, a cytokine that would in turn stimulate the survival of the MM cells[35,36]. Therefore, the soluble part of this secretome has a key role in the progression of tumor. Moreover, in the last few years, several molecules (miRNAs) that are present in the cargo in the extracellular vesicles (EVs) produced by BM-MSCs upon MM cells stimulation also seem to have a key role in the disease promotion. Although the soluble proteins and EVs produced by the BM-MSCs are the main actors in the communication between BM-MSCs and MM cells, other ways of communication have also been implicated. This will be discussed in the following sections.
Current available treatments for MM patients mainly target MM cells but have none or limited effect on other cells in the BM or de BM microenvironment. Knowledge of the different interactions between BM-MSCs and MM cells is key to understand how MM cells behave and grow within the BM and how osteolytic lesions are formed. In this work, we will address key aspects of the different ways of communication between MSCs and MM cells as well as the outcome of this crosstalk.
The multiple cellular interactions taking place in the BM, make this microenvironment a dynamic compartment with a myriad of soluble factors that would affect the behavior of the various cell types concurring at that microenvironment. Although many of those cells have paracrine activity, BM-MSCs are the ones that have a stronger impact in the BM microenvironment due to the wide variety of soluble and non-soluble factors secreted by these cells. Various constituents of the, so called, BM-MSC secretome orchestrate the fate of the MM cells, from the first step encompassing the homing of those cells to the BM, onwards.
A key factor in the communication between BM cells and MM cells during the first stages of BM colonization, is the cytokine stromal cell derived factor 1α (SDF1α), also known as CXCL12. This factor, produced by BM-MSCs, works as a chemoattractant, being responsible of the homing of HSCs to the BM once they abandon the fetal liver during development[37]. SDF1α activity is mediated by the binding to a specific G-protein 7-span transmembrane receptor (CXCR4) at the target cells. CXCR4 is expressed at the surface of different cells in the BM microenvironment[38], and also at the surface of MM cells and other tumor cells[39]. Thus, SDF1α/CXCR4 interaction might have a relevant role in directing de metastasis of hematopoietic malignancies. Similar to its effect on HSCs, the interaction of SDF1α with its receptor at the MM cells, increases their migration, homing and adhesion towards the BM, in fact, knock down of CDCR4 in BM-MSCs or the use of the CXCR4 inhibitor AMD3100 (AnorMED), that blocks the binding of SDF1α to its receptor[40], seems to inhibit the migration of MM towards the BM[41]. The binding of SDF1α to its receptor at the MM cells, also triggers the activation of the phosphatidylinositol 3-kinase (PI3K) and the MAPK kinase (MEK)-extracellular signal regulated kinase (ERK, MEK/ERK) pathways, inducing a rearrangement in the cytoskeleton of MM cells that facilitates BM colonization[41]. SDF1α has also been described to act in a more indirect way, not mediated by the binding to CXCR4. SDF1α interacts with other molecules including matrix metalloproteinases (MMPs), integrins or growth factors such as hepatocyte growth factor (HGF), insulin like growth factor-1 (IGF-1) or molecules of the GTPases family. All of these effects elicited by SDF1α, in one way or another, lead to a promotion in MM cells migration, homing or adhesion into the BM[38].
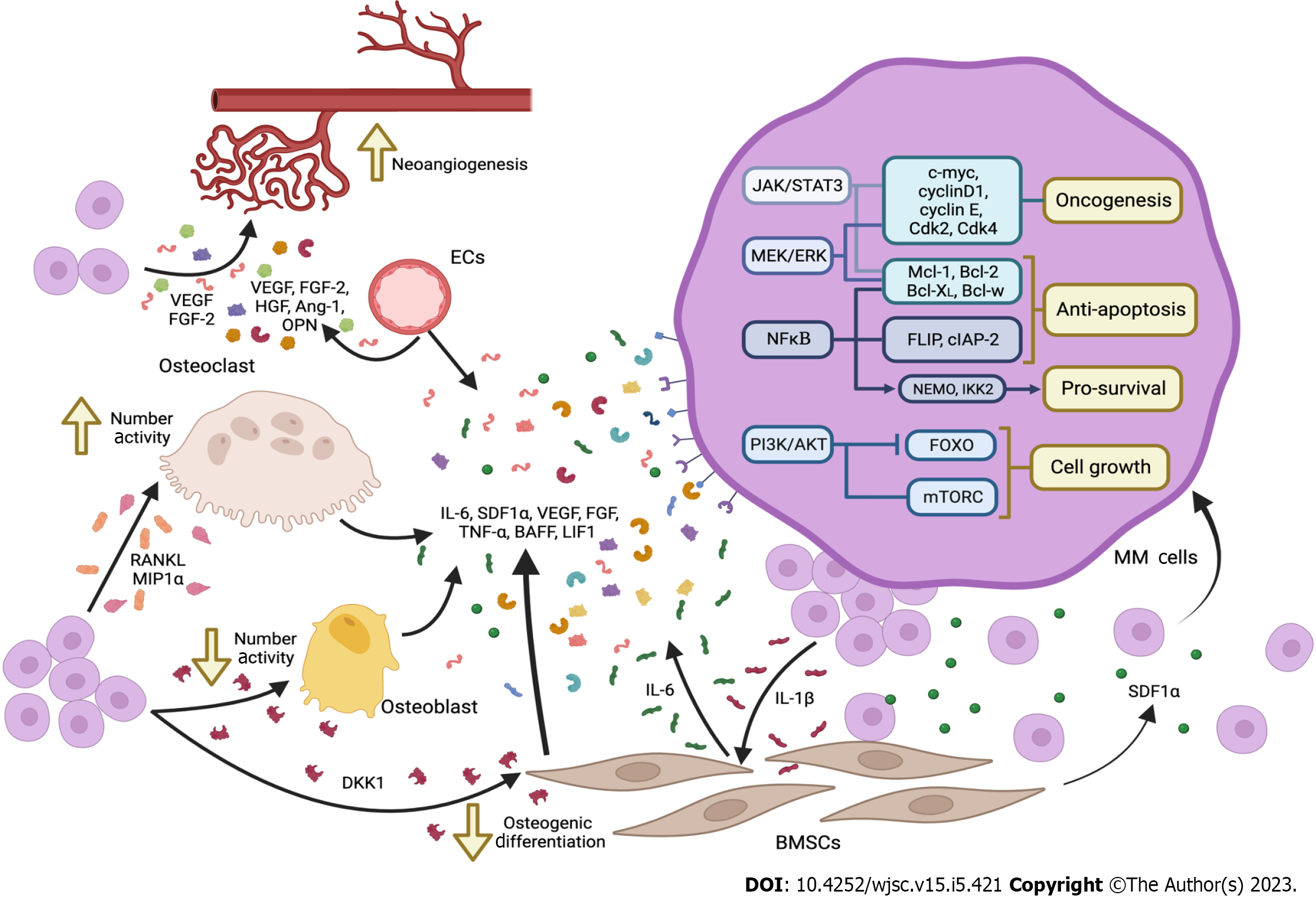
Many of the factors secreted by BM-MSCs and by other cells of the BM microenvironment, activate key signaling pathways in the MM cells that would increase their chances to survive and proliferate in the BM microenvironment. A summary of these factors as well as the signaling pathways involved in this communication are shown in Figure 1. In fact, some mutations activating those pathways have also been found in patients with MM. We will address some of those key pathways in this section.
Once in the BM, for the tumor to progress further, MM cells would need a permissive microenvironment. This microenvironment would be created by multiple soluble factors secreted by the different cell types present at the BM. The soluble factors produced by the BM-MSCs seem to be the main, but not the only, effectors of the changes elicited in the MM cells. Besides SDF1α, BM-MSCs seem to secrete other important soluble factors such as IL-6, IL-17, vascular endothelial growth factor (VEGF), fibroblast growth factors (FGF), tumor necrosis factor-α (TNF-α), BAFF or leukemia inhibitory factor-1; osteoclasts mainly secrete IL-6 and VEGF; and vascular endothelial cells secrete cyclophilin-A[42,43]. These factors will activate specific signaling pathways in the MM cells such as PI3K/Akt, MEK/ERK, Janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3, JAK2/STAT3) pathways, related to cell survival, proliferation and drug resistance[43]. It is important to highlight that this communication is bi-directional, since MM cells would also produce cytokines such as IL-1β, VEGF, and transforming growth factor-beta (TGF-β) that would exert their effect on BM-MSCs, activating the nuclear factor kappa-Β (NFκΒ) pathway and thus, inducing further secretion of cytokines by the BM-MSCs into the BM microenvironment, particularly IL-6[44,45].
IL-6 is the main activator of the JAK2/STAT3 pathway, known to be implicated in the pathogenicity of cancer. JAK2/STAT3 pathway activation promoted by IL-6 leads in MM cells to the expression not only of potent proto-oncogenes such as c-myc and cyclin D1, but also of anti-apoptotic genes like Mcl-1, Bcl-XL and Bcl-2. Moreover, STAT3 activation has also a immunosuppressive effect since it regulates T-cell mediated cytotoxic immune response[46], contributing to the establishment of a immunosuppressed microenvironment that would contribute to the survival and proliferation of the MM cells in the BM. On the other hand, IL-6 activation of JAK2/STAT3 pathways, also has an important role in bone destruction, a hallmark of MM. IL-6/JAK2/STAT3 axis induces the expression of the receptor activator of NFκΒ ligand (RANKL)[36,47] whose binding to its receptor at the surface of pre-osteoclasts, promotes their differentiation towards mature osteoclasts, activating bone resorption and thus, promoting the formation of osteolytic lesions.
It is important to highlight that the NFκΒ signaling pathway also has an important role in the survival of MM cells and in the maintenance of the tumorigenic microenvironment at the BM. Both canonical and non-canonical NF-kB pathways are activated by different factors present in the BM microenvironment, including IL-6, IGF-1, TNF-α or BAFF[48]. While IGF-1 is able to activate NFκΒ pathway, inducing the expression of anti-apoptotic, caspase-8 inhibitors FLIP and cIAP-2[49], TNF-α has a pro-survival effect through NFκΒ pathway mediators such as NFκΒ (NEMO) and IκB kinase subunit 2[44]. On the other hand, BAFF activates NFκΒ non-canonical pathway upregulating the expression of antiapoptotic proteins including Mcl-1, Bcl-XL, Bcl-w and Bcl-2[50]. There is also evidence indicating that IL-6 is linked to the expression of VEGF in MM cells, being some of the VEFG isoform expression driven by the NFκΒ pathway[51,52].
The MEK/ERK pathway is the signaling pathway most found activated in MM patients, with a prevalence in between 43% and 53% of the patients[53]. Changes in MEK/ERK pathway have important effects in cell cycle, due to the alteration in the expression of molecules such as cyclin D1, cyclin E, Cdk2 and Cdk4 and in apoptosis prevention by the induction of the phosphorylation of the pro-apoptotic protein Bim. This phosphorylation results in the release of anti-apoptotic molecules such as Mcl-1, Bcl-XL and Bcl-2, also related to Akt pathway[54]. In the absence of mutations that activate this pathway, the stimulation of the MEK/ERK pathway in the MM cells might also occur by the action of different soluble factors present in the BM microenvironment such as BAFF, IL-6, SDF1α, VEGF or TNF-α among others[42]. As with other relevant signaling pathways that become activated in MM, the MEK/ERK is also studied as a potential therapeutic target.
PI3K/Akt signaling pathway also has a relevant role in cell proliferation, cell cycle and apoptosis. Alteration of the PI3K/Akt/mTOR pathway due to genetic modifications or its hyper-activation contributes to carcinogenesis, metastasis, invasion, proliferation and drug resistance of tumor cells. However, no activating mutations have been described in MM cells yet. Despite this fact, PI3K/Akt/mTOR pathway is important for MM cells survival[55,56].
Up to this point, we have mentioned some of the effects of the pro-tumorigenic microenvironment in the BM on the MM cell survival and growth. However, once modified by the BM microenvironment, MM cells will start to release different soluble factors that will not only perpetuate that tumorigenic microenvironment, but also will have a deep impact in angiogenesis and bone homeostasis.
Neovascularization in the bone is an essential feature for MM progression and the presence of high density of micro-vessels in the BM microenvironment is characteristic in MM. Cells residing at BM, such as BM-MSCs, osteoblasts, HSCs, or endothelial precursor cells, commonly express various angiogenic factors, such as VEGF, FGF-2, TNF-α, HGF, IL-6, BAFF, SDF-1α, angiopoietin-1 or osteopontin (OPN). Also, MM cells are able to directly produce VEGF stablishing a VEGF autocrine loop where the produced VEGF would stimulate MM cells proliferation through the MEK-1/ERK pathway[57,58]. FGF-2 is another key pro-angiogenic molecule that would be produced by both MM cells and BM-MSCs[59]. However, contrary to VEGF, which is produced by all MM cells, FGF-2 production by MM does not seem to be a general feature in all MM cases[59]. Other molecules with pro-angiogenic activity such as MMPs[60,61] or OPN, also produced by MM cells, have also a relevant role in promoting micro-vessels formation in the BM microenvironment. The overall increase in the production of such angiogenic factors is elicited by the MM cells. The activation of angiogenesis linked to tumor progression is known as “angiogenic switch”[62] .
Bone homeostasis is a dynamic process driven by osteoclasts, osteoblast and osteocytes. Alterations in the balance between these cell types will lead to the remodeling of the bone. The characteristic bone lesions found in MM derive from the disruption of bone homeostasis initiated by the activation of JAK2/STAT3 pathway by IL-6 and the subsequent induction of RANKL expression by MM cells. Not only this but, as will be discussed later, cell-to-cell interaction of MM cells with BMSCs also induce the expression of the macrophage inflammatory protein (MIP)-1α[63]. Both RANKL and MIP-1α are mediators in the bone destruction driven by MM as they have an both in the number of osteoclasts and in their activity. MIP-1α is a chemoattractant for osteoclasts and stimulates osteoclast formation[64], while RANKL after being recognized by its receptor RANK, will induce the commitment of the macrophage/monocyte precursor cells to the osteoclast lineage[65].
Secreted by MM cell in response to the activation of the JNK pathway, Dickkopf-1 (DKK-1) is also a disruptor in bone homeostasis[66]. DKK-1 is an extracellular inhibitor of the Wnt pathway. DKK-1 interacts with membrane receptors as transmembrane proteins Kremen 1/2 and the human low-density lipoprotein receptor-related protein 5/6, thus competing with Wnt[67]. As one of the main regulatory pathways for osteogenic differentiation of BM-MSCs into osteoblasts[68], the inhibition of the Wnt/β-catenin pathway by DKK-1 will result in a reduced number of osteoblasts. By the action of these factors, RANKL, MIP-1α and DKK-1, the balance between bone formation and bone resorption driven by osteoblasts and osteoclasts is disrupted, resulting in the characteristic bone lesions present in MM patients.
A table summarizing the latest scientific evidence regarding key factors involved in MM/BM-MSCs communication and their effect is shown (Table 1).
| Soluble factors | Origin | Function | Ref. |
| SDF1α | BMSCs | Chemoattractant of MM cell towards the BM microenvironment | [38] |
| IL-1β | MM cells | Act over BMSCs inducing the secretion of soluble factors, mainlyIL-6 | [45] |
| IL-6 | BMSCs | Closely related with cancer pathogenicity due to it proto-oncogenic and anti-apoptotic effect over MM cells | [46,47] |
| Immunosuppressive effect over T cells | |||
| Also related with bone destruction by inducing the expression of RANKL by the MM cells | |||
| VEGF | BM cells, MM cells | Promotes bone neovascularization, essential for tumour progression | [58] |
| RANKL | MM cells | Induce the commitment of the macrophage/monocyte precursor cells to the osteoclast lineage. Promoting indirectly bone destruction | [63] |
| DKK-1 | MM cells | Disruptor of bone homeostasis by inhibiting BMSCs differentiation into osteoblasts | [66,67] |
Under non-pathological conditions, BM homeostasis is maintained by cell-to-cell contact, soluble molecules, and EVs. Whereas, over the years solid evidence has accumulated about the relevance of the first two, the involvement of EVs-mediated communication in the maintenance of BM homeostasis has started to be contemplated only in the last few decades[69]. Despite being a fairly new field, important advances have been made in the knowledge of EVs, such as their classification, in terms of their size and biogenesis, into three major categories (exosomes, micro-vesicles, and apoptotic bodies) and the fact that its content varies according to the state of their parental cells[31].
As we have previously discussed, MM cells have the capacity to alter the environment in which they reside[70] as well as the characteristics of cells present in that microenvironment. Thus, it is not surprising that the EVs produced by MM cells also play a key role in disease progression. In fact, it has recently been shown that, exosomes (a particular class of EVs) produced by both BM-MSCs and MM cells are largely responsible for MM pathogenesis[71]. This recent demonstration of the relevance of EVs in MM progression has resulted in several studies in the lats few years, however, the multitude of agents and interactions involved in the development and progression of this disease has made it difficult to fully understand the molecular mechanisms involved. In this section we aim to gather the available information so far.
As previously mentioned, osteolysis, one of the main hallmarks of MM disease, is linked to the negative effect of MM cells on cells responsible for bone homeostasis, such as MSCs, osteoblasts and osteoclasts[72]. In particular, myeloma bone disease (MBD) has a unique feature compared to other diseases that encompass bone destruction, since in MBD osteoblast activity is also severely impaired[24]. Several authors have suggested that an essential part of this bone damage is related to EVs directly produced by MM cells (MM-EVs). Zhang et al[73] demonstrated that the cargo of MM-EVs was enriched in various molecules which negatively regulate osteogenesis. They confirmed that MM-EVs induced high expression of miR-103a-3p in BM-MSCs, which led to impaired osteogenesis in vitro. Moreover, they showed that injection of MM-EVs in mouse tibia resulted in defective bone formation. Interestingly, in vitro assays also revealed that MM-EVs were also able to influence MM cells increasing viability and IL-6 production, known to regulate MM cell proliferation thus, establishing an autocrine feedback. MM-EVs also increased miR103a-3p expression in MM cells however, in those cells the increased proliferation of MM cells after exposures to MM-EVs does not seem to be related to miR103a-3p but to other miRNAs also present in the MM-EVs cargo, such as miR107 and miR181a-3p[24].
Among the different biomolecules found as part of the exosome cargo, long non-coding RNAs (lncRNAs) and miRNAs have been the focus of attention due to their key regulatory roles. Various miRNAs found in MM-EVs have been studied for their involvement in the disruption of osteogenesis. miR-129-5p was identified as a player in vesicle-mediated bone disease[74]. In particular, miR-129-5p seemed to inhibit the transcription factor specificity protein 1, leading to a reduction of ALPL, both at the mRNA and protein levels, during the early osteogenic differentiation of MSCs. On the other hand, the long non coding RNA Long Intergenic Non-Protein Coding RNA 461, found as part of the MM-exosomes cargo, has also been found to inhibit osteoblast differentiation by reducing the activity of Wnt/β-Catenin pathways, responsible for osteoblast proliferation, differentiation and activity[75]. Other molecules, such as soluble proteins present in the MM-EVs cargo also showed anti-osteogenic activity Faict et al[72] revealed that Wnt/β-Catenin inhibitor DKK-1 is present in MM-EVs and observed a lower expression of Osterix (OSX), Collagen 1A1 and alkaline phosphatase in differentiated MC3T3-E1 cells after MM-EVs treatment.
Runx2 is the master regulator of early osteogenic differentiation, and therefore a possible target for the anti-osteogenic effect of MM-EVs. In fact, lncRNA RUNX2-AS1 present in the MM-EVs cargo was identified as a bioactive molecule able to reach MSCs and form a transcriptionally repressed RNA duplex with RUNX2 premRNA, reducing the osteogenic activity[76]. In addition, a MM-EVs impact in osteoblastic differentiation through reduction of Runx2, together with OSX and OCN, has been described by Liu et al[77]. These authors also record increased levels of IL-6 secretion via APE1/NF-kB which, as aforementioned, is an important survival factor of MM cells.
Once the EVs produced by MM cells reach the BM-MSCs, their cargo modifies the BM-MSCs behaviour in the benefit of MM cells. A clear example of this is miR-146a which acts in a positive loop to favor disease progression[19]. Once this miRNA targets BM-MSCs, it produces an increase in the secretion of several cytokines and chemokines from those cells, including CXCL1, IL-6, IL-8, inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), and CCL-5, which, in turn, once released into the BM microenvironment, would favor MM cell viability and migration. In addition, MM-EVs cargo miR-146a and miR-21, participate in proliferation and transformation of MSCs into cancer associated fibroblasts (CAFs). This is a type of cell which could contribute to a tumour-supportive microenvironment through secretion of cytokines, including IL-6 and TGF-β[78].
Interestingly, it has been shown that conventional chemotherapeutic agents including melphalan, and anti-proteases such as bortezomib and carfilzomib can stimulate a considerable MM-EVs release. The EVs produced under these circumstances are called “chemoexosomes”. These chemoexosomes are characterized by the high presence of the heparanase enzyme in their surface. This heparanase is implied in several cellular changes leading to chemoresistance and the subsequent relapse of the patient. Heparanase EVs content is delivered in MM cells and activate ERK pathway as well as TNF-α production by macrophages, matrix degradation and migration promotion[71].
So far, we have analyzed the influence of MM-EVs on BM-MSCs, however, this communication, as previously mentioned, is bidirectional. In 2016, Wang et al[69] showed that BM-MSC-EVs from MM patients contained a lower level of the tumor suppressor miR-15a, and higher levels of oncogenic proteins, cytokines, and adhesion molecules, when compared to EVs from healthy BM-MSCs. Cytokines such as IL-1ra, interferon-IP-10, MCP-1, MIP-1α, MIP-1β, and SDF1α were detected in murine BM-MSC-EVs. They confirmed that BM-MSC-EVs from MM patients act on MM cells activating proliferation, survival, and migration, as well as drug resistance to bortezomib, a widely used clinical drug for MM treatment.
In a similar study, a reduction of mir-15a levels in the cargo of BM-MSCs-EVs from MM patients was also detected. This change was shown to promote cell proliferation and dissemination or metastasis to other niches, which is a hallmark of MM. The same authors also revealed the importance of some of the proteins present in BM-MSCs-EVs cargo, as they detected higher content levels of IL-6, CCL2, γ-catenin and fibronectin, which are key to MM pathogenesis[70]. Other miRNAs cargo were also implicated in these processes. miR-483-5p was found packed in BM-MSCs-EVs and was responsible for promoting MM cell proliferation and reduced apoptosis via the miR-483-5p/TIMP2 axis[79]. Umezu et al[80] highlighted the role of miR-10a in MM disease since its transference via BM-MSC-EVs promoted cell proliferation in several MM cell lines (RPMI 8226, KMS-11, and U266) compared to BM-MSC-EVs with miR-10a blocked. Moreover, Gao et al[81] studied miR-155 present in BM-MSC-EVs cargo, which turned out to be involved in viability, stemness and drug resistance in MM cells. The role of miR-155 was underscored by the fact that incubation of the MM cells line mitochondrial pyruvate carrier 11 (MPC-11) with miR-155-mimics for 24 h resulted in a significantly reduced cell apoptosis in vitro and augmented expression of stemness maintenance markers OCT-4 and Nanog and drug resistance-associated proteins MRP1, ABCG2 and P-g.
As in the previous section, a table summarizing the main works referred to the relevance of communication between MM cells and BM-MSCs through EVs and the role of their cargo is shown (Table 2).
| Function | Ref. | |
| MM-EVs cargo | ||
| lncRNA RUNX2-AS1 | Form a RNA duplex with RUNX2 premRNA, reducing the osteogenic activity in MSCs | [76] |
| miR-146a | Increase the secretion of several cytokines in BM-MSCs that favor MM cell viability and migration and induce CAF transformation | [78] |
| DKK-1 | Lower expression of OSX, COL1A1 and ALP in osteoblast precusor cell line (MC3t3-E1) | [72] |
| MSC-EVs cargo | ||
| mir-15a | Promote MM cell proliferation and dissemination to other niches | [70] |
| miR-483-5p | Induce MM cell proliferation and reduced apoptosis | [79] |
| miR-155 | Reduce MM cell apoptosis and augment expression of stemness maintenance and drug resistance markers | [81] |
The resistance to treatment is precisely one of the major problems in MM at the clinical level, as this is directly responsible for the relapses. Some studies investigating the mechanisms behind this resistance have highlighted the implication of the activation of several signaling pathways, including p38, p53, c-Jun N-terminal kinases and Akt through the assessment of bortezomib treatment. The role of BM-MSC-EVs in interfering with the antitumor effect developed by bortezomib in MM was confirmed through different experiments. BM-MSC-EVs were able to alter apoptosis-related proteins Bcl-2, Bax, caspase-8, caspase-9, and caspase-3 promoting an antiapoptotic profile in both murine and human cells. These EVs blocked the significant reduction of Bcl-2 expression caused by bortezomib and reduced cleaved caspase-9, caspase-3, and PARP either in the absence or presence of bortezomib. Moreover, the use of GW4869, a neutral sphingomyelinase inhibitor of the formation of exosomes by the ceramide pathway, in combination with bortezomib treatment led to a significant effect on tumor load reduction[71,82].
In conclusion, the two-way communication between MM cells and BM-MSCs mediated by EVs is extremely intricate and plays a pivotal role in the progression of the disease. Since BM-MSCs-EVs have a key role in supporting MM development, this could become a key target to develop new therapies for the treatment of this hematological disease.
As well as the already described interactions through paracrine secretion of different cytokines and EVs, MM cells also interact with BM-MSCs by direct cell-to-cell contact. These cell-to-cell interactions are not restricted to MM and BM-MSCs since MM cells also interact with other cells of the BM microenvironment such as osteoclasts and osteoblasts, endothelial cells, and lymphocytes. It is known that these contacts are also key to protect MM cells against chemotherapy, helping them to accumulate inside the BM[83], to adhere to endothelium, and to spread into the bloodstream[84], although the detailed mechanisms involved in those processes have not been completely elucidated[85].
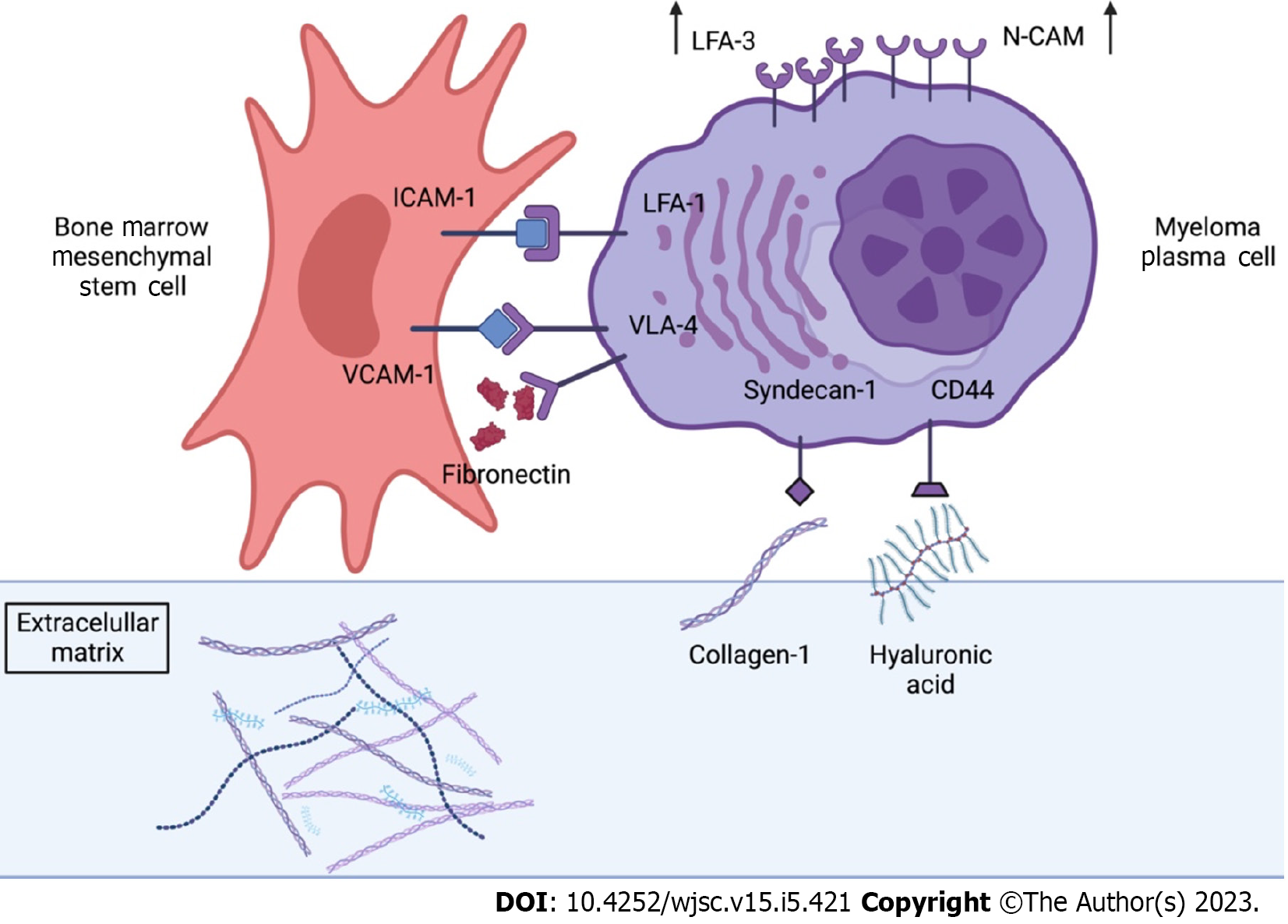
Direct cell-to-cell adhesion and communication mechanisms have been known for more than 40 years[86,87]. These cell-to-cell communication is mediated by Cell Adhesion Molecules (CAMs), a subcategory of adhesion proteins located at the cell surface, involved in binding either to other cells, or in attaching cells to proteins of the ECM[88], suchas fibronectin, laminin or collagen (Figure 2). While it has been well documented that the ECM promotes the survival of different types of tumors, much less is known about the influence of the direct contact of BM-MSCs in their progression.
CAMs play a central role in cell communication and the maintenance of tissue homeostasis[89]. There are different superfamilies or groups of CAMs with different specificities and distributions. These families would include the Immunoglobulin superfamily CAMs (IgCAMs), integrins, cadherins and one superfamily of proteins that contain a C-type lectin-like domain (C-type lectin domain proteins or CTLDs)[89]. Following other criteria, CAMs can be classified into calcium-independent or calcium-dependent molecules[90], meaning that these molecules would need Ca2+ ions binding to different domains of the protein in order to rigidify their extracellular domains and enable interaction[91]. Integrins and IgCAMs belong to the calcium independent group whereas CLTDs and selectins belong to calcium dependent group[92]. Cell adhesion molecules bind to different ligands. Cadherins, selectins and IgCAMs are associated with the cell-to-cell contact, while integrins are involved in the attachment of MM cells to the ECM[93]. All these molecules are integral in modeling cellular mechanisms such as growth, contact inhibition and apoptosis. In fact, changes in cell adhesion, involving these molecules, can be the defining event in a wide range of diseases, including cancer development[94], as lower intercellular adhesiveness allows malignant cells to scape from their site, thus, destroying the architecture of the original tissue, commonly the first step leading to cancer[94].
As well as the already described interactions through paracrine secretion of different cytokines and EVs, MM cells also interact with BM-MSCs by direct cell-to-cell contact. In fact, recent studies revealed that many of the changes undergone by BM-MSCs supporting the progression of MM, are acquirable by physical contact with MM cells[95]. In MM, this cell-to-cell interactions are not restricted to MM and BM-MSCs since MM cells also interact with other cells of the BM microenvironment such as osteoclasts and osteoblasts, endothelial cells, and lymphocytes. All these interactions are regulated by CAMs. It is known that these contacts are key to protect MM cells against chemotherapy, helping them to accumulate inside the BM[83], to adhere to endothelium, and to spread into the bloodstream[84], although the detailed mechanisms involved in those processes have not been completely elucidated[85].
The most relevant role of CAMs in MM pathophysiology is related to the homing of malignant PCs to the BM. To complete the process of homing, mediated by CXCL12, MM cells need to adhere to either ECM proteins or BM-MSCs. This is mediated by CAMs such as very late antigen (VLA)4, VLA5, CD44, leukocyte function-associated antigen 1 (LFA-1), intercellular adhesion molecule 1 (ICAM-1), MPC-1 and syndecan (Figure 2).
One way of ensuring adhesion of MM to the ECM is the binding of its integrin VLA-4 to fibronectin, a common component of the ECM. VLA-4, which is in fact a heterodimer of two integrins CD49d(a4) and CD29(b1), also mediates the interaction of MM cells with BM-MSCs, through the vascular cell adhesion molecule-1 (VCAM-1), located at the BM-MSCs[96]. This interaction activates the secretion of MIP-1α and MIP-1β in MM cells, leading to an increase of osteoclastogenic activity[97]. Moreover, the direct contact of these two types of cells through VLA-4 also induces the production of DKK-1 by MM cells, which inhibits osteoblastic differentiation of BM-MSCs. Thus, these two actions, promotion of osteoclastogenesis and inhibition of osteogenesis, would have a detrimental effect on bone structure, contributing to the typical osteolytic lesions in MM. In addition, BM-MSCs unable to undergo osteoblastic differentiation would produce higher levels of IL-6, a cytokine that would stimulate the proliferation of DKK-1-secreting MM cells[25]. Moreover, it has been observed that VLA-4–fibronectin binding is an essential step that supports the IL-6-mediated induction of PCs in normal BM, since antibodies against VLA-4 were found to inhibit the secretion of IL-6 in co-cultures of MM cells and BM-MSCs cells[96,98,99].
The interaction between MM cells and BM-MSCs is also mediated by ICAM-1 (CD54) and LFA-1 (CD11α/CD18) expressed in BM-MSCs and MM respectively. The glycoprotein ICAM-1 is the main ligand for b2 integrins and its expression is induced in response to an inflammatory microenvironment[100], such as the one resulting in the BM following the colonization by MM cells. The ICAM-1/LFA-1 interaction seems to have a key role in the progression of MM since the blocking of LFA-1 through the use of monoclonal antibodies, inhibits the production of IL-6 by BM-MSCs. Thus, this interaction is focus of various studies aimed to the development of treatments for MM[101].
Syndecan (CD138) is the principal transmembrane proteoglycan expressed in the surface of MM cells and has in fact been used as a marker for the detection of this pathology. Syndecan has multiple functions in MM. This molecule mediates de adhesion of MM cells to the collagen in the ECM through direct interaction with collagen molecules but can also mediate myeloma cell-cell adhesion[102]. Syndecan-1 also plays a broad role in cells signaling since heparan sulfate chains on syndecan-1 can bind to and sequester growth factors and cytokines, regulating their availability to cells. Also, a recent study has shown that syndecan contributes to the survival of mature MM cells by enhancing IL-6 signaling[103]. Finally, the binding of syndecan to VEGF and other angiogenic factors, has been shown to promote angiogenesis in MM[104].
Finally, CD44, a transmembrane glycoprotein, interacts mainly although not exclusively with hyaluronic acid in the ECM[105]. CD44 signaling has been shown to activate various signaling pathways in different types of cancer including PI3k/AKT, MAPK/ERK and NF-kB[106], which, as we have seen, promote MM cell survival.
Although normal PC and MM cells express basically the same set of CAMS, some of these molecules were found to be more significantly overexpressed in MM cells when compared to healthy patients. In this group we can include, leukocyte adhesion molecule LFA-3 (CD58)[107] and neural cell adhesion molecule (CD56)[108]. MM cells can also express the lymphocyte function-associated antigen LFA-1 (CD11α/CD18) which was associated with tumor growth and homotypic tumor cell adhesion or aggregation[109]. It is also worth mentioning that some homing molecules could not be detected on MPCs: Selectin molecule L-selectin and collagen receptor VLA-2[89]. Although this study provides relevant information, for this information to be biologically relevant, ligands of these receptors had to be available within the tumor environment.
Overall, given the importance of some of these CAMs in the process of MM cells homing, these molecules could be important targets for designing antitumoral treatments. Several approaches have been explored, including antibodies specifically targeting these molecules on the cell surface, as well as small molecule inhibitors that interfere with the binding of the CAMs to their ligands. Moreover, receptor-blocking antibodies against most of these CAMs (VLA-4, CD56, MPC-1, CD21) were found to partially block MM cells adhesion to the BM stroma. This partial effect could be attributed to an additional adhesion mechanism yet to be discovered[110,111].
In MM a specific type of drug resistance seems to be mediated by CAMs, the so called, CAMs mediated drug resistance[112]. CAMs can activate intracellular pathways that promote cell survival, promote cancer cell adhesion to the ECM and regulate the expression of drug transporters that could pump chemotherapy drug out of cancer cells and reduce their efficacy. It is also important to highlight that MM spreading in the last stages of the diseases also involve important changes in cell adhesion. MM can abandon de BM microenvironment and become stroma independent because of different processes involving changes in the expression levels of CAM and certain cytokines. Once this happens, cells can be found to spread extramedullary at different sites such as lungs, liver, or pleural fluid[113,114].
As we have seen, communication between MM and BM-MSCs cells can take place through mechanisms that can be classified as contact-dependent and/or contact-independent mechanisms[115]. While the previous section has been dedicated to direct communication mediated by cell adhesion molecules, in this last section we will briefly discuss transport via tunneling nanotubes (TNTs), another form of contact-dependent interaction.
TNTs are transient intercellular structures formed by the polymerization of F-actin which provide an important and general mechanism of cell-to-cell communication[116,117] and constitute a reliable infrastructure for vesicle and protein trafficking[118]. Numerous examples of communication between MSCs and malignant hematological cells such as B cells, MM and chronic lymphocytic leukemia, are already known, as well as the effects of this communication, such as increased drug resistance. This has already been demonstrated in acute myeloid leukemia (AML), B-cell precursor acute lymphocytic leukemia (ALL) or CML[119,120]. Therefore, TNTs are considered one of the key pharmacological targets in current research.
The role of TNTs is to deliver autophagosomes, mitochondria and other lipophiles to MSCs. This induces the secretion of specific cytokines, including interferon-γ-IP-10, CXCL10, IL-8, MCP-1 and CCL2 and other growth factors which, in turn, induce tumor cell survival, enhanced growth and even drug resistance[121]. This has been checked in AML, where increased survival of cells against chemotherapy treatments is observed by means of mitochondrial transfer from MSCs routed by TNT. In this case, the mitochondrial transfer translates into an increase of up to 14% in mitochondrial mass in co-cultures of tumor cells with MSCs and a 1.5-fold increase in mitochondrial adenosine triphosphate production (ATP), making them less prone to mitochondrial depolarization and thus resulting in increased survival against chemotherapy treatments[122].
Numerous lines of treatment are currently under development for various hematological diseases that reduce the formation of TNT by blocking actin polymerization. This inhibits the cellular commun
Although the fate of mitochondria transferred into tumor cells remains unclear, there is evidence indicating that MSCs play a key role in the progression of AML, ALL, MM and mitochondrial transfer chemoresistance. It is well known that the initiation of cancer requires metabolic adjustments, since rapid proliferation cancer cells have high metabolic requirements. This mitochondrial and/or mitochondrial DNA transfer to cancer cells increases mitochondrial content and enhances the mitochondrial process of oxidative phosphorylation (Oxphos), which generates a larger quantity of ATP than glycolysis, thus, promoting cell proliferation and invasion[125]. Therefore, targeting mitochondrial respiration and Oxphos is also a treatment option, FOXM1 is known to regulate myeloma cell metabolism by increasing glycolysis and Oxphos. NB73 is a FOXM1 inhibitor that promotes FOXM1 degradation and thus growth of MM cells, making it a potential drug targeting Oxphos[126].
Studies to date have elucidated that mitochondrial transfer dynamically induced resistance occurs between MM cells and other cells in the BM microenvironment via TNT, providing a starting point for the development of new targeted therapies[127]. An example of this line of treatment for MM is the use of anti-CD38 monoclonal antibodies[128]. This antibodies have different mechanisms of action, including cell apoptosis[129]. Moreover, their administration in mice has shown inhibition of mitochondrial transfer, a reduction in tumor volume and, in general, increased survival[1]. However, it should be noted that, although patients who have received this treatment show increased survival, it has been observed that resistance to these treatments can be acquired in the long term.
Conditions at the BM microenvironment are essential for the establishment and progression of MM. The complex BM microenvironment encompasses hematopoietic cells, immune cells, and cells involved in bone homeostasis such as osteoclasts, osteoblasts and BM-MSCs. Thus, it is understandable that the disruption of microenvironment homeostasis by MM cells results in angiogenesis, osteolysis, immune suppression and anemia[69].
As key regulators of this microenvironment, BM-MSCs play an important role in the progression of the disease. The crosstalk between MM cells and BM-MSCs takes place at different levels, through soluble cytokines, EVs, and direct cell-to-cell contact.
The interaction between these two cell types can have both positive and negative effects on the proliferation and survival of MM cells. The communication between MM cells and BM-MSCs can promote tumor growth. The survival and proliferation of MM cells once they reach the BM is associated with immune suppression, eliminating the possibility of an effective antitumor response. Although it is the interaction between all cells in the BM what produces this immunosuppressive microenvironment, BM-MSCs have a relevant role in the construction of this particular microenvironment due not only to their important paracrine activity, but also to their ability to establish direct communication with other cells in that microenvironment. All these direct or indirect interactions activate a pleiotropic proliferative and antiapoptotic cascades favoring disease progression.
On the other hand, the communication between MM cells and BM-MSCs can also have a negative impact on cancer cell growth and survival. BM-MSCs can secrete factors that inhibit the growth and survival of MM cells.
Currently, therapeutic advances in the treatment of this disease are based on targeted therapies using monoclonal antibodies or CAR-T. These treatments have improved patient prognosis, although long-term resistance is still observed, and further research is needed into the specific mechanisms by which cells acquire this resistance. In the quest for new effective treatments for MM, the importance of communication between MM cells and BM-MSCs cannot be overstated. Understanding the molecular mechanisms involved in this two-way communication can provide valuable insights into MM pathogenesis and help identify key targets involved in the survival and proliferation of MM cells in the BM microenvironment and thus, opening new opportunities for the design of targeted therapies to avoid disease progression.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L, China; Sheykhhasan M, Iran; Liu AF, China S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15469] [Article Influence: 2578.2] [Reference Citation Analysis (2)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64538] [Article Influence: 16134.5] [Reference Citation Analysis (176)] |
| 3. | Padala SA, Barsouk A, Rawla P, Vakiti A, Kolhe R, Kota V, Ajebo GH. Epidemiology, Staging, and Management of Multiple Myeloma. Med Sci (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 4. | Abramson HN. Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Yamamoto C, Minakata D, Koyama S, Sekiguchi K, Fukui Y, Murahashi R, Nakashima H, Matsuoka S, Ikeda T, Kawaguchi SI, Toda Y, Ito S, Nagayama T, Umino K, Nakano H, Morita K, Yamasaki R, Ashizawa M, Ueda M, Hatano K, Sato K, Ohmine K, Fujiwara SI, Kanda Y. Daratumumab in first-line therapy is cost-effective in transplant-eligible patients with newly diagnosed myeloma. Blood. 2022;140:594-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, Gay F, Anderson KC. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 814] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 7. | Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975;50:29-40. [PubMed] |
| 8. | Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 181] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 9. | Mundy GR, Raisz LG, Cooper RA, Schechter GP, Salmon SE. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med. 1974;291:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 413] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 11. | Lomas OC, Tahri S, Ghobrial IM. The microenvironment in myeloma. Curr Opin Oncol. 2020;32:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Moschetta M, Kawano Y, Sacco A, Belotti A, Ribolla R, Chiarini M, Giustini V, Bertoli D, Sottini A, Valotti M, Ghidini C, Serana F, Malagola M, Imberti L, Russo D, Montanelli A, Rossi G, Reagan MR, Maiso P, Paiva B, Ghobrial IM, Roccaro AM. Bone Marrow Stroma and Vascular Contributions to Myeloma Bone Homing. Curr Osteoporos Rep. 2017;15:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Hoang PH, Cornish AJ, Dobbins SE, Kaiser M, Houlston RS. Mutational processes contributing to the development of multiple myeloma. Blood Cancer J. 2019;9:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Visram A, Dasari S, Anderson E, Kumar S, Kourelis TV. Relapsed multiple myeloma demonstrates distinct patterns of immune microenvironment and malignant cell-mediated immunosuppression. Blood Cancer J. 2021;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | García-Ortiz A, Rodríguez-García Y, Encinas J, Maroto-Martín E, Castellano E, Teixidó J, Martínez-López J. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 16. | Chu VT, Berek C. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev. 2013;251:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Lindquist RL, Niesner RA, Hauser AE. In the Right Place, at the Right Time: Spatiotemporal Conditions Determining Plasma Cell Survival and Function. Front Immunol. 2019;10:788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19:626-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 483] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 19. | De Veirman K, Wang J, Xu S, Leleu X, Himpe E, Maes K, De Bruyne E, Van Valckenborgh E, Vanderkerken K, Menu E, Van Riet I. Induction of miR-146a by multiple myeloma cells in mesenchymal stromal cells stimulates their pro-tumoral activity. Cancer Lett. 2016;377:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Fu J, Li S, Feng R, Ma H, Sabeh F, Roodman GD, Wang J, Robinson S, Guo XE, Lund T, Normolle D, Mapara MY, Weiss SJ, Lentzsch S. Multiple myeloma-derived MMP-13 mediates osteoclast fusogenesis and osteolytic disease. J Clin Invest. 2016;126:1759-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Colombo M, Thümmler K, Mirandola L, Garavelli S, Todoerti K, Apicella L, Lazzari E, Lancellotti M, Platonova N, Akbar M, Chiriva-Internati M, Soutar R, Neri A, Goodyear CS, Chiaramonte R. Notch signaling drives multiple myeloma induced osteoclastogenesis. Oncotarget. 2014;5:10393-10406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Roodman GD. Osteoblast function in myeloma. Bone. 2011;48:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Yaccoby S. Osteoblastogenesis and tumor growth in myeloma. Leuk Lymphoma. 2010;51:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Reagan MR, Liaw L, Rosen CJ, Ghobrial IM. Dynamic interplay between bone and multiple myeloma: emerging roles of the osteoblast. Bone. 2015;75:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Berenstein R, Blau O, Nogai A, Waechter M, Slonova E, Schmidt-Hieber M, Kunitz A, Pezzutto A, Doerken B, Blau IW. Multiple myeloma cells alter the senescence phenotype of bone marrow mesenchymal stromal cells under participation of the DLK1-DIO3 genomic region. BMC Cancer. 2015;15:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Berenstein R, Nogai A, Waechter M, Blau O, Kuehnel A, Schmidt-Hieber M, Kunitz A, Pezzutto A, Dörken B, Blau IW. Multiple myeloma cells modify VEGF/IL-6 levels and osteogenic potential of bone marrow stromal cells via Notch/miR-223. Mol Carcinog. 2016;55:1927-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 228] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 29. | Dewald JH, Colomb F, Bobowski-Gerard M, Groux-Degroote S, Delannoy P. Role of Cytokine-Induced Glycosylation Changes in Regulating Cell Interactions and Cell Signaling in Inflammatory Diseases and Cancer. Cells. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 1070] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 31. | González-González A, García-Sánchez D, Dotta M, Rodríguez-Rey JC, Pérez-Campo FM. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J Stem Cells. 2020;12:1529-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (6)] |
| 32. | Atsuta I, Liu S, Miura Y, Akiyama K, Chen C, An Y, Shi S, Chen FM. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res Ther. 2013;4:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Kumar S, Witzig TE, Timm M, Haug J, Wellik L, Kimlinger TK, Greipp PR, Rajkumar SV. Bone marrow angiogenic ability and expression of angiogenic cytokines in myeloma: evidence favoring loss of marrow angiogenesis inhibitory activity with disease progression. Blood. 2004;104:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, Schlossman RL, Richardson P, Ralph P, Wu L, Payvandi F, Muller G, Stirling DI, Anderson KC. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 400] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | Xiong Y, Donovan KA, Kline MP, Gornet MK, Moon-Tasson LL, Lacy MQ, Dispenzieri A, Gertz MA, Greipp PR, Lust JA. Identification of two groups of smoldering multiple myeloma patients who are either high or low producers of interleukin-1. J Interferon Cytokine Res. 2006;26:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Harmer D, Falank C, Reagan MR. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front Endocrinol (Lausanne). 2018;9:788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 37. | Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1111] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 38. | Bouyssou JM, Ghobrial IM, Roccaro AM. Targeting SDF-1 in multiple myeloma tumor microenvironment. Cancer Lett. 2016;380:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 492] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 40. | De Clercq E. Potential clinical applications of the CXCR4 antagonist bicyclam AMD3100. Mini Rev Med Chem. 2005;5:805-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, Spencer JA, Kimlinger T, Ghobrial JM, Jia X, Lu G, Timm M, Kumar A, Côté D, Veilleux I, Hedin KE, Roodman GD, Witzig TE, Kung AL, Hideshima T, Anderson KC, Lin CP, Ghobrial IM. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 42. | Hideshima T, Anderson KC. Signaling Pathway Mediating Myeloma Cell Growth and Survival. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 43. | Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 717] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 44. | Musolino C, Allegra A, Innao V, Allegra AG, Pioggia G, Gangemi S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediators Inflamm. 2017;2017:1852517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 45. | Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, Donovan KA. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Chong PSY, Chng WJ, de Mel S. STAT3: A Promising Therapeutic Target in Multiple Myeloma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 47. | Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford). 2008;47:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Roy P, Sarkar UA, Basak S. The NF-κB Activating Pathways in Multiple Myeloma. Biomedicines. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG, Anderson KC. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673-5683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 361] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 50. | Hengeveld PJ, Kersten MJ. B-cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J. 2015;5:e282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Chilov D, Kukk E, Taira S, Jeltsch M, Kaukonen J, Palotie A, Joukov V, Alitalo K. Genomic organization of human and mouse genes for vascular endothelial growth factor C. J Biol Chem. 1997;272:25176-25183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Kumar S, Witzig TE, Timm M, Haug J, Wellik L, Fonseca R, Greipp PR, Rajkumar SV. Expression of VEGF and its receptors by myeloma cells. Leukemia. 2003;17:2025-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | John L, Krauth MT, Podar K, Raab MS. Pathway-Directed Therapy in Multiple Myeloma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1793] [Cited by in RCA: 1763] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 55. | Ramakrishnan V, Kumar S. PI3K/AKT/mTOR pathway in multiple myeloma: from basic biology to clinical promise. Leuk Lymphoma. 2018;59:2524-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Rascio F, Spadaccino F, Rocchetti MT, Castellano G, Stallone G, Netti GS, Ranieri E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 57. | Ria R, Vacca A, Russo F, Cirulli T, Massaia M, Tosi P, Cavo M, Guidolin D, Ribatti D, Dammacco F. A VEGF-dependent autocrine loop mediates proliferation and capillarogenesis in bone marrow endothelial cells of patients with multiple myeloma. Thromb Haemost. 2004;92:1438-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Ribatti D, Vacca A. New Insights in Anti-Angiogenesis in Multiple Myeloma. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 59. | Colla S, Morandi F, Lazzaretti M, Polistena P, Svaldi M, Coser P, Bonomini S, Hojden M, Martella E, Chisesi T, Rizzoli V, Giuliani N. Do human myeloma cells directly produce basic FGF? Blood. 2003;102:3071-2; author reply 3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 676] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 61. | Terpos E, Anargyrou K, Katodritou E, Kastritis E, Papatheodorou A, Christoulas D, Pouli A, Michalis E, Delimpasi S, Gkotzamanidou M, Nikitas N, Koumoustiotis V, Margaritis D, Tsionos K, Stefanoudaki E, Meletis J, Zervas K, Dimopoulos MA; Greek Myeloma Study Group, Greece. Circulating angiopoietin-1 to angiopoietin-2 ratio is an independent prognostic factor for survival in newly diagnosed patients with multiple myeloma who received therapy with novel antimyeloma agents. Int J Cancer. 2012;130:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2465] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 63. | Abe M, Hiura K, Ozaki S, Kido S, Matsumoto T. Vicious cycle between myeloma cell binding to bone marrow stromal cells via VLA-4-VCAM-1 adhesion and macrophage inflammatory protein-1alpha and MIP-1beta production. J Bone Miner Metab. 2009;27:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, Grubbs B, Zhao M, Chen D, Sherry B, Mundy GR. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003;102:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Park JH, Lee NK, Lee SY. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol Cells. 2017;40:706-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 66. | Colla S, Zhan F, Xiong W, Wu X, Xu H, Stephens O, Yaccoby S, Epstein J, Barlogie B, Shaughnessy JD Jr. The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood. 2007;109:4470-4477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Dun X, Jiang H, Zou J, Shi J, Zhou L, Zhu R, Hou J. Differential expression of DKK-1 binding receptors on stromal cells and myeloma cells results in their distinct response to secreted DKK-1 in myeloma. Mol Cancer. 2010;9:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Houschyar KS, Tapking C, Borrelli MR, Popp D, Duscher D, Maan ZN, Chelliah MP, Li J, Harati K, Wallner C, Rein S, Pförringer D, Reumuth G, Grieb G, Mouraret S, Dadras M, Wagner JM, Cha JY, Siemers F, Lehnhardt M, Behr B. Wnt Pathway in Bone Repair and Regeneration - What Do We Know So Far. Front Cell Dev Biol. 2018;6:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 69. | Wang J, Faict S, Maes K, De Bruyne E, Van Valckenborgh E, Schots R, Vanderkerken K, Menu E. Extracellular vesicle cross-talk in the bone marrow microenvironment: implications in multiple myeloma. Oncotarget. 2016;7:38927-38945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, Anderson KC, Scadden DT, Ghobrial IM. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 642] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 71. | Moloudizargari M, Abdollahi M, Asghari MH, Zimta AA, Neagoe IB, Nabavi SM. The emerging role of exosomes in multiple myeloma. Blood Rev. 2019;38:100595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 72. | Faict S, Muller J, De Veirman K, De Bruyne E, Maes K, Vrancken L, Heusschen R, De Raeve H, Schots R, Vanderkerken K, Caers J, Menu E. Exosomes play a role in multiple myeloma bone disease and tumor development by targeting osteoclasts and osteoblasts. Blood Cancer J. 2018;8:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 73. | Zhang L, Lei Q, Wang H, Xu C, Liu T, Kong F, Yang C, Yan G, Sun L, Zhao A, Chen W, Hu Y, Xie H, Cao Y, Fu F, Yuan G, Chen Z, Guo AY, Li Q. Tumor-derived extracellular vesicles inhibit osteogenesis and exacerbate myeloma bone disease. Theranostics. 2019;9:196-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 74. | Raimondo S, Urzì O, Conigliaro A, Bosco GL, Parisi S, Carlisi M, Siragusa S, Raimondi L, Luca A, Giavaresi G, Alessandro R. Extracellular Vesicle microRNAs Contribute to the Osteogenic Inhibition of Mesenchymal Stem Cells in Multiple Myeloma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Wu Y, Zhang Z, Wu J, Hou J, Ding G. The Exosomes Containing LINC00461 Originated from Multiple Myeloma Inhibit the Osteoblast Differentiation of Bone Mesenchymal Stem Cells via Sponging miR-324-3p. J Healthc Eng. 2022;2022:3282860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Li B, Xu H, Han H, Song S, Zhang X, Ouyang L, Qian C, Hong Y, Qiu Y, Zhou W, Huang M, Zhuang W. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene. 2018;37:5508-5519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 77. | Liu Z, Liu H, Li Y, Shao Q, Chen J, Song J, Fu R. Multiple myeloma-derived exosomes inhibit osteoblastic differentiation and improve il-6 secretion of bmscs from multiple myeloma. J Investig Med. 2020;68:45-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Cheng Q, Li X, Liu J, Ye Q, Chen Y, Tan S. Multiple Myeloma-Derived Exosomes Regulate the Functions of Mesenchymal Stem Cells Partially via Modulating miR-21 and miR-146a. Stem Cells Int. 2017;2017:9012152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Gu J, Wang M, Wang X, Li J, Liu H, Lin Z, Yang X, Zhang X. Exosomal miR-483-5p in Bone Marrow Mesenchymal Stem Cells Promotes Malignant Progression of Multiple Myeloma by Targeting TIMP2. Front Cell Dev Biol. 2022;10:862524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 80. | Umezu T, Imanishi S, Yoshizawa S, Kawana C, Ohyashiki JH, Ohyashiki K. Induction of multiple myeloma bone marrow stromal cell apoptosis by inhibiting extracellular vesicle miR-10a secretion. Blood Adv. 2019;3:3228-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 81. | Gao X, Zhou J, Wang J, Dong X, Chang Y, Jin Y. Mechanism of exosomal miR-155 derived from bone marrow mesenchymal stem cells on stemness maintenance and drug resistance in myeloma cells. J Orthop Surg Res. 2021;16:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Wang J, Hendrix A, Hernot S, Lemaire M, De Bruyne E, Van Valckenborgh E, Lahoutte T, De Wever O, Vanderkerken K, Menu E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124:555-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 83. | Katz BZ. Adhesion molecules--The lifelines of multiple myeloma cells. Semin Cancer Biol. 2010;20:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 84. | Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2222] [Cited by in RCA: 2145] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 85. | Zhang X, Sun Y, Wang Z, Huang Z, Li B, Fu J. Up-regulation of connexin-43 expression in bone marrow mesenchymal stem cells plays a crucial role in adhesion and migration of multiple myeloma cells. Leuk Lymphoma. 2015;56:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 875] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 87. | Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 470] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 88. | Edelman GM. Cell adhesion molecules. Science. 1983;219:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 628] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 89. | Bou Zerdan M, Nasr L, Kassab J, Saba L, Ghossein M, Yaghi M, Dominguez B, Chaulagain CP. Adhesion molecules in multiple myeloma oncogenesis and targeted therapy. Int J Hematol Oncol. 2022;11:IJH39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 90. | Brackenbury R, Rutishauser U, Edelman GM. Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc Natl Acad Sci U S A. 1981;78:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 101] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 527] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 92. | Hale JS, Li M, Lathia JD. The malignant social network: cell-cell adhesion and communication in cancer stem cells. Cell Adh Migr. 2012;6:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Samanta D, Almo SC. Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell Mol Life Sci. 2015;72:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 94. | Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 313] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 95. | Garcia-Gomez A, De Las Rivas J, Ocio EM, Díaz-Rodríguez E, Montero JC, Martín M, Blanco JF, Sanchez-Guijo FM, Pandiella A, San Miguel JF, Garayoa M. Transcriptomic profile induced in bone marrow mesenchymal stromal cells after interaction with multiple myeloma cells: implications in myeloma progression and myeloma bone disease. Oncotarget. 2014;5:8284-8305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Roldán E, García-Pardo A, Brieva JA. VLA-4-fibronectin interaction is required for the terminal differentiation of human bone marrow cells capable of spontaneous and high rate immunoglobulin secretion. J Exp Med. 1992;175:1739-1747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S, Wakatsuki S, Kosaka M, Kido S, Inoue D, Matsumoto T. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195-2202. [PubMed] |
| 98. | Asosingh K, Vankerkhove V, Van Riet I, Van Camp B, Vanderkerken K. Selective in vivo growth of lymphocyte function- associated antigen-1-positive murine myeloma cells. Involvement of function-associated antigen-1-mediated homotypic cell-cell adhesion. Exp Hematol. 2003;31:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Lokhorst HM, Lamme T, de Smet M, Klein S, de Weger RA, van Oers R, Bloem AC. Primary tumor cells of myeloma patients induce interleukin-6 secretion in long-term bone marrow cultures. Blood. 1994;84:2269-2277. [PubMed] |
| 100. | Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 451] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 101. | Sherbenou DW, Su Y, Behrens CR, Aftab BT, Perez de Acha O, Murnane M, Bearrows SC, Hann BC, Wolf JL, Martin TG, Liu B. Potent Activity of an Anti-ICAM1 Antibody-Drug Conjugate against Multiple Myeloma. Clin Cancer Res. 2020;26:6028-6038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Ridley RC, Xiao H, Hata H, Woodliff J, Epstein J, Sanderson RD. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood. 1993;81:767-774. [PubMed] |
| 103. | McCarron MJ, Park PW, Fooksman DR. CD138 mediates selection of mature plasma cells by regulating their survival. Blood. 2017;129:2749-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 104. | Andersen NF, Kristensen IB, Preiss BS, Christensen JH, Abildgaard N. Upregulation of Syndecan-1 in the bone marrow microenvironment in multiple myeloma is associated with angiogenesis. Eur J Haematol. 2015;95:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | van Riet I, de Greef C, del Favero H, Demanet C, Van Camp B. Production of fibronectin and adherence to fibronectin by human myeloma cell lines. Br J Haematol. 1994;87:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front Immunol. 2015;6:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 107. | Barker HF, Hamilton MS, Ball J, Drew M, Franklin IM. Expression of adhesion molecules LFA-3 and N-CAM on normal and malignant human plasma cells. Br J Haematol. 1992;81:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Van Camp B, Durie BG, Spier C, De Waele M, Van Riet I, Vela E, Frutiger Y, Richter L, Grogan TM. Plasma cells in multiple myeloma express a natural killer cell-associated antigen: CD56 (NKH-1; Leu-19). Blood. 1990;76:377-382. [PubMed] |
| 109. | Ahsmann EJ, Lokhorst HM, Dekker AW, Bloem AC. Lymphocyte function-associated antigen-1 expression on plasma cells correlates with tumor growth in multiple myeloma. Blood. 1992;79:2068-2075. [PubMed] |
| 110. | Huang N, Kawano MM, Harada H, Harada Y, Sakai A, Kuramoto A, Niwa O. Heterogeneous expression of a novel MPC-1 antigen on myeloma cells: possible involvement of MPC-1 antigen in the adhesion of mature myeloma cells to bone marrow stromal cells. Blood. 1993;82:3721-3729. [PubMed] |
| 111. | Huang N, Kawano MM, Mahmoud MS, Mihara K, Tsujimoto T, Niwa O, Kuramoto A. Expression of CD21 antigen on myeloma cells and its involvement in their adhesion to bone marrow stromal cells. Blood. 1995;85:3704-3712. [PubMed] |
| 112. | Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658-1667. [PubMed] |
| 113. | Klimienė I, Radzevičius M, Matuzevičienė R, Sinkevič-Belliot K, Kučinskienė ZA, Pečeliūnas V. Adhesion molecule immunophenotype of bone marrow multiple myeloma plasma cells impacts the presence of malignant circulating plasma cells in peripheral blood. Int J Lab Hematol. 2021;43:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 114. | Pellat-Deceunynck C, Barillé S, Puthier D, Rapp MJ, Harousseau JL, Bataille R, Amiot M. Adhesion molecules on human myeloma cells: significant changes in expression related to malignancy, tumor spreading, and immortalization. Cancer Res. 1995;55:3647-3653. [PubMed] |
| 115. | Goodarzi A, Valikhani M, Amiri F, Safari A. The mechanisms of mutual relationship between malignant hematologic cells and mesenchymal stem cells: Does it contradict the nursing role of mesenchymal stem cells? Cell Commun Signal. 2022;20:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Gerdes HH, Rustom A, Wang X. Tunneling nanotubes, an emerging intercellular communication route in development. Mech Dev. 2013;130:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 117. | Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 118. | Kolba MD, Dudka W, Zaręba-Kozioł M, Kominek A, Ronchi P, Turos L, Chroscicki P, Wlodarczyk J, Schwab Y, Klejman A, Cysewski D, Srpan K, Davis DM, Piwocka K. Tunneling nanotube-mediated intercellular vesicle and protein transfer in the stroma-provided imatinib resistance in chronic myeloid leukemia cells. Cell Death Dis. 2019;10:817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 119. | Mangolini M, Ringshausen I. Bone Marrow Stromal Cells Drive Key Hallmarks of B Cell Malignancies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | de Rooij B, Polak R, van den Berk LCJ, Stalpers F, Pieters R, den Boer ML. Acute lymphoblastic leukemia cells create a leukemic niche without affecting the CXCR4/CXCL12 axis. Haematologica. 2017;102:e389-e393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood. 2015;126:2404-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 122. | Moschoi R, Imbert V, Nebout M, Chiche J, Mary D, Prebet T, Saland E, Castellano R, Pouyet L, Collette Y, Vey N, Chabannon C, Recher C, Sarry JE, Alcor D, Peyron JF, Griessinger E. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 333] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 123. | Mittal R, Karhu E, Wang JS, Delgado S, Zukerman R, Mittal J, Jhaveri VM. Cell communication by tunneling nanotubes: Implications in disease and therapeutic applications. J Cell Physiol. 2019;234:1130-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 124. | Zampieri LX, Silva-Almeida C, Rondeau JD, Sonveaux P. Mitochondrial Transfer in Cancer: A Comprehensive Review. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 125. | Suzuki R, Ogiya D, Ogawa Y, Kawada H, Ando K. Targeting CAM-DR and Mitochondrial Transfer for the Treatment of Multiple Myeloma. Curr Oncol. 2022;29:8529-8539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 126. | Cheng Y, Sun F, Thornton K, Jing X, Dong J, Yun G, Pisano M, Zhan F, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS, Hari P, Janz S. FOXM1 regulates glycolysis and energy production in multiple myeloma. Oncogene. 2022;41:3899-3911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 127. | Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, Wei X, Ke Q, Sui X, Wang Y, Huang Y, Li H, Wang T, Lin R, Liu Q, Xiang AP. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 128. | Leleu X, Martin T, Weisel K, Schjesvold F, Iida S, Malavasi F, Manier S, Chang-Ki Min, Ocio EM, Pawlyn C, Perrot A, Quach H, Richter J, Spicka I, Yong K, Richardson PG. Anti-CD38 antibody therapy for patients with relapsed/refractory multiple myeloma: differential mechanisms of action and recent clinical trial outcomes. Ann Hematol. 2022;101:2123-2137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 129. | D'Agostino M, Mina R, Gay F. Anti-CD38 monoclonal antibodies in multiple myeloma: another cook in the kitchen? Lancet Haematol. 2020;7:e355-e357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |