Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.354
Peer-review started: January 14, 2023
First decision: March 21, 2023
Revised: March 31, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: May 26, 2023
Processing time: 132 Days and 5.2 Hours
The mammalian intestinal epithelium constitutes the largest barrier against the external environment and makes flexible responses to various types of stimuli. Epithelial cells are fast-renewed to counteract constant damage and disrupted barrier function to maintain their integrity. The homeostatic repair and rege
Core Tip: The homeostatic repair and regeneration of the intestinal epithelium upon injury are governed by the Lgr5+ intestinal stem cells (ISCs) located at the base of crypts, which fuel rapid renewal and give rise to different epithelial cell types. We review the current understanding of the intrinsic niche signaling and extrinsic stimulating factors that control homeostasis and regeneration of the ISCs. Deciphering the regulatory machinery that modulates stem cell fate, and formulating strategies for better repair and regeneration would aid in the development of novel therapeutics that facilitate mucosal healing and restore epithelial barrier function.
- Citation: Wang Z, Qu YJ, Cui M. Modulation of stem cell fate in intestinal homeostasis, injury and repair. World J Stem Cells 2023; 15(5): 354-368
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/354.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.354
Intestinal epithelium serves as the first line of defense against the external environment. As an outward single-layered epithelial structure, the intestinal mucosa withstands continuous mechanical, physicochemical, and biological insults[1,2]. To counteract intestinal injury and preserve their barrier function, epithelial cells are renewed every 2–5 d in most adult mammals[3]. The epithelial turnover is coordinated by Lgr5+ intestinal stem cells (ISCs) residing at the base of the crypts where they are kept in a multipotent state and produce transit amplifying (TA) progenitor cells. TA cells will undergo several cycles of division before migrating to the villi and ultimately differentiate into multiple lineages[4,5]. The disrupted barrier function and defective mucosal healing are the predominant biological features of intestinal pathology, and particularly, chronic gastrointestinal inflammation such as inflammatory bowel disease (IBD), which is represented by ulcerative colitis and Crohn’s disease[6-8]. Current clinical strategies focus on the symptomatic relief and blockade of inflammatory progression[9,10], while better solutions should emphasize the motivation of regenerative response orchestrated by ISCs for complete mucosal healing.
Mucosal healing is an integrated network initiated by a series of biological processes and signals[11]. Intestinal homeostasis is characterized by constant regeneration which demands a fine-tuned balance between ISC proliferation and differentiation[12,13]. In response to diverse insults, the cellular response, combined with the stem cell niche adaptions, synthetically modulates the fate of ISCs to restore homeostasis by replenishment of damaged epithelial cells, or to hasten cell demise by impairment of cell function and vitality[14,15]. Therefore, understanding the cellular response and niche adaptations during injury-induced intestinal regeneration is therefore of importance for ISC biology.
Constant efforts have been made to exploit the regulatory mechanisms of critical components that seal the fate of ISCs. In this review, we give an overview of ISCs and review the adaptations and signals required for homeostasis maintenance. We focus on the cell fate specification and biological alterations of ISCs upon diverse insults and provide insights into intestinal regeneration.
Two distinct ISC populations located at the crypts have been proposed: Crypt base columnar (CBC) cells, the active cycling stem cells that facilitate homeostatic self-renewal[16], and +4 cells, the quiescent stem cells reserved for injury-induced repair[17].
CBC cells have been the centerpiece of stem cell research since they were initially identified in 1974 as continuously cycling cells at the base of the crypts[18]. Radionucleotide labeling and autoradiography have been used to state that the cells derived from the crypts migrate upward along the villi to be extruded at the villus tips[19,20]. This conveyor belt mechanism confirms stem cell fueling this rapid self-renewal process resides at the base of the crypts[21]. The generation of Lgr5EGFP−IRES−CreERT2 mice reveals that Lgr5, a receptor for WNT signaling-associated R-spondins, is a highly suitable candidate for CBC cell recognition and specification[3]. Single-sorted Lgr5+ stem cells are also able to form these crypt–villus organoids and the Lgr5 hierarchy is maintained in organoids[22]. Recent studies have identified that p27 and Mex3a label the slowly cycling subpopulation of Lgr5+ ISCs based on single-cell transcriptome profiling[23,24].
In addition to Lgr5+ CBC cells, Bmi1+ cells localized at the fourth position of the crypt base and discovered by in vivo lineage tracing and transcriptome analyses, are a possible candidate stem cell population[4,25]. Functionally distinct from Lgr5+ ISCs, the quiescent +4 stem cells are considered reserved stem cells that replenish the continuously cycling CBC cells pool when required, and are highly resistant to radiation and insensitive to Wnt signal[17,26]. In face of chemoradiotherapy, Lgr5+ stem cells are vulnerable to chemical- or irradiation-induced injury, due to their predominantly cycling nature[24,27]. Bmi1+ cells quickly revert to ISCs and the de novo-generated Lgr5+ ISCs are vital for epithelial regeneration[28]. The evidence summarizes the relationship between active and quiescent stem cells and identifies Lgr5+ stem cells as a substantial contributor to homeostatic regeneration[29].
The identification of new ISC markers and the dedication each subpopulation of ISCs commit to regeneration have improved the understanding of stem cell biology during homeostasis and disease. The emergence of new technologies has promoted the decoding of many key problems in intestinal diseases and tumors[4].
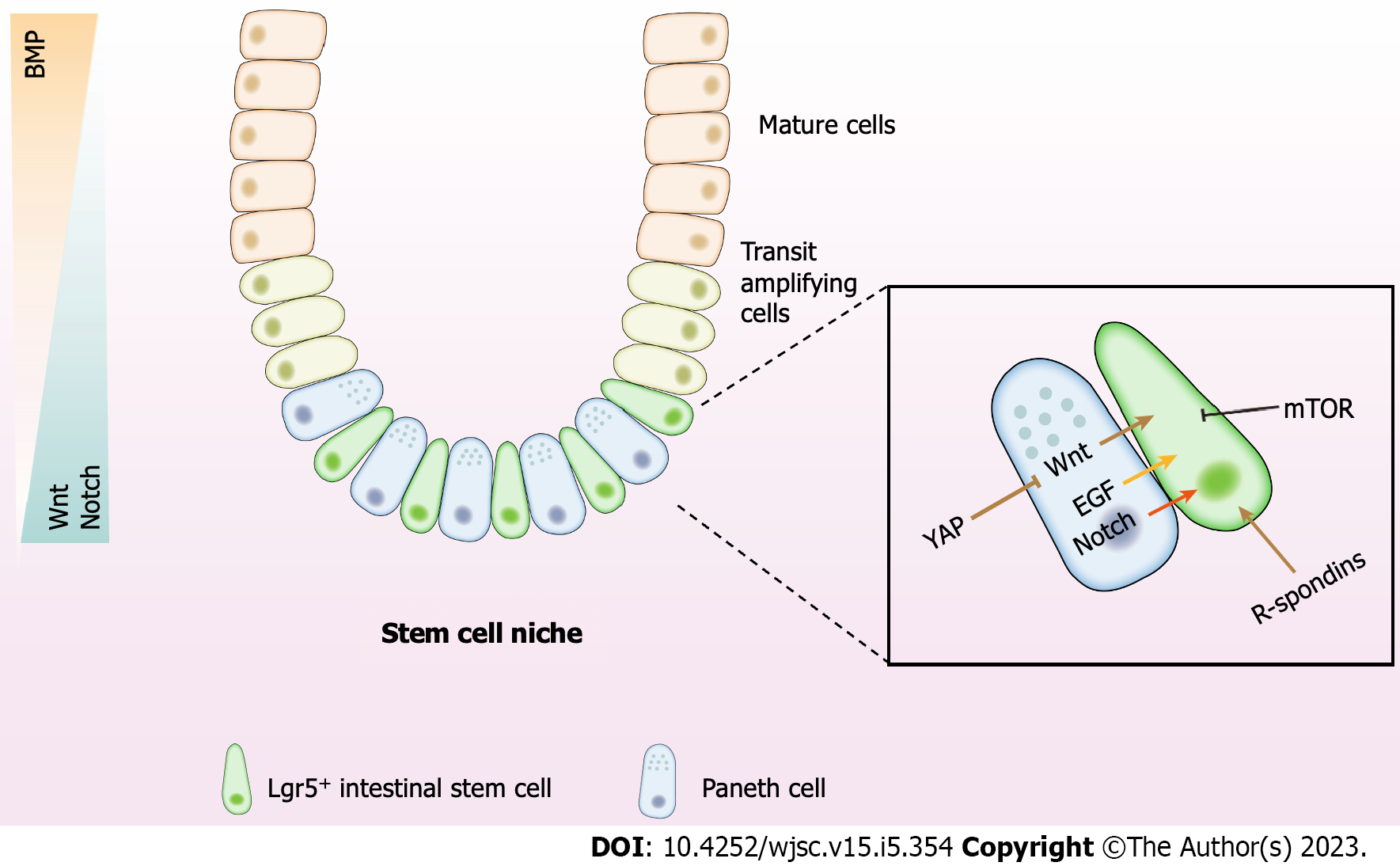
The niche in which ISCs reside can be defined as the microenvironment essential for self-renewal and stemness maintentance[13]. ISCs are strongly linked with adjacent cells of both epithelial and mesenchymal origin. These components, along with their communications, comprise the ISC niche[30,31]. The specific instructive microenvironment offers a native source of signals that fuel ISCs to maintain tissue homeostasis[32]. Various cell types of the niche elaborate typical paracrine signals containing Wnt, R-spondin, Notch, mammalian target of rapamycin (mTOR), bone morphogenetic protein (BMP), epidermal growth factor (EGF) and Hippo, which fine-tune the balance between differentiation and proliferation of ISCs, and ensure the production of an adequate number of cells in homeostatic and injury conditions[1,33] (Figure 1).
The canonical Wnt signaling acts as the prominent driver for ISC proliferation. Synchronous Wnt binding to Frizzled and to LRP5/6 suppresses APC-related ubiquitination of β-catenin which mediates its nuclear translocation, the association with lymphoid enhancer binding factor/T cell factor transcription factors, and the succeeding transactivation of Wnt target genes[34-36]. Multiple Wnts such as Wnt2b, Wnt4, and Wnt5a are abundantly expressed in intestinal stroma[30]. A subset of Foxl1+ mesenchymal stromal cells that form a subepithelial plexus around the crypt is a crucial source of intestinal Wnt[37]. Genetic elimination of Foxl1+ cells triggers the loss of Wnt family expression in the epithelium and an abrupt cessation of proliferation of both epithelial stem cell and TA progenitor cell populations, but not Paneth cell[38]. Wnt2b is highly expressed in Gli1+ or αSMA+ subepithelial stromal cells, which is sufficient to restore epithelial integrity when injected into mice which is devoid of Wnt secretion[39]. Gli1+ subepithelial cells are essential contributors to the integrity of the colonic epithelium for Lgr5+ ISC self-renewal in the colon[40]. As a noncanonical Wnt ligand, Wnt5a deficiency causes a failure to develop new crypts at the wound site and limits the proliferation of crypt cells after injury in a transforming growth factor (TGF)β-dependent manner[41]. These findings reveal the essential role of Wnt signal for the stemness and proliferation of ISC and highlight the contribution of stromal cells in the ISC niche.
The R-spondins comprise one of crucial elements of the niche. R-spondins are secretory glycoproteins which firmly cement the capacity of Wnt ligands for the activation of β-catenin-dependent transcription and canonical Wnt signaling, while R-spondins themselves have no intrinsic Wnt signaling activity[29,42]. Overexpressed R-spondins in vivo forcefully induce the expansion of ISCs and maintain the epithelial integrity against damage induced by the chemotherapeutic agent 5-fluorouracil, dextran sulfate sodium (DSS), or irradiation[43-45]. Wnt proteins are reported to be insufficient to directly regulate ISC self-renewal, while alternatively grant a fundamental competency through motivating R-spondin ligands to actively motivate ISC[46].
Notch signaling plays a dominant role in the stem cell niche by preserving the quiescent state of ISCs[47]. Integration between Notch ligands (Notch1–4) and receptors (Jag1–2 and Dll1–4) in adjacent cells is required for Notch activation[48]. Different from Wnt signaling that is mainly generated from a stromal microenvironment, Notch signaling may function via neighboring epithelial cells or even stromal subpopulations contacting with ISCs, thus featuring an epithelial niche[49].
Disruption of Notch activity leads to the exhaustion of ISC and differentiation from proliferating TA cells to secretory cells[50]. Simultaneous Notch1/2 deletion recapitulates the global Notch inhibition phenotype of Lgr5+ ISC loss, while the single deletion does not change ISC activity, which suggests the synthetical effect of Notch1/2 in stemness maintenance[51,52].
The mTOR signaling is a vital pathway for cellular development and metabolism in mammals. mTOR signaling directly modulates stemness and proliferation of ISCs, functioning as a crucial determinant of cell status within the ISC lineage and modulating differentiation in a nutrient-dependent way[53]. Inhibiting mTOR signaling helps to maintain stemness of ISCs, whereas activation of mTOR facilitates ISCs differentiation and proliferation[54,55]. In the case of caloric restriction, the activity of mTOR complex 1 is inhibited in Paneth cells, resulting in the paracrine release of cyclic ADP ribose that increases self-renewal of ISCs at the cost of differentiation[56]. In high relevance to diet, the mTOR pathway controls stem cell fate possibly by regulating mitochondrial metabolic states.
BMP signaling acts as an initiator of differentiation in the crypt. Wnt and BMP signalings are deemed as opposite forces along the crypt–villus axis with counteractive gradients of activity[57]. BMP activity is lower in the bottom and higher towards the top of the villus[58]. To offset the inhibitory effects of BMP signaling on ISC fate, BMP antagonists like Noggin, Gremlin-1, and Gremlin-2 are highly expressed in the crypts, permitting the proliferation of ISCs. The BMP antagonists that enhance ISCs self-renewal are secreted by intestinal subepithelial myofibroblasts and smooth muscle cells[59,60].
EGF is a vital component of the ISC niche[61]. The EGF receptor is abundantly expressed in CBCs, whereas its ligands are expressed in Paneth cells[62]. The activity of ErbB signaling is monitored by the negative regulation of Lrig1, a transmembrane protein coexpressed with Lgr5 in CBCs[62]. Loss of Lrig1 leads to the activation of receptors and a concomitant rapid expansion of crypts and cell numbers. Blockade of EGF signaling in intestinal organoids drives proliferative ISCs into quiescent state and stops organoid budding[63]. The evidence suggests the requirement of EGF in epithelial regeneration.
The Hippo pathway, a highly conserved signaling first described in Drosophila as an organ size control pathway, is comprised of a core kinase cascade, Mst1/2 and Lats1/2, which phosphorylate and suppress transcriptional coactivators Yes1 associated transcriptional regulator (YAP) and Tafazzin (TAZ), thereby modulating TEA domain transcription factor 1 (TEAD)-mediated transcriptional activation[64,65]. YAP/TAZ are the core components for stem cell-based regeneration. YAP overexpression in mice accelerates the self-renewal of colonic epithelium, and augments the number of proliferative cells and the cell migration along the crypt-villus axis, as detected by BrdU marker[43]. While YAP depletion causes a significant decrease in crypt proliferation, extensive crypt loss and consequently regeneration failure upon DSS or irradiation[66,67]. Loss of YAP activity contributes to higher sensitivity of ISCs to apoptosis and lower proliferative capacity during regeneration[68]. Moreover, the core Hippo kinases Lats1/2 are essential to maintain ISC activity and their deletion leads to the loss of ISCs[69]. This demonstrates their essential effects on epithelial proliferation and tissue regeneration. YAP may actively block Wnt signaling and thus apply negative feedback on Wnt signaling via β-catenin inhibition[43], indicating the complex interaction between YAP and other niche signals which may need further investigation.
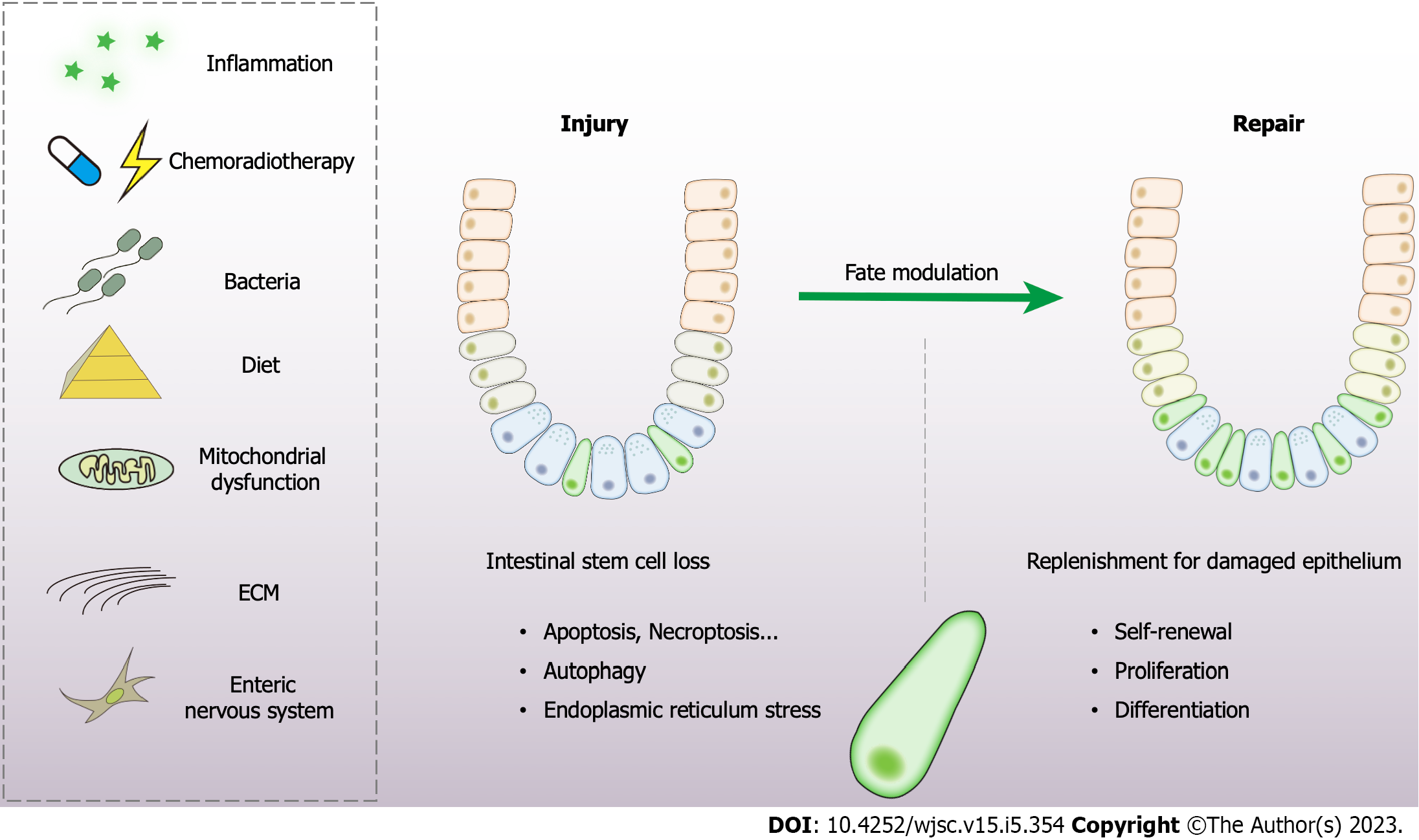
The intestinal epithelium is exposed to a hostile luminal environment, thus resulting in Lgr5+ ISCs continuously encountering sources of stress to maintain dynamic homeostasis. We summarize the major endogenous and environmental stimuli that influence stem cell regenerative potential (Figure 2).
Niche signals: Damage to the crypts can result from infections, chronic inflammation, chemoradiotherapy, or traumatic injury and motivate a series of actions in the stem cell niche[31]. The microenvironment monitors the regenerative response via regulating a series of signaling pathways, such as Wnt, Notch, BMP, and Hippo. This has been discussed above.
Extracellular matrix: The state of stem cells largely depends on the properties of the extracellular matrix (ECM)[31]. The integrin complex assists cells in sensing the stiffness of the ECM and directs the fate of ISCs via adhesion signaling[70,71]. In this way, the ECM affects cellular behavior, including proliferation and differentiation. ECM stiffness is also a vital endogenous factor of mesenchymal stem cells to differentiate into osteoblasts, myoblasts, or neurons[72]. In particular, YAP/TAZ lay the foundation for ECM stiffness sensing and play a prominent role in intestinal repair and regeneration[73]. High matrix stiffness significantly enhances ISC expansion in a YAP-dependent manner[74]. These clues speculate that ECM sensation is capable of modulating self-renewal in ISCs.
Mitochondrial function: Mitochondrial function emerges as a central player in cell fate determination and extensive control of cellular stress responses, metabolism, immunity, and apoptosis[75]. Mito
Enteric nervous system: In spite of the limited studies into the underlying relationship between the nervous system and intestinal epithelial regeneration, some novel indications are pointed out that the enteric nervous system exerts a potential role with great value. Enteric glial cells are closely connected with the intestinal epithelium and depletion of enteric glial cells will exacerbate DSS-induced injury[79]. The administration of hepatocyte growth factor from neural cells of the enteric nervous system attenuates the hostile effects of DSS[80]. Several reports also unveil a potential effect of the enteric glial cells in mucosal healing through the release of the specific niche factors like glial-derived neurotropic factor, TGF-β1 or 15-deoxy-12,14-prostaglandin J2[41,81,82].
Diet: The biological behavior of stem cells is largely affected by nutritional state[31]. The mTOR signaling is responsible for sensing the nutritional state[83]. Mice fed with a calorie-restricted diet exhibit an augmented function in Lgr5+ ISCs and Paneth cells compared with mice with a normal diet[56,84]. Moreover, the calorie-restricted diet diminishes mTOR activity in quiescent stem cells, improving their resistance to radiation damage and promoting intestinal repair[85]. In contrast, a high-fat diet can increase ISC activity despite decreasing Paneth cells activity. ISCs of high-fat diet mice exhibit higher resistance to irradiation and more efficient organoid budding potential than control mice. The high-fat diet activates Wnt signaling in ISCs dependent on the nuclear peroxisome proliferator-activated receptor δ[86]. Another study also reveals that an obesogenic diet induces ISCs and progenitor cells hyperproliferation, triggers ISC differentiation and cell turnover, and alters the regional characteristics of ISCs and enterocytes in mice[87]. Acute fasting has been shown to lead to transient phosphatase and tensin homolog (PTEN) phosphorylation within quiescent ISCs and render quiescent ISCs functionally poised to contribute to the regenerative response during refeeding[88].
Microorganisms: Microorganisms play an indispensable role in gut homeostasis, but the underlying mechanisms are complicated and elusive. Small molecules and metabolites produced by gut microbiota significantly contribute to the host intestinal development, function, and homeostasis[89]. Lactate from lactic acid-producing bacteria plays a pivotal role in promoting ISC proliferation and epithelial development[90]. Butyrate within the crypts conveys a growth-inhibiting effect on Lgr5+ ISCs via Forkhead box O3[91]. The bacterial product muramyl dipeptide has been reported to decrease the level of ROS in ISCs, and promote intestinal organoid growth and tissue repair[92,93]. Salmonella can enter the crypts during infection and cause a significant decrease in Lgr5+ ISCs[94,95]. The enteric pathogen rotavirus specifically invades and deteriorates differentiated cells at villus tips, and then motivates Lgr5+ ISCs, crypt expansion, and hyperproliferation[96]. Gut pathogens are thus distinctive elements capable of tuning the stem cell fate.
Inflammatory signaling: In addition to the local niche signals and physicochemical stimuli, the activation of the immune system is also involved in the interactions between epithelial cells and the niche to guarantee proper initiation and continuation of the regenerative response. Interleukin (IL)-22, which derives from the intestine, contains group 3 innate lymphoid cells that reside in close proximity to intestinal crypts and are upregulated after injury and support subsequent epithelial regeneration[97]. Recombinant IL-22 has been shown to directly target ISCs, thus facilitating the growth of human and mice intestinal organoids and promoting ISC self-renewal[98,99]. A recent study also indicates that the symmetric division of ISCs can be triggered by inflammatory signals to prevent excessive expansion in the process of epithelial repair[100].
Chemoradiotherapy: Intestinal mucosal damage occurs in 40%–60% of patients receiving chemotherapy or radiotherapy[101]. Chemotherapy- or radiotherapy-induced cellular apoptosis can be the primary factor initiating the gastrointestinal syndrome[102,103]. The injury response of intestinal epithelium after chemoradiotherapy has been the most extensively characterized model of Lgr5+ ISC loss and proliferation to date, due to its hypersensitivity to radiation and chemotherapy. Targeting p53-dependent stem cell death is the core strategy for intestinal chemo- or radioprotection[102,104,105]. The Toll-like receptor 4 signaling pathway[106], Slit guidance ligand 2 (Slit2)/ Roundabout guidance receptor 1 (Robo1) signaling[45], gut microbiota[90,107], and dietary components such as green tea derivative (-)-epigallocatechin-3-gallate[108], aspartate[109], pectin[110], and vitamin D[111] have been shown to mitigate the loss of ISCs and alleviate intestinal injury. The deletion of CREPT suppresses the proliferation and differentiation of ISCs and reduces Lgr5+ cell numbers after X-ray irradiation[112]. Therapeutic strategies based on the inhibition of ISC apoptosis without compromising the efficacy of cancer treatment are of great potential.
The injury response of intestinal epithelium is critical to restore epithelial integrity upon diverse insults[13]. The immediate response of intestinal damage is the loss of Lgr5+ ISCs, while it is generally adaptive modulation since the reserved subpopulations are activated to replenish the defects. However, excessive damage may cause ISC depletion and militate against epithelial regeneration. Critical cellular adaptations have been made to restore homeostasis in ISCs.
As the best-understood form of programmed cell death, apoptosis has been largely clarified in the field of stem cells. Lgr5+ ISCs are more vulnerable to apoptosis than Bmi1+ stem cells are[113]. Considering the critical role of mitochondria in stemness maintenance, regenerative capacity determination, and modulation between self-renewal and cell death programs, mitochondrial function is the vital determinant of stem cell fate[75]. For Lgr5+ ISCs, mitochondrial dysfunction is the major cause of apoptosis. A series of molecules such as Bcl-2, Puma, Survivin, Phosphoribosyl pyrophosphate synthetase 1 (PRPS1), and X-linked inhibitor of apoptosis have been characterized for modulating ISC apoptosis. Other biological processes, including immune response, hormone response, post-translational modification, and signaling such as Hippo and G protein coupled receptor, are crucial to controlling Lgr5+ ISC apoptosis. The pivotal molecules regulating stem cell apoptosis are shown in Table 1. The excavation of novel strategies based on ISC survival is of great significance to epithelial regeneration.
| Biological process or signaling | Molecule | Role | Evidence |
| Mitochondrial dysfunction | Puma | Pro-apoptotic | Puma depletion reduces chemoradiotherapy- induced apoptosis in a p53-dependent manner[102,136] |
| Bcl-2 | Anti-apoptotic | Bcl-2 is highly expressed in ISCs and alleviating radiation-induced damage[137] | |
| Survivin | Anti-apoptotic | An essential guardian of ISC during mucosal healing[138] | |
| PRPS1 | Pro-apoptotic | PRPS1 deficiency exhibit resistance against intestinal damage in a manner dependent upon Lgr5+ ISCs[139] | |
| Immune response | IL-22 | Anti-apoptotic | IL-22 deficiency led to increased crypt apoptosis, depletion of ISCs[97] |
| NOD2 | Anti-apoptotic | Nod2 stimulation triggers stem cell survival against oxidative stress-mediated cell death[93] | |
| Hippo | YAP | Anti-apoptotic | Loss of YAP activity results in sensitivity of crypt stem cells to apoptosis and reduced cell proliferation during regeneration[140] |
| GPCR | β-Arrestin1/2 | Anti-apoptotic | βArr reduced the chemotherapy- induced Lgr5+ stem cell apoptosis by inhibiting endoplasmic reticulum stress[141,142] |
| Hormone | GLP-2 | Anti-apoptotic | GLP-2 expanded intestinal organoids and downregulated apoptosis-related genes[143] |
| Ghrelin | Anti-apoptotic | Ghrelin treatment accelerated the reversal of radiation-induced epithelial damage and defective self-renewing property of ISCs[144] | |
| Methylation | Mettl14 | Anti-apoptotic | Specific deletion of the Mettl14 gene resulted in colonic stem cell apoptosis[145] |
| GsdmC | Anti-apoptotic | GsdmC N6-adenomethylation protects mitochondrial homeostasis and is essential for Lgr5+ cell survival[146] | |
| Glycosylation | HYOU1 | Anti-apoptotic | HYOU1 glycosylation modulated by FUT2 protects ISCs against apoptosis[147] |
Necroptosis is also involved in crypt damage. The loss of SETDB1 in ISCs, a histone methyltransferase that induces the trimethylation of histone H3 at lysine 9, triggers Z-DNA-binding protein 1-dependent necroptosis, which irreversibly disrupts the integrity of the epithelial barrier and promotes the progression of IBD[114]. Intestinal organoids lacking ATG16L1 are more prone to initiate tumor necrosis factor (TNF)α-mediated necroptosis, and therapeutic blockage of necroptosis through TNFα or RIPK1 inhibition ameliorates the severity of IBD[115]. TNFα exacerbates necroptosis of differentiated cells and mediates the expansion of LGR5+ ISCs[116]. Therefore, necroptosis inhibitors could be used to promote mucosal healing in IBD patients.
Autophagy is a highly conserved process during evolution in eukaryotes, by which the cytoplasmic materials are degraded inside the autolysosome. Three distinct forms of autophagy, including microautophagy, chaperone-mediated autophagy, and macroautophagy have been described. Autophagy has been demonstrated crucial in modulating the interactions between gut microbiota and innate and adaptive immunity, in host defense against intestinal pathogens, and in maintaining intestinal homeostasis[117].
In the Drosophila intestine, autophagy downregulates the sensitivity of differentiated enterocytes to ROS when exposed to commensal bacteria. Mechanistically, the autophagic substrate Ref (2)P/p62 accumulates upon autophagy deficiency, thus inactivating Hippo signaling and leading to stem cell over-proliferation[118]. Autophagy can also protect ISCs against irradiation-induced oxidative stress by preserving mitochondrial health and function. Accordingly, stem cell-based intestinal regeneration after radiotherapy is impaired in mice with Atg5 deficiency. Another recent work has highlighted the role of ATG16L1-dependent autophagy in protecting ISCs from irradiation-induced ROS[92,119]. In a Drosophila model, Atg6 deficiency impairs the inhibitory effect of metformin on ISC aging[120]. A recent study has confirmed the role of Atg7 in maintaining epithelial integrity against DNA damage and cell death[121]. With the rapid progress of Lgr5+ ISC isolation and detection, the role of autophagy in ISCs will be further elucidated.
As the primary organelle for protein folding and quality control, endoplasmic reticulum (ER) is sensitive to multiple intrinsic cellular disturbances and extrinsic environmental changes, which would alter ER homeostasis and cause misfolded protein accumulation, leading to activation of unfolded protein response (UPR)[122]. Previous studies have shown that UPR exerts a significant role in the pathogenesis and progression of IBD[123]. Human genetic studies of IBD have identified primary genetic abnormalities in several genes, including Xbp1, Agr2, and Ormdl3, that encode proteins associated with ER stress[124-126]. More importantly, the control of ISC fate is coordinated by ER stress and UPR. Activation of ER stress leads to the loss of stemness of ISCs in a PERK–eIF2α-dependent manner[127]. XBP1, a stress sensor involved in the UPR, acts as a signaling hub to regulate stem cell function and epithelial DNA damage responses in a p53–DDIT4L-dependent manner[128]. XBP1 is also demonstrated to maintain ISC quiescence and control ISC activity[129]. Intestinal epithelium-specific deletion of glycoprotein 96, an ER-resident master chaperone, causes rapid destruction of stem cell niche, followed by complete eradication of the mucosal layer and epithelial cell death[130]. In summary, UPR is indispensable for stemness maintenance and fate determination of ISCs.
Stem-cell-based therapy holds great promise for the complete mucosal healing of gastrointestinal diseases. Related studies have applied exogenous stem cells such as mesenchymal stem cells and placental-derived stem cells for treating intestinal inflammation and injury[131,132], and achieved encouraging outcomes. With the boost of research in the field of ISCs, the intestinal organoid models, especially those of human origin, offer a unique platform to explore the mystery of ISC fate decisions and lineage specification in physiological and pathological conditions[14], and excavate novel strategies to facilitate the regenerative capacity of ISCs. Integration with novel nanomaterials can provide a more effective strategy for facilitating intestinal repair targeting at ISCs, such as grape exosome-like nanoparticles[133], polydopamine nanoparticles[134], and carbon nanoparticles[135]. Thus, one important future direction in the ISCs field is to precisely tune the fate of stem cells for better regeneration.
Intestinal epithelial regeneration is a complex network that is based on the function of ISCs. The dynamic balance between stemness and self-renewal is fine-tuned by stem cell niche and various endogenous or extrinsic factors. Great strides have been made in our understanding of the function and fate specification of ISCs in health and disease. In this review, we summarize the different components and signals that function in ISCs in the process of intestinal epithelial injury and repair. Cellular adaptations including apoptosis, necroptosis, autophagy, and UPR have been extensively investigated. Modulating the essential niche signaling or facilitating beneficial elements in the stem cell microenvironment provides novel insights into the regenerative process and opens an avenue for stem cell-based therapies for diseases caused by intestinal epithelial injury.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: George TA, Taiwan; Zhang JW, United Kingdom S-Editor: Li L L-Editor: A P-Editor: Zhang XD
| 1. | Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 649] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 2. | Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1682] [Cited by in RCA: 2132] [Article Influence: 193.8] [Reference Citation Analysis (0)] |
| 3. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4331] [Article Influence: 240.6] [Reference Citation Analysis (0)] |
| 4. | Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 926] [Article Influence: 77.2] [Reference Citation Analysis (1)] |
| 5. | Sorrentino G, Perino A, Yildiz E, El Alam G, Bou Sleiman M, Gioiello A, Pellicciari R, Schoonjans K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology. 2020;159:956-968.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 6. | Horiguchi H, Endo M, Kawane K, Kadomatsu T, Terada K, Morinaga J, Araki K, Miyata K, Oike Y. ANGPTL2 expression in the intestinal stem cell niche controls epithelial regeneration and homeostasis. EMBO J. 2017;36:409-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 480] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 8. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1794] [Article Influence: 224.3] [Reference Citation Analysis (111)] |
| 9. | Deng F, Wu Z, Zou F, Wang S, Wang X. The Hippo-YAP/TAZ Signaling Pathway in Intestinal Self-Renewal and Regeneration After Injury. Front Cell Dev Biol. 2022;10:894737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 10. | Shah SC, Colombel JF, Sands BE, Narula N. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1245-1255.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, Sandborn WJ, Hardiman G, Raz E, Maehara Y, Yoshimura A, Zucman-Rossi J, Guan KL, Karin M. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 521] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 12. | Chen Y, Ye Z, Seidler U, Tian D, Xiao F. Microenvironmental regulation of intestinal stem cells in the inflamed intestine. Life Sci. 2021;273:119298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Santos AJM, Lo YH, Mah AT, Kuo CJ. The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol. 2018;28:1062-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 14. | Beumer J, Clevers H. Cell fate specification and differentiation in the adult mammalian intestine. Nat Rev Mol Cell Biol. 2021;22:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 15. | Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 16. | Date S, Sato T. Mini-gut organoids: reconstitution of the stem cell niche. Annu Rev Cell Dev Biol. 2015;31:269-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 935] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 18. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1038] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | LEBLOND CP, MESSIER B. Renewal of chief cells and goblet cells in the small intestine as shown by radioautography after injection of thymidine-H3 into mice. Anat Rec. 1958;132:247-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 223] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | WALKER BE, LEBLOND CP. Sites of nucleic acid synthesis in the mouse visualized by radioautography after administration of C14-labelled adenine and thymidine. Exp Cell Res. 1958;14:510-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 893] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 22. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4394] [Cited by in RCA: 5160] [Article Influence: 322.5] [Reference Citation Analysis (0)] |
| 23. | Barriga FM, Montagni E, Mana M, Mendez-Lago M, Hernando-Momblona X, Sevillano M, Guillaumet-Adkins A, Rodriguez-Esteban G, Buczacki SJA, Gut M, Heyn H, Winton DJ, Yilmaz OH, Attolini CS, Gut I, Batlle E. Mex3a Marks a Slowly Dividing Subpopulation of Lgr5+ Intestinal Stem Cells. Cell Stem Cell. 2017;20:801-816.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 24. | Ishikawa K, Sugimoto S, Oda M, Fujii M, Takahashi S, Ohta Y, Takano A, Ishimaru K, Matano M, Yoshida K, Hanyu H, Toshimitsu K, Sawada K, Shimokawa M, Saito M, Kawasaki K, Ishii R, Taniguchi K, Imamura T, Kanai T, Sato T. Identification of Quiescent LGR5(+) Stem Cells in the Human Colon. Gastroenterology. 2022;163:1391-1406.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 965] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 26. | Tetteh PW, Farin HF, Clevers H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2015;25:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | Girish N, Liu CY, Gadeock S, Gomez ML, Huang Y, Sharifkhodaei Z, Washington MK, Polk DB. Persistence of Lgr5+ colonic epithelial stem cells in mouse models of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2021;321:G308-G324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 29. | Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 656] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 30. | Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518-1529.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 508] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 31. | Hageman JH, Heinz MC, Kretzschmar K, van der Vaart J, Clevers H, Snippert HJG. Intestinal Regeneration: Regulation by the Microenvironment. Dev Cell. 2020;54:435-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 32. | Sailaja BS, He XC, Li L. The regulatory niche of intestinal stem cells. J Physiol. 2016;594:4827-4836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Andersson-Rolf A, Zilbauer M, Koo BK, Clevers H. Stem Cells in Repair of Gastrointestinal Epithelia. Physiology (Bethesda). 2017;32:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 1029] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 35. | Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 648] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 36. | Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1685] [Cited by in RCA: 1664] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 37. | Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Tóth B, Kondo A, Massasa EE, Itzkovitz S, Kaestner KH. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557:242-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 390] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 38. | Aoki R, Shoshkes-Carmel M, Gao N, Shin S, May CL, Golson ML, Zahm AM, Ray M, Wiser CL, Wright CV, Kaestner KH. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol. 2016;2:175-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 39. | Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantù C, Aguet M, Basler K. Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep. 2016;15:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 40. | Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature. 2018;558:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 280] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 41. | Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 378] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 42. | de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13:242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 43. | Harnack C, Berger H, Antanaviciute A, Vidal R, Sauer S, Simmons A, Meyer TF, Sigal M. R-spondin 3 promotes stem cell recovery and epithelial regeneration in the colon. Nat Commun. 2019;10:4368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 44. | Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, Abo A, Funk WD. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Zhou WJ, Geng ZH, Spence JR, Geng JG. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature. 2013;501:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 46. | Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, Ootani A, Roelf K, Lee M, Yuan J, Li X, Bolen CR, Wilhelmy J, Davies PS, Ueno H, von Furstenberg RJ, Belgrader P, Ziraldo SB, Ordonez H, Henning SJ, Wong MH, Snyder MP, Weissman IL, Hsueh AJ, Mikkelsen TS, Garcia KC, Kuo CJ. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature. 2017;545:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 47. | Gjorevski N, Nikolaev M, Brown TE, Mitrofanova O, Brandenberg N, DelRio FW, Yavitt FM, Liberali P, Anseth KS, Lutolf MP. Tissue geometry drives deterministic organoid patterning. Science. 2022;375:eaaw9021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 235] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 48. | Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2980] [Cited by in RCA: 2802] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 49. | Liang SJ, Li XG, Wang XQ. Notch Signaling in Mammalian Intestinal Stem Cells: Determining Cell Fate and Maintaining Homeostasis. Curr Stem Cell Res Ther. 2019;14:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 51. | Khaminets A, Ronnen-Oron T, Baldauf M, Meier E, Jasper H. Cohesin controls intestinal stem cell identity by maintaining association of Escargot with target promoters. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 333] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 53. | Jasper H, Jones DL. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 2010;12:561-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Chen T, Shen L, Yu J, Wan H, Guo A, Chen J, Long Y, Zhao J, Pei G. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. 2011;10:908-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 55. | Sampson LL, Davis AK, Grogg MW, Zheng Y. mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB J. 2016;30:1263-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 56. | Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 585] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 57. | McCarthy N, Kraiczy J, Shivdasani RA. Cellular and molecular architecture of the intestinal stem cell niche. Nat Cell Biol. 2020;22:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 58. | McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, Kapoor VN, Madha S, Gaynor LT, Cox C, Keerthivasan S, Wucherpfennig K, Yuan GC, de Sauvage FJ, Turley SJ, Shivdasani RA. Distinct Mesenchymal Cell Populations Generate the Essential Intestinal BMP Signaling Gradient. Cell Stem Cell. 2020;26:391-402.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 59. | Malijauskaite S, Connolly S, Newport D, McGourty K. Gradients in the in vivo intestinal stem cell compartment and their in vitro recapitulation in mimetic platforms. Cytokine Growth Factor Rev. 2021;60:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104:15418-15423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 460] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 61. | Abud HE, Chan WH, Jardé T. Source and Impact of the EGF Family of Ligands on Intestinal Stem Cells. Front Cell Dev Biol. 2021;9:685665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 62. | Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 1930] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 63. | Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell. 2017;20:177-190.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 64. | Dobens LL, Nauman C, Fischer Z, Yao X. Control of Cell Growth and Proliferation by the Tribbles Pseudokinase: Lessons from Drosophila. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13:324-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 66. | Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 475] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 67. | Wang Y, Yu A, Yu FX. The Hippo pathway in tissue homeostasis and regeneration. Protein Cell. 2017;8:349-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 68. | Konsavage WM Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730-11739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 69. | Li Q, Sun Y, Jarugumilli GK, Liu S, Dang K, Cotton JL, Xiol J, Chan PY, DeRan M, Ma L, Li R, Zhu LJ, Li JH, Leiter AB, Ip YT, Camargo FD, Luo X, Johnson RL, Wu X, Mao J. Lats1/2 Sustain Intestinal Stem Cells and Wnt Activation through TEAD-Dependent and Independent Transcription. Cell Stem Cell. 2020;26:675-692.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 70. | Chen S, Zheng Y, Ran X, Du H, Feng H, Yang L, Wen Y, Lin C, Wang S, Huang M, Yan Z, Wu D, Wang H, Ge G, Zeng A, Zeng YA, Chen J. Integrin αEβ7(+) T cells direct intestinal stem cell fate decisions via adhesion signaling. Cell Res. 2021;31:1291-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Won JH, Choi JS, Jun JI. CCN1 interacts with integrins to regulate intestinal stem cell proliferation and differentiation. Nat Commun. 2022;13:3117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 72. | Even-Ram S, Artym V, Yamada KM. Matrix control of stem cell fate. Cell. 2006;126:645-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 73. | Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4707] [Cited by in RCA: 4178] [Article Influence: 298.4] [Reference Citation Analysis (0)] |
| 74. | Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 978] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 75. | Rath E, Moschetta A, Haller D. Mitochondrial function - gatekeeper of intestinal epithelial cell homeostasis. Nat Rev Gastroenterol Hepatol. 2018;15:497-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 76. | Gao Y, Yan Y, Tripathi S, Pentinmikko N, Amaral A, Päivinen P, Domènech-Moreno E, Andersson S, Wong IPL, Clevers H, Katajisto P, Mäkelä TP. LKB1 Represses ATOH1 via PDK4 and Energy Metabolism and Regulates Intestinal Stem Cell Fate. Gastroenterology. 2020;158:1389-1401.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Schell JC, Wisidagama DR, Bensard C, Zhao H, Wei P, Tanner J, Flores A, Mohlman J, Sorensen LK, Earl CS, Olson KA, Miao R, Waller TC, Delker D, Kanth P, Jiang L, DeBerardinis RJ, Bronner MP, Li DY, Cox JE, Christofk HR, Lowry WE, Thummel CS, Rutter J. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol. 2017;19:1027-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 78. | Ludikhuize MC, Meerlo M, Gallego MP, Xanthakis D, Burgaya Julià M, Nguyen NTB, Brombacher EC, Liv N, Maurice MM, Paik JH, Burgering BMT, Rodriguez Colman MJ. Mitochondria Define Intestinal Stem Cell Differentiation Downstream of a FOXO/Notch Axis. Cell Metab. 2020;32:889-900.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 79. | Van Landeghem L, Chevalier J, Mahé MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G976-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | Avetisyan M, Wang H, Schill EM, Bery S, Grider JR, Hassell JA, Stappenbeck T, Heuckeroth RO. Hepatocyte Growth Factor and MET Support Mouse Enteric Nervous System Development, the Peristaltic Response, and Intestinal Epithelial Proliferation in Response to Injury. J Neurosci. 2015;35:11543-11558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Bach-Ngohou K, Mahé MM, Aubert P, Abdo H, Boni S, Bourreille A, Denis MG, Lardeux B, Neunlist M, Masson D. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2. J Physiol. 2010;588:2533-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Zhang DK, He FQ, Li TK, Pang XH, Cui DJ, Xie Q, Huang XL, Gan HT. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol. 2010;222:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1229] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 84. | Igarashi M, Guarente L. mTORC1 and SIRT1 Cooperate to Foster Expansion of Gut Adult Stem Cells during Calorie Restriction. Cell. 2016;166:436-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 85. | Yousefi M, Nakauka-Ddamba A, Berry CT, Li N, Schoenberger J, Bankler-Jukes D, Simeonov KP, Cedeno RJ, Yu Z, Lengner CJ. Calorie Restriction Governs Intestinal Epithelial Regeneration through Cell-Autonomous Regulation of mTORC1 in Reserve Stem Cells. Stem Cell Reports. 2018;10:703-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Beyaz S, Mana MD, Yilmaz ÖH. High-fat diet activates a PPAR-δ program to enhance intestinal stem cell function. Cell Stem Cell. 2021;28:598-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Aliluev A, Tritschler S, Sterr M, Oppenländer L, Hinterdobler J, Greisle T, Irmler M, Beckers J, Sun N, Walch A, Stemmer K, Kindt A, Krumsiek J, Tschöp MH, Luecken MD, Theis FJ, Lickert H, Böttcher A. Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat Metab. 2021;3:1202-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 88. | Richmond CA, Shah MS, Deary LT, Trotier DC, Thomas H, Ambruzs DM, Jiang L, Whiles BB, Rickner HD, Montgomery RK, Tovaglieri A, Carlone DL, Breault DT. Dormant Intestinal Stem Cells Are Regulated by PTEN and Nutritional Status. Cell Rep. 2015;13:2403-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 89. | Xing PY, Pettersson S, Kundu P. Microbial Metabolites and Intestinal Stem Cells Tune Intestinal Homeostasis. Proteomics. 2020;20:e1800419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 90. | Lee YS, Kim TY, Kim Y, Lee SH, Kim S, Kang SW, Yang JY, Baek IJ, Sung YH, Park YY, Hwang SW, O E, Kim KS, Liu S, Kamada N, Gao N, Kweon MN. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe. 2018;24:833-846.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 91. | Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016;165:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 476] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 92. | Levy A, Stedman A, Deutsch E, Donnadieu F, Virgin HW, Sansonetti PJ, Nigro G. Innate immune receptor NOD2 mediates LGR5(+) intestinal stem cell protection against ROS cytotoxicity via mitophagy stimulation. Proc Natl Acad Sci U S A. 2020;117:1994-2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 93. | Nigro G, Rossi R, Commere PH, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 94. | Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1429] [Cited by in RCA: 1181] [Article Influence: 147.6] [Reference Citation Analysis (1)] |
| 95. | Santos AJM, Durkin CH, Helaine S, Boucrot E, Holden DW. Clustered Intracellular Salmonella enterica Serovar Typhimurium Blocks Host Cell Cytokinesis. Infect Immun. 2016;84:2149-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Zou WY, Blutt SE, Zeng XL, Chen MS, Lo YH, Castillo-Azofeifa D, Klein OD, Shroyer NF, Donowitz M, Estes MK. Epithelial WNT Ligands Are Essential Drivers of Intestinal Stem Cell Activation. Cell Rep. 2018;22:1003-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 97. | Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, van den Brink MR. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft vs host disease. Immunity. 2012;37:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 98. | Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O'Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, Hanash AM. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 756] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (1)] |
| 99. | Tan C, Hong G, Wang Z, Duan C, Hou L, Wu J, Qian W, Han C, Hou X. Promoting Effect of L-Fucose on the Regeneration of Intestinal Stem Cells through AHR/IL-22 Pathway of Intestinal Lamina Propria Monocytes. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Bu P, Wang L, Chen KY, Srinivasan T, Murthy PK, Tung KL, Varanko AK, Chen HJ, Ai Y, King S, Lipkin SM, Shen X. A miR-34a-Numb Feedforward Loop Triggered by Inflammation Regulates Asymmetric Stem Cell Division in Intestine and Colon Cancer. Cell Stem Cell. 2016;18:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 101. | Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer. 2006;14:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 102. | Leibowitz BJ, Yang L, Wei L, Buchanan ME, Rachid M, Parise RA, Beumer JH, Eiseman JL, Schoen RE, Zhang L, Yu J. Targeting p53-dependent stem cell loss for intestinal chemoprotection. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Zeng H, Li H, Yue M, Fan Y, Cheng J, Wu X, Xu R, Yang W, Li M, Tang J, Chen H, Kuang B, Fan G, Zhu Q, Shao L. Isoprenaline protects intestinal stem cells from chemotherapy-induced damage. Br J Pharmacol. 2020;177:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Fu G, Chen S, Liang L, Li X, Tang P, Rao X, Pan M, Xu X, Li Y, Yao Y, Zhou Y, Gao J, Mo S, Cai S, Peng J, Zhang Z, Clevers H, Hua G. SIRT1 inhibitors mitigate radiation-induced GI syndrome by enhancing intestinal-stem-cell survival. Cancer Lett. 2021;501:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Zhang C, Zhou Y, Zheng J, Ning N, Liu H, Jiang W, Yu X, Mu K, Li Y, Guo W, Hu H, Li J, Chen D. Inhibition of GABAA receptors in intestinal stem cells prevents chemoradiotherapy-induced intestinal toxicity. J Exp Med. 2022;219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Feng Z, Xu Q, He X, Wang Y, Fang L, Zhao J, Cheng Y, Liu C, Du J, Cai J. FG-4592 protects the intestine from irradiation-induced injury by targeting the TLR4 signaling pathway. Stem Cell Res Ther. 2022;13:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 107. | Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V, Thotala D, Ciorba MA, Stenson WF. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2019;68:1003-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 108. | Xie LW, Cai S, Zhao TS, Li M, Tian Y. Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic Biol Med. 2020;161:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 109. | Wang D, Kuang Y, Wan Z, Li P, Zhao J, Zhu H, Liu Y. Aspartate Alleviates Colonic Epithelial Damage by Regulating Intestinal Stem Cell Proliferation and Differentiation via Mitochondrial Dynamics. Mol Nutr Food Res. 2022;66:e2200168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 110. | Sureban SM, May R, Qu D, Chandrakesan P, Weygant N, Ali N, Lightfoot SA, Ding K, Umar S, Schlosser MJ, Houchen CW. Dietary Pectin Increases Intestinal Crypt Stem Cell Survival following Radiation Injury. PLoS One. 2015;10:e0135561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Li W, Lin Y, Luo Y, Wang Y, Lu Y, Li Y, Guo H. Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 112. | Yang L, Yang H, Chu Y, Song Y, Ding L, Zhu B, Zhai W, Wang X, Kuang Y, Ren F, Jia B, Wu W, Ye X, Wang Y, Chang Z. CREPT is required for murine stem cell maintenance during intestinal regeneration. Nat Commun. 2021;12:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Zhu Y, Huang YF, Kek C, Bulavin DV. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell. 2013;12:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Wang R, Li H, Wu J, Cai ZY, Li B, Ni H, Qiu X, Chen H, Liu W, Yang ZH, Liu M, Hu J, Liang Y, Lan P, Han J, Mo W. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature. 2020;580:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 115. | Matsuzawa-Ishimoto Y, Shono Y, Gomez LE, Hubbard-Lucey VM, Cammer M, Neil J, Dewan MZ, Lieberman SR, Lazrak A, Marinis JM, Beal A, Harris PA, Bertin J, Liu C, Ding Y, van den Brink MRM, Cadwell K. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. J Exp Med. 2017;214:3687-3705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 232] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 116. | Lee C, An M, Joung JG, Park WY, Chang DK, Kim YH, Hong SN. TNFα Induces LGR5+ Stem Cell Dysfunction In Patients With Crohn's Disease. Cell Mol Gastroenterol Hepatol. 2022;13:789-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018;20:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 509] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 118. | Nagai H, Tatara H, Tanaka-Furuhashi K, Kurata S, Yano T. Homeostatic Regulation of ROS-Triggered Hippo-Yki Pathway via Autophagic Clearance of Ref(2)P/p62 in the Drosophila Intestine. Dev Cell. 2021;56:81-94.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 119. | Asano J, Sato T, Ichinose S, Kajita M, Onai N, Shimizu S, Ohteki T. Intrinsic Autophagy Is Required for the Maintenance of Intestinal Stem Cells and for Irradiation-Induced Intestinal Regeneration. Cell Rep. 2017;20:1050-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 120. | Na HJ, Pyo JH, Jeon HJ, Park JS, Chung HY, Yoo MA. Deficiency of Atg6 impairs beneficial effect of metformin on intestinal stem cell aging in Drosophila. Biochem Biophys Res Commun. 2018;498:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 121. | Trentesaux C, Fraudeau M, Pitasi CL, Lemarchand J, Jacques S, Duche A, Letourneur F, Naser E, Bailly K, Schmitt A, Perret C, Romagnolo B. Essential role for autophagy protein ATG7 in the maintenance of intestinal stem cell integrity. Proc Natl Acad Sci U S A. 2020;117:11136-11146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 122. | Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 840] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 123. | Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, Kaufman RJ. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144:989-1000.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 124. | Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 Links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1177] [Cited by in RCA: 1138] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 125. | McGovern DP, Gardet A, Törkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagacé C, Li C, Green T, Stevens CR, Beauchamp C, Fleshner PR, Carlson M, D'Amato M, Halfvarson J, Hibberd ML, Lördal M, Padyukov L, Andriulli A, Colombo E, Latiano A, Palmieri O, Bernard EJ, Deslandres C, Hommes DW, de Jong DJ, Stokkers PC, Weersma RK; NIDDK IBD Genetics Consortium, Sharma Y, Silverberg MS, Cho JH, Wu J, Roeder K, Brant SR, Schumm LP, Duerr RH, Dubinsky MC, Glazer NL, Haritunians T, Ippoliti A, Melmed GY, Siscovick DS, Vasiliauskas EA, Targan SR, Annese V, Wijmenga C, Pettersson S, Rotter JI, Xavier RJ, Daly MJ, Rioux JD, Seielstad M. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 126. | Zheng W, Rosenstiel P, Huse K, Sina C, Valentonyte R, Mah N, Zeitlmann L, Grosse J, Ruf N, Nürnberg P, Costello CM, Onnie C, Mathew C, Platzer M, Schreiber S, Hampe J. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun. 2006;7:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 127. | Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M, Ferrante M, Lee AS, Onderwater JJ, Paton JC, Paton AW, Mommaas AM, Kodach LL, Hardwick JC, Hommes DW, Clevers H, Muncan V, van den Brink GR. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013;3:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 128. | Welz L, Kakavand N, Hang X, Laue G, Ito G, Silva MG, Plattner C, Mishra N, Tengen F, Ogris C, Jesinghaus M, Wottawa F, Arnold P, Kaikkonen L, Stengel S, Tran F, Das S, Kaser A, Trajanoski Z, Blumberg R, Roecken C, Saur D, Tschurtschenthaler M, Schreiber S, Rosenstiel P, Aden K. Epithelial X-Box Binding Protein 1 Coordinates Tumor Protein p53-Driven DNA Damage Responses and Suppression of Intestinal Carcinogenesis. Gastroenterology. 2022;162:223-237.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 129. | Wang L, Zeng X, Ryoo HD, Jasper H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. 2014;10:e1004568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 130. | Häfliger J, Schwarzfischer M, Atrott K, Stanzel C, Morsy Y, Wawrzyniak M, Lang S, Valenta T, Basler K, Rogler G, Scharl M, Spalinger MR. Glycoprotein (GP)96 Is Essential for Maintaining Intestinal Epithelial Architecture by Supporting Its Self-Renewal Capacity. Cell Mol Gastroenterol Hepatol. 2023;15:717-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 131. | Shi Y, Zhang X, Wan Z, Liu X, Chen F, Zhang J, Leng Y. Mesenchymal stem cells against intestinal ischemia-reperfusion injury: a systematic review and meta-analysis of preclinical studies. Stem Cell Res Ther. 2022;13:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 132. | Weis VG, Deal AC, Mekkey G, Clouse C, Gaffley M, Whitaker E, Peeler CB, Weis JA, Schwartz MZ, Atala A. Human placental-derived stem cell therapy ameliorates experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2021;320:G658-G674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 133. | Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, Roth M, Welti R, Mobley J, Jun Y, Miller D, Zhang HG. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 545] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 134. | Jia S, Dong S, Liu H, Yu H, Chen Z, Wang S, Li W, Peng R, Li F, Jiang Q, Liu J. Dopamine-derived nanoparticles for the protection of irradiation-induced intestinal injury by maintaining intestinal homeostasis. Biomater Sci. 2022;10:3309-3322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 135. | Wang C, Xie J, Dong X, Mei L, Zhao M, Leng Z, Hu H, Li L, Gu Z, Zhao Y. Clinically Approved Carbon Nanoparticles with Oral Administration for Intestinal Radioprotection via Protecting the Small Intestinal Crypt Stem Cells and Maintaining the Balance of Intestinal Flora. Small. 2020;16:e1906915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 136. | Yuan Q, Peng R, Yu H, Wang S, Chen Z, Dong S, Li W, Cheng B, Jiang Q, Cong Y, Li F, Li C. Disulfiram Protects Against Radiation-Induced Intestinal Injury in Mice. Front Pharmacol. 2022;13:852669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | van der Heijden M, Zimberlin CD, Nicholson AM, Colak S, Kemp R, Meijer SL, Medema JP, Greten FR, Jansen M, Winton DJ, Vermeulen L. Bcl-2 is a critical mediator of intestinal transformation. Nat Commun. 2016;7:10916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 138. | Martini E, Schneider E, Neufert C, Neurath MF, Becker C. Survivin is a guardian of the intestinal stem cell niche and its expression is regulated by TGF-β. Cell Cycle. 2016;15:2875-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 139. | Koren E, Yosefzon Y, Ankawa R, Soteriou D, Jacob A, Nevelsky A, Ben-Yosef R, Bar-Sela G, Fuchs Y. ARTS mediates apoptosis and regeneration of the intestinal stem cell niche. Nat Commun. 2018;9:4582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 140. | Guillermin O, Angelis N, Sidor CM, Ridgway R, Baulies A, Kucharska A, Antas P, Rose MR, Cordero J, Sansom O, Li VSW, Thompson BJ. Wnt and Src signals converge on YAP-TEAD to drive intestinal regeneration. EMBO J. 2021;40:e105770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 141. | Zhan Y, Xu C, Liu Z, Yang Y, Tan S, Jiang J, Liu H, Chen J, Wu B. β-Arrestin1 inhibits chemotherapy-induced intestinal stem cell apoptosis and mucositis. Cell Death Dis. 2016;7:e2229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 142. | Liu Z, Tian H, Jiang J, Yang Y, Tan S, Lin X, Liu H, Wu B. β-Arrestin-2 modulates radiation-induced intestinal crypt progenitor/stem cell injury. Cell Death Differ. 2016;23:1529-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 143. | Norona J, Apostolova P, Schmidt D, Ihlemann R, Reischmann N, Taylor G, Köhler N, de Heer J, Heeg S, Andrieux G, Siranosian BA, Schmitt-Graeff A, Pfeifer D, Catalano A, Frew IJ, Proietti M, Grimbacher B, Bulashevska A, Bhatt AS, Brummer T, Clauditz T, Zabelina T, Kroeger N, Blazar BR, Boerries M, Ayuk F, Zeiser R. Glucagon-like peptide 2 for intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans. Blood. 2020;136:1442-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 144. | Kwak SY, Shim S, Park S, Kim H, Lee SJ, Kim MJ, Jang WS, Kim YH, Jang H. Ghrelin reverts intestinal stem cell loss associated with radiation-induced enteropathy by activating Notch signaling. Phytomedicine. 2021;81:153424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Zhang T, Ding C, Chen H, Zhao J, Chen Z, Chen B, Mao K, Hao Y, Roulis M, Xu H, Kluger Y, Zou Q, Ye Y, Zhan M, Flavell RA, Li HB. m(6)A mRNA modification maintains colonic epithelial cell homeostasis via NF-κB-mediated antiapoptotic pathway. Sci Adv. 2022;8:eabl5723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 146. | Du J, Sarkar R, Li Y, He L, Kang W, Liao W, Liu W, Nguyen T, Zhang L, Deng Z, Dougherty U, Kupfer SS, Chen M, Pekow J, Bissonnette M, He C, Li YC. N(6)-adenomethylation of GsdmC is essential for Lgr5(+) stem cell survival to maintain normal colonic epithelial morphogenesis. Dev Cell. 2022;57:1976-1994.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 147. | Wang Z, Tan C, Duan C, Wu J, Zhou D, Hou L, Qian W, Han C, Hou X. FUT2-dependent fucosylation of HYOU1 protects intestinal stem cells against inflammatory injury by regulating unfolded protein response. Redox Biol. 2023;60:102618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |