Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.196
Peer-review started: December 28, 2022
First decision: January 9, 2023
Revised: January 25, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: April 26, 2023
Processing time: 118 Days and 14.9 Hours
Osteoarthritis (OA) is the most common musculoskeletal disease, and it is a major cause of pain, disability and health burden. Pain is the most common and bothersome presentation of OA, but its treatment is still suboptimal, due to the short-term action of employed analgesics and their poor adverse effect profile. Due to their regenerative and anti-inflammatory properties, mesenchymal stem cells (MSCs) have been extensively investigated as a potential therapy for OA, and numerous preclinical and clinical studies found a significant improvement in joint pathology and function, pain scores and/or quality of life after administration of MSCs. Only a limited number of studies, however, addressed pain control as the primary end-point or investigated the potential mechanisms of analgesia induced by MSCs. In this paper, we review the evidence reported in literature that support the analgesic action of MSCs in OA, and we summarize the potential mechanisms of these antinociceptive effects.
Core Tip: Osteoarthritis (OA) is the most common musculoskeletal disease, and it is a major cause of pain, disability and economic burden. Pain is the most common and bothersome presentation of OA, but its treatment is still suboptimal, which highlights the need for new analgesic agents for OA. Mesenchymal stem cells (MSCs) have been extensively investigated as a potential therapy for OA due to their regenerative and anti-inflammatory properties. The administration of MSCs resulted in significant improvement in joint pathology and function, pain scores and/or quality of life in numerous preclinical and clinical studies. Only a limited number of studies, however, addressed pain control as the primary end-point or investigated the potential mechanisms of analgesia induced by MSCs. So, this paper reviews literature for evidence of analgesic actions of MSCs in OA, and summarizes the potential mechanisms of these anti-nociceptive effects
- Citation: Almahasneh F, Abu-El-Rub E, Khasawneh RR. Mechanisms of analgesic effect of mesenchymal stem cells in osteoarthritis pain. World J Stem Cells 2023; 15(4): 196-208
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/196.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.196
Osteoarthritis (OA) is a disease of movable joints characterized by anatomic and/or physiologic derangements including cartilage degradation, bone remodeling, osteophyte formation, joint inflammation and loss of normal joint function. It is initiated by micro- and macro-injury of the joint, which activates maladaptive repair responses producing abnormal tissue metabolism[1].
Clinical manifestations of OA include joint pain, tenderness, limitation of movement, coarse crepitus and, occasionally, effusion and mild local inflammation[2]. Diagnosis depends on the observation of signs and symptoms through clinical examination[3]. X-ray can be performed to help with differential diagnosis and in case of atypical features[4]. However, radiologic findings do not always complement the clinical presentation of pain[5].
Pathophysiology of OA is complex and is based on cartilage degeneration preceded by subchondral bone lesions. Historically, OA had been considered a disease of joint wear and tear, but recently, low-grade chronic inflammation has been found to play a key role in OA pathology. Both innate and adaptive central immunological mechanisms are involved in inflammation. Formation of ectopic bone and osteophytes are a characteristic pathologic features of OA[6]. Neuroinflammation and central sensitization mechanisms contribute to the development of chronic pain[7].
OA is the most common musculoskeletal disorder worldwide, and it poses a huge health and economic burden. It is considered a major cause of chronic pain, disability -due to diminished joint mobility and function-, and decreased quality of life[8,9]. Thus, finding effective and safe therapies for the treatment of OA is a significant clinical need.
Despite the recent progresses in understanding the pathophysiology of OA[7], the treatment of this condition is still suboptimal[10]. For low-grade OA, pain management and lifestyle changes are the only available therapeutic options, with total joint replacement considered the end-stage therapy. However, no effective therapeutic options are available that can stop the progress of the disease[10]. Analgesia may be achieved using nonsteroidal anti-inflammatory drugs (NSAIDs) (topical or systemic) as a first line, followed by paracetamol or tramadol. Less used medications include duloxetine and topical capsaicin. Intra-articular steroids are effective and recommended if other agents do not provide sufficient pain control, although their effect is short-termed[3]. Pharmacological therapies should be always accompanied by physical and psychosocial interventions, such as exercise, weight management and manual therapy. Patients whose joint symptoms are substantially impacting their quality of life and in which non-surgical management is ineffective or unsuitable should be referred for joint replacement surgery[3]. Table 1 summarizes the treatment guidelines for OA as recommended by the American College of Rheumatology and Arthritis Foundation[11].
| Hand | Knee | Hip | |
| Physical and psychosocial approaches | |||
| Exercise1 | √ | √ | √ |
| Self-Efficacy and Self-Management Programs1 | √ | √ | √ |
| Weight loss1 | √ | √ | |
| Tai Chi1 | √ | √ | |
| Heat, therapeutic cooling2 | √ | √ | √ |
| Cognitive Behavioral Therapy2 | √ | √ | √ |
| Acupuncture2 | √ | √ | √ |
| Paraffin2 | √ | ||
| Yoga2 | √ | ||
| Pharmacological approach | |||
| Oral NSAIDs1 | √ | √ | √ |
| Topical NSAIDs | √2 | √1 | |
| IA corticosteroids | √2 | √1 | √1 (image-guided) |
| Acetaminophen2 | √ | √ | √ |
| Tramadol2 | √ | √ | √ |
| Duloxetine2 | √ | √ | √ |
| Chondroitin2 | √ | ||
| Topical capsaicin2 | √ |
Pain is the major manifestation of OA and it significantly affects the function and quality of life of patients[12]. Both peripheral and central mechanisms contribute to OA pain. Peripherally, nociceptive signals may arise from the synovium, bone marrow, soft tissues and even cartilage in the advanced stages of the disease[13]. These inputs are modulated at the central level through mechanisms involving spinal and cortical sensitization, and the activity of discrete areas of the brain. Central sensitization may explain the poor correlation between pain severity in OA and the extent of cartilage damage, and the persistence of pain after joint replacement in some patients[14].
In the periphery, sensitization of afferent neurons is mediated by cytokines, chemokines and neuropeptides and is associated with low-grade inflammation and innate immunity[15], immune cell infiltration and activation, and damage-associated molecular patterns. These mechanisms involve early post-translational changes to receptor ion channels, followed by late transcription-dependent mechanisms, which produce changes to the chemical phenotype of the cell[16]. Animal models of OA demonstrated the role of nerve growth factor (NGF) in nociceptor sensitization after tissue injury, mainly through the tropomyosin receptor kinase A (TrkA) receptor. In addition, increased NGF levels were found in the synovial fluid of OA patients and were associated with pain[17,18]. Other molecules associated with the peripheral component of OA pain include the neuropeptide calcitonin gene-related peptide (CGRP)[19], interleukin (IL)-1β, IL-6 and tumor-necrosis factor α (TNF-α)[20].
Central sensitization occurs both at the spinal and supraspinal levels. In the spinal cord, acute pain is accompanied with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor activation by glutamate, followed by an early phase of sensitization, mediated by substance P (SP) acting on neurokinin 1 receptors, in which N-methyl-D-aspartate (NMDA) receptors are activated. The late phase sensitization witnesses gene induction with enhanced synthesis of prostaglandins and other local inflammatory mediators[14]. In models of OA, spinal cord exhibits enhanced transient receptor potential cation channel subfamily V member 1 (TRPV1) activity and increased levels of SP, CGRP, IL-1α/β, IL-13, IL-17, TNF-α, L-selectin, tissue inhibitor of metallopeptidase inhibitor 1 and vascular endothelial growth factors[21]. In addition, activation of glial cells was found to contribute to spinal sensitization, indicating a strong neuropathic component of the OA associated pain[22]. Supraspinal sensitization occurs in several areas of the brain, including the rostral ventral medulla and the periaqueductal gray and it involves inflammatory mediators, such as prostaglandins, as well as serotoninergic and noradrenergic facilitation[23].
Pain management in OA is still considered suboptimal. The use of paracetamol, once recommended as the first-line analgesic for OA pain, has now been reviewed after meta-analysis suggested that monotherapy with paracetamol may be ineffective[24] and that long-term treatment provides no more pain relief than placebo for most patients[11]. NSAIDs are effective on the short term to control OA pain and are thus strongly recommended. Their use, however, is limited by the adverse effect profile[11]. Intra-articular injections of steroids also showed short-term effectiveness (2-10 wk)[3], but they are limited in frequency of administration and may cause cartilage damage if used repeatedly[25]. The use of other analgesics, such as duloxetine, tramadol, and non-tramadol opioids is not supported by strong evidence of efficacy, as well as other supplements, such as hyaluronic acid, glucosamine, chondroitin, fish oil and vitamin D[11]. These factors highlight the need for the development of novel therapies that are both effective and safe on the long term.
Mesenchymal stem cells (MSCs) are multipotent adult stem cells likely derived from diverse embryonic lineages and isolated from different sources. They were discovered by Friedenstein in 1970[26]. The International Society for Cellular Therapy has proposed a set of standards to define MSCs: (1) Expression of a certain set of cluster of differentiation (CD) markers (CD105, CD73, and CD90); (2) Lack of expression of hematopoietic lineage CD markers (CD45, CD34, CD14 or CD11b, CD79α or CD19 and histocompatibility complex (HLA)-DR surface molecules); (3) Differentiation into osteoblasts (bone), adipocytes (fat) and chondroblasts (cartilage) in vitro; and (4) Plastic adherence and ability to form colony-forming unit fibroblasts[27]. MSCs from different sources showed differences in differentiation potential, immunophenotype, immunomodulatory activity, proteome, and transcriptome, producing their specific characteristics and features in their application.
MSCs are an attractive option and among the most frequently used stem cell types for clinical application and regenerative medicine due to numerous advantages, including self-renewal and differentiation, mostly due to their secreted trophic factors that mediate cell-to-cell communication. Moreover, immune rejection is an important concern with allogeneic cell-based therapy, but the lack of cell surface HLA class II molecules and T cell costimulatory molecules and their paracrine-mediated immunomodulatory activity, and secretion of immunomodulatory factors indicate that the MSCs have broad anti-inflammatory properties and active in tissue repair[28-31].
A large number of studies demonstrated the beneficial effects of MSC-based therapies to treat different pathologies, including neurological disorders, cardiac ischemia, diabetes, and bone and cartilage diseases[32,33], which indicate that MSCs holds great promise for cell therapies and tissue engineering.
Studies showed the ability of MSCs to migrate toward damaged tissues, which functionally influence the repairing of these tissues[34]. MSCs act to accelerate healing and reduce inflammation, which is essential to remove dead cells, and facilitate cell migration and proliferation in the injury site[35,36]. For instance, a study using a rat model of cardiomyopathy showed that MSC transplantation significantly improved cardiac function by induction of myogenesis and angiogenesis, resulting in decreased left ventricular end-diastolic pressure and increased left ventricular maximum[37]. Another study showed that basic fibroblast growth factor (bFGF) can promote the migration and survival of bone marrow MSCs in vitro, as the perfusion of the coronary vein with retrograde bFGF can enhance the graft transplantation of MSCs, promote the differentiation of MSCs to cardiomyocytes, and restore cardiac function[38]. Some studies used a laboratory-grown cell sheet patches of MSCs to repair large damaged areas instead of intravenous infusion of MSCs. Kim et al[39] transplanted adipose-derived stem cells (AD-MSCs) sheet to treat myocardial infarction in a rat model and showed that the stem cell sheet promoted cellular engraftment and upregulated growth factor and cytokine expression. An in vivo study of neonatal stroke rat model proved that intranasal delivery of MSC reduced ischemic brain damage and reduced white and gray matters loss[40]. The study attributed the healing in the injury site to an increase in cell proliferation after transplantation of MSCs.
In skeletal disorders, MSCs could be helpful in tissue repairing and regeneration through several mechanisms, including homing, angiogenesis, differentiation, and response to inflammatory condition[41]. Liu et al[42] used umbilical cord-MSCs (UC-MSCs) to treat rheumatoid arthritis in collagen-induced arthritis mice through suppression of T follicular helper cell differentiation partly via the production of indoleamine 2,3-dioxygenase. In addition, MSCs prevented arthritis progression by inhibiting both the number and function of follicular helper cell in vivo. Another study showed that transplantation of MSCs from allogeneic related donors treated severe progressive systemic sclerosis[43]. A third in vitro study treated bone marrow derived-MSCs (BM-MSCs) with all-trans retinoic acid, then co-cultured them with CD3/28-activated peripheral blood mononuclear cells derived from ankylosing spondylitis (AS) patients. The results showed that BM-MSCs treated with all-trans retinoic acid significantly decreased pathogenic cytokine, TNF-α, IL-17A and interferon-γ (IFN-γ) in AS[44].
MSCs are the most studied stem cells for treating bone related diseases and the associated inflammation. The effects of treatment by MSCs on OA pathology and presentation was widely investigated in vitro and in different preclinical models of OA using different routes of administration of multiple types and amounts of MSCs. Numerous clinical trials also addressed the potential therapeutic role of MSCs in OA.
MSCs have the ability to differentiate into mesoderm-derived cells, including osteoblasts and chondrocytes. More importantly, MSCs are considered powerful immunomodulators and inflammation combatants rendering them suitable for many immunological and bone diseases where inflammation is a prominent component. OA is the most common form of arthritis and clinically variable in its severity. Patients with symptomatic form of OA suffer from intermittent attacks which are usually associated with joint pain. OA pathology encompasses the inflammation and degeneration of articular cartilage that defects its integrity and leads to subsequent changes in the subchondral bone. Many preclinical and clinical studies have corroborated the therapeutic potential of MSCs in alleviating the inflammation associated with OA and initiating the regeneration of the defective articular cartilage. A pilot study conducted by Song et al[45] reported positive outcomes of injecting autologous human adipose-derived MSCs (haMSCs) intra-articularly in patients with OA. Song et al[45] reported that a dosage of 5 × 107 haMSCs produced the desired improvement in the pain scale and restored the volume and the function of knee cartilage. Similarly, Lee et al[46] reported that single intra-articular injection of 1 × 108 AD-MSCs for patients with knee OA produced a significant reduction in the Western Ontario and McMaster Universities OA (WOMAC) index total score and the test related sub-scores for pain, stiffness and physical function at 6 mo post-injection. The range of articular motion remarkably enhanced after MSCs transplantation with no change in the joint space width of medial and lateral compartment and size of the cartilage damage[46]. On the other hand, Matas et al[47] found that intra-articular injection of two repeated doses of umbilical cord-derived MSCs (UC-MSCs) (20 × 106per dose) at baseline and 6 mo was superior than using a single dose in lowering the WOMAC pain scores. Patients with knee OA who were recruited in Matas et al study experienced 86% reduction in pain and 89% reduction in disability with no progression in the chondral damage and intra-articular calcifications examined by magnetic resonance imaging at 12 mo[47]. Bastos et al[48] compared the effect of injecting autologous bone marrow-derived culture-expanded MSCs (hBM-MSCs) intra-articularly with or without the addition of platelet-rich plasma to intra-articular corticosteroid injections in patients with symptomatic knee OA. MSCs alone or in combination with platelet rich plasma were superior to corticosteroids in improving the Knee Injury and OA Outcome Score, increasing the range of motion and reducing the expression of IL-10, which is usually increased in OA knees, at 12 mo follow up[48].
The extracellular vesicles (EVs) derived from different MSCs have currently gained a wide interest due to their high therapeutic efficacy and safety profile. Li et al[49] injected UC-MSCs-EVs in the articular lumen of a rat model of OA created by the surgical transection of the anterior cruciate ligament (ACLT), and reported that the UC-MSCs derived EVs can deliver therapeutically effective miRNAs, including has-miR-122-5p, has-miR-148a-3p, has-miR-486-5p, has-miR-let-7a-5p, and has-miR-100-5p, which were able to promote the reprogramming of macrophages into anti-inflammatory M2-macrophages and increasing the level of inflammation inhibitory cytokine IL-10. Moreover, these miRNAs were effective in inducing the phosphoinositide-3-kinase-Akt signaling pathway, which is integral to prevent further degeneration of the knee cartilage and seize the progression of OA[49]. Duan et al[50] conducted an interesting study by isolating synovial MSCs and chondrocytes from the knee cartilage of patients getting total knee arthroplasty. After culturing these cells, they primed the MSCs with lipopolysaccharide (LPS) and isolated EVs from these preconditioned synovial MSCs. The EVs were injected in the right knees of mice model of OA created by surgical removal of anterior cruciate ligament and medial meniscus. It was found that the presence of Let-7b miRNA in the EVs isolated from LPS primed synovial MSCs were effective in reducing the cartilage damage, increasing the thickness of cartilage layer, and decreasing the level of the inflammatory and matrix lysis protein disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) while increasing the levels of aggrecan and type II collagen alpha 1 chain, which are essential for the interaction with hyaluronan and enhancing the load-bearing characteristics of the knee[50]. Likewise, Jin et al[51] reported that lncRNA MEG-3 existed in the exosomes derived from bone marrow MSCs (BM-MSCs) and prevents the apoptosis and senescence of chondrocytes through lowering the level of IL-1β and decreasing the inflammatory damage of knee cartilage, which helps in restoring the trabecular bone volume and density of the knee joint in OA rat model. Mao et al[52] observed that miR-92a-3p was significantly less in the chondrocytes isolated from the cartilage of OA patients who underwent total knee replacement. BM-MSCs derived exosomes can deliver miR-92a-3p following their injection in the knees of collagenase-induced OA mouse model, which enhances the differentiation potential of resident stem cells to chondrocytes and increases the synthesis of cartilage matrix. These protective and regenerative effects are mediated by the inhibition of Wnt Family Member 5A[52]. Huang et al[53] demonstrated that miR-206 was downregulated and E74-like factor 3 (Elf3) was upregulated in the femoral tissues of OA mouse model. The administration of BM-MSCs derived exosomes that have sufficient amount of miR-206 was effective in downregulating Elf3 and ameliorating the inflammation and apoptosis of resident osteoblasts besides increasing the expression of osteocalcin and bone morphogenetic protein 2 and enhancing the deposition of calcium and the activity of alkaline phosphatase[53]. In the rat model of temporomandibular joint OA, Zhang et al[54] injected exosomes derived from MSCs. They reported a significant suppression of inflammation mediated by decreasing the level of IL-1β and increasing the levels of nitric oxide and matrix metalloproteinase 13 (MMP13), which promoted the synthesis of glycosaminoglycans required for matrix restoration and regeneration[54].
Genetic modification of MSCs is one of the most studied approaches to enhance their therapeutic and regenerative potential. It has been found that the transplantation of MSCs overexpressing platelet-derived growth factor (PDGF) or heme oxygenase-1 in the surgery-induced canine OA model can considerably suppress the destructive inflammation and increase the levels of aggrecan and collagen type 2 in chondrocytes. PDGF-MSCs were more effective in improving the limb function and reducing pain[55]. The accumulative evidence supported by the above mentioned studies highlights the importance of MSCs in mitigating OA inflammation and restoring the integrity and density of volume of articular cartilage and its associated matrix, which indicates that MSCs can be a possible new therapeutic tool for OA.
Despite the large number of preclinical studies investigating the effects of MSC administration on OA pathophysiology, only a small part of them included the effects on pain, and even less had pain control as the main outcome. This may be due to the difficulty of pain assessment in animal models of OA[56] and the complexity of the pain phenomenon[57]. In addition, reported results were contrasting, probably due to the variability in animal models and the methods of pain evaluation, as well as types of MSCs used and routes of their administration[56].
A number of in vivo studies, however, found an improvement in OA associated pain following treatment with MSCs. Transforming growth factor β1 (TGF-β1)-modified MSC-derived exosome was found to attenuate cartilage damage and pain behaviors in the ACLT model of OA by inhibiting angiogenesis, suppressing calcification of the cartilage zone and osteoclastogenesis[58]. Intra-articular injection of adipose derived MSCs (ADSCs) in rats diminished monoiodoacetate (MIA)-induced OA cartilage lesions by paracrine-based mechanisms and restored the OA associated mechanical allodynia and thermal hyperalgesia. In patients, ADSCs also reduced OA pain, as measured by the visual analog scale and WOMAC[59].
In a study by Zeng et al[60], bone marrow-derived MSCs (BMSCs) were enhanced by kartogenin (KGN) nanoparticles and administered to osteoarthritic rats. In addition to articular cartilage repair and enhanced chondrogenesis, KGN-enhanced BMSCs also ameliorated OA pain, as shown by increased weight bearing on the injured leg and decreased latency period after hot plate exposure. Administration of different concentrations of MSCs into OA rat knees improved both the histological damage and weight bearing distribution in the ACLT model[61].
Sakamoto et al[62] found that early intra-articular injection of adipose-derived MSCs resulted in significant suppression of inflammation and pain and prevented degenerative OA changes, but did not promote cartilage repair. In another study involving the MIA model of OA, STAT3 signaling pathway was suppressed by treatment of MSCs with STA21. Both intravenous (IV) and intra-articular (IA) administration of STA21-treated MSCs decreased expression of proinflammatory cytokines in the joint, which improved pain severity and cartilage damage[63].
A trial of adult human bone marrow-derived mesenchymal stromal cells in the MIA model of OA caused significant pain reduction, along with articular damage repair. In patients, the same cells did not produce the significant improvement in pain scores[64].
Intra-articular injection of a large variety of MSCs in patients with knee OA was found to significantly improve joint pathology, disease symptoms, pain score and quality of life[65]. Human umbilical cord MSCs (hUC-MSCs) was found to improve pain scores and quality of life in OA patients. hUC-MSCs increase the expression of chondrocytes and activate anti-inflammatory mechanisms, preventing degradation of cartilage and bone[66]. A clinical trial of autologous bone marrow stem cells (BM-SC) concluded that a single IA injection of these stem cells significant reduced knee pain and improved quality of life[67]. Other clinical trials reviewed by Hwang et al[68] also resulted in improved joint function, reduction of pain severity and improved life satisfaction.
Data from literature suggest that the analgesic action of MSCs in OA involves a peripheral component, originating in the joint tissues, and a central component related to central hypersensitization. This reflects the mechanisms of nociception reported in OA[69].
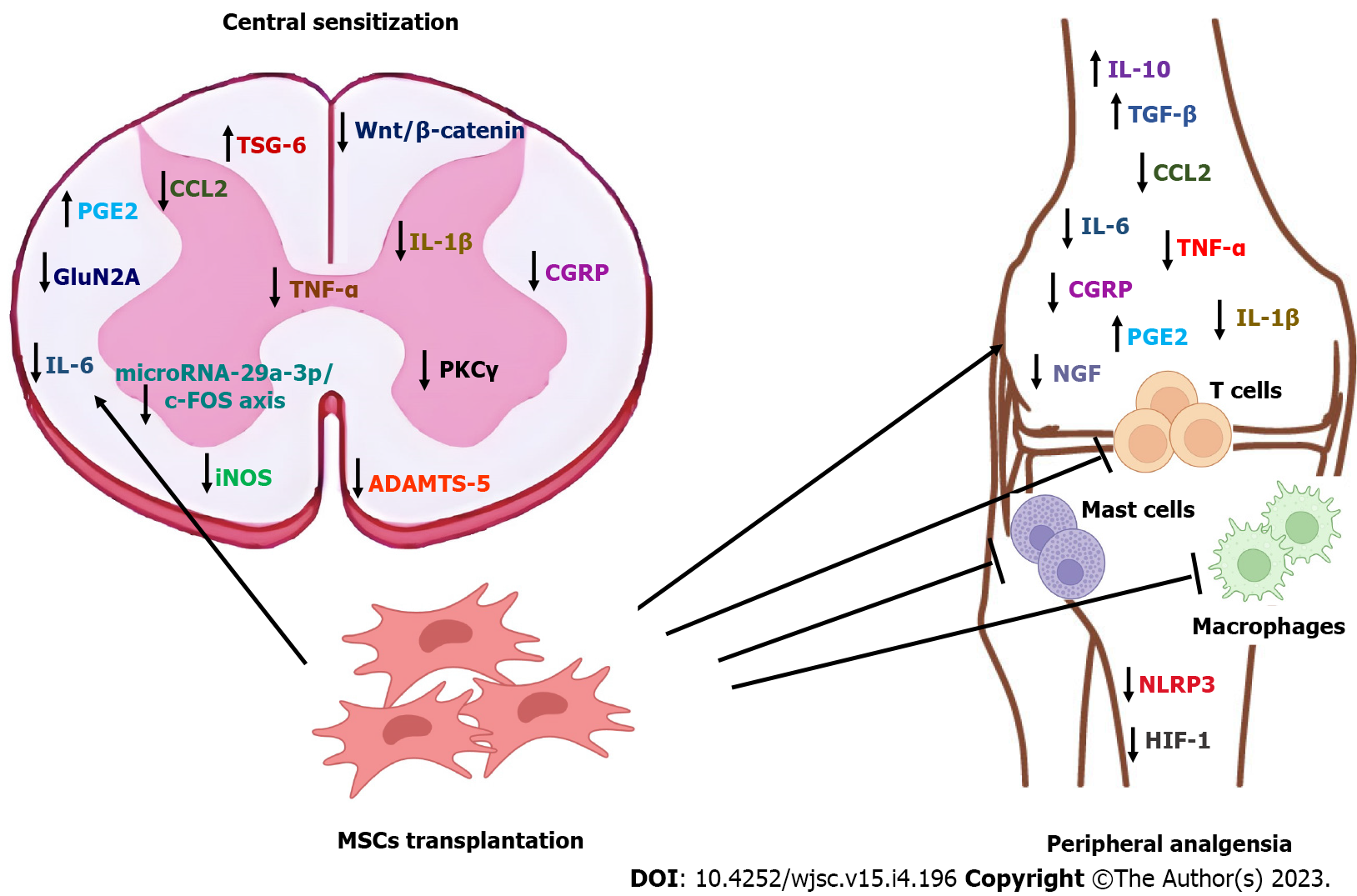
It can be speculated that a major antinociceptive mechanism of MSCs in OA is through inhibition of inflammation. MSCs secrete anti-inflammatory and growth factors that support their immunomodulatory, immunosuppressive and trophic capacities. These properties contribute to the regeneration of damaged cartilage and joint homeostasis, improving inflammatory and catabolic aspects of OA[70,71]. Initially, the bioactive substances secreted by MSCs change the inflammatory milieu in the joint from pro-inflammatory to anti-inflammatory, producing analgesia[65]. On the longer term, MSCs are inserted in the joint tissues and trigger the repair and regeneration of damaged tissues, including cartilage[72]. Numerous molecules have been reported to contribute to the antiinflammatory effects of MSCs in OA, including nitric oxide (NO), inducible nitric oxide synthase (iNOS)-27, IL-10, TGF-β, IL-6, IFN-γ, CCL2, hepatocyte growth factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and TNF-α[73-75]. Cyclooxygenase 2/prostaglandin E2 (PGE2) pathway plays a key role in the anti-inflammatory effect of MSCs in OA[76], where MSCs increase the levels of the antiinflammatory mediator PGE2[75]. Similar antiinflammatory results were also produced by MSC EVs[77]. Since these molecules and their related pathways are well established contributors to OA pain, their involvement in the analgesic mechanisms of MSCs in OA can be hypothesized.
Chemokine (C-C motif) ligand 2 (CCL2)/C-C chemokine receptor type 2 (CCR2) signaling is crucial in the development of knee OA pain. Neuronal CCL2 and CCR2 from dorsal root ganglion (DRG) mediate macrophage infiltration, while local CCL2/CCR2 signaling in the joint directly stimulates intra-articular CCR2 positive sensory nerves, producing knee hyperalgesia[78]. Interestingly, the analgesic effect of MSCs in different models of pain was associated with decreased levels of CCL2, produced by the downregulation of the NF-κB and c-Jun N-terminal kinase/mitogen activated protein kinase pathways[79,80], which suggests that a similar mechanism could be involved in the analgesic effect of MSC in OA.
Other molecules and pathways relevant to OA pain include: (1) NGF / TrkA; (2) CGRP; (3) IL-1β; (4) Pyrin domain-containing protein 3 (NLRP3) inflammasome; and (5) Wnt/β-Catenin[69]. NLRP3 inflammasome is a component of the innate immune system and is involved in the regulation of active IL-1β. NF-κB increases the expression of inactive NLRP3 and pro-IL-1 mRNA, followed by the assembly of the inflammasome, which results in the activation of caspase 1 and release of IL-1, IL-18, MMP13 and ADAMTS5. In OA, dysregulation of NLRP3 inflammasome in OA contributes to chronic pain[69]. MSCs were found to inhibit the NLRP3 inflammasome in macrophages[81], inflammatory cardiomyopathy[82] and inflammatory renal disease[83], which could indicate inhibition of NLRP3 inflammasome as a potential mechanism of MSCs induced analgesia in OA.
In OA, high levels of Wnt are associated with progressive joint damage[84] and hyperalgesia[69]. Since MSCs were found to inhibit the Wnt/β-Catenin pathway in a number of disorders[85,86], this pathway could be involved in MSC analgesic mechanism and should be investigated further. In the study by Lee et al[63], administration of signal transducer and activator of transcription 3-inhibited MSCs reduced the levels of inflammatory mediators and chemokines in the OA joint, and this was associated with improvement in pain behavior and decreased TRPV1 expression in the dorsal root ganglion. This effect was more pronounced with IV administration, indicating a systemic immunomodulatory effect on inflammation.
The interaction of MSCs with immune cells, including macrophage, dendritic cells, T lymphocytes, and natural killer cells, also contributes to MSC antiinflammatory properties[87]. MSCs induce polarization of macrophages to an antiinflammatory M2 phenotype through: (1) Cellular interaction and paracrine factor-mediated mechanisms; and (2) exosome-mediated mechanisms. The first involves cytokines and hormones, and the latter depends on RNAs and other molecules[88]. Following an intra-articular injection of BM-MSCs in patients with knee OA, a decrease in synovial fluid levels of pro-inflammatory monocytes/macrophages and IL-12p40 was recorded[89].
Ai et al[90] investigated the effects of MSCs and derived EVs (MSC-EVs) on pain behaviors in the destabilization of the medial meniscus murine model of OA. It was found that treated OA mice did not display pain behaviors observed in untreated counterparts, and that did not result from reduced joint damage, but rather from a lack of knee-innervating sensory neuron hyperexcitability. MSC-EV treatment also prevented NGF-induced sensory neuron hyperexcitability. These results suggest that MSCs and MSC-EVs may reduce pain in OA by direct action on peripheral sensory neurons[90]. Another study[91] found that the intrathecal administration of umbilical cord blood MSCs (UCBMSCs) improved both pain behavior and inflammation in the MIA model of OA. This effect is regulated by LncRNA H19 and involves microRNA-29a-3p/ FOS axis. microRNA29a-3p, the target gene of LncRNA H19, and FOS mRNA were down-regulated after stem cell therapy, suggesting that microRNA-29a-3p and FOS might play a role in pain improvement. c-fos in spinal dorsal horn of rats was significantly down-regulated after UCBMSCs treatment, which may be the reason for pain improvement. Phosphorylation levels of NMDA receptor 1 (NR1), NR2B, protein kinase C γ (PKCγ), extracellular signal-regulated kinase in spinal dorsal horn of rats with OA pain decreased significantly after intervention of UCBMSCs, indicating that the central sensitization of rats with advanced OA pain decreased and the pain symptoms improved. Similar results were observed in astrocytes.
Intra-articular injection of human adipose tissue-derived MSCs was reported to improve pain behavior in a medial meniscal transection (MMT) rat model of OA[92]. These effects were attributed to the recruitment of endogenous cells through paracrine communication[93], and to a lesser extent, to direct engraftment coordinated with the local environment[94]. Paracrine factors excreted by MSCs help recruitment of stem and progenitor cells, repair of degraded tissue and, most importantly, counteracting inflammation. A similar result was reported after administration of bone marrow MSC (BMSC)-derived exosome in the MIA model of OA[95], which was found to inhibit CGRP and iNOS expression in the DRG, indicating relief of both inflammatory and neuropathic aspects of OA pain[95]. BMSC-derived exosome also attenuates the inhibitory effect of IL-1β on the upregulated inflammatory mediators.
In the meniscal transection (MNX) model of OA, different effects were reported on pain behavior and joint pathology with the use of early and late passage MSCs. Late passage MSCs attenuated established pain behavior, while early passage MSCs exacerbated it. Interestingly, none of them modified MNX-induced joint pathology, which suggests an analgesic mechanism not related to articular pathology[96]. SiMAG-labelled MSCs were detected within the synovial cavity at 29 d postinjection, indicating a peripheral site of analgesic action of the MSCs. The recorded decrease in serum TNFα indicated inhibition of systemic inflammation, which is the expected cause of pain relief[97]. Similarly, van Buul et al[56] reported that intra-articular injection of bone marrow mononuclear cells in the MIA model of OA significantly improved pain behavior – measured as weight bearing distribution - but did not affect cartilage damage, subchondral bone changes and synovial inflammation. Similar results were observed with the administration of MSCs and MSC-EVs in a mouse collagenase-induced OA model[98]. MSCs were also found to downregulate ADAMTS-5 expression, inhibit the expression of anti- CGRP and increase the expression of TNF-stimulated gene/protein-6 (TSG-6)[99]. These changes indicate the suppression of the central sensitization of pain.
Several pain pathways were found to be inhibited by MSCs in other types or models of pain. Small EVs from induced pluripotent stem cell-derived MSCs alleviated acute pain in tendinopathy by inhibition of mast cell degranulation and infiltration and the expression of proinflammatory cytokines and genes involved in the HIF-1 signaling pathway[100]. Human BMSCs relieved pain behavior in rodents by inhibition of neuronal hyperexcitability and primary afferent input, as well as suppression of GluN2A (N-methyl-D-aspartate receptor subunit 2A) tyrosine phosphorylation and PKC gamma (PKCg) immunoreactivity in the rostral ventromedial medulla[101]. In murine chronic constriction injury and spared nerve injury models, TGF-β1 was found to suppress spinal synaptic plasticity and DRG neuronal hyperexcitability via TGF-β receptor 1-mediated noncanonical signaling. This effect was mediated by paracrine mechanism by which BMSCs target C-X-C motif chemokine ligand 12-producing DRGs[102]. We thus anticipate that MSCs use in OA may exhibit their analgesic effects through the HIF-1, GluN2A tyrosine, PKCg and TGF-β signaling pathways. Figure 1 summarizes the established and proposed mechanisms of analgesia exerted by MSCs or MSC related EVs in OA.
Numerous in vitro, in vivo and clinical studies demonstrated the capability of MSCs in halting and/or reversing the progression of joint tissue damage in OA, as well as mitigating joint inflammation, improving pain sensation and enhancing overall patient quality of life. MSCs were able to produce an analgesic effect in models of OA through both peripheral mechanisms, mainly involving antiinflammatory processes, and on a central level, by preventing or reversing central sensitization. This evidence further reinforces the potential of MSCs as a safe and effective treatment for OA pain. Additional pathways which were observed in other types of pain may contribute to the analgesic mechanisms of MSCs in OA, and require further investigations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Jordan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shengwen Calvin Li, United States; Chen H, China; Shuang W, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage. 2015;23:1233-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 428] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 2. | Abhishek A, Doherty M. Diagnosis and clinical presentation of osteoarthritis. Rheum Dis Clin North Am. 2013;39:45-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Taruc-Uy RL, Lynch SA. Diagnosis and treatment of osteoarthritis. Prim Care. 2013;40:821-836, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Wang X, Oo WM, Linklater JM. What is the role of imaging in the clinical diagnosis of osteoarthritis and disease management? Rheumatology (Oxford). 2018;57:iv51-iv60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51:844-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 405] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Roelofs AJ, Kania K, Rafipay AJ, Sambale M, Kuwahara ST, Collins FL, Smeeton J, Serowoky MA, Rowley L, Wang H, Gronewold R, Kapeni C, Méndez-Ferrer S, Little CB, Bateman JF, Pap T, Mariani FV, Sherwood J, Crump JG, De Bari C. Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis. Ann Rheum Dis. 2020;79:1625-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Coaccioli S, Sarzi-Puttini P, Zis P, Rinonapoli G, Varrassi G. Osteoarthritis: New Insight on Its Pathophysiology. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 8. | Trouvin AP, Perrot S. Pain in osteoarthritis. Implications for optimal management. Joint Bone Spine. 2018;85:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Chen A, Gupte C, Akhtar K, Smith P, Cobb J. The Global Economic Cost of Osteoarthritis: How the UK Compares. Arthritis. 2012;2012:698709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Primorac D, Molnar V, Matišić V, Hudetz D, Jeleč Ž, Rod E, Čukelj F, Vidović D, Vrdoljak T, Dobričić B, Antičević D, Smolić M, Miškulin M, Ćaćić D, Borić I. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies' Guidelines. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 11. | Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, Gellar K, Harvey WF, Hawker G, Herzig E, Kwoh CK, Nelson AE, Samuels J, Scanzello C, White D, Wise B, Altman RD, DiRenzo D, Fontanarosa J, Giradi G, Ishimori M, Misra D, Shah AA, Shmagel AK, Thoma LM, Turgunbaev M, Turner AS, Reston J. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken). 2020;72:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 906] [Article Influence: 181.2] [Reference Citation Analysis (0)] |
| 12. | Adatia A, Rainsford KD, Kean WF. Osteoarthritis of the knee and hip. Part I: aetiology and pathogenesis as a basis for pharmacotherapy. J Pharm Pharmacol. 2012;64:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 13. | Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Fransès RE, Mapp PI, Wilson D. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49:1852-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 14. | Kidd B. Mechanisms of pain in osteoarthritis. HSS J. 2012;8:26-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Syx D, Tran PB, Miller RE, Malfait AM. Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr Rheumatol Rep. 2018;20:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723-7730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 410] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Aloe L, Tuveri MA, Carcassi U, Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992;35:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 216] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2014;66:3018-3027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Walsh DA, Mapp PI, Kelly S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br J Clin Pharmacol. 2015;80:965-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1054] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 21. | Zhang RX, Ren K, Dubner R. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthritis Cartilage. 2013;21:1308-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. 2010;22:499-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Haywood AR, Hathway GJ, Chapman V. Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci Rep. 2018;8:7122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 324] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 25. | Pavone V, Vescio A, Turchetta M, Giardina SMC, Culmone A, Testa G. Injection-Based Management of Osteoarthritis of the Knee: A Systematic Review of Guidelines. Front Pharmacol. 2021;12:661805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 949] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 27. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12678] [Article Influence: 704.3] [Reference Citation Analysis (2)] |
| 28. | Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour A, Yousefi M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: A novel therapeutic paradigm. J Cell Physiol. 2020;235:706-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 30. | Asgarpour K, Shojaei Z, Amiri F, Ai J, Mahjoubin-Tehran M, Ghasemi F, ArefNezhad R, Hamblin MR, Mirzaei H. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 31. | Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol Rev. 2016;96:1127-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 32. | Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 33. | Han L, Zhou Y, Zhang R, Wu K, Lu Y, Li Y, Duan R, Yao Y, Zhu D, Jia Y. MicroRNA Let-7f-5p Promotes Bone Marrow Mesenchymal Stem Cells Survival by Targeting Caspase-3 in Alzheimer Disease Model. Front Neurosci. 2018;12:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Shojaei F, Rahmati S, Banitalebi Dehkordi M. A review on different methods to increase the efficiency of mesenchymal stem cell-based wound therapy. Wound Repair Regen. 2019;27:661-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Toh WS, Zhang B, Lai RC, Lim SK. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy. 2018;20:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, Huang J, Zhang Y, Tao Y, Zang X, Li D, Du M. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16:908-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 37. | Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 38. | Wang X, Zhen L, Miao H, Sun Q, Yang Y, Que B, Lopes Lao EP, Wu X, Ren H, Shi S, Lau WB, Ma X, Ma C, Nie S. Concomitant Retrograde Coronary Venous Infusion of Basic Fibroblast Growth Factor Enhances Engraftment and Differentiation of Bone Marrow Mesenchymal Stem Cells for Cardiac Repair after Myocardial Infarction. Theranostics. 2015;5:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Kim JH, Joo HJ, Kim M, Choi SC, Lee JI, Hong SJ, Lim DS. Transplantation of Adipose-Derived Stem Cell Sheet Attenuates Adverse Cardiac Remodeling in Acute Myocardial Infarction. Tissue Eng Part A. 2017;23:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | van Velthoven CT, Sheldon RA, Kavelaars A, Derugin N, Vexler ZS, Willemen HL, Maas M, Heijnen CJ, Ferriero DM. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. 2013;44:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 41. | Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, Razmkhah M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 2020;11:492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 42. | Liu R, Li X, Zhang Z, Zhou M, Sun Y, Su D, Feng X, Gao X, Shi S, Chen W, Sun L. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Sci Rep. 2015;5:12777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Keyszer G, Christopeit M, Fick S, Schendel M, Taute BM, Behre G, Müller LP, Schmoll HJ. Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: report of five cases. Arthritis Rheum. 2011;63:2540-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Li D, Wang P, Li Y, Xie Z, Wang L, Su H, Deng W, Wu Y, Shen H. All-Trans Retinoic Acid Improves the Effects of Bone Marrow-Derived Mesenchymal Stem Cells on the Treatment of Ankylosing Spondylitis: An In Vitro Study. Stem Cells Int. 2015;2015:484528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 46. | Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med. 2019;8:504-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 47. | Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, Figueroa FE, Espinoza F. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med. 2019;8:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 48. | Bastos R, Mathias M, Andrade R, Amaral RJFC, Schott V, Balduino A, Bastos R, Miguel Oliveira J, Reis RL, Rodeo S, Espregueira-Mendes J. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2020;28:1989-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 49. | Li K, Yan G, Huang H, Zheng M, Ma K, Cui X, Lu D, Zheng L, Zhu B, Cheng J, Zhao J. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J Nanobiotechnology. 2022;20:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 50. | Duan A, Shen K, Li B, Li C, Zhou H, Kong R, Shao Y, Qin J, Yuan T, Ji J, Guo W, Wang X, Xue T, Li L, Huang X, Sun Y, Cai Z, Liu W, Liu F. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res Ther. 2021;12:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 51. | Jin Y, Xu M, Zhu H, Dong C, Ji J, Liu Y, Deng A, Gu Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med. 2021;25:9281-9294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 52. | Mao G, Zhang Z, Hu S, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 344] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 53. | Huang Y, Zhang X, Zhan J, Yan Z, Chen D, Xue X, Pan X. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J Cell Mol Med. 2021;25:7734-7745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 54. | Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 400] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 55. | Oh J, Son YS, Kim WH, Kwon OK, Kang BJ. Mesenchymal stem cells genetically engineered to express platelet-derived growth factor and heme oxygenase-1 ameliorate osteoarthritis in a canine model. J Orthop Surg Res. 2021;16:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | van Buul GM, Siebelt M, Leijs MJ, Bos PK, Waarsing JH, Kops N, Weinans H, Verhaar JA, Bernsen MR, van Osch GJ. Mesenchymal stem cells reduce pain but not degenerative changes in a mono-iodoacetate rat model of osteoarthritis. J Orthop Res. 2014;32:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, Kamoda H, Arai G, Toyone T, Aoki Y, Kubo T, Takahashi K, Ohtori S. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 58. | Wang R, Xu B. TGFβ1-modified MSC-derived exosome attenuates osteoarthritis by inhibiting PDGF-BB secretion and H-type vessel activity in the subchondral bone. Acta Histochem. 2022;124:151933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 59. | Yan B, Lv S, Tong P, Yan L, Chen Z, Zhou L, Yuan Q, Guo L, Shan L. Intra-Articular Injection of Adipose-Derived Stem Cells Ameliorates Pain and Cartilage Anabolism/Catabolism in Osteoarthritis: Preclinical and Clinical Evidences. Front Pharmacol. 2022;13:854025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 60. | Zeng WN, Zhang Y, Wang D, Zeng YP, Yang H, Li J, Zhou CP, Liu JL, Yang QJ, Deng ZL, Zhou ZK. Intra-articular Injection of Kartogenin-Enhanced Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Knee Osteoarthritis in a Rat Model. Am J Sports Med. 2021;49:2795-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Ueki H, Katagiri H, Tsuji K, Miyatake K, Watanabe T, Sekiya I, Muneta T, Koga H. Effect of transplanted mesenchymal stem cell number on the prevention of cartilage degeneration and pain reduction in a posttraumatic osteoarthritis rat model. J Orthop Sci. 2021;26:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 62. | Sakamoto T, Miyazaki T, Watanabe S, Takahashi A, Honjoh K, Nakajima H, Oki H, Kokubo Y, Matsumine A. Intraarticular injection of processed lipoaspirate cells has anti-inflammatory and analgesic effects but does not improve degenerative changes in murine monoiodoacetate-induced osteoarthritis. BMC Musculoskelet Disord. 2019;20:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Lee SY, Lee SH, Na HS, Kwon JY, Kim GY, Jung K, Cho KH, Kim SA, Go EJ, Park MJ, Baek JA, Choi SY, Jhun J, Park SH, Kim SJ, Cho ML. The Therapeutic Effect of STAT3 Signaling-Suppressed MSC on Pain and Articular Cartilage Damage in a Rat Model of Monosodium Iodoacetate-Induced Osteoarthritis. Front Immunol. 2018;9:2881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D, Viswanathan P, Thej C, Balasubramanian S, Majumdar AS. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 65. | Shoukrie SI, Venugopal S, Dhanoa RK, Selvaraj R, Selvamani TY, Zahra A, Malla J, Hamouda RK, Hamid PF. Safety and Efficacy of Injecting Mesenchymal Stem Cells Into a Human Knee Joint To Treat Osteoarthritis: A Systematic Review. Cureus. 2022;14:e24823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Dhillon J, Kraeutler MJ, Belk JW, Scillia AJ. Umbilical Cord-Derived Stem Cells for the Treatment of Knee Osteoarthritis: A Systematic Review. Orthop J Sports Med. 2022;10:23259671221104409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 67. | Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, Pérez-Garza DM, Acosta-Olivo C, Vilchez-Cavazos F, Diaz-Hutchinson C, Gómez-Almaguer D, Jaime-Pérez JC, Mancías-Guerra C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis. 2018;21:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Hwang JJ, Rim YA, Nam Y, Ju JH. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front Immunol. 2021;12:631291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 69. | Yu H, Huang T, Lu WW, Tong L, Chen D. Osteoarthritis Pain. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 70. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1178] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 71. | Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14:1307-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 72. | Kuah D, Sivell S, Longworth T, James K, Guermazi A, Cicuttini F, Wang Y, Craig S, Comin G, Robinson D, Wilson J. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med. 2018;16:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 73. | Petri RM, Hackel A, Hahnel K, Dumitru CA, Bruderek K, Flohe SB, Paschen A, Lang S, Brandau S. Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem Cell Reports. 2017;9:985-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 74. | Zha K, Li X, Yang Z, Tian G, Sun Z, Sui X, Dai Y, Liu S, Guo Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. NPJ Regen Med. 2021;6:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 75. | Kwon DG, Kim MK, Jeon YS, Nam YC, Park JS, Ryu DJ. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 76. | Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, Peyrafitte JA, Jorgensen C, Bourin P, Fleury-Cappellesso S, Facchini A, Noël D, Lisignoli G. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65:1271-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 77. | Bryk M, Karnas E, Mlost J, Zuba-Surma E, Starowicz K. Mesenchymal stem cells and extracellular vesicles for the treatment of pain: Current status and perspectives. Br J Pharmacol. 2022;179:4281-4299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012;109:20602-20607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 79. | Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WE, Baba H. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 2015;33:1902-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 80. | Liu H, Zhu X, Cao X, Chi A, Dai J, Wang Z, Deng C, Zhang M. IL-1β-primed mesenchymal stromal cells exert enhanced therapeutic effects to alleviate Chronic Prostatitis/Chronic Pelvic Pain Syndrome through systemic immunity. Stem Cell Res Ther. 2021;12:514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Kouroupis D, Bowles AC, Greif DN, Leñero C, Best TM, Kaplan LD, Correa D. Regulatory-compliant conditions during cell product manufacturing enhance in vitro immunomodulatory properties of infrapatellar fat pad-derived mesenchymal stem/stromal cells. Cytotherapy. 2020;22:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Miteva K, Pappritz K, Sosnowski M, El-Shafeey M, Müller I, Dong F, Savvatis K, Ringe J, Tschöpe C, Van Linthout S. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of Coxsackievirus B3-induced inflammatory cardiomyopathy. Sci Rep. 2018;8:2820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Zhu Q, Li XX, Wang W, Hu J, Li PL, Conley S, Li N. Mesenchymal stem cell transplantation inhibited high salt-induced activation of the NLRP3 inflammasome in the renal medulla in Dahl S rats. Am J Physiol Renal Physiol. 2016;310:F621-F627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Lories RJ, Monteagudo S. Review Article: Is Wnt Signaling an Attractive Target for the Treatment of Osteoarthritis? Rheumatol Ther. 2020;7:259-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 85. | Zhao YT, Qin Y, Yang JS, Huang DG, Hu HM, Wang XD, Wu SF, Hao DJ. Wharton's Jelly-derived mesenchymal stem cells suppress apoptosis of nucleus pulposus cells in intervertebral disc degeneration via Wnt pathway. Eur Rev Med Pharmacol Sci. 2020;24:9807-9814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Aslam N, Abusharieh E, Abuarqoub D, Ali D, Al-Hattab D, Wehaibi S, Al-Kurdi B, Jamali F, Alshaer W, Jafar H, Awidi AS. Anti-oncogenic activities exhibited by paracrine factors of MSCs can be mediated by modulation of KITLG and DKK1 genes in glioma SCs in vitro. Mol Ther Oncolytics. 2021;20:147-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 88. | Kuppa SS, Kim HK, Kang JY, Lee SC, Seon JK. Role of Mesenchymal Stem Cells and Their Paracrine Mediators in Macrophage Polarization: An Approach to Reduce Inflammation in Osteoarthritis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 89. | Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, Chisholm J, Weston A, Chiovitti J, Keating A, Kapoor M, Ogilvie-Harris DJ, Syed KA, Gandhi R, Mahomed NN, Marshall KW, Sussman MS, Naraghi AM, Viswanathan S. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl Med. 2019;8:746-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 90. | Ai M, Hotham WE, Pattison LA, Ma Q, Henson FMD, Smith ESJ. Role of Human Mesenchymal Stem Cells and Derived Extracellular Vesicles in Reducing Sensory Neuron Hyperexcitability and Pain Behaviors in Murine Osteoarthritis. Arthritis Rheumatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 91. | Yang Q, Yao Y, Zhao D, Zou H, Lai C, Xiang G, Wang G, Luo L, Shi Y, Li Y, Yang M, Huang X. LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am J Transl Res. 2021;13:1245-1256. [PubMed] |
| 92. | Wang Z, Zhu H, Dai S, Liu K, Ge C. Alleviation of medial meniscal transection-induced osteoarthritis pain in rats by human adipose derived mesenchymal stem cells. Stem Cell Investig. 2020;7:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | McKinney JM, Doan TN, Wang L, Deppen J, Reece DS, Pucha KA, Ginn S, Levit RD, Willett NJ. Therapeutic efficacy of intra-articular delivery of encapsulated human mesenchymal stem cells on early stage osteoarthritis. Eur Cell Mater. 2019;37:42-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, Roberts S, Baba H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 95. | He L, He T, Xing J, Zhou Q, Fan L, Liu C, Chen Y, Wu D, Tian Z, Liu B, Rong L. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 96. | Chapman V, Markides H, Sagar DR, Xu L, Burston JJ, Mapp P, Kay A, Morris RH, Kehoe O, El Haj AJ. Therapeutic Benefit for Late, but Not Early, Passage Mesenchymal Stem Cells on Pain Behaviour in an Animal Model of Osteoarthritis. Stem Cells Int. 2017;2017:2905104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, Turner JM, Hathway GJ, Bennett AJ, Walsh DA, Kendall DA, Lichtman A, Chapman V. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS One. 2013;8:e80440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 98. | Khatab S, van Osch GJ, Kops N, Bastiaansen-Jenniskens YM, Bos PK, Verhaar JA, Bernsen MR, van Buul GM. Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur Cell Mater. 2018;36:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 99. | Ichiseki T, Shimazaki M, Ueda Y, Ueda S, Tsuchiya M, Souma D, Kaneuji A, Kawahara N. Intraarticularly-Injected Mesenchymal Stem Cells Stimulate Anti-Inflammatory Molecules and Inhibit Pain Related Protein and Chondrolytic Enzymes in a Monoiodoacetate-Induced Rat Arthritis Model. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 100. | Gao R, Ye T, Zhu Z, Li Q, Zhang J, Yuan J, Zhao B, Xie Z, Wang Y. Small extracellular vesicles from iPSC-derived mesenchymal stem cells ameliorate tendinopathy pain by inhibiting mast cell activation. Nanomedicine (Lond). 2022;17:513-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 101. | Guo W, Chu YX, Imai S, Yang JL, Zou S, Mohammad Z, Wei F, Dubner R, Ren K. Further observations on the behavioral and neural effects of bone marrow stromal cells in rodent pain models. Mol Pain. 2016;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest. 2015;125:3226-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |