Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.209
Peer-review started: January 2, 2023
First decision: February 7, 2023
Revised: February 25, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: April 26, 2023
Processing time: 114 Days and 0.5 Hours
Cancer stem cells (CSCs) are the main cause of tumor growth, invasion, metastasis and recurrence. Recently, CSCs have been extensively studied to identify CSC-specific surface markers as well as signaling pathways that play key roles in CSCs self-renewal. The involvement of CSCs in the pathogenesis of gastrointestinal (GI) cancers also highlights these cells as a priority target for therapy. The diagnosis, prognosis and treatment of GI cancer have always been a focus of attention. Therefore, the potential application of CSCs in GI cancers is receiving increasing attention. This review summarizes the role of CSCs in GI cancers, focusing on esophageal cancer, gastric cancer, liver cancer, colorectal cancer, and pancreatic cancer. In addition, we propose CSCs as potential targets and therapeutic strategies for the effective treatment of GI cancers, which may provide better guidance for clinical treatment of GI cancers.
Core Tip: This review summarizes the role of cancer stem cells (CSCs) in gastrointestinal (GI) cancers, focusing on esophageal cancer, gastric cancer, liver cancer, colorectal cancer, and pancreatic cancer. In addition, we propose CSCs as potential targets and therapeutic strategies for the effective treatment of GI cancers, which may provide better guidance for clinical treatment of GI cancers.
- Citation: Xuan SH, Hua ML, Xiang Z, He XL, Huang L, Jiang C, Dong P, Wu J. Roles of cancer stem cells in gastrointestinal cancers. World J Stem Cells 2023; 15(4): 209-220
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/209.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.209
Cancer stem cells (CSCs) are small subgroups of undifferentiated cancer cells. CSCs possess an infinite self-renewal capability and a set of unique surface biomarkers[1]. Gastrointestinal (GI) cancer is the most common major malignancy, and includes esophageal cancer (EC), gastric cancer (GC), liver cancer (LC), colorectal cancer (CRC), pancreatic cancer and other related diseases[2]. The incidence of GI cancers is high. Recently, a growing number of studies have been conducted on the important role of CSCs in GI cancers. Research shows that CSCs are mainly involved in the growth, initiation, maintenance, survival, metastasis and recurrence of GI cancer. There are certain limitations in the methods of treatment for currently accepted GI cancers, often leading to treatment failure. This is due to CSCs resistance to chemotherapy and radiation therapy. In current treatment, CSCs cannot be erased, causing metastasis and recurrence of the tumor. Recently, studies have been conducted to clarify the signaling pathway which plays a critical role in the specific surface markers of CSCs and the self-renewal of CSCs. These cell surface markers as well as signaling pathways are potential targets for the treatment of GI cancer that provide the environment necessary for tumor growth[3-5]. Thus, certain therapies for CSCs will potentially help to eliminate the tumor.
This review aims to summarize the mechanism and treatment of CSCs in GI cancer, and to propose a potential target and therapeutic strategy for the treatment of GI cancer.
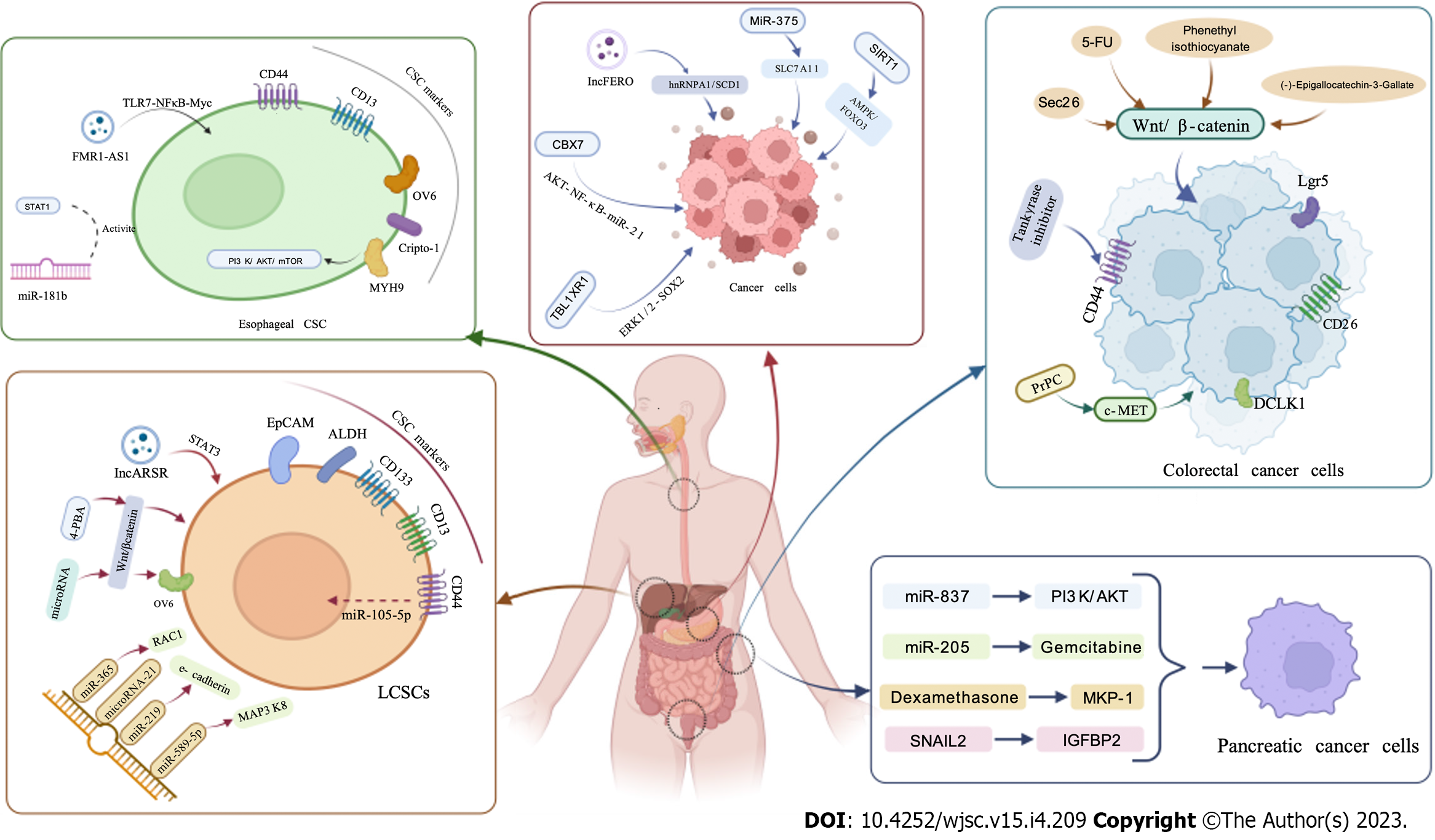
EC, a common tumor of the digestive tract, causes a majority of cancer deaths[6]. EC includes esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). EAC may be associated with obesity and ESCC may be associated with drinking and narcotics. Approximately 300000 people die of EC worldwide every year. The incidence and mortality rates of EC vary from country to country[7]. Recently, the relationship between CSCs and the occurrence and development of EC has attracted more and more attention. Chen et al[8] discovered that esophageal CSCs activate matrix metalloproteinase 9 in EC cells and promotes cancer metastasis by the expression of placental growth factor. Moreover, CSCs expressing placental growth factor can promote[9] or suppress tumor angiogenesis by stimulating vascular endothelial growth factor[10]. Wang et al[11] found that by acting on ATG7-dependent β-catenin, OV6 CSCs can stably promote the progression of ESCC. In addition, studies have shown that exosomal O-GlcNAc transferase derived from esophageal CSCs can promote the suppression of cancer immunity by increasing programmed death-1 (PD-1) in CD8 T cells[12].
CSCs play a critical role in the treatment of EC. CSCs markers could help to identify the functions of CSCs in EC and can be used as targets for the treatment of EC. It was found that CD44, a CSC surface marker, can isolate and detect ESCC[13]. In addition, Lu et al[14] found CD133 and CXCR4 markers on the surface of ESCC, and the high expression of CD133-CXCR4 may become a marker for forecasting poor prognosis in patients with ESCC. Liu et al[15] found that Cripto-1 is a functional marker of CSC-like cells (CSLCs) and can predict the prognosis of patients with ESCC. In addition, studies have shown that MYH9 is a novel esophageal CSC marker and prognostic indicator, which promotes tumorigenesis through the PI3K/AKT/mTOR axis[16]. Studies have demonstrated that esophageal CSLCs can resist ferroptosis induced cell death through the active HSP27-GPX4 pathway. Hence, targeting HSP27 or GPX4 blockade is a promising therapeutic strategy to eradicate CSCs in ESCC[17]. Li et al[18] found that exosomal FMR1-AS1 can promote CSLCs homeostasis through TLR7-NFκB-Myc signaling in female EC. Song et al[19] analyzed clinical specimens and found that BCL-2 inhibitor AT101 could overcome drug resistance by targeting the CSC pathway and had good antitumor activity. In addition, Zarei et al[20] found that salinomycin could destabilize the low-pathway sensor TAZ in CSLCS and reduce the viability of esophageal CSLCs. Studies have shown that TRPV2 can maintain the growth of CSCs, and its specific inhibitor tranilast may be used as a targeted therapeutic agent for ESCC[21]. STAT3 and miR-181b activate each other via the CYLD pathway, thereby regulating the proliferation of esophageal CSLCs[22]. Metformin can inhibit EC cell growth and sensitize EC cells to the cytotoxic effects of 5-FU by targeting components of CSCs and mTOR[23].
CSCs play an important role in the development, progress and future treatment of EC, but the associated research is inadequate, and the underlying mechanisms have not been fully explored. Therefore, more studies are needed to determine the mechanism and support the corresponding conclusions.
GC is a malignant tumor derived from gastric mucosal epithelium. Most GCs are early adenocarcinomas with no apparent symptoms and are easily overlooked[24]. GC is the main cause of global cancer deaths. The treatment of GC involves surgery in combination with chemotherapy and radiotherapy, but the prognosis of terminal GC is still poor[25]. Over the past decade there is increasing evidence to show that CSCs have a significant role in GC development.
The concept that CSCs cause GC and may lead to invasion, metastasis, and treatment resistance has profound implications for anticancer therapy. Takaishi et al[26] implanted gastric CSCs (GCSCs) in the skin of immunodeficient mice, and a few weeks later observed that these GCSCs showed strong tumorigenic ability. Yang screened out GCSCs with serum-free medium, and found that these GCSCs had high tumorigenesis ability in nude mice, and high expression of GCSCs surface markers OCT4 and SOX2[27]. E-cadherin is a vital adhesion molecule, and its expression is closely related to the degree of cell adhesion[28]. Studies have found that the expression of E-cadherin in GCSCs is down-regulated, resulting in a decrease in the adhesion of tumor cells; thus, GCSCs are highly aggressive and easily metastasize to local lymph nodes or distant metastasis[29]. CSC Lgr5+ can promote the growth and proliferation potential of GC[30]. In addition, CSCs may maintain their viability through autophagy[31].
Identifying and targeting CSCs play a vital role in the treatment of GC. Chemotoxicity-induced exosomal lncFERO can regulate ferroptosis and stemness in GCSCs. Therefore, chemotherapy targeting the lncFERO/hnRNPA1/SCD1 axis could be an implicit strategy for CSCs-based GC therapy[32]. In addition, microRNA (miR)-375 can trigger ferroptosis by targeting SLC7A11, thereby attenuating the stemness of GCSCs[33]. CBX7 was found to positively regulate the stem-like characteristics of GC cells by inhibiting p16 and activating the AKT-NF-κB-miR-21 pathway[34]. Sezer et al[35] found that lymphatic metastasis-associated TBL1XR1 promotes the metastasis of gastric CSLCs by sensitizing ERK1/2-SOX2 signaling. Huang et al[36] found that SIRT1 inhibits both chemoresistance and cancer stemness in GC by launching a positive feedback loop of AMPK/FOXO3. The m6A methyltransferase METTL3 was found to promote oxaliplatin resistance in CD133+ GCSCs by improving PARP1 mRNA stability to increase base excision repair pathway activity[37]. Celastrus orbiculatus ethyl acetate extract can prevent GC growth by limiting the stemness of GCSCs by changing PDCD4 and EIF3H expression[38]. Methionine has been found to inhibit autophagy in GCSCs by promoting RAB37 methylation and phosphorylation. Therefore, supplementation with methionine γ-lyase can induce autophagy in GCSCs and inhibit tumor growth[36].
The role of CSCs in growth, metastasis, treatment and prognosis of GC is increasingly important. In the past few years, an increasing number of studies have been conducted on the mechanism of action of CSCs against GC and to offer promising targets and therapeutic tactics for GC treatment. Many treatments targeting CSCs have been developed, but there are limits to CSCs targeting therapy. It affects normal stem cells and causes tissue renewal problems. For example, Lgr5 is a marker associated with common stem cells in gastric tissue. mTORC1 can maintain self-renewal of the Lgr5 population, preventing cell differentiation and causing gastric tumorigenesis. However, the use of mTORC1 inhibitors may cause gastric glandular atrophy due to tissue malfunction, limiting its therapeutic use[39]. Therefore, more efforts are needed to treat GC based on CSCs.
LC is the third most common cause of cancer associated deaths worldwide. This is due to its high recurrence rate, which after normal treatment, can reach 70%. Hepatocellular carcinoma (HCC) is the main pathology and causes approximately 80% of LC cases[40]. Liver CSCs (LCSCs) are now known to be responsible for HCC growth, metastasis and recurrence, in addition to failure of chemotherapy and radiation therapy[41]. These findings indicate that LC therapy kills most of the tumor cells, but cannot eliminate LCSCs and treatment can eventually fail as LCSCs survive and generate new tumors. Therefore, CSCs theory has provided new findings in the diagnosis, treatment and prevention of LC (Table 1).
| Ref. | Genes/transcription factors/protein | Inducing way | Role |
| Park et al[43] | EpCAM-high HCC stem cells | Upregulating CEACAM1 | Promoting the growth of LC |
| Deng et al[44] | Histone demethylase JMJD2D | Enhancing Sox9 expression | Promoting the growth of LC |
| Yang et al[45] | lncARSR | Targeting STAT3 signaling | Promoting LCSCs expansion |
| Chen et al[47] | 4-PBA | Activating β-catenin signaling pathway | Promoting the growth of LC |
| Galizia et al[49] | CD133 | - | Indicator of tumor recurrence |
| Haraguchi et al[51] | CD13 | - | Causing drug resistance of LCSCs |
| Yang et al[52] | OV6 | - | Causing drug resistance of LCSCs |
| Wei et al[53] | CD44 | Regulating PES1 | Promoting the growth of LC |
| Chen et al[55] | FOXM1 | Reducing the expression of ALDH2 | Inducing the apoptosis of LCSCs |
| Dou et al[56] | BC-02 | Inhibiting CD13 | Eradicate LCSCs |
| Zhou et al[57] | CD133-apt-Dox | Targeting CD133-expressing cells | Inhibiting the growth of LC |
| Feng et al[58] | MiR-124 | - | Inhibiting LCSCs self-renewal |
| Jiang et al[59] | MiR-365 | Regulating RAC1 pathway | Inhibiting the proliferation and invasion of HCC cells |
| Li et al[60] | MicroRNA-21 | - | Inhibiting highly invade LCSCs |
| Si et al[61] | MiR-219 | E-cadherin pathway | regulates the expansion of LCSCs |
| Dou et al[62] | MicroRNA-6838-5p | Down-regulating CBX4 expression and Inactivating ERK signaling | Inhibiting self-renewal and metastasis of Human LCSCs |
| Zhang et al[63] | MiR-589-5p | Inhibiting MAP3K8 | Inhibiting the growth of LC |
| Wang et al[64] | HAND2-AS1 | - | Promoting the growth of LC |
| Li et al[66] | Neuropilin1 | The loss of neuropilin1 | Inhibiting LCSCs |
| Wang et al[67] | ZBP-89 | Inhibiting Notch1 signaling pathway | Regulating self-renewal of LCSCs |
LCSCs play a vital role in the development, progression, recurrence and drug tolerance of HCC. Studies have shown that LCSCs can accelerate tumor growth in primary cancer cells and metastasis of secondary tumors, causing the recurrence of HCC[42]. Studies have found that EpCAM-high HCC stem cells can promote tumor growth by upregulating CEACAM1 to weaken the capacity of natural killer cells to recognize and kill cancer cells[43]. Histone demethylase JMJD2D can enhance EpCAM and Sox9 expression to promote the self-renewal of liver CSLCs, promoting tumor growth[44]. Yang et al[45] found that lncARSR promoted HCC cell dedifferentiation and LCSCs expansion by targeting STAT3 signaling. Moreover, LCSCs release exosomes in a RAB27A-dependent manner which can lead to resistance to regorafenib in LC cells[46]. Chen et al[47] found that activating the β-catenin signaling pathway leads to upregulation of PPAR-α by 4-PBA, which in turn initiates LCSCs and promotes early HCC. Cao et al[48] found that RACK1 advances self-renewal and chemoresistance of CSCs in HCC by stabilizing nanoparticles.
The biomarkers of LCSCs are important targets in the treatment of LC. CD133 is one of the common surface markers of LCSCs, and studies have found that CD133 isolated from HCC cell lines has high proliferative and tumorigenic potential; thus, enhanced CD133 expression can also serve as a prognostic indicator for survival and tumor recurrence in patients with LC[49]. Yin et al[50] found that aldehyde dehydrogenase was expressed in LCSCs and was positively correlated with CD133 expression. CD13 is a marker of LCSCs, which has the function of dormancy and slow growth, and is the main reason for drug resistance of LCSCs[51]. The LCSCs marker OV6 was found to be chemoresistant, but this was reversed when lentivirus-delivered miRNAs targeting β-catenin were stably expressed. Therefore, targeting Wnt/β-catenin signaling could be a potential strategy to reverse the drug resistance properties of OV6 LCSCs[52]. Wei et al[53] found that CD44 regulated PES1 in LCSCs through miR-105-5p to promote tumor growth. Twist2 advances self-renewal of liver CSLCs by changing CD24[54].
LCSCs play a vital role in the treatment of LC. It was found that the transcription factor FOXM1 inhibits the dryness of LCSCs by reducing the expression of ALDH2, and inhibits proliferation, migration, invasion and tumorigenesis, while inducing apoptosis of LCSCs[55]. The CD13 inhibitor BC-02 can target CD13 and upregulate intracellular reactive oxygen species (ROS) and ROS-induced DNA damage to damage LCSCs. Therefore, BC-02 may be a potential therapeutic strategy to eradicate LCSCs and overcome chemoresistance in LC[56]. Studies have found that aptamer-based drug delivery agents (CD133-apt-Dox) targeting CD133-expressing cells can impair the self-renewal ability of liver CSLCs and inhibit the growth of LC[57]. MiRNAs are important regulators of CSCs therapy in LC. Feng et al[58] found that forced expression of miR-124 can inhibit LCSCs self-renewal and tumorigenesis. It was shown that miR-365 regulated LCSCs through the RAC1 pathway and prevented the proliferation and invasion of HCC cells[59]. In addition, miR-21 downregulation can inhibit cell proliferation and highly invade LCSCs[60]. Si et al[61] found that miR-219 regulates the expansion of LCSCs through the E-cadherin pathway. Dou et al[62] showed that miR-6838-5p inhibited self-renewal and metastasis of LCSCs by down-regulating CBX4 expression and inhibiting ERK signaling. In addition, miR-589-5p inhibited MAP3K8 in HCC and inhibited CD90 CSCs[63]. It was shown that HAND2-AS1 can promote self-renewal of LCSCs and drive liver tumorigenesis, providing a potential new target for HCC treatment[64]. In addition, tumor-associated macrophages produce interleukin-6 and signal through STAT3 to improve the expansion of LCSCs[65]. Li et al[66] found that the loss of neuropilin 1 inhibits the LCSC population and blocks metastasis in HCC through epithelial-mesenchymal transition. Wang et al[67] found that ZBP-89 negatively regulated self-renewal of LCSCs by inhibiting the Notch1 signaling pathway.
Traditional HCC treatment mainly targets fast growing and differentiated HCC cells. However, a part of the emerging CSCs concept explains the failure of these therapies. The research progress of LCSCs has provided a new viewpoint on the possible adhibition in the clinical treatment of LCSCs. Detection of LCSCs is useful for predicting postoperative survival of patients. The development of treatment strategies for LCSCs may greatly improve the treatment of LC[68].
CRC is one of the most common cancers and the fourth most frequent cause of cancer death worldwide[69]. Despite the great progress in surgery and chemotherapy over the past decade, the five year survival rate of CRC patients is 50%-65%[70]. In the past few years, an increasing number of studies have shown that CSCs are closely associated with the occurrence and development of CRC, which provides promising directions for the diagnosis and treatment of CRC (Table 2).
| Ref. | Genes/transcription factors/protein | Inducing way | Role |
| Fumagalli et al[72] | Lgr5 | - | Promoting the growth of CRC |
| Cheung et al[74] | CD16 | - | Promoting the growth of CRC |
| Razi et al[75] | DCLK1 | MiR-137 and Mir-15a-dependent manner | Promoting the growth of CRC |
| Park et al[76] | JAK2/STAT3/CCND2 axis | - | Causing drug resistance of CCSCs |
| Liu et al[77] | Sec62 | Activating the Wnt/β-catenin pathway | Causing drug resistance of CCSCs |
| Izumi et al[78] | F-Box/WD repeat-containing protein 7 | - | Causing drug resistance of CCSCs |
| Wei et al[79] | PD-L1 | Activating HMGA1-dependent Signaling pathway | Maintaining CSCs self-renewal |
| Cho et al[80] | 5-FU | Activing p53-mediated WNT/β-catenin pathway | Promoting the stemness of CRC |
| Chen et al[81] | Phenethyl isothiocyanate | Suppressing Wnt/β-catenin pathway | Inhibiting CCSCs |
| Chen et al[82] | (-)-Epigallocatechin-3-Gallate | Suppressing Wnt/β-catenin pathway | Inhibiting CCSCs |
| Dahal et al[83] | AGR2 | Regulating Wnt/β-catenin pathway | Regulating the stemness maintenance of CCSCs |
| Jang et al[84] | Tankyrase inhibitors | Downregulating c-KIT tyrosine kinase | Inhibiting the growth of CD44-positive CCSCs |
| Liu et al[85] | PTK6 interacts with JAK2 | Activating JAK2/STAT3 signaling | Reversing chemoresistance in CRC |
| Quarni et al[86] | Mitramycin A | - | Inhibiting CCSCs |
| Lim et al[88] | PrPC | Interacting with c-MET | Inhibiting CCSCs |
CSCs are the major cause of drug resistance and disease recurrence in CRC. Studies have found that Lgr5 CSCs have a vital role in primary and metastatic colon cancer, and can promote tumor growth and metastasis[71]. In addition, most colorectal CSCs (CCSCs) express Lgr5 and form distant metastasis, which is a major factor driving CRC metastasis[72]. Recent studies have found that human CCSCs can give rise to vascular endothelial cells and constitute the vasculature in cancer tissue, which provides a new mechanism for tumor angiogenesis in cancer[73]. CD26 CCSCs can lead to CRC metastasis[74]. Razi et al[75] found that DCLK1 is a promising CCSC marker that changes tumor progression and invasion in a miR-137- and miR-15a-dependent manner. The JAK2/STAT3/CCND2 axis promotes the persistence and radioresistance of CRCs, which is the drug resistance mechanism for the continuous growth of CSCs after radiotherapy[76]. Liu et al[77] found that Sec62 promotes stemness and chemoresistance in human CRC by sensitizing the Wnt/β-catenin pathway. Studies have shown that CCSCs acquire chemoresistance by the upregulation of F-Box/WD repeat-containing protein 7 and the consequent degradation of c-Myc[78]. In addition, PD-L1 can maintain CSCs self-renewal by activating the HMGA1-dependent signaling pathway[79]. 5-FU improves the stemness of CRC through p53-mediated WNT/β-catenin pathway activation[80].
Whereas conventional therapies target proliferating and mature cancer cells, CSCs are mostly quiescent and poorly differentiated, so they could easily survive the chemotherapy attack. Therefore, novel therapies targeting CSCs are necessary. Chen et al[81] found that phenethyl isothiocyanate inhibits CCSCs by suppressing the Wnt/β-catenin pathway. In addition, (-)-Epigallocatechin-3-Gallate can also inhibit CCSCs by suppressing the Wnt/β-catenin pathway[82]. Studies have shown that AGR2 is a new stem cell marker that is changed by the canonical Wnt/β-catenin pathway in CCSCs and is important for stemness maintenance of CCSCs[83]. Jang et al[84] reported that tankyrase inhibitors downregulated c-KIT tyrosine kinase and prevented the growth of CD44-positive CCSCs. Liu et al[85] found that PTK6 interacts with JAK2 and phosphorylates to activate JAK2/STAT3 signaling, which can improve stemness and chemoresistance of CRC cells and reverse chemoresistance in CRC. Studies have shown that mithramycin A inhibits CRC growth by targeting CSCs[86]. Studies have also found that disruption of endolysosome RAB5/7 effectively eliminates CCSCs[87]. PrPC inhibits CSCs properties by interacting with c-MET in CRC cells[88].
According to the data accumulated during cancer research, CSCs have become a fundamental cause of cancer progression and resistance to treatment. Therefore, it is important to understand the biological, functional and clinical significance of CSCs in CRC tolerance in order to develop an effective treatment model for CRC patients. However, the practical clinical application of CSCs in CRC is still limited, and further studies and efforts are needed for clinical applications.
Pancreatic cancer is one of the most deadly human malignancies, the survival rate is 8% and the prognosis is the worst of all GI tumors[89-91]. It is now the fourth most common cause of cancer associated deaths worldwide. The tumorigenesis capacity of pancreatic cancer cells is different, and the proliferation and growth of pancreatic cancer are highly dependent on the presence of a limited subgroup of pancreatic cancer cells, called pancreatic CSCs (PCSCs)[92]. The concept of CSCs is recognized and some of the identified molecules and signaling pathways are associated with cancer diagnosis and treatment.
PCSCs contribute to the development and invasion of pancreatic cancer. It has been found that PCSC CD9 can promote the plasma membrane localization of glutamine transporter ASCT2, improving glutamine uptake in pancreatic cancer cells and promoting tumor growth[93]. Leng et al[94] found that SIRT1 coordinating with CRL4B can regulate PCSCs to promote tumorigenesis. In PCSCs, PAF1 interacts with DDX3 and PHF5A to regulate the expression of NANOG and other genes that regulate stemness. Therefore, knockdown of PAF1 reduced the development and progression ability of in situ pancreatic cancer in mice and its CSCs[95]. Bao et al[96] found that pancreatic CSLCs can promote tumor formation and rapid tumor growth by activating FoxQ1. Masuo et al[97] demonstrated that SNAIL2 can promote the tumorigenicity and chemotherapy resistance of PCSCs by regulating IGFBP2.
PCSCs have an important role in pancreatic cancer therapy. Studies have shown that PCSCs have longer telomeres and higher telomerase activity than tumor cells, which is associated with the expression of pluripotent genes (Nanog, Sox2, Oct3/4). Therefore, telomerase inhibition can lead to apoptosis of PCSCs, which is a suitable therapeutic approach against CSCs, especially in pancreatic cancer[98]. Yang et al[99] found that miR-873 could inhibit self-renewal and proliferation of PCSCs by blocking the PLEK2-dependent PI3K/AKT pathway. In addition, miR-205 can resensitize gemcitabine-resistant pancreatic cancer cells to gemcitabine and act as a tumor suppressor miRNA[100]. JNK is required for PCSCs self-renewal and tumor initiation, as well as its survivin expression. Dexamethasone was found to induce the expression of MKP-1 through glucocorticoid receptor activation, thereby inactivating JNK and inhibiting tumor growth[101]. Urtasun et al[102] showed that simultaneous blockade of IGF-IR and EGFR/Her-2 using NVP-AEW541 and lapatinib could inhibit drug resistance in pancreatic cancer.
The role of CSCs in pancreatic cancer metastasis, recurrence and treatment has become very important. A large number of studies have allowed tumor stem cell markers to achieve metastasis, progression and resistance of pancreatic cancer cells, which provide potential targets and therapeutic directions for the treatment of pancreatic cancer. Nevertheless, there are still few practical clinical applications of PCSCs, and further studies are needed for clinical applications.
Recently, many studies have shown that CSCs are closely related to tumor recurrence, metastasis and drug resistance, especially in EC, GC, LC, CRC and pancreatic cancer (Figure 1). CSCs can be identified by a series of surface markers, including OV6, EpCAM, CD13, CD133 and CD44. Surface markers of CSCs are useful for cancer diagnosis and prognosis prediction. In addition, CSCs regulate tumor progression and therapeutic resistance through multiple mechanisms, including Notch, Wnt/β-catenin and other signaling pathways. There are many other elements that make CSC impressions, such as the tumor microenvironment and non-coding RNAs, including miRNAs and long non-coding RNAs.
Currently, we have developed a combination of chemotherapeutic agents and small molecule inhibitors to reduce CSCs and effectively treat GI cancer. As CSCs have many similar features to stem cells, the molecular signal pathway or mechanism that distinguishes the two cell subgroups is still unknown. Therefore, stem cell therapy is limited, and it is necessary to further study the biological difference between normal stem cells and LCSCs. Research on the therapy of GI cancer with regard to CSCs is still at the in vitro and animal experimental stage, and the precise molecular mechanism of CSCs in GI cancer requires further study. Therefore, more research is needed to promote the application of CSCs in clinical practice.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ikram D, Indonesia; Tang ZP, China S-Editor: Yan JP L-Editor: A P-Editor: Zhang XD
| 1. | Puglisi MA, Tesori V, Lattanzi W, Gasbarrini GB, Gasbarrini A. Colon cancer stem cells: controversies and perspectives. World J Gastroenterol. 2013;19:2997-3006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Wolfe W, Xiang Z, Yu X, Li P, Chen H, Yao M, Fei Y, Huang Y, Yin Y, Xiao H. The Challenge of Applications of Probiotics in Gastrointestinal Diseases. Adv Gut Microbiome Res. 2023;2023. [DOI] [Full Text] |
| 3. | Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, Clarke MF, Hoey T, Lewicki J, Gurney AL. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 440] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 4. | Wu J, Zhang JR, Qin J. Clinical significance of methylation of E-cadherin and p14ARF gene promoters in skin squamous cell carcinoma tissues. Int J Clin Exp Med. 2014;7:1808-1812. [PubMed] |
| 5. | Wu J, Cui LL, Yuan J, Wang Y, Song S. Clinical significance of the phosphorylation of MAPK and protein expression of cyclin D1 in human osteosarcoma tissues. Mol Med Rep. 2017;15:2303-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 499] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (4)] |
| 7. | Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg. 2017;6:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Chen Y, Jiang T, Mao A, Xu J. Esophageal cancer stem cells express PLGF to increase cancer invasion through MMP9 activation. Tumour Biol. 2014;35:12749-12755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1198] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 10. | Eriksson A, Cao R, Pawliuk R, Berg SM, Tsang M, Zhou D, Fleet C, Tritsaris K, Dissing S, Leboulch P, Cao Y. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Wang C, Yan FH, Zhang JJ, Huang H, Cui QS, Dong W, Zhang WW, Zhao Y, Chen HZ, Zhao TJ. OV6(+) cancer stem cells drive esophageal squamous cell carcinoma progression through ATG7-dependent β-catenin stabilization. Cancer Lett. 2017;391:100-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Yuan Y, Wang L, Ge D, Tan L, Cao B, Fan H, Xue L. Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8(+) T cells. Cancer Lett. 2021;500:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Zhao JS, Li WJ, Ge D, Zhang PJ, Li JJ, Lu CL, Ji XD, Guan DX, Gao H, Xu LY, Li EM, Soukiasian H, Koeffler HP, Wang XF, Xie D. Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44. PLoS One. 2011;6:e21419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Lu C, Xu F, Gu J, Yuan Y, Zhao G, Yu X, Ge D. Clinical and biological significance of stem-like CD133(+)CXCR4(+) cells in esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2015;150:386-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Liu Q, Cui X, Yu X, Bian BS, Qian F, Hu XG, Ji CD, Yang L, Ren Y, Cui W, Zhang X, Zhang P, Wang JM, Cui YH, Bian XW. Cripto-1 acts as a functional marker of cancer stem-like cells and predicts prognosis of the patients in esophageal squamous cell carcinoma. Mol Cancer. 2017;16:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Kai JD, Cheng LH, Li BF, Kang K, Xiong F, Fu JC, Wang S. MYH9 is a novel cancer stem cell marker and prognostic indicator in esophageal cancer that promotes oncogenesis through the PI3K/AKT/mTOR axis. Cell Biol Int. 2022;46:2085-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 17. | Liu CC, Li HH, Lin JH, Chiang MC, Hsu TW, Li AF, Yen DH, Hsu HS, Hung SC. Esophageal Cancer Stem-like Cells Resist Ferroptosis-Induced Cell Death by Active Hsp27-GPX4 Pathway. Biomolecules. 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 18. | Li W, Zhang L, Guo B, Deng J, Wu S, Li F, Wang Y, Lu J, Zhou Y. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc signaling in female esophageal carcinoma. Mol Cancer. 2019;18:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Song S, Chen Q, Li Y, Lei G, Scott A, Huo L, Li CY, Estrella JS, Correa A, Pizzi MP, Ma L, Jin J, Liu B, Wang Y, Xiao L, Hofstetter WL, Lee JH, Weston B, Bhutani M, Shanbhag N, Johnson RL, Gan B, Wei S, Ajani JA. Targeting cancer stem cells with a pan-BCL-2 inhibitor in preclinical and clinical settings in patients with gastroesophageal carcinoma. Gut. 2021;70:2238-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Zarei M, Jazi MS, Tajaldini M, Khosravi A, Asadi J. Selective Inhibition of Esophageal Cancer Stem-like Cells with Salinomycin. Anticancer Agents Med Chem. 2020;20:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Shiozaki A, Kudou M, Ichikawa D, Fujiwara H, Shimizu H, Ishimoto T, Arita T, Kosuga T, Konishi H, Komatsu S, Okamoto K, Marunaka Y, Otsuji E. Esophageal cancer stem cells are suppressed by tranilast, a TRPV2 channel inhibitor. J Gastroenterol. 2018;53:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Xu DD, Zhou PJ, Wang Y, Zhang L, Fu WY, Ruan BB, Xu HP, Hu CZ, Tian L, Qin JH, Wang S, Wang X, Li YC, Liu QY, Ren Z, Zhang R, Wang YF. Reciprocal activation between STAT3 and miR-181b regulates the proliferation of esophageal cancer stem-like cells via the CYLD pathway. Mol Cancer. 2016;15:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, Johnson RL, Song S. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int J Oncol. 2014;45:567-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 256] [Reference Citation Analysis (0)] |
| 25. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 727] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 26. | Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 817] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 27. | Yang L, Ping YF, Yu X, Qian F, Guo ZJ, Qian C, Cui YH, Bian XW. Gastric cancer stem-like cells possess higher capability of invasion and metastasis in association with a mesenchymal transition phenotype. Cancer Lett. 2011;310:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Delektorskaia VV, Perevoshchikov AG, Golovkov DA, Kushlinskiĭ NE. [Immunohistochemical study of E-cadherin, beta-catenin and CD-44v6 expression in the cells of primary colon cancer and its metastases]. Arkh Patol. 2005;67:34-38. [PubMed] |
| 29. | Zhu Z, Xu J, Li L, Ye W, Xu G, Chen B, Zeng J, Li J, Huang Z. Effect of gastric cancer stem cell on gastric cancer invasion, migration and angiogenesis. Int J Med Sci. 2020;17:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Li XB, Yang G, Zhu L, Tang YL, Zhang C, Ju Z, Yang X, Teng Y. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res. 2016;26:838-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Togano S, Yashiro M, Masuda G, Sugimoto A, Miki Y, Yamamoto Y, Sera T, Kushiyama S, Nishimura S, Kuroda K, Okuno T, Ohira M. Gastric cancer stem cells survive in stress environments via their autophagy system. Sci Rep. 2021;11:20664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Zhang H, Wang M, He Y, Deng T, Liu R, Wang W, Zhu K, Bai M, Ning T, Yang H, Liu Y, Wang J, Ba Y. Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells. Cell Death Dis. 2021;12:1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 33. | Ni H, Qin H, Sun C, Liu Y, Ruan G, Guo Q, Xi T, Xing Y, Zheng L. MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res Ther. 2021;12:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 34. | Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19:1468-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 35. | Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O, Clingan P, Sriuranpong V, Rizvi N, Gao B, Li S, Lee S, McGuire K, Chen CI, Makharadze T, Paydas S, Nechaeva M, Seebach F, Weinreich DM, Yancopoulos GD, Gullo G, Lowy I, Rietschel P. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 513] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 36. | Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, Chen J, Zhang H, Niu Z, Fan Q, Lin L, Gu K, Liu Y, Ba Y, Miao Z, Jiang X, Zeng M, Fu Z, Gan L, Wang J, Zhan X, Liu T, Li Z, Shen L, Shu Y, Zhang T, Yang Q, Zou J; ESCORT Study Group. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 37. | Li H, Wang C, Lan L, Yan L, Li W, Evans I, Ruiz EJ, Su Q, Zhao G, Wu W, Zhang H, Zhou Z, Hu Z, Chen W, Oliveira JM, Behrens A, Reis RL, Zhang C. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability. Cell Mol Life Sci. 2022;79:135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 38. | Zhu YD, Ba H, Chen J, Zhang M, Li P. Celastrus orbiculatus Extract Reduces Stemness of Gastric Cancer Stem Cells by Targeting PDCD4 and EIF3H. Integr Cancer Ther. 2021;20:15347354211058168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Li K, Wu H, Wang A, Charron J, Mishina Y, Habib SL, Liu H, Li B. mTOR signaling regulates gastric epithelial progenitor homeostasis and gastric tumorigenesis via MEK1-ERKs and BMP-Smad1 pathways. Cell Rep. 2021;35:109069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1215] [Article Influence: 202.5] [Reference Citation Analysis (1)] |
| 41. | Zhao Y, Li Y, Sheng J, Wu F, Li K, Huang R, Wang X, Jiao T, Guan X, Lu Y, Chen X, Luo Z, Zhou Y, Hu H, Liu W, Du B, Miao S, Cai J, Wang L, Zhao H, Ying J, Bi X, Song W. P53-R273H mutation enhances colorectal cancer stemness through regulating specific lncRNAs. J Exp Clin Cancer Res. 2019;38:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg. 2011;254:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Park DJ, Sung PS, Kim JH, Lee GW, Jang JW, Jung ES, Bae SH, Choi JY, Yoon SK. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 44. | Deng Y, Li M, Zhuo M, Guo P, Chen Q, Mo P, Li W, Yu C. Histone demethylase JMJD2D promotes the self-renewal of liver cancer stem-like cells by enhancing EpCAM and Sox9 expression. J Biol Chem. 2021;296:100121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Yang C, Cai WC, Dong ZT, Guo JW, Zhao YJ, Sui CJ, Yang JM. lncARSR promotes liver cancer stem cells expansion via STAT3 pathway. Gene. 2019;687:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Huang H, Hou J, Liu K, Liu Q, Shen L, Liu B, Lu Q, Zhang N, Che L, Li J, Jiang S, Wang B, Wen Q, Hu L, Gao J. RAB27A-dependent release of exosomes by liver cancer stem cells induces Nanog expression in their differentiated progenies and confers regorafenib resistance. J Gastroenterol Hepatol. 2021;36:3429-3437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Chen SZ, Ling Y, Yu LX, Song YT, Chen XF, Cao QQ, Yu H, Chen C, Tang JJ, Fan ZC, Miao YS, Dong YP, Tao JY, Monga SPS, Wen W, Wang HY. 4-phenylbutyric acid promotes hepatocellular carcinoma via initiating cancer stem cells through activation of PPAR-α. Clin Transl Med. 2021;11:e379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Cao J, Zhao M, Liu J, Zhang X, Pei Y, Wang J, Yang X, Shen B, Zhang J. RACK1 Promotes Self-Renewal and Chemoresistance of Cancer Stem Cells in Human Hepatocellular Carcinoma through Stabilizing Nanog. Theranostics. 2019;9:811-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 49. | Galizia G, Gemei M, Del Vecchio L, Zamboli A, Di Noto R, Mirabelli P, Salvatore F, Castellano P, Orditura M, De Vita F, Pinto M, Pignatelli C, Lieto E. Combined CD133/CD44 expression as a prognostic indicator of disease-free survival in patients with colorectal cancer. Arch Surg. 2012;147:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 51. | Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard GF, Doki Y, Mori M. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 52. | Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, Kong XN, Chen C, Liu SQ, Wu MC, Wang HY. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 53. | Wei S, Liu K, He Q, Gao Y, Shen L. PES1 is regulated by CD44 in liver cancer stem cells via miR-105-5p. FEBS Lett. 2019;593:1777-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Liu AY, Cai Y, Mao Y, Lin Y, Zheng H, Wu T, Huang Y, Fang X, Lin S, Feng Q, Huang Z, Yang T, Luo Q, Ouyang G. Twist2 promotes self-renewal of liver cancer stem-like cells by regulating CD24. Carcinogenesis. 2014;35:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Chen L, Wu M, Ji C, Yuan M, Liu C, Yin Q. Silencing transcription factor FOXM1 represses proliferation, migration, and invasion while inducing apoptosis of liver cancer stem cells by regulating the expression of ALDH2. IUBMB Life. 2020;72:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Dou C, Fang C, Zhao Y, Fu X, Zhang Y, Zhu D, Wu H, Liu H, Zhang J, Xu W, Liu Z, Wang H, Li D, Wang X. BC-02 eradicates liver cancer stem cells by upregulating the ROS-dependent DNA damage. Int J Oncol. 2017;51:1775-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Zhou G, Da Won Bae S, Nguyen R, Huo X, Han S, Zhang Z, Hebbard L, Duan W, Eslam M, Liddle C, Yuen L, Lam V, Qiao L, George J. An aptamer-based drug delivery agent (CD133-apt-Dox) selectively and effectively kills liver cancer stem-like cells. Cancer Lett. 2021;501:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 58. | Feng Y, Jiang W, Zhao W, Lu Z, Gu Y, Dong Y. miR-124 regulates liver cancer stem cells expansion and sorafenib resistance. Exp Cell Res. 2020;394:112162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Jiang ZB, Ma BQ, Liu SG, Li J, Yang GM, Hou YB, Si RH, Gao P, Yan HT. miR-365 regulates liver cancer stem cells via RAC1 pathway. Mol Carcinog. 2019;58:55-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Li YC, Xu FM, Zhang GQ, Li SB, Wen YY, Zeng F. Down-regulation of microRNA-21 inhibits cell proliferation and invasion of high-invasion liver cancer stem cells. Eur Rev Med Pharmacol Sci. 2018;22:7832-7840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 61. | Si A, Wang L, Miao K, Zhang R, Ji H, Lei Z, Cheng Z, Fang X, Hao B. miR-219 regulates liver cancer stem cell expansion via E-cadherin pathway. Cell Cycle. 2019;18:3550-3561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Dou Z, Lu F, Hu J, Wang H, Li B, Li X. MicroRNA-6838-5p suppresses the self-renewal and metastasis of human liver cancer stem cells through downregulating CBX4 expression and inactivating ERK signaling. Biol Chem. 2023;404:29-39. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Zhang X, Jiang P, Shuai L, Chen K, Li Z, Zhang Y, Jiang Y, Li X. miR-589-5p inhibits MAP3K8 and suppresses CD90(+) cancer stem cells in hepatocellular carcinoma. J Exp Clin Cancer Res. 2016;35:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Wang Y, Zhu P, Luo J, Wang J, Liu Z, Wu W, Du Y, Ye B, Wang D, He L, Ren W, Sun X, Chen R, Tian Y, Fan Z. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. EMBO J. 2019;38:e101110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 65. | Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 531] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 66. | Li X, Zhou Y, Hu J, Bai Z, Meng W, Zhang L, Song X, Wei Y, Yan J. Loss of neuropilin1 inhibits liver cancer stem cells population and blocks metastasis in hepatocellular carcinoma via epithelial-mesenchymal transition. Neoplasma. 2021;68:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Wang N, Li MY, Liu Y, Yu J, Ren J, Zheng Z, Wang S, Yang S, Yang SL, Liu LP, Hu BG, Chong CC, Merchant JL, Lai PB, Chen GG. ZBP-89 negatively regulates self-renewal of liver cancer stem cells via suppression of Notch1 signaling pathway. Cancer Lett. 2020;472:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Chan LH, Luk ST, Ma S. Turning hepatic cancer stem cells inside out--a deeper understanding through multiple perspectives. Mol Cells. 2015;38:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21366] [Article Influence: 2136.6] [Reference Citation Analysis (3)] |
| 70. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2291] [Article Influence: 208.3] [Reference Citation Analysis (1)] |
| 71. | de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, Dijkgraaf GJ, Piskol R, de Sauvage FJ. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature. 2017;543:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 582] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 72. | Fumagalli A, Oost KC, Kester L, Morgner J, Bornes L, Bruens L, Spaargaren L, Azkanaz M, Schelfhorst T, Beerling E, Heinz MC, Postrach D, Seinstra D, Sieuwerts AM, Martens JWM, van der Elst S, van Baalen M, Bhowmick D, Vrisekoop N, Ellenbroek SIJ, Suijkerbuijk SJE, Snippert HJ, van Rheenen J. Plasticity of Lgr5-Negative Cancer Cells Drives Metastasis in Colorectal Cancer. Cell Stem Cell. 2020;26:569-578.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 73. | Shangguan W, Fan C, Chen X, Lu R, Liu Y, Li Y, Shang Y, Yin D, Zhang S, Huang Q, Li X, Meng W, Xu H, Zhou Z, Hu J, Mo X. Endothelium originated from colorectal cancer stem cells constitute cancer blood vessels. Cancer Sci. 2017;108:1357-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Cheung AH, Iyer DN, Lam CS, Ng L, Wong SKM, Lee HS, Wan T, Man J, Chow AKM, Poon RT, Pang R, Law WL. Emergence of CD26+ Cancer Stem Cells with Metastatic Properties in Colorectal Carcinogenesis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Razi S, Sadeghi A, Asadi-Lari Z, Tam KJ, Kalantari E, Madjd Z. DCLK1, a promising colorectal cancer stem cell marker, regulates tumor progression and invasion through miR-137 and miR-15a dependent manner. Clin Exp Med. 2021;21:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Park SY, Lee CJ, Choi JH, Kim JH, Kim JW, Kim JY, Nam JS. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res. 2019;38:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 77. | Liu X, Su K, Sun X, Jiang Y, Wang L, Hu C, Zhang C, Lu M, Du X, Xing B. Sec62 promotes stemness and chemoresistance of human colorectal cancer through activating Wnt/β-catenin pathway. J Exp Clin Cancer Res. 2021;40:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 78. | Izumi D, Ishimoto T, Miyake K, Eto T, Arima K, Kiyozumi Y, Uchihara T, Kurashige J, Iwatsuki M, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Watanabe M, Goel A, Tan P, Baba H. Colorectal Cancer Stem Cells Acquire Chemoresistance Through the Upregulation of F-Box/WD Repeat-Containing Protein 7 and the Consequent Degradation of c-Myc. Stem Cells. 2017;35:2027-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Wei F, Zhang T, Deng SC, Wei JC, Yang P, Wang Q, Chen ZP, Li WL, Chen HC, Hu H, Cao J. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett. 2019;450:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 80. | Cho YH, Ro EJ, Yoon JS, Mizutani T, Kang DW, Park JC, Il Kim T, Clevers H, Choi KY. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat Commun. 2020;11:5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 81. | Chen Y, Li Y, Wang XQ, Meng Y, Zhang Q, Zhu JY, Chen JQ, Cao WS, Xie CF, Li XT, Geng SS, Wu JS, Zhong CY, Han HY. Phenethyl isothiocyanate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Phytother Res. 2018;32:2447-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 82. | Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y, Xie CF, Li XT, Wu JS, Geng SS, Zhong CY, Han HY. (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 83. | Dahal Lamichane B, Jung SY, Yun J, Kang S, Kim DY, Lamichane S, Kim YJ, Park JH, Jang WB, Ji ST, Dehua L, Ha JS, Kim YH, Kwon SM. AGR2 is a target of canonical Wnt/β-catenin signaling and is important for stemness maintenance in colorectal cancer stem cells. Biochem Biophys Res Commun. 2019;515:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 84. | Jang MK, Mashima T, Seimiya H. Tankyrase Inhibitors Target Colorectal Cancer Stem Cells via AXIN-Dependent Downregulation of c-KIT Tyrosine Kinase. Mol Cancer Ther. 2020;19:765-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Liu C, Pan Z, Chen Q, Chen Z, Liu W, Wu L, Jiang M, Lin W, Zhang Y, Zhou R, Zhao L. Pharmacological targeting PTK6 inhibits the JAK2/STAT3 sustained stemness and reverses chemoresistance of colorectal cancer. J Exp Clin Cancer Res. 2021;40:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 86. | Quarni W, Dutta R, Green R, Katiri S, Patel B, Mohapatra SS, Mohapatra S. Mithramycin A Inhibits Colorectal Cancer Growth by Targeting Cancer Stem Cells. Sci Rep. 2019;9:15202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 87. | Takeda M, Koseki J, Takahashi H, Miyoshi N, Nishida N, Nishimura J, Hata T, Matsuda C, Mizushima T, Yamamoto H, Ishii H, Doki Y, Mori M, Haraguchi N. Disruption of Endolysosomal RAB5/7 Efficiently Eliminates Colorectal Cancer Stem Cells. Cancer Res. 2019;79:1426-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 88. | Lim JH, Go G, Lee SH. PrPC Regulates the Cancer Stem Cell Properties via Interaction With c-Met in Colorectal Cancer Cells. Anticancer Res. 2021;41:3459-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Zhang Y, Yang C, Cheng H, Fan Z, Huang Q, Lu Y, Fan K, Luo G, Jin K, Wang Z, Liu C, Yu X. Novel agents for pancreatic ductal adenocarcinoma: emerging therapeutics and future directions. J Hematol Oncol. 2018;11:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Cui J, Tian J, Wang W, He T, Li X, Gu C, Wang L, Wu J, Shang A. IGF2BP2 promotes the progression of colorectal cancer through a YAP-dependent mechanism. Cancer Sci. 2021;112:4087-4099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 91. | Lu H, Li Z, Liu L, Tao Y, Zhou Y, Mao X, Zhu A, Wu H, Zheng X. A Pan-Cancer Analysis of the Oncogenic Roles of RAD51 in Human Tumors. Adv Gut Microbiome Res. 2022;2022:1591377. [DOI] [Full Text] |
| 92. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2428] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 93. | Wang VM, Ferreira RMM, Almagro J, Evan T, Legrave N, Zaw Thin M, Frith D, Carvalho J, Barry DJ, Snijders AP, Herbert E, Nye EL, MacRae JI, Behrens A. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol. 2019;21:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 94. | Leng S, Huang W, Chen Y, Yang Y, Feng D, Liu W, Gao T, Ren Y, Huo M, Zhang J, Wang Y. SIRT1 coordinates with the CRL4B complex to regulate pancreatic cancer stem cells to promote tumorigenesis. Cell Death Differ. 2021;28:3329-3343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 95. | Karmakar S, Rauth S, Nallasamy P, Perumal N, Nimmakayala RK, Leon F, Gupta R, Barkeer S, Venkata RC, Raman V, Rachagani S, Ponnusamy MP, Batra SK. RNA Polymerase II-Associated Factor 1 Regulates Stem Cell Features of Pancreatic Cancer Cells, Independently of the PAF1 Complex, via Interactions With PHF5A and DDX3. Gastroenterology. 2020;159:1898-1915.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 96. | Bao B, Azmi AS, Aboukameel A, Ahmad A, Bolling-Fischer A, Sethi S, Ali S, Li Y, Kong D, Banerjee S, Back J, Sarkar FH. Pancreatic cancer stem-like cells display aggressive behavior mediated via activation of FoxQ1. J Biol Chem. 2014;289:14520-14533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Masuo K, Chen R, Yogo A, Sugiyama A, Fukuda A, Masui T, Uemoto S, Seno H, Takaishi S. SNAIL2 contributes to tumorigenicity and chemotherapy resistance in pancreatic cancer by regulating IGFBP2. Cancer Sci. 2021;112:4987-4999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 98. | Walter K, Rodriguez-Aznar E, Ferreira MSV, Frappart PO, Dittrich T, Tiwary K, Meessen S, Lerma L, Daiss N, Schulte LA, Najafova Z, Arnold F, Usachov V, Azoitei N, Erkan M, Lechel A, Brümmendorf TH, Seufferlein T, Kleger A, Tabarés E, Günes C, Johnsen SA, Beier F, Sainz B Jr, Hermann PC. Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Yang XL, Ma YS, Liu YS, Jiang XH, Ding H, Shi Y, Jia CY, Lu GX, Zhang DD, Wang HM, Wang PY, Lv ZW, Yu F, Liu JB, Fu D. microRNA-873 inhibits self-renewal and proliferation of pancreatic cancer stem cells through pleckstrin-2-dependent PI3K/AKT pathway. Cell Signal. 2021;84:110025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 100. | Chaudhary AK, Mondal G, Kumar V, Kattel K, Mahato RI. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microRNA-205. Cancer Lett. 2017;402:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 101. | Suzuki S, Okada M, Sanomachi T, Togashi K, Seino S, Sato A, Yamamoto M, Kitanaka C. Therapeutic targeting of pancreatic cancer stem cells by dexamethasone modulation of the MKP-1-JNK axis. J Biol Chem. 2020;295:18328-18342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Urtasun N, Vidal-Pla A, Pérez-Torras S, Mazo A. Human pancreatic cancer stem cells are sensitive to dual inhibition of IGF-IR and ErbB receptors. BMC Cancer. 2015;15:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |