Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.221
Peer-review started: December 15, 2022
First decision: January 18, 2023
Revised: January 19, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 26, 2023
Processing time: 132 Days and 7.4 Hours
Allogeneic hematopoietic stem cell transplantation is a deterministic curative procedure for various hematologic disorders and congenital immunodeficiency. Despite its increased use, the mortality rate for patients undergoing this proce
Core Tip: This article provides insights into the use of validated mesenchymal stem/stromal cells (MSCs) as a potential treatment strategy for graft-versus-host disease (GVHD) in hematopoietic stem cell transplantation (HSCT). Current prevention and treatment options involve immunosuppression, which can hinder immune recovery and limit the graft-versus-tumor effect. By using MSCs, clinicians can effectively treat GVHD, identify high-risk patients, and stratify patients based on disease severity. Therefore, MSCs can aid in promoting engraftment, ameliorating acute GVHD, and preventing chronic GVHD, making them an attractive option for HSCT.
- Citation: Jaing TH, Chang TY, Chiu CC. Harnessing and honing mesenchymal stem/stromal cells for the amelioration of graft-versus-host disease. World J Stem Cells 2023; 15(4): 221-234
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/221.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.221
Mesenchymal stem/stromal cells (MSCs) are multipotent cells with self‐renewal abilities[1] that can be derived from different tissue sources. They attach to tissue culture dishes and express CD73, CD90, and CD105 but lack the expression of CD45, CD34, CD14, or CD11b, CD79α or CD19, and HLA-DR surface molecules. In vitro, MSCs can differentiate into osteoblasts, adipocytes, or chondroblasts[2,3]. MSCs can be effectively harvested without significant ethical concerns and have low immunogenicity. They have emerged as a promising cell source due to their regenerative and immunomodulatory potentials, limited ethical concerns, and low risk of tumor formation[4-6].
Malignancy relapse is a significant challenge in allogeneic hematopoietic stem cell transplantation (HSCT). Chronic graft-versus-host disease (GVHD) is associated with lower relapse rates, but the diagnosis, staging, and risk stratification of GVHD are challenging[7]. In this scoping review, we highlight recent evidence on different types of MSCs studied for GVHD, including bone marrow (BM), umbilical cord blood, placenta, adipose tissue, and others. MSCs have been found to inhibit immune cell proliferation and cytotoxic action, making them a potential treatment option for GVHD[8].
This review aims to provide a critical overview of the mechanisms by which MSC can treat GVHD, including immunomodulation, migration, homing, and clinical applications of MSC therapy. We searched peer-reviewed literature in PubMed and Embase to gather the latest information on this topic.
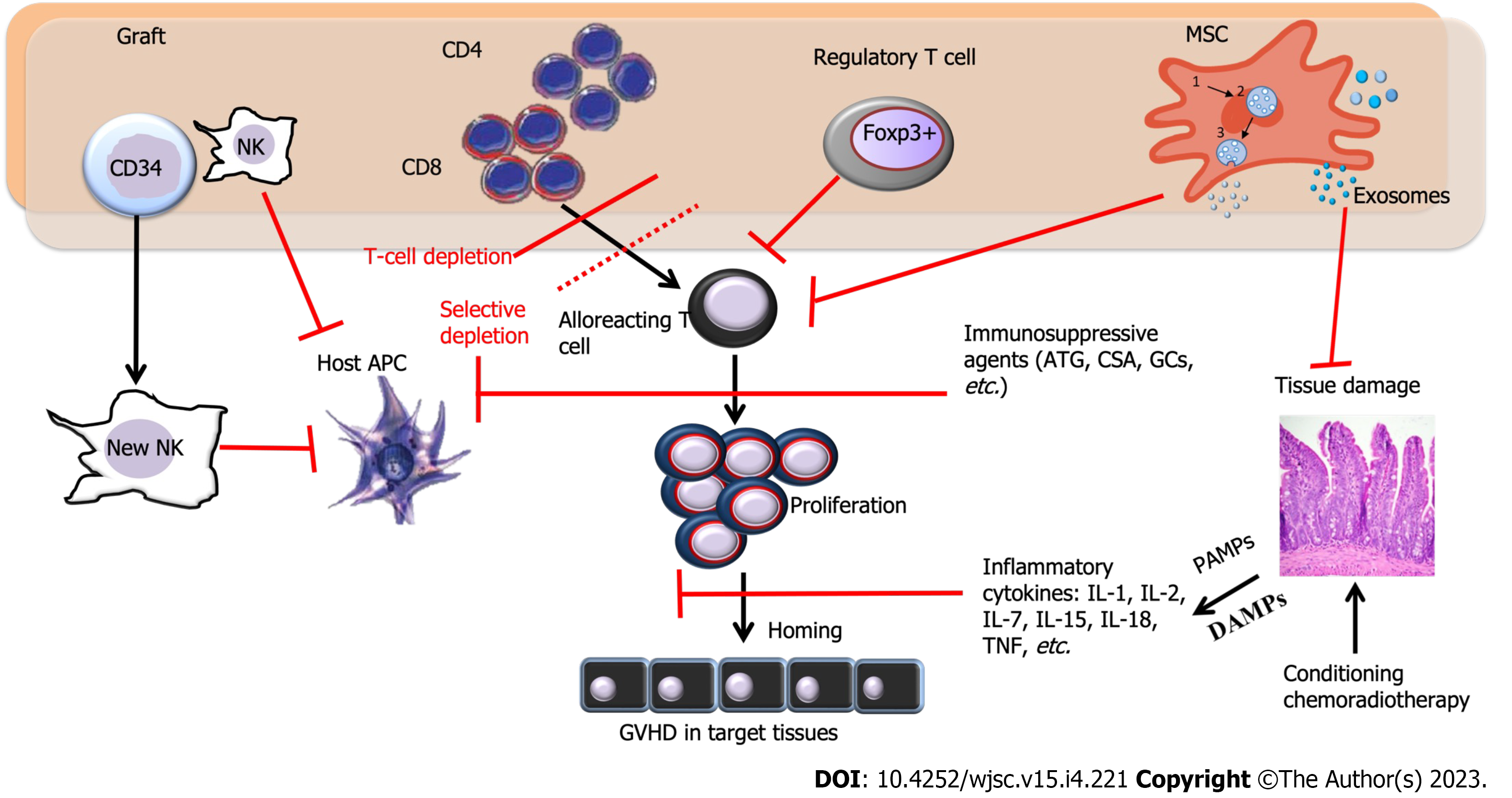
One of the most significant challenges in improving the prognosis for patients undergoing allogeneic HSCT is GVHD. This condition can be characterized as a rapid escalation in immune activation caused by massive target tissue apoptosis. The prevention of GVHD is primarily based on the use of calcineurin inhibitors and methotrexate, while the treatment of ongoing GVHD involves the use of corticosteroids. GVHD manifests as acute GVHD (aGVHD) in 53%-62.5% of the patients and chronic GVHD (cGVHD) in 20%-50.4% of patients[9,10], and the development of this complication may contribute to 6.3% of deaths following HSCT[9]. Although the administration of calcium inhibitors such as calcium sulphoaluminate can prevent the development of GVHD in some cases, about 19% of aGVHD II-IV cases are often resistant to all conventional therapy, resulting in a high mortality rate for these patients. Several potential second-line options have been proposed, including the use of MSCs. MSCs have attracted significant interest because they can actively undergo apoptosis by recipient cytotoxic cells[11]. Figure 1 illustrates the immune pathways involved in GVHD and the sites where therapy is used to block GVHD development.
In a typical case of aGVHD, which occurs following a triptych course, symptoms begin with the prodromal phase caused by the underlying disease and conditioning regimens that secrete proinflammatory cytokines, mainly tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6)[12]. Host conditioning facilitates donor cell grafting. Donor allograft T-cells are the primary effector cells for GVHD. However, tissue damage leads to the release of alarmins and the expression of pathogen-recognition receptors, triggering the next phase. This phase activates the innate immune system and, in turn, the adaptive immune system. Alarmins and exogenous pathogen-associated molecular patterns (PAMPs) elicit similar responses to relevant signals, and they belong to the group of damage-associated molecular patterns (DAMPs)[13]. DAMPs and PAMPs are potent stimulators for host and donor-derived antigen-presenting cells (APCs), which activate and enhance the responses of alloreactive donor T cells[14].
The immunosuppressive effects of MSCs are classified into soluble factor-mediated effects and cell-cell contact-mediated effects. MSCs suppress the proliferation and survival of activated T lymphocytes and reduce the release of inflammatory factors such as IL-2, TNF-α, IL-1β, and IFN-γ. By the same means, MSCs also reduce the number of Th1/Th2 and Th17 cells. Through cell-to-cell contacts, MSCs can stimulate the expression of transcription complexes related to Runt 1 (RUNX1), RUNX3, and CBFβ in Treg-specific regulatory regions to improve the stability of Foxp3[15]. MSCs have also been shown to be highly effective in inhibiting the cytotoxic effect, proliferation, and secretion of different cytokines of NK cells by directly contacting these cells and transforming their phenotype.
The effects of MSCs on B cells involve inhibiting their cell cycle progression by inducing G0/G1 cell cycle arrest and suppressing their proliferation. Additionally, the differentiation of B cells into IgM-, IgG-, and IgA-secreting cells is impaired by MSCs, thereby limiting their antibody production. Furthermore, MSCs can affect the chemotactic function of B cells[16].
Most relevant models for studying human adaptive immune responses use immunocompromised mice whose immune system is reconstituted with human immune cells and immune system components. Lee et al[17] used a model of NSG mice reconstituted with human CD34 cells to evaluate the immunological safety of therapeutically compromising human MSCs. As major histocompatibility complex (MHC) molecules are the primary mediators of the allogeneic immune response, MHC expression levels are critical in the potential immunogenicity of cells. To investigate MSCs as a cellular therapy in GVHD, Tobin et al[18] treated NSG-PBMC humanized mice with human MSCs as a GVHD model. MSC treatment resulted in a reduction in liver and intestinal pathology and a significant increase in the survival of the GVHD NSG mouse.
In contrast to aGVHD, some MHC-mismatched animal models may mimic the features of cGVHD. However, due to the pathological resemblance between cGVHD and autoimmune diseases, there is a clear connection between the two entities, and the difference in cGVHD is primarily caused by the donor lymphoid graft[19]. These findings provide compelling evidence for the essential role of human leukocyte antigen (HLA) disparity in both aGVHD and cGVHD. The expression pattern of minor histocompatibility antigens (miHAs) determines the target organ involvement in aGVHD. The miHAs exhibit hierarchical immunodominance, which may contribute to the variability in GVHD variability[20].
aGVHD: HLA mismatching is one of the most significant risk factors for aGVHD and cGVHD risk. HLA proteins are specifically encoded by MHC. In vitro studies have demonstrated that most T cells associated with GVHD are naïve T cells, whereas memory T cells mediate immunity against pathogens and the graft-versus-leukemia (GVL) effect[21]. Regardless of the graft source or conditioning intensity, the incidence of aGVHD is closely related to the number of HLA disparities. Although the impact of HLA disparity has been analyzed in the outcomes following allogeneic HSCT, relatively few studies have tried to correlate it with the incidence and severity of cGVHD. Some studies reported an association between HLA-A, -B, and -C disparity and aGVHD[22].
Although MHC antigens guarantee HLA matching, the donor and recipient may differ in various proteins presented in the form of HLA-peptide complexes to T cells that act as miHAs. The genomes include more than 107 polymorphic sequences outside HLA, and the role of miHAs is supported by genome-wide analysis of single-nucleotide polymorphisms[7]. The disparity in a single immuno
cGVHD: In contrast, cGVHD has been considered an autoimmune disease based on its clinical features[25]. Some experimental studies have shown that T cells from animals with cGVHD are specific for a public determinant of MHC class II molecules and are therefore considered autoreactive. These autoreactive cells of cGVHD are often associated with an injured thymus and adverse selection.
Recent clinical data has highlighted a significant link between immune responses against ubiquitous miHAs and cGVHD. Since cGVHD usually occurs after allogeneic HSCT, aGVHD is its related risk factor. Unlike syngeneic GVHD, which results from deficient thymic selection[26], cGVHD typically arises after allogeneic HSCT and is characterized by chronic T-cell activation due to continuous exposure to miHAs. This chronic stimulation can cause target organ damage that resembles auto
Epitope spreading and the failure of appropriate regulatory mechanisms in aGVHD may result in donor T cells recognizing both non-polymorphic and miHA epitopes, perpetuating cGVHD. In contrast, T cells directed against miHAs with hematopoietic restriction may also mediate a GVL response in the absence of GVHD[29]. However, the relevant immunogenic targets for cGVHD remain speculative and confidential.
The safety and effectiveness questions regarding using MSCs remain unresolved, and conflicting effects have been noted due to the heterogeneity observed among MSCs. MSCs-derived exosomes (MSCs-Exo), a subgroup of extracellular vesicles released by MSCs, have shown therapeutic benefits for inflammatory diseases and cancers due to their ability to transport proteins and nucleic acids from donor cells to recipient cells of the same or different tissues, making it a suitable candidate for cell-free therapy. MSCs-Exo have been found to reduce inflammation and fibrosis in the skin, lungs, and liver, and inhibit Th17 cells while inducing Treg cells, making it a potential alternative method for the treatment of cGVHD. The activation of CD4+ T cells and their infiltration into the inflamed mouse lung were reduced in MSCs-Exo-treated mice[30]. MSCs-Exo, extracted from healthy donors’ BM, suppress the expression of pro-inflammatory factors TNF-α and IL-1β but increase the level of anti-inflammatory factor TGF-β during in vitro culture[31].
Typically, MSCs-Exo are characterized by endosomes that bud inward and package into multi-vesicular bodies (MVBs). These MVBs fuse with the plasma membrane and deliver the exosomes into the intracellular space. However, exosomes can enrich several molecules as cargo, such as proteins/cytokines, DNA, RNA, and other nucleic acids. Exosomes, as secretory components of MSCs, transport cytokines, and growth factors of immunoregulation, such as transforming growth factor beta-1 (TGF-β1), IL-6, IL-10, hepatocyte growth factor, signaling lipids, mRNAs, and regulatory miRNAs, which exert biological effects on recipient cells, such as cell-to-cell communication, tissue regeneration, metabolism, immune modulation, and homing of immune cells[32,33]. Diverse immune cells establish complex interactions with each other. MSCs-Exo might represent a novel cell-free therapy with unique competitive advantages over parent MSCs, such as no apparent risk of tumor formation or lower immunogenicity.
Antigen-presenting cells (APCs) play a critical role in inducing aGVHD, with dendritic cells (DCs) being one of the most formidable cells in this regard[26]. Innate immunity activation during acute inflammation leads to DCs maturation and subsequent T cell priming, which is central to the potential antitumor benefits of aGVHD. Experimental data suggest that modulating perceptible DC subsets can influence aGVHD[34]. For instance, the absence of RelB signaling in host DCs or enhancing host CD8+ lymphoid DC subsets following HSCT significantly reduces aGVHD[35]. Other APCs, such as monocytes/macrophages, also play a crucial role in this phase. Some data suggest that the host B cells may reduce aGVHD in specific contexts. Although the precise mechanisms remain unclear when acting as APCs, MSCs from the donor, or host also reduce aGVHD.
Natural killer (NK) cells can directly kill tumor cells without specific immunization and also have a modulatory effect on aGVHD. In an allo-HSCT donor-to-F1 model, NK cells recognize the absence of donor class I on host APCs and eliminate them, resulting in a reduction of aGVHD reduction. Upon activation, NK cells may induce apoptosis of target cells through contact-dependent cytotoxicity primarily via perforin and granzyme[36]. Pro-apoptotic granzymes enter through perforin pores in the plasma membrane of target cells. Besides the cytotoxic activity, NK cell activation increases the secretion of various cytokines and chemokines, such as IFN-γ. However, the role of NK cells in GVHD remains controversial.
The infusion of donor γδ T cells may increase aGVHD, while the absence of host γδ T cells may reduce APC activation and aGVHD in an MHC-mismatched model. Conversely, in the absence of host γδ T cells, GVHD severity was not modified in an MHC-matched, miHA-disparate model of cGVHD. aGVHD could be more significant in patients with more considerable donor γδ T cells. The significance of γδ T cells in aGVHD and cGVHD is not fully understood and may reflect differences in immunobiology between the two or be solely a consequence of variation in the experimental models.
NKT cells, which are CD1d-reactive, are believed to play an immunoregulatory role in suppressing dysfunctional immune reactions, including GVHD[37]. The cumulative frequency of regulatory T cells (Tregs) is negatively correlated with GVHD development[38], and exogenous NKT cell infusion can reduce the degree of GVHD[39]. However, Treg populations have unstable Foxp3 expression, particularly those expanded in vitro. Because the expression Foxp3 is needed for the suppressive function, further research is necessary to determine if Foxp3 expression can be simplified, especially under pro-inflammatory conditions characteristic of the GVHD milieu[40].
The complex interactions between MSCs and T cells have been extensively studied, particularly in vitro culture techniques. MSCs may facilitate activated T cells in the phase G0/G1 cell cycle, yet apoptosis is not applicable[41-43]. MSCs may suppress or downregulate the proliferation of both naïve and memory T cells through cell-cell contact or mitogenic stimuli. This suppression is generally not MHC-restricted. MSCs can further decrease IFN-γ producing T cells and contribute to the T-cell skewing toward Th2 cells producing IL-4. cGVHD is a Th2 cell dominant disease process[12].
MSCs activate immune responses that induce the expression of Tregs, which are a cluster of cells with a CD4+CD25+ Foxp3+ phenotype that regulate the body's immune response. Tregs highly and constitutively express CTLA-4, which binds to CD80, and CD86 on DCs, leading to impaired DC maturation and blocking CD80/CD86 to CD28 on conventional T cells, thereby preventing costimulation, and T-cell activation. Lower Tregs and deficient Foxp3 expression have been associated with cGVHD in peripheral blood and mucosal biopsies. However, levels of Foxp3 mRNA in the CD25+ T cell compartment do not predict the development of cGVHD, demonstrating that the presence, or absence of Tregs must be considered in the context of their impact on aGVHD and cGVHD. An intriguing possibility is that the negative impact of calcineurin inhibitors on Tregs could exacerbate cGVHD as a consequence of the suppression of the alloreactive donor cytopathic and Tregs.
Host B cells attenuate aGVHD in an IL-10-dependent manner. Recent data provide a rationale for the pathogenic role of donor B cells in cGVHD[12], including a robust correlation between cGVHD and (1) The effects of antibodies against Y-chromosome-encoded miHA; (2) higher numbers of B cells with altered TLR9 responses; (2) levels of a B-cell-activating factor, which enhances survival and differentiation of activated B cells; and (4) in animal models, levels of autoantibodies. Besides, emerging data from the depletion of B cells with rituximab further supports the theory of the pathogenic action of B cells in cGVHD[44]. However, whether B cells are the effectors or inducers of cGVHD remains unknown.
We systematically searched the electronic bibliographic databases MEDLINE, EMBASE, and Google Scholar for studies published before November 2022 using the keywords: “graft-versus-host disease” OR “acute GVHD” OR “chronic GVHD”AND “mesenchymal stem cells” AND “mesenchymal stromal cells” AND “treatment response” AND “outcome.” Publications were included if they met the following inclusion criteria: (1) Original research; (2) published in 2002 or later; and (3) specifically reporting on the use of MSCs in GVHD patients. Publications were excluded based on the following criteria: (1) Non-English literature; (2) small populations (n < 20 patients) or case studies; and (3) mixed population with non-GVHD patients. A meta-analysis was not performed for the limited number of published studies meeting the inclusion criteria. Pre-post design studies and case series were not included for lack of sustainability of the results. Additionally, reference lists of retrieved articles were cross-referenced for additional eligible articles.
This review provides an overview of clinical studies, animal models, and limited human patient trials regarding MSCs. MSCs have been widely studied and increasingly used in GVHD treatment since the first report of promising results by Le Blanc et al[45] However, the studies have reported varying outcomes, which could be contributed to differences in cell concentration and MSC infusion dose. While MSC infusion has shown quite promising results following GVHD prophylaxis failure, some clinicians still prefer using methylprednisolone and calcineurin inhibitors before cell therapy with MSCs.
In addition to suppressing inflammation, MSCs have other beneficial effects, including increased angiogenesis, reduced apoptosis, and modified extracellular matrix dynamics. These cells mediate immune system components like macrophages and neutrophils, improving tissue microenvironments. After the injury, MSCs can either promote or suppress the immune system to guide the whole-tissue regeneration process[1]. Clinical responses to MSC infusion assessed as early as one week after treatment may predict patients' overall survival, indicating the potential of MSCs in treating GVHD[45].
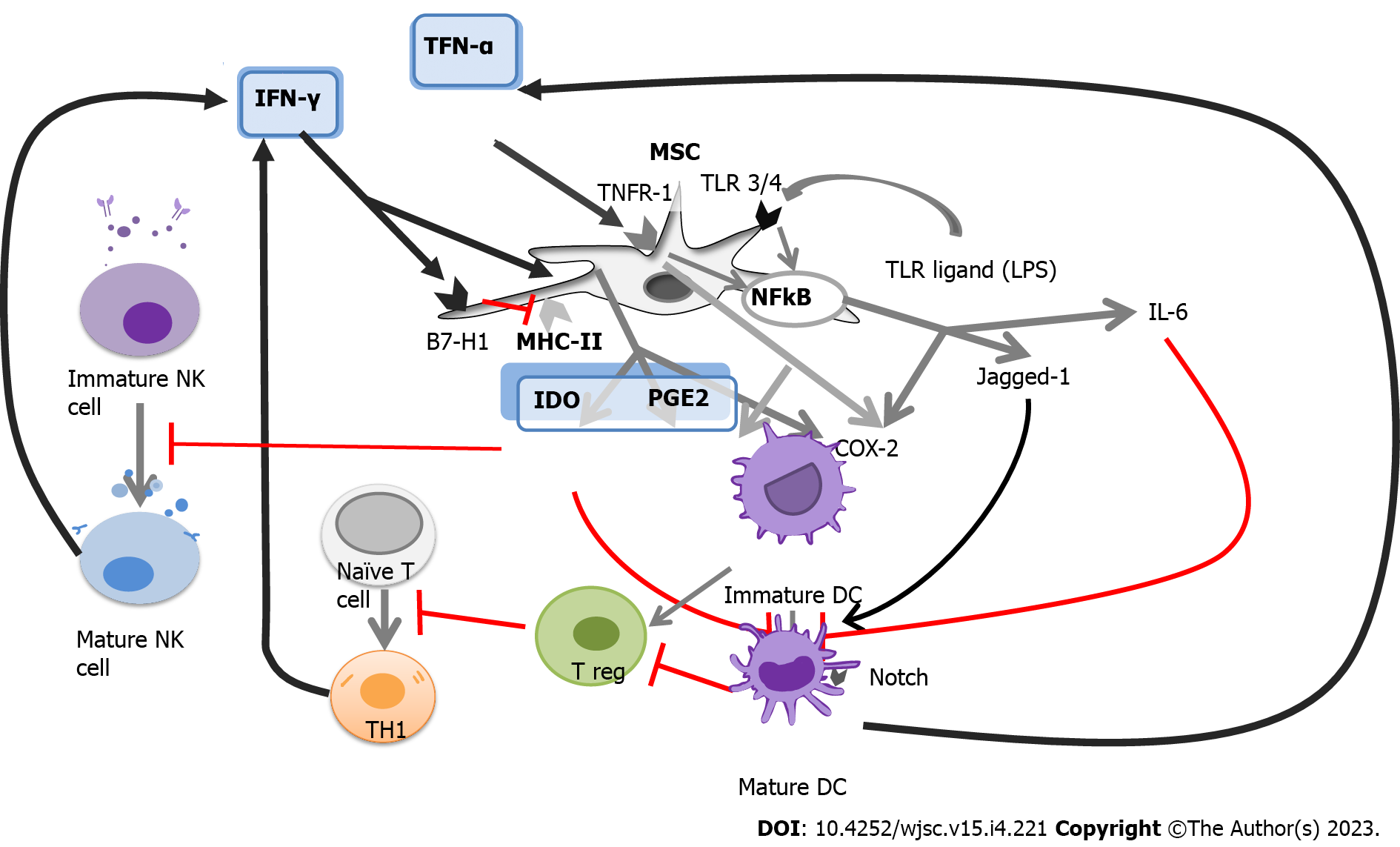
Although the paracrine effects of MSCs are known to mediate the modulation of the immune response, the mechanisms underlying this modulation are not yet fully understood. However, it has been found that under conditions of chronic hypoxia or co-stimulation with IFN-γ, MSCs express proteins that have the immunosuppressive capacity, such as IDO, HLA-G, PGE2, and FasL, which can modulate the immune response[46]. While other cytokines play a crucial role in immunosuppression, blocking highly expressed proteins can result in the setback of the human immunosuppressed state, leading to the growth, and proliferation of immune cells. Moreover, MSCs do not trigger the activation of immune cells as they lack CD40, CD80, CD86, and HLA-DR-stimulating molecules. Given that GVHD occurs following the infusion of immune cells donated by the same donors, suppressing the immune activity can improve the patient’s prognosis. MSCs’ expression of paracrine effects can regulate these donor immune cells through various mechanisms (Table 1)[47-62].
| MSC types | Mechanism of immunosuppressive effect | Ref. |
| BM-MSCs | Recipient-derived MSCs from patients with GVHD are analogous to MSCs from healthy volunteers | Copland et al[47] |
| After MSC infusion, the ratio of Th1 cells to Th2 cells was reversed, with an increase in Th1 and a decrease in Th2 achieving a new balance | Zhou et al[48] | |
| BM-MSCs reduce the incidence and severity of GVHD by improving thymic function and induction of Tregs but not increase the risks of infections and tumor relapse | Zhao et al[49]; Selmani et al[50] | |
| HLA-G5 secreted by MSCs is critical to the suppressive functions of MSCs | Selmani et al[51] | |
| MenSCs | MenSCs exhibit a higher capacity to migrate into the intestine and liver and not to their anti-inflammatory capacities | Luz-Crawford et al[52] |
| FL-MSCs | FL-MSCs demonstrates much longer-lasting immunomodulatory properties by inhibiting directly the proliferation and activation of CD4+ and CD8+ T cells | Yu et al[53] |
| UC-MSCs | UC-MSCs showed minimal expression of HLA-DR after activation and posed minimal risk of initiating an allogeneic immune | Kim et al[54] |
| UC-MSCs alleviate SLE through upregulating Treg cells, which was partly dependent on HLA-G | Chen et al[55] | |
| UC-MSCs ameliorate GVHD and spare GVL effect via immunoregulations | Wu et al[56] | |
| WJ-MSCs | WJ-MSCs exert immunosuppressive effects by cell-cell contact with activated T cells and in part through the soluble factor indoleamine 2,3-dioxygenase | He et al[57] |
| MC-, WJ- and BM-MSCs | The mixed populations of MSCs displayed all of the positive attributes of WJ-MSC and BM-MSC | Mennan et al[58] |
| AT-MSCs | The use of AT-MSC rather than BM-MSC could further preserve NK cell activity and favor GVL | Blanco et al[59] |
| hG-MSCs | hG-MSC treatment inhibited local inflammation of injured skin by suppressing inflammatory cells, reducing pro-inflammatory cytokine tumor necrosis factor-α, and increasing anti-inflammatory cytokine interleukin-10, which was promoted by hypoxia | Jiang et al[60] |
| CP-, BM- and AT-MSCs | CP-MSCs may have additional advantage over the other MSCs in terms of immunomodulation | Lee et al[61] |
| DP-MSCs | Immunomodulation and expression of trophic factors by dental MSCs increase their resistance to allogeneic NK cell lysis and their potential in vivo lifespan | Martinez et al[62] |
When MSCs are exposed to an insult, such as injury, or bacterial infection, MHC-II molecules facilitate the presentation of bacterial antigens, which induces further activation of T cells expressing IFN-γ. MHC-II is downregulated at high levels of IFN-γ, while B7-H1 is upregulated[45]. These presentation pathways are illustrated in Figure 2.
MSCs have been used to treat various conditions, including diabetes mellitus (DM), cardiovascular diseases, GVHD, and autoimmune diseases. Despite persistent questions, the immunomodulatory effects of MSCs make them a top choice for cell therapy. MSCs are early multipotent progenitors and non-hematopoietic cell populations that can be expanded ex-vivo to achieve large numbers necessary for in vivo use. Recently, adipose tissues, umbilical cord, placenta, and dental pulp have been recognized as multipotent sources of MSCs. MSCs can differentiate into a variety of cell types capable of osteogenic, chondrogenic, adipogenic, myogenic, and neurogenic differentiation. However, not all individual cells cultivated in tissue culture flasks result in the same degree of multipotency. Self-renewing progenitors can be identified in human BM, and it is currently unknown whether MSCs from other tissues exhibit this property. BM-MSCs are a critical source of multipotent stem cells and serve as a standard for comparing MSCs from different sources (Table 2)[49,63-85].
| Study type | Patient No. | Indication | MSC type | Response criteria | Main findings | Ref. |
| Phase 2 | 55 | Steroid-resistant, severe, aGVHD | BM | Glucksberg | CR: 30/55, better OS/TRM for complete responder | Le Blanc et al[63], 2008 |
| Phase 2 | 31 | Gr. II-IV aGVHD | BM | Glucksberg | CR: 77%; PR: 16% | Kebriaei et al[64], 2009 |
| Pilot study | 20 | Co-transplantation with NMA mismatched HSCT | BM | Glucksberg | Decreased 1 yr GVHD death (10% vs 31%, P = 0.04). Better NRM & OS | Baron et al[65], 2010 |
| Retrospective | 37 | Resistant Gr. III-IV aGVHD | BM | Glucksberg | CR: 65%, better TRM and OS | Ball et al[66], 2013 |
| Multicenter trial | 50 | Resistant Gr. IV aGVHD | BM | Not mentioned | OR: 33%, CR: 17%, initial response and young age have better survival | Resnick et al[67], 2013 |
| Prospective, single-arm, open-label | 75 | Severe refractory aGVHD | BM | IBMTR SI | OR on day +28: 61.3%, better OS for responder on day +100 (78.1% vs 31.0%; P < 0.001) | Kurtzberg et al[68], 2014 |
| Phase 1 | 40 | Resistant Gr. II-IV aGVHD | BM | Glucksberg | CR: 27.5%, OR: 67.5% on day +28; more CR in pediatric group | Introna et al[69], 2014 |
| Phase 2 | 25 | Refractory aGVHD | BM | Glucksberg | 71% responded, CR 11/24, better OS for CR | Sánchez-Guijo et al[70], 2014 |
| Prospective, nonrandomized | 28 vs 19 without MSC | Refractory aGVHD | BM | Glucksberg | Decreased incidence and severity of cGVHD. Better OR and CR. | Zhao et al[49], 2015 |
| Phase 2 | 48 | Steroid-resistant aGVHD | BM | Glucksberg | CR: 25% on day 28, 50% lasting > 1 mo, with better OS | Te Boome et al[71], 2015 |
| Compassionate use | 58 | Steroid-resistant aGVHD | BM | IBMTR SI | OR: 47%, but no improvement in OS | von Dalowski et al[72], 2016 |
| Phase 2/3 | 25 | Refractory Gr. III-IV aGVHD | BM | Glucksberg | Better OS for OR at 4-wk (CR: 6/25, PR: 9/25) | Muroi et al[73], 2016 |
| Pilot study | 33 | Refractory aGVHD | BM | IBMTR SI | CR: 18/33, PR: 7/33, better OS in CR, no TRM in CR | Erbey et al[74], 2016 |
| Compassionate use | 26 | Severe resistant aGVHD | BM | Not mentioned | OR: 77% on day +28 (CR: 5/26, PR: 15/26) | Kuçi et al[75], 2016 |
| Phase 2 prospective RCT | 62 vs 62 without MSC | cGVHD prophylaxis in haplo | Cord | NIH score | cGVHD: 27% (MSC) vs 49% in 2 yr (P = 0.021) | Gao et al[76], 2016 |
| Phase 1/2 | 26 | Steroid-refractory aGVHD | BM | Glucksberg | OR: 62% on day 28. Higher response rate in children. High NRM in adults | Salmenniemi et al[77], 2017 |
| Pilot study | 22 | Refractory GVHD (Gr. 2-4 a or cGVHD) | BM or adipose tissue | Glucksberg/NIH score | CR: 45.8%, PR: 33.3%, better OS in CR/PR | Cetin et al[78], 2017 |
| Retrospective | 46 | Refractory Gr. III/IV aGVHD | BM | Not mentioned | 50% responded with better OS (P = 0.0004) | Dotoli et al[79], 2017 |
| Phase 1/2 | 33 | Steroid-refractory aGVHD | BM | Glucksberg | CR: 34%, PR: 50% on day 28. Better OS on day 90 and 1 yr (P = 0.006, 0.002) | Fernández-Maqueda et al[80], 2017 |
| Phase 1/2 | 69 | Refractory aGVHD | BM | Glucksberg | OR: 83% on day 28 | Bader et al[81], 2018 |
| Observational study | 34 vs 34 without MSC | aGVHD | BM or adipose tissue | IBMTR SI | Better OS compared with historical control, P = 0.0678. MSC has no association with risk of infectious complication | Stoma et al[82], 2018 |
| Retrospective | 11 (study group 2) | Severe refractory aGVHD | Placenta derived decidual stromal cell | Glucksberg | 73% 1 yr OS in study group 2 (albumin), 47% in group 1 (AB plasma), P = 0.016 | Ringden et al[83], 2018 |
| Retrospective | 22 | Severe refractory aGVHD | Cord | IBMTR SI | CR: 45.5%, PR: 13.6% | Bozkurt et al[84], 2019 |
| Phase 3 RCT | 151 vs 72 placebo | Severe refractory aGVHD | BM | IBMTR SI | Difference of durable CR (lasting > 28 d) not achieved (35% vs 30%, P = 0.42); Pediatric pts had better OR (64% vs 23%, P = 0.05) | Kebriaei et al[85], 2020 |
The term "mesenchymal stem cells" has been proposed as a more appropriate term than MSCs. These cells possess not only multipotency but also significant immunomodulatory and engraftment-promoting properties. They create a specialized microenvironment for HSCs by promoting the secretion of various inflammatory cytokines, chemokines, growth factors, extracellular matrix, and extracellular vesicles that are crucial for HSC differentiation, proliferation, and maintenance[86-88]. After in vivo biological application, MSCs secrete a range of cytokines and regulatory molecules with anti-inflammatory, wound healing, and regenerative effects, promoting the repair of endogenous tissues or tissue replacement. Beres et al[40] demonstrated that even in otherwise immunocompetent humans, allogeneic MSCs may graft, and differentiate through significant histocompatible barriers.
Similar to hematopoietic stem cells, MSCs have multi-organ specificity, and plasticity. In 2006, the International Society for Cellular Therapy officially defined MSCs as plastic practitioners under standard growing conditions, expressing CD73, and CD90 surface molecules while lacking CD11b, CD14, CD19, CD34, CD45, CD79a, and HLA-DR[2]. In addition, MSCs can differentiate into various mesodermal lineages including osteoblast, adipocyte, and chondroblast, to different degrees.
MSCs are capable of modulating both innate and adaptive immunity through the release of various soluble factors, including indoleamine 2,3-dioxygenase[11], IL-10, prostaglandin 2, nitric oxide, transforming growth factor-β, HLA-G5, and anti-inflammatory molecule TNF-α-induced gene/protein 6[89]. These molecules are believed to play a key role in the immunomodulating effects of MSCs, which have been shown to be beneficial in certain immunopathological diseases, such as aGVHD, and type 1 DM. However, the precise mechanisms underlying this therapeutic potential are not yet fully understood. The literature suggests that the immunomodulating potential of MSCs involves interactions with both humoral and cellular components of the innate and adaptive immune systems. The literature refers to several fundamental cellular interactions. An integrated perspective on the utility of MSCs for GVHD has been strengthened by the recent findings that MSCs are induced to undergo necrosis/apoptosis by the recipient’s cytotoxic cells and that this process is assumed to elicit MSC-induced immunosuppression[90]. This finding made it possible to reconcile the dilemma between the effectiveness of MSC and its apparent lack of engraftment and highlighted the crucial role of the patient in the promotion and administration of immunosuppression of MSCs. Recent research has shed light on the role of the patient in promoting and administering immunosuppression of MSCs, with evidence suggesting that MSCs are induced to undergo necrosis/apoptosis by the recipient’s cytotoxic cells, leading to MSC-induced immunosuppression[90]. Table 2 provides an overview of recent studies on this topic, with 97 articles selected for full-text evaluation based on agreed-upon title and abstract criteria.
Innate immunity is primarily centered around the complementary system, with C3, and C5 being cleaved into anaphylatoxins C3a and C5a by convertases at the sites of inflammation. The labile C3 convertases cleave C3 into C3a and C3b which can thereafter participate in forming distinct complexes and activate pathways for proliferation and protection against apoptosis through receptor binding. MSCs also secrete the factor H, which inhibits complement activation by limiting the activity of C3 and C5 convertases. In mice, MSCs promote pro-inflammatory repolarization and produce chemostatic cytokines, including IL-6, IL-8, GM-CSF, and macrophage inhibitory factors. IL-8, in particular, is a pro-inflammatory chemokine produced by multiple cell types that recruits leukocytes to sites of infection or tissue injury. Additionally, MSCs can inhibit mast cell degranulation and histamine release by binding allergens to allergen-specific IgE via FcRε on mast cells, providing a potential therapeutic benefit for allergic reactions[91].
The molecular interaction between NK cells and MSCs is complex and depends on the immune microenvironment and NK cell activation status. MSC can inhibit cytokine proliferation and production and interfere with NK cell cytotoxicity. They also inhibit monocyte maturation and differentiation into DCs, which are the primary type of APC and play a key role in T lymphocyte activation through antigen presentation. Monocytes and macrophages are important for tissue development, homeostasis, and injury repair. Activated MSCs produce chemokines that attract circulating monocytes to sites of inflammation and injury[92].
MSCs can regulate the adaptive immune system through multiple redundant pathways. They suppress the proliferation of T cells, IFNγ production, CD4 T cell differentiation, and CD8 T-cell cytotoxicity. Di Nicola et al[41] reported that MSCs can suppress T lymphocyte proliferation in vitro with autologous and allogeneic MSCs, including T lymphocytes cultured with DCs or lymphocytes in mixed lymphocyte reactions. MSCs can express and secrete programmed death-ligand 1 and 2, which suppress T-cell proliferation in the presence of MSCs, secrete IL-2, induce apoptosis, and promote the induction of an irreversible hyporeactive state[93]. In vivo studies suggested that MSCs may restore the balance between T helper 1 and 2 cells in diseases associated with a shift to dominance of these T cell subpopulations[94]. In vitro models have shown that MSCs induce Tregs and maintain survival and suppressive phenotypes[95].
This article provides insights into the use of validated MSCs as a potential treatment strategy for GVHD in HSCT. Current prevention and treatment options involve immunosuppression, which can hinder immune recovery and limit the graft-versus-tumor effect. By using MSCs, clinicians can effectively treat GVHD, identify high-risk patients, and stratify patients based on disease severity. Therefore, MSCs can aid in promoting engraftment, ameliorating aGVHD, and preventing cGVHD, making them an attractive option for HSCT.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kode JA, India; Li SC, United States; Zhang XF, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 386] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 2. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12641] [Article Influence: 702.3] [Reference Citation Analysis (2)] |
| 3. | Harichandan A, Bühring HJ. Prospective isolation of human MSC. Best Pract Res Clin Haematol. 2011;24:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Fričová D, Korchak JA, Zubair AC. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. NPJ Regen Med. 2020;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 584] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 6. | Saas P, Daguindau E, Perruche S. Concise Review: Apoptotic Cell-Based Therapies-Rationale, Preclinical Results and Future Clinical Developments. Stem Cells. 2016;34:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Giaccone L, Faraci DG, Butera S, Lia G, Di Vito C, Gabrielli G, Cerrano M, Mariotti J, Dellacasa C, Felicetti F, Brignardello E, Mavilio D, Bruno B. Biomarkers for acute and chronic graft versus host disease: state of the art. Expert Rev Hematol. 2021;14:79-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 527] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 9. | Paz A, Rigoni L, Fischer G, Schittler M, Pezzi A, Valim V, Dahmer A, Zambonato B, Amorin B, Sehn F, Silva MAD, Daudt L, Silla L. Donor characteristics and hematopoietic stem cell transplantation outcome: experience of a single center in Southern Brazil. Hematol Transfus Cell Ther. 2018;40:136-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Baker M, Wang H, Rowley SD, Cai L, Pecora AL, Skarbnik A, Vesole DH, Adler-Brecher B, Kim D, Donato ML. Comparative Outcomes after Haploidentical or Unrelated Donor Bone Marrow or Blood Stem Cell Transplantation in Adult Patients with Hematological Malignancies. Biol Blood Marrow Transplant. 2016;22:2047-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 490] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 12. | Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 947] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 13. | Ramadan A, Paczesny S. Various forms of tissue damage and danger signals following hematopoietic stem-cell transplantation. Front Immunol. 2015;6:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Toubai T, Mathewson ND, Magenau J, Reddy P. Danger Signals and Graft-versus-host Disease: Current Understanding and Future Perspectives. Front Immunol. 2016;7:539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Zhou X, Jin N, Wang F, Chen B. Mesenchymal stem cells: a promising way in therapies of graft-versus-host disease. Cancer Cell Int. 2020;20:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1269] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 17. | Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim JS, Jeon HB. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Tobin LM, Healy ME, English K, Mahon BP. Human mesenchymal stem cells suppress donor CD4(+) T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease. Clin Exp Immunol. 2013;172:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Boieri M, Shah P, Dressel R, Inngjerdingen M. The Role of Animal Models in the Study of Hematopoietic Stem Cell Transplantation and GvHD: A Historical Overview. Front Immunol. 2016;7:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Choi EY, Christianson GJ, Yoshimura Y, Sproule TJ, Jung N, Joyce S, Roopenian DC. Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity. 2002;17:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Gatza E, Reddy P, Choi SW. Prevention and Treatment of Acute Graft-versus-Host Disease in Children, Adolescents, and Young Adults. Biol Blood Marrow Transplant. 2020;26:e101-e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Rimando J, Slade M, DiPersio JF, Westervelt P, Gao F, Liu C, Romee R. The Predicted Indirectly Recognizable HLA Epitopes (PIRCHE) Score for HLA Class I Graft-versus-Host Disparity Is Associated with Increased Acute Graft-versus-Host Disease in Haploidentical Transplantation with Post-Transplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2020;26:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Dickinson AM, Wang XN, Sviland L, Vyth-Dreese FA, Jackson GH, Schumacher TN, Haanen JB, Mutis T, Goulmy E. In situ dissection of the graft-versus-host activities of cytotoxic T cells specific for minor histocompatibility antigens. Nat Med. 2002;8:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Sweeney C, Vyas P. The Graft-Versus-Leukemia Effect in AML. Front Oncol. 2019;9:1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Tyndall A, Dazzi F. Chronic GVHD as an autoimmune disease. Best Pract Res Clin Haematol. 2008;21:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105:4885-4891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C, Ho V, Soiffer RJ, Antin JH, Ritz J. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973-2978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 307] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4:318-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | MacDonald KP, Shlomchik WD, Reddy P. Biology of graft-versus-host responses: recent insights. Biol Blood Marrow Transplant. 2013;19:S10-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Lai P, Chen X, Guo L, Wang Y, Liu X, Liu Y, Zhou T, Huang T, Geng S, Luo C, Huang X, Wu S, Ling W, Du X, He C, Weng J. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. 2018;11:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 31. | Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, Li H, Liu Z, Shi C, Duan F, Xiao Y. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 32. | Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 543] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 33. | Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 34. | Yu H, Tian Y, Wang Y, Mineishi S, Zhang Y. Dendritic Cell Regulation of Graft-Vs.-Host Disease: Immunostimulation and Tolerance. Front Immunol. 2019;10:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | MacDonald KP, Kuns RD, Rowe V, Morris ES, Banovic T, Bofinger H, O'Sullivan B, Markey KA, Don AL, Thomas R, Hill GR. Effector and regulatory T-cell function is differentially regulated by RelB within antigen-presenting cells during GVHD. Blood. 2007;109:5049-5057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. 2013;4:499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Leveson-Gower DB, Olson JA, Sega EI, Luong RH, Baker J, Zeiser R, Negrin RS. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood. 2011;117:3220-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Zhang P, Yang S, Zou Y, Yan X, Wu H, Zhou M, Sun YC, Zhang Y, Zhu H, Xu K, Wang Y, Sheng LX, Mu Q, Sun L, Ouyang G. NK cell predicts the severity of acute graft-versus-host disease in patients after allogeneic stem cell transplantation using antithymocyte globulin (ATG) in pretreatment scheme. BMC Immunol. 2019;20:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol. 2013;4:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2362] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 42. | Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1087] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 43. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 832] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 44. | Olivieri J, Coluzzi S, Attolico I, Olivieri A. Tirosin kinase inhibitors in chronic graft versus host disease: from bench to bedside. ScientificWorldJournal. 2011;11:1908-1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 2027] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 46. | Galleu A, Milojkovic D, Deplano S, Szydlo R, Loaiza S, Wynn R, Marks DI, Richardson D, Orchard K, Kanfer E, Tholouli E, Saif M, Sivaprakasam P, Lawson S, Bloor A, Pagliuca A, Potter V, Mehra V, Snowden JA, Vora A, Kishore B, Hunter H, Apperley JF, Dazzi F. Mesenchymal stromal cells for acute graft-versus-host disease: response at 1 week predicts probability of survival. Br J Haematol. 2019;185:89-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Copland IB, Qayed M, Garcia MA, Galipeau J, Waller EK. Bone Marrow Mesenchymal Stromal Cells from Patients with Acute and Chronic Graft-versus-Host Disease Deploy Normal Phenotype, Differentiation Plasticity, and Immune-Suppressive Activity. Biol Blood Marrow Transplant. 2015;21:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, Ai H, Zhao RC. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant. 2010;16:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Zhao K, Lou R, Huang F, Peng Y, Jiang Z, Huang K, Wu X, Zhang Y, Fan Z, Zhou H, Liu C, Xiao Y, Sun J, Li Y, Xiang P, Liu Q. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 50. | Selmani Z, Naji A, Gaiffe E, Obert L, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 2009;87:S62-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 772] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 52. | Luz-Crawford P, Torres MJ, Noël D, Fernandez A, Toupet K, Alcayaga-Miranda F, Tejedor G, Jorgensen C, Illanes SE, Figueroa FE, Djouad F, Khoury M. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells. 2016;34:456-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Yu Y, Valderrama AV, Han Z, Uzan G, Naserian S, Oberlin E. Human fetal liver MSCs are more effective than adult bone marrow MSCs for their immunosuppressive, immunomodulatory, and Foxp3(+) T reg induction capacity. Stem Cell Res Ther. 2021;12:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Kim JH, Jo CH, Kim HR, Hwang YI. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018;2018:8429042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Chen C, Liang J, Yao G, Chen H, Shi B, Zhang Z, Zhao C, Zhang H, Sun L. Mesenchymal stem cells upregulate Treg cells via sHLA-G in SLE patients. Int Immunopharmacol. 2017;44:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Wu QL, Liu XY, Nie DM, Zhu XX, Fang J, You Y, Zhong ZD, Xia LH, Hong M. Umbilical cord blood-derived mesenchymal stem cells ameliorate graft-versus-host disease following allogeneic hematopoietic stem cell transplantation through multiple immunoregulations. J Huazhong Univ Sci Technolog Med Sci. 2015;35:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | He H, Nagamura-Inoue T, Takahashi A, Mori Y, Yamamoto Y, Shimazu T, Tsunoda H, Tojo A. Immunosuppressive properties of Wharton's jelly-derived mesenchymal stromal cells in vitro. Int J Hematol. 2015;102:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Mennan C, Brown S, McCarthy H, Mavrogonatou E, Kletsas D, Garcia J, Balain B, Richardson J, Roberts S. Mesenchymal stromal cells derived from whole human umbilical cord exhibit similar properties to those derived from Wharton's jelly and bone marrow. FEBS Open Bio. 2016;6:1054-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Blanco B, Herrero-Sánchez MD, Rodríguez-Serrano C, García-Martínez ML, Blanco JF, Muntión S, García-Arranz M, Sánchez-Guijo F, Del Cañizo C. Immunomodulatory effects of bone marrow versus adipose tissue-derived mesenchymal stromal cells on NK cells: implications in the transplantation setting. Eur J Haematol. 2016;97:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Jiang CM, Liu J, Zhao JY, Xiao L, An S, Gou YC, Quan HX, Cheng Q, Zhang YL, He W, Wang YT, Yu WJ, Huang YF, Yi YT, Chen Y, Wang J. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J Dent Res. 2015;94:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Lee JM, Jung J, Lee HJ, Jeong SJ, Cho KJ, Hwang SG, Kim GJ. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 62. | Martinez VG, Ontoria-Oviedo I, Ricardo CP, Harding SE, Sacedon R, Varas A, Zapata A, Sepulveda P, Vicente A. Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells. Stem Cell Res Ther. 2017;8:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 63. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2027] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 64. | Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, Monroy R, Uberti J. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 65. | Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L, Vanbellinghen JF, Hafraoui K, Lejeune M, Gothot A, Fillet G, Beguin Y. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2010;16:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 66. | Ball LM, Bernardo ME, Roelofs H, van Tol MJ, Contoli B, Zwaginga JJ, Avanzini MA, Conforti A, Bertaina A, Giorgiani G, Jol-van der Zijde CM, Zecca M, Le Blanc K, Frassoni F, Egeler RM, Fibbe WE, Lankester AC, Locatelli F. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 67. | Resnick IB, Barkats C, Shapira MY, Stepensky P, Bloom AI, Shimoni A, Mankuta D, Varda-Bloom N, Rheingold L, Yeshurun M, Bielorai B, Toren A, Zuckerman T, Nagler A, Or R. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res. 2013;3:225-238. [PubMed] |
| 68. | Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, Horn B, Yu L, Talano JA, Nemecek E, Mills CR, Chaudhury S. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 69. | Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, Longoni D, Pavan F, Masciocchi F, Algarotti A, Micò C, Grassi A, Deola S, Cavattoni I, Gaipa G, Belotti D, Perseghin P, Parma M, Pogliani E, Golay J, Pedrini O, Capelli C, Cortelazzo S, D'Amico G, Biondi A, Rambaldi A, Biagi E. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. 2014;20:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 70. | Sánchez-Guijo F, Caballero-Velázquez T, López-Villar O, Redondo A, Parody R, Martínez C, Olavarría E, Andreu E, Prósper F, Díez-Campelo M, Regidor C, Villaron E, López-Corral L, Caballero D, Cañizo MC, Pérez-Simon JA. Sequential third-party mesenchymal stromal cell therapy for refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 71. | Te Boome LC, Mansilla C, van der Wagen LE, Lindemans CA, Petersen EJ, Spierings E, Thus KA, Westinga K, Plantinga M, Bierings M, Broers AE, Cuijpers ML, van Imhoff GW, Janssen JJ, Huisman C, Zeerleder S, Huls G, Boelens JJ, Wulffraat NM, Slaper-Cortenbach IC, Kuball J. Biomarker profiling of steroid-resistant acute GVHD in patients after infusion of mesenchymal stromal cells. Leukemia. 2015;29:1839-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | von Dalowski F, Kramer M, Wermke M, Wehner R, Röllig C, Alakel N, Stölzel F, Parmentier S, Sockel K, Krech M, Schmitz M, Platzbecker U, Schetelig J, Bornhäuser M, von Bonin M. Mesenchymal Stromal Cells for Treatment of Acute Steroid-Refractory Graft Versus Host Disease: Clinical Responses and Long-Term Outcome. Stem Cells. 2016;34:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 73. | Muroi K, Miyamura K, Okada M, Yamashita T, Murata M, Ishikawa T, Uike N, Hidaka M, Kobayashi R, Imamura M, Tanaka J, Ohashi K, Taniguchi S, Ikeda T, Eto T, Mori M, Yamaoka M, Ozawa K. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol. 2016;103:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 74. | Erbey F, Atay D, Akcay A, Ovali E, Ozturk G. Mesenchymal Stem Cell Treatment for Steroid Refractory Graft-versus-Host Disease in Children: A Pilot and First Study from Turkey. Stem Cells Int. 2016;2016:1641402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Kuçi Z, Bönig H, Kreyenberg H, Bunos M, Jauch A, Janssen JW, Škifić M, Michel K, Eising B, Lucchini G, Bakhtiar S, Greil J, Lang P, Basu O, von Luettichau I, Schulz A, Sykora KW, Jarisch A, Soerensen J, Salzmann-Manrique E, Seifried E, Klingebiel T, Bader P, Kuçi S. Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: a multicenter survey. Haematologica. 2016;101:985-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, Su Y, Yang T, Li H, Liu Y, Zhang C, Gao L, Zhu L, Wen Q, Wang P, Chen X, Zhong J, Zhang X. Phase II Multicenter, Randomized, Double-Blind Controlled Study of Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cells in the Prophylaxis of Chronic Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation. J Clin Oncol. 2016;34:2843-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 77. | Salmenniemi U, Itälä-Remes M, Nystedt J, Putkonen M, Niittyvuopio R, Vettenranta K, Korhonen M. Good responses but high TRM in adult patients after MSC therapy for GvHD. Bone Marrow Transplant. 2017;52:606-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Cetin M, Akyol G, Gonen ZB, Keklik M, Zararsiz G, Unal A, Tiren-Verbeet NL, Kaynar L. Additional infusions of mesenchymal stem cells improve response rate in multidrug-resistant GvHD patients. Bone Marrow Transplant. 2017;52:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Dotoli GM, De Santis GC, Orellana MD, de Lima Prata K, Caruso SR, Fernandes TR, Rensi Colturato VA, Kondo AT, Hamerschlak N, Simões BP, Covas DT. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 80. | Fernández-Maqueda C, Gonzalo-Daganzo R, Regidor C, Martín-Donaire T, Sánchez R, Bueno JL, Bautista G, De Liglesia A, Gutiérrez Y, García-Berciano M, Forés R, Royuela A, Fernández MN, Duarte RF, Cabrera-Marín JR. Mesenchymal stromal cells for steroid-refractory acute GvHD. Bone Marrow Transplant. 2017;52:1577-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Bader P, Kuçi Z, Bakhtiar S, Basu O, Bug G, Dennis M, Greil J, Barta A, Kállay KM, Lang P, Lucchini G, Pol R, Schulz A, Sykora KW, von Luettichau I, Herter-Sprie G, Uddin MA, Jenkin P, Alsultan A, Buechner J, Stein J, Kelemen A, Jarisch A, Soerensen J, Salzmann-Manrique E, Hutter M, Schäfer R, Seifried E, Klingebiel T, Bonig H, Kuçi S. Effective treatment of steroid and therapy-refractory acute graft-versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM). Bone Marrow Transplant. 2018;53:852-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 82. | Stoma I, Karpov I, Krivenko S, Iskrov I, Milanovich N, Koritko A, Uss A. Mesenchymal stem cells transplantation in hematological patients with acute graft-versus-host disease: characteristics and risk factors for infectious complications. Ann Hematol. 2018;97:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Ringden O, Baygan A, Remberger M, Gustafsson B, Winiarski J, Khoein B, Moll G, Klingspor L, Westgren M, Sadeghi B. Placenta-Derived Decidua Stromal Cells for Treatment of Severe Acute Graft-Versus-Host Disease. Stem Cells Transl Med. 2018;7:325-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 84. | Bozkurt C, Karaöz E, Adaklı Aksoy B, Aydoğdu S, Fışgın T. The Use of Allogeneic Mesenchymal Stem Cells in Childhood Steroid-Resistant Acute Graft-Versus-Host Disease: A Retrospective Study of a Single-Center Experience. Turk J Haematol. 2019;36:186-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, Waller EK, Burke E, Skerrett D, Shpall E, Martin PJ. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020;26:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 86. | Zoehler B, Fracaro L, Senegaglia AC, Bicalho MDG. Infusion of Mesenchymal Stem Cells to Treat Graft Versus Host Disease: the Role of HLA-G and the Impact of its Polymorphisms. Stem Cell Rev Rep. 2020;16:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Cominal JG, da Costa Cacemiro M, Pinto-Simões B, Kolb HJ, Malmegrim KCR, de Castro FA. Emerging Role of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Pathogenesis of Haematological Malignancies. Stem Cells Int. 2019;2019:6854080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Fernández-García M, Yañez RM, Sánchez-Domínguez R, Hernando-Rodriguez M, Peces-Barba M, Herrera G, O'Connor JE, Segovia JC, Bueren JA, Lamana ML. Mesenchymal stromal cells enhance the engraftment of hematopoietic stem cells in an autologous mouse transplantation model. Stem Cell Res Ther. 2015;6:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Lin QM, Zhao S, Zhou LL, Fang XS, Fu Y, Huang ZT. Mesenchymal stem cells transplantation suppresses inflammatory responses in global cerebral ischemia: contribution of TNF-α-induced protein 6. Acta Pharmacol Sin. 2013;34:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Cheung TS, Bertolino GM, Giacomini C, Bornhäuser M, Dazzi F, Galleu A. Mesenchymal Stromal Cells for Graft Versus Host Disease: Mechanism-Based Biomarkers. Front Immunol. 2020;11:1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 91. | Kim HS, Yun JW, Shin TH, Lee SH, Lee BC, Yu KR, Seo Y, Lee S, Kang TW, Choi SW, Seo KW, Kang KS. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-β1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells. 2015;33:1254-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 92. | Harrell CR, Djonov V, Volarevic V. The Cross-Talk between Mesenchymal Stem Cells and Immune Cells in Tissue Repair and Regeneration. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 93. | Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017;35:766-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 94. | Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 747] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 95. | Negi N, Griffin MD. Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells. 2020;38:596-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |