Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.136
Peer-review started: December 19, 2022
First decision: January 6, 2023
Revised: January 18, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 26, 2023
Processing time: 128 Days and 0.7 Hours
Since dental pulp stem cells (DPSCs) were first reported, six types of dental SCs (DSCs) have been isolated and identified. DSCs originating from the craniofacial neural crest exhibit dental-like tissue differentiation potential and neuro-ectodermal features. As a member of DSCs, dental follicle SCs (DFSCs) are the only cell type obtained at the early developing stage of the tooth prior to eruption. Dental follicle tissue has the distinct advantage of large tissue volume compared with other dental tissues, which is a prerequisite for obtaining a sufficient number of cells to meet the needs of clinical applications. Furthermore, DFSCs exhibit a significantly higher cell proliferation rate, higher colony-formation capacity, and more primitive and better anti-inflammatory effects than other DSCs. In this respect, DFSCs have the potential to be of great clinical significance and translational value in oral and neurological diseases, with natural advantages based on their origin. Lastly, cryopreservation preserves the biological properties of DFSCs and enables them to be used as off-shelf products for clinical applications. This review summarizes and comments on the properties, application potential, and clinical transformation value of DFSCs, thereby inspiring novel perspectives in the future treatment of oral and neurological diseases.
Core Tip: This review is intended to summarize and comment on the properties, application potentials, and clinical transformation value of dental follicle stem cells (DFSCs). Stem cells derived from dental SCs (DSCs) originating from the craniofacial neural crest exhibit dental-like tissue differentiation potentials and neuro-ectodermal features, making them a promising alternative for the treatment of oral and neurological diseases. Moreover, in contrast to other DSCs, DFSCs from the early-developing tissues exhibit a number of superior properties, including larger tissue volume, higher cell proliferation rate, more similar biological profiles to progenitor cells of origin, and better anti-inflammatory effects, etc. These advantages are part of the critical mechanism by which DFSCs exert therapeutic effects and are relevant for large scale scaling and industrial generation for clinical applications. Moreover, cryopreservation preserves the biological properties of DFSCs and enables them to be used as off-shelf products for clinical applications. Therefore, DFSCs could have great clinical prospects and translational value in oral and neurological diseases with natural advantages.
- Citation: Yang C, Du XY, Luo W. Clinical application prospects and transformation value of dental follicle stem cells in oral and neurological diseases. World J Stem Cells 2023; 15(4): 136-149
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/136.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.136
Stem cells are undifferentiated cells characterized by the ability of self-renewal, clonality, and differentiation into various types of cells[1]. With the development of cell biology and modern medicine, the application of stem cells has brought a new approach for restoring tissue defects and treatment of some refractory diseases[2,3]. To date, regenerative medicine has become an important branch of modern medical science and has played an increasingly important role in clinical treatment. Stem cells are a vital element of regenerative medicine, and different stem cell types have their own advantages and drawbacks[4]. With stem cell research deepening, it is crucial to explore the appropriate stem cells to solve clinical problems and obtain better clinical outcomes.
Stem cells for application in regenerative medicine are divided into the following two categories: Pluripotent and multipotent. Pluripotent stem cells include natural embryonic stem cells (ESCs) originating from the inner cell mass of the blastocyte and artificially induced pluripotent SCs (iPSCs)[5]. Multipotent stem cells refer to adult stem cells that exist in different tissues of the body, and their main function is general homeostasis and repair of injured tissues by differentiation[6]. Adult stem cells are also known as postnatal stem cells and mainly originate from either epithelial cells or mesenchymal cells[4].
Mesenchymal SCs (MSCs) are typical adult stem cells derived from mesenchymal tissues. In addition to the ability of self-renewal, high proliferation, and multidirectional differentiation, MSCs also present high immunomodulation and antiapoptotic capacity, to achieve the purpose of promoting tissue regeneration and disease treatment. Compared with ESCs, MSCs are multipotent but limited in terms of differentiation ability. However, the acquisition and clinical application of ESCs are also dramatically restricted to ethical, legal, safety, and source constraints[7]. Moreover, since iPSCs were first generated in 2006 by Takahashi et al[8] with four factors, a growing number of researchers have focused on the clinical application prospects and transformation value of iPSCs and their specialized differentiation cells. Nevertheless, iPSCs also present some worrying aspects as follows: (1) iPSCs have pluripotency, similar to ESCs, as well as the ability to cause possible teratomas while the specialized differentiated final product contains undifferentiated cells[9]; (2) there is possibility of tumor formation by integrated oncogenes, insertional mutagenesis, and disrupting tumor suppressor genes[9]; (3) epigenetic memories and genomic aberrations have been detected in reprogrammed cells[10]; and (4) human skin fibroblast-derived iPSCs have a 72% ultraviolet light-related damage, and human blood-derived iPSCs have a high prevalence of acquired BCL6 corepressor mutations (26.9% of lines)[11].
The first human trial using retinal pigment epithelium derived from iPSCs for the treatment of age-related macular degeneration was started in Japan in 2014 but was later suspended[12]. Recently, another study has reported a distinctive case of immature teratoma after the patient underwent autologous iPSC-derived cell therapy for diabetes. Two months after the cells had been injected into the deltoid muscle, a teratoma formed in the injection area[13]. Safety remains the most important criterion of a cellular product for clinical applications[9]; thus, more safety-related quality detections with iPSC-derived cell therapy should be performed. In contrast to ESCs, iPSCs, and iPSCs specialized differentiation cells, several studies have demonstrated that MSCs exhibit good safety profiles, making MSCs the most widely used cell type for clinical applications at present[14-16]. To date, various MSCs have been discovered in different tissues, including bone marrow, umbilical cord, umbilical cord blood, placenta, amniotic fluid, hair follicle, adipose tissue, and dental tissues[17-26].
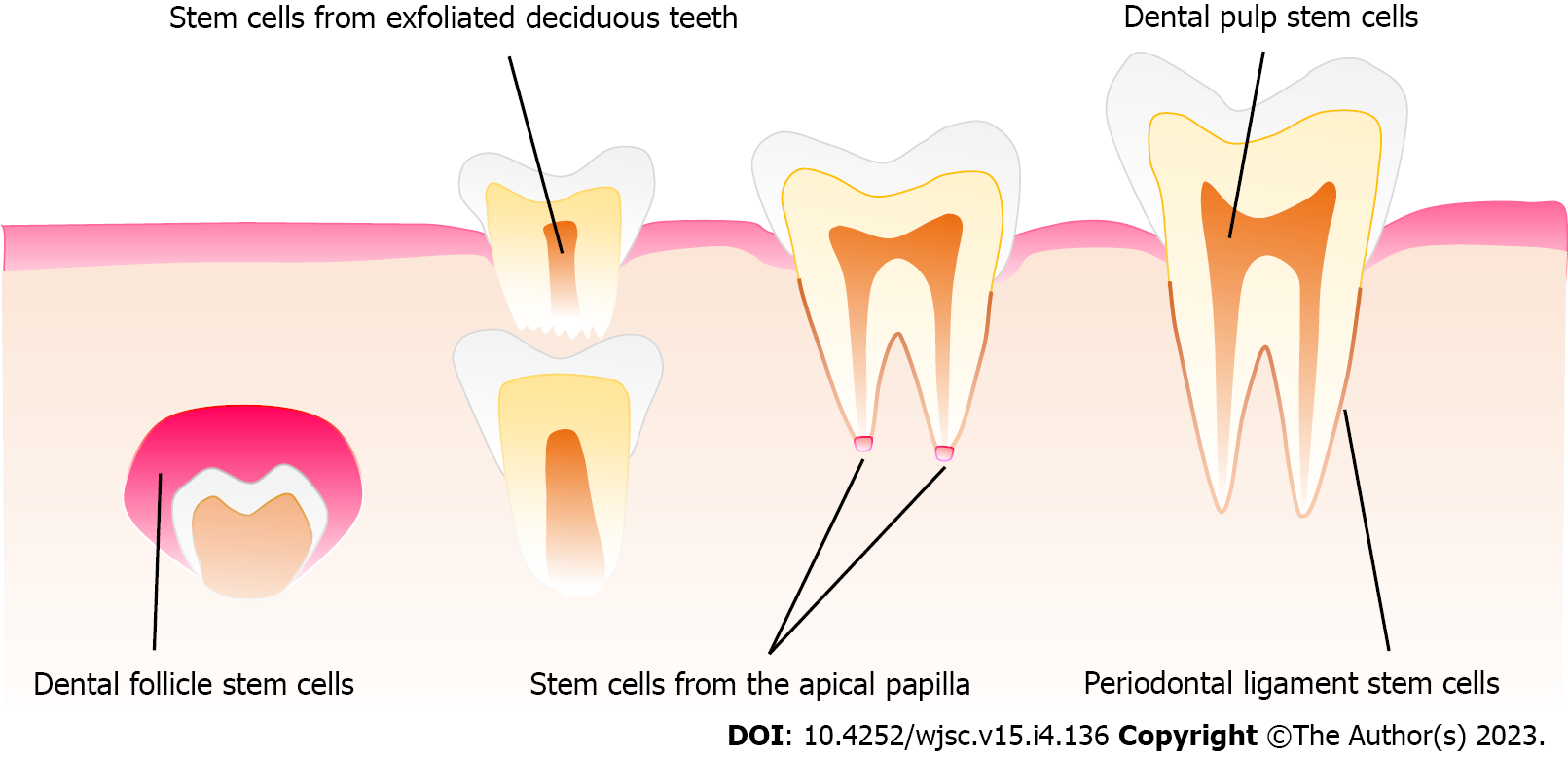
Since dental pulp stem cells (DPSCs) were first reported by Gronthos et al[27] in 2000, a growing interest has been observed toward the potential of dental stem cells (DSCs) for the treatment of oral and neurological diseases[28,29]. Six types of human DSCs have been isolated and identified at different stages of tooth development[30]. For instance, DPSCs, stem cells from apical papilla (SCAPs)[31], stem cells from human exfoliated deciduous teeth (SHEDs)[32], periodontal ligament stem cells (PDLSCs)[33], and gingival mesenchymal stem cells (GMSCs)[34] can be gained after tooth eruption, while dental follicle stem cells (DFSCs) are a special kind of DSCs, which can be obtained at the early developing stage of the tooth prior to eruption[35] (Figure 1).
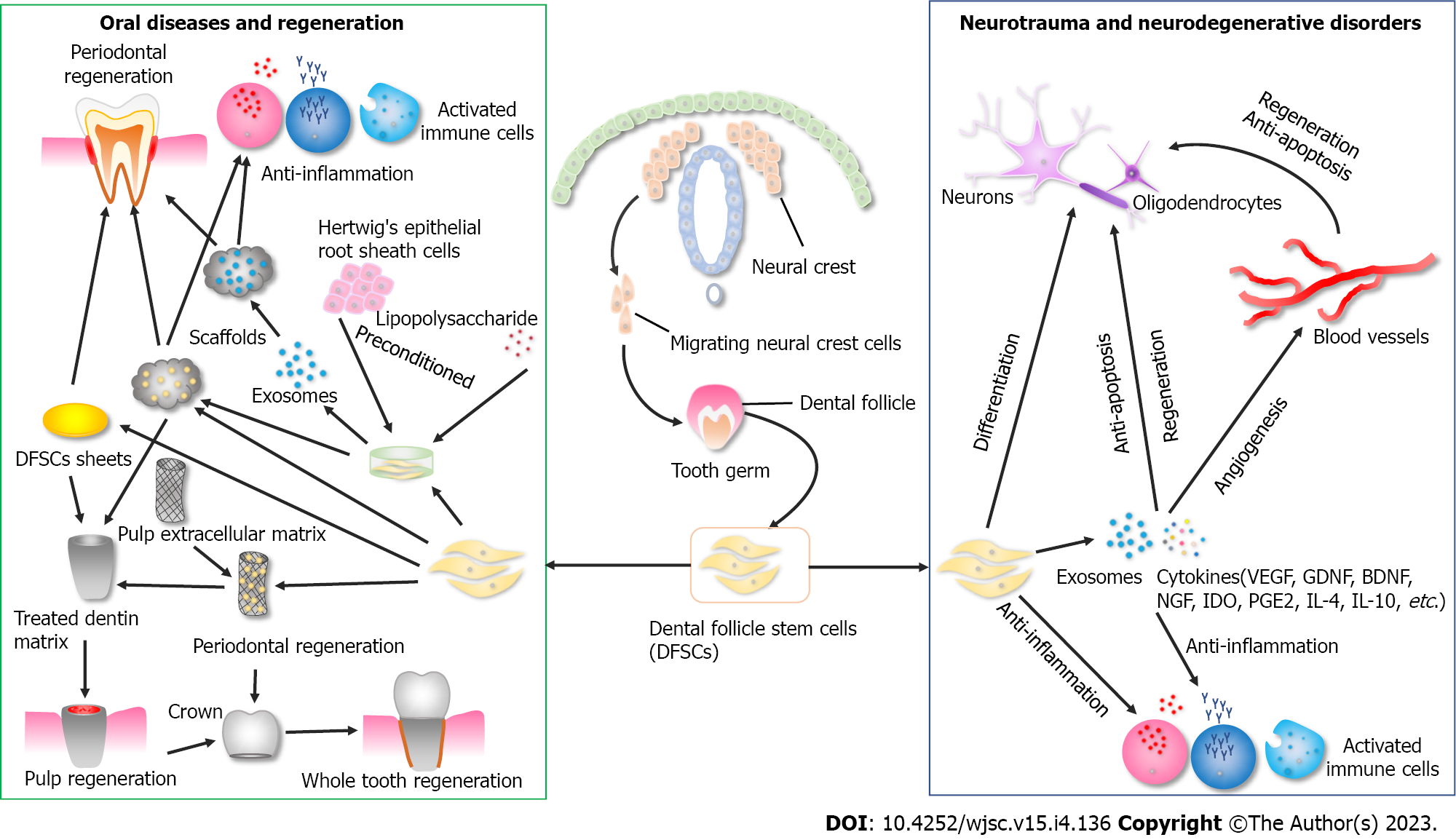
The dental follicle is an ectomesenchyme-derived and loose connective tissue originating from the cranial neural crest. During the bud stage of tooth development, the dental follicle is formed and surrounds the dental papilla and enamel organ, which plays a critical role in tooth eruption via regulating bone resorption and formation[36]. In the late bell stage, the dental follicle gives rise to the supporting tissues of the tooth-periodontium, including cementum, alveolar bone, and periodontal ligament[37]. Compared with other dental tissues, dental follicle tissue has the obvious advantage of large tissue volume, which is the premise of obtaining a sufficient number of cells to meet the needs of clinical application. Meanwhile, the number of DFSCs obtained in the same passage is far greater than that of DPSCs, accordingly being more suitable for large-scale expansion and industrial generation[38]. Due to the origin of dental follicle tissue in the early stage of tooth development, it has been demonstrated that DFSCs have the following advantages over DPSCs. First, DFSCs exhibit a significantly higher cell proliferation rate and colony-formation capacity than DPSCs[38], which further suggests that DFSCs may be better able to meet the needs of clinical transformation in terms of quantity and quality. Second, DSCs originate from neural crest cells. DFSCs have more similar protein profiles with cranial neural crest cells (CNCCs) than DPSCs, and possess high potency in odontogenic differentiation in vitro[39], which demonstrates that DFSCs may have better transformation advantages for the treatment of neurological and oral diseases. Third, DFSCs have better inhibitory effects on the proliferation of proinflammatory lymphocytes and better promote the proliferation of anti-inflammatory Treg cells than SHEDs and DPSCs[40], which indicates that DFSCs have better immunoregulation capacity. Of note, the advantages in terms of quantity, quality, differentiation, and immunoregulatory properties, as mentioned above, are part of the critical mechanism by which MSCs exert therapeutic effects. In this regard, DFSCs appear to be the candidate cells with natural advantages for regenerative medicine compared with stem cells from other dental tissues. Indeed, as a kind of dental tissue-derived stem cells originating from the neural crest, DFSCs may be more advantageous in promoting oral tissue regeneration, including periodontium, dental pulp, and tooth regeneration, as well as in treating neurological injury and neurodegenerative diseases, such as spinal cord and brain injury, as well as Parkinson’s disease (PD) and Alzheimer’s disease.
The periodontium is a complex functional unit that plays a critical role in the oral cavity[41]. Periodontitis is a common and chronic inflammatory disease caused by plaque in the teeth[42]. As the disease progresses, gingival recession, loss of soft tissue attachment, and even intrabony defects can occur, ultimately resulting in premature tooth loss[43]. To date, while numerous conventional clinical treatments for periodontitis have been shown to control inflammation and aggressive progression, these strategies have not been able to achieve periodontal regeneration. Previous preclinical studies have demonstrated that the transplantation of stem cells presented the potential and provided new hope for periodontal regeneration[44,45].
The combination of DFSCs and hydroxyapatite scaffold forms a cementum-like matrix in vivo after transplantation into mice[35]. Scaffold plays a critical role in tissue engineering, providing support for transplanted stem cells and enhancing the therapeutic effects of tissue regeneration. The cell sheet technique prevents extracellular matrix degradation and provides a novel scaffold-free cell delivery strategy[46]. The extracellular matrix contains numerous growth factors and provides support to the cells without the need for additional scaffolds[47]. In addition, cell viability and function can be restored without the digestion operation step. A complex of dental follicle cell sheets forms periodontal tissue-like structures, including cementum-like structures and periodontal ligament with abundant blood vessels after transplantation into the subcutaneous areas of nude mice[48], demonstrating that DFSC sheets have the potential to achieve the goal of periodontal regeneration.
The tooth root development requires stimulation from Hertwig’s epithelial root sheath (HERS), and DFSCs differentiate into cementoblasts during the epithelial-mesenchymal interaction process[49]. Accordingly, it has been demonstrated that the formation of cementum and periodontal ligament-like tissue was enhanced at 5 wk after implantation into rat submentum when DFSCs had been pre-exposed to HERSCs[50], suggesting that establishing a microenvironment similar to tooth root development is important for periodontal regeneration. Moreover, appropriate microinflammation preconditioning is important for improving the regeneration and immunoregulation capacity of DFSCs and their secreted exosomes. Lipopolysaccharides (LPS) upregulated the expression of osteogenic and adhesion-related proteins in DFSC sheets, which showed good performance in canine periodontal regeneration[51]. Furthermore, LPS enhanced the paracrine activity and immunomodulatory effect of DFSCs, and LPS-preconditioned DFSC-derived small extracellular vesicles (sEV) were beneficial for repairing lost alveolar bone in rats[52]. A later study further clarified that LPS-preconditioned DFC-sEV inhibited intracellular reactive oxygen species (ROS) as an antioxidant; it reduced the NF-κB receptor activator ligand/osteoprotegerin ratio of PDLSCs by inhibiting ROS/Jun amino-terminal kinase (JNK) signaling under inflammatory conditions and promoted macrophages to polarize toward the M2 phenotype via ROS/extracellular signal-regulated kinase (ERK) signaling[53].
For clinical applications, autologous PDLSCs have been used in clinical studies (ClinicalTrials.gov Identifier: NCT01357785; www.isrctn.com Identifier: ISRCTN13093912), and the results showed no significant differences in the effect on intrabony lesions between PDLSCs groups and scaffold-only groups[54,55]. Although some previous studies have demonstrated that PDLSCs might be the first choice for periodontal regeneration[28,43], DFSCs can give rise to periodontal supporting tissues, including cementum, alveolar bone, and periodontal ligaments. Moreover, previous comparative studies have revealed that DFSCs exhibit a stronger capacity for the regeneration of cementum and periodontal attachment than PDLSCs[48,56]. Thus, DFSCs can be considered a better candidate cell source for periodontal regeneration.
Dental pulp necrosis is an irreversible inflammatory dental disease that causes destruction and loss of the pulp tissue, resulting in the loss of teeth and even abscesses of the jaw[57]. Bacterial infections play a key role in the development of dental pulp necrosis. Bacterial invasion and colonization were observed in the pulp necrotic areas with caries exposure and symptomatic irreversible pulpitis. Additionally, bacterial penetration of blood vessels occurred, which may spread bacterial infections[58]. Before it leads to more serious consequences, endodontic treatment must be performed to remove the damaged pulp[59,60]. However, the tooth becomes more fragile and susceptible to caries, periapical infection, and fracture after endodontic treatment because of the loss of blood and nutrition supply[59].
Stem cell-based dental pulp regeneration has the objective of developing new methods to replace the conventional treatment of dental pulp necrosis. Scaffolds, stem cells, and growth factors have been used for dental pulp regeneration. Findings from animal studies have shown that DPSCs seeded in a collagen scaffold with dentin matrix protein 1 were able to induce the formation of dental pulp-like tissues in immunodeficient mice[61], and DPSCs pellets stimulated by bone morphogenetic protein 2 promoted the dentin formation onto the amputated pulp of dog teeth[62]. Later, autologous pulp stem cells and granulocyte colony-stimulating factor with a clinical-grade atelocollagen scaffold were transplanted into the dog pulpectomized teeth, promoting pulp/dentin regeneration[63]. A pilot clinical study using a similar strategy has also observed complete pulp regeneration in humans[64]. Moreover, a scaffold-free translation strategy has also been used for pulp regeneration. DPSC aggregates derived from the autologous canine tooth pulp induced the regeneration of three-dimensional pulp tissue equipped with blood vessels and sensory nerves 12 mo after treatment[65]. Consequently, DPSCs are derived from the pulp tissue and it appears that DPSCs have the potential to promote dentin-pulp regeneration, while DFSCs have a similar capacity.
Previous studies have demonstrated that providing an inductive microenvironment with a suitable scaffold could achieve dentin and even dental pulp regeneration. Treated dentin matrix (TDM) is derived from animal or human dentin matrix treated with ethylenediamine tetraacetic acid, containing abundant collagen, noncollagenous proteins, and growth factors. Both rat and human TDM induce and support complete dentin regeneration, in addition to inducing transplanted DFSCs to differentiate into odontoblasts and express dentin sialoprotein and matrix protein 1[66,67]. The cell sheet technique promotes dental pulp regeneration. DFSC sheets were substituted for DFSCs and, in combination with TDM, were implanted subcutaneously into the dorsum of mice. New dentin pulp-like tissues were observed after eight weeks post-transplantation[68]. Moreover, native dental pulp extracellular matrix (NDPE) can be used to obtain prefabricated-shaped dental pulp. Dentin-pulp complex-like tissues and columnar odontoblasts-like layers arranged along the interface between newly formed predentin matrix could be found after DFSCs-NDPE-TDM transplantation to the jaw of miniature swine for 12 wk[69]. Therefore, DFSCs could also exhibit the capacity to regenerate the dentin-pulp tissues with suitable inductive scaffolds.
The regeneration of whole teeth is a major objective and promise of oral regenerative medicine. To date, two main strategies have been used to achieve this goal. The first strategy is a combination of mesenchymal and epithelial cells to construct a bioengineered tooth germ, which is then transplanted into the alveolar socket. Parts of animals showed whole-tooth eruption around 3.5 mo after tooth-germ implantation[70]. However, several barriers should be addressed in future studies of bioengineered teeth, such as indiscriminate shape and smaller size than natural teeth[4,70-73]. The second strategy includes the direct reconstruction of the functional units, such as bio-tooth root with periodontal tissue-like structures and dentin-pulp-like tissues.
As with the combination of implants and crowns to replace missing teeth, researchers have explored implant-like scaffolds to reconstruct the root. As mentioned above, TDM has the potential to be a suitable scaffold for root reconstruction. Yang et al[68] used calcified human dentin to model an alveolar microenvironment and used DFSC sheets-TDM-DFSC sheets to reconstruct the pulp-root-periodontium structure. TDM could induce and support DFSC sheets to develop new dentin-pulp and cementum-periodontium-like tissues after subcutaneous transplantation into nude mice for 8 wk[68]. Moreover, with seed DFSCs, the complex of aligned PLGA/Gelatin electrospun sheet/TDM/NDPE generated tooth root-like tissues after 12 wk of transplantation in porcine jaws[69]. Shape-optimized TDM scaffolds with DFSCs were transplanted into the alveolar bone of swine, and ceramic crowns were installed. These bio-tooth roots not only regenerated histologically but also allowed masticatory functions and remained stable for 3 mo[74]. In nonhuman primates, a novel functional biological root complex was constructed based on DFSC sheets and in vitro three dimensional (3D) suspension culture. This complex was then transplanted into rhesus monkeys and gradually restored occlusal function and long-term masticatory function for 2 years during the evaluation period[75]. In this regard, DFSCs with suitable scaffolds have the potential to be suitable stem cells for whole-tooth reconstruction based on bio-booth root regeneration.
As mentioned above, dental follicle is an ectomesenchyme tissue that originates from the cranial neural crest. DFSCs not only express the markers typical of MSCs [e.g., cluster of differentiation (CD) 44, CD90, CD105] but also express neural cell markers (e.g., Nestin, β-III tubulin, and CNpase)[76-78] and even embryonic stem cell markers (octamer-binding transcription factor 4 and sex-determining region Y-box 2)[79], indicating that DFSCs may retain some of the neural and embryonic features and can differentiate into neural-like cells. DFSCs display neural-like cell morphology with small neurite-like cell extrusions with a neuronal differentiation strategy[76]. Dental pulp comprises blood vessels, neural fibers, and connective tissue, and DPSCs exhibit extraordinary capacity to differentiate into neural-like cells and represent a potential source for neuronal regeneration therapies[80]. However, a comparative study has demonstrated that DFSCs possess more similar protein profiles to CNCCs than DPSCs[39]. Moreover, compared with DPSCs, the expression of CNpase, neurofilament protein, Nestin, and β-III tubulin of DFSCs was upregulated significantly after treatment in the same neural-induction condition[78]. From this perspective, DFSCs may be a better candidate cell type for neural differentiation and even for the treatment of neurological diseases based on pre-differentiation.
Spinal cord injury (SCI) is a severe neurological trauma that causes the impairment of sensory and motor functions[81]. The acute stage of injury is directly caused by trauma, including compression, contusion, and shear injury forces. Proinflammatory cells are then activated, releasing abundant inflammatory cytokines, which induce a cascade of secondary injury[82]. Many neurons, astrocytes, and other neural cells die in the injured area due to necrosis or apoptosis during the secondary injury[83]. Until now, current clinical strategies have not achieved satisfactory outcomes due to irreversible damage to neural cells. Stem cell-based therapies hold the promise of developing new approaches for the treatment of SCI. Several types of stem cells have been used for transplantation, such as neural SCs (NSCs), ES/iPS-derived NSCs, and MSCs[84]. However, the sources of NSCs are limited and also face ethical issues. ES/iPS-derived cells exhibit some of the aforementioned worrying aspects. Thus, MSCs may now be a better candidate cell type for SCI. Researchers have compared the therapeutic effects of MSCs from bone marrow and dental pulp. Transplantation of DPSCs promoted marked recovery of locomotor function in the hind limbs, while transplantation of bone marrow SCs (BMSCs) resulted in substantially less recovery of locomotor function in rats with complete SCI. The main mechanisms include inhibition of apoptosis in neurons, astrocytes, and oligodendrocytes; promotion of regeneration of disjunct axons; and differentiation into mature oligodendrocytes to replace lost cells[85]. Later, another comparative study explored the differences among DFSCs, SCAPs, and DPSCs for the treatment of SCI. Findings from an animal study demonstrated that all three types of DSCs, especially DFSCs, have the potential to promote functional recovery after SCI by reducing the inflammatory response, promoting neurite regeneration, reducing progressive hemorrhagic necrosis, and differentiation into mature neurons and oligodendrocytes but not astrocytes[77]. Moreover, scaffolds with DFSCs were also used to repair the spinal cord defect; for example, aligned poly-ε-caprolactone/poly-lactide-co-glycolic acid electrospun material allowed nerve fibers to pass through, and induced DFSCs to differentiate in vivo[86].
PD is a common and progressive neurodegenerative disorder characterized by tremors, rigidity, and bradykinesia[87,88]. The aggregates of ubiquitin and α-synuclein-positive protein, Lewy bodies, and the loss of dopaminergic neurons in the substantia nigra pars compacta are the main characteristics that define PD[89]. The incidence of the disease rises steeply with age, affecting approximately 1% of the population between the ages of 70 years and 79 years[90]. Although pharmacological approaches (such as amantadine and levodopa) and nonpharmacologic strategies (deep brain stimulation, exercise, and physical therapy) have been used in PD treatment[91,92], these therapeutic strategies only delay the progression of the disease and relieve the symptoms but do not achieve regeneration of dopaminergic neurons. There has been considerable excitement about the use of MSCs to treat neurodegenerative diseases via secretion of anti-inflammatory factors [e.g., indoleamine (2,3)-dioxygenase (IDO), prosta
Both in oral and neurological diseases, the immunomodulatory capacity is one of the most crucial functions of MSCs to facilitate the repair or regeneration of damaged tissues[51,77]. MSCs can regulate the proliferation, activation, maturation, and function of innate and adaptive immune cells via cell-to-cell direct contact, soluble cytokines, and exosomes[102]. As a kind of MSCs, DFSCs also present immunomodulatory characteristics. In acute lung injury models, DFSCs could suppress the production of proinflammatory cytokines, such as monocyte chemoattractant protein (MCP)-1, interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α; decrease the proportion of proinflammatory macrophage M1 phenotype; increase the level of anti-inflammatory cytokine IL-10 and the proportion of anti-inflammatory M2 phenotype both in bronchoalveolar lavage fluid in vivo and in vitro experiments[103]. Furthermore, DFSCs also exhibit great therapeutic potential in autoimmune diseases and chronic inflammatory disorders. DFSCs suppress the proliferation of T lymphocytes and lymphocyte apoptosis but increase the number of Tregs. DFSCs also reduce the secretion level of TNF-α but upregulate the level of IL-10 in peripheral blood mononuclear cells (PBMCs) of patients with rheumatoid arthritis[104]. Moreover, in inflamed mononuclear cell samples of patients with Crohn’s disease, DFSCs also downregulate lymphocyte proliferation, CD4 + IL22BP T cell ratio, and the secretion of TNF-α and IL-6, but increase the frequency of Tregs and the level of IL-10[105]. From the case studies mentioned above, we can briefly summarize that DFSCs exert the common immune modulatory capacity via suppressing the proinflammatory immune cells [e.g., T-helper (Th) 1, Th17, macrophage M1] and cytokines (e.g., TNF-α, IL-1, IL-6) and increasing the number of anti-inflammatory immune cells (e.g., Tregs, macrophage M2) and cytokines (e.g., IL-4 and IL-10). Therefore, it is necessary to design experiments to examine the ratio or level changes in inflammatory cells and cytokines after co-culture of DFSCs with activated PBMCs, which can help us to screen the cells with superior immune properties and obtain better clinical outcomes.
Stem cell-based therapies have been investigated for tissue engineering and treatment of many diseases for several decades, and cryopreservation can be used to effectively preserve stem cells. Cryopreservation is the process of gradually cooling cells or tissues to sub-zero temperatures, and finally preserving them in the gas phase liquid nitrogen (-150°C to -196°C) for an extended period. In this state, the biological activity of the cells is stopped, and their viability can be restored by careful thawing when needed[106]. Human DFSCs isolated from fresh and cryopreserved dental follicles show similar biological characteristics, such as proliferation ability, surface markers, and tri-linage differentiation capacities[107]. Moreover, the two types of DFSCs possess the same osteogenic differentiation potential and immunomodulatory properties for bone tissue engineering, resulting in the inhibition of adaptive immune response, which demonstrates that the stemness and immunomodulatory capacity of long-term-preserved dental follicle tissues can be restored[108]. Another study compared the biological characteristics between cryopreserved DFSCs and the cells from cryopreserved dental follicles. After 3 mo of cryopreservation, the cells from the cryopreserved dental follicles showed similar levels of stemness and apoptosis-related genes and exhibited similar osteogenic and adipogenic differentiation capabilities to cryopreserved DFSCs[109]. In this regard, both cryopreservation of DFSCs from fresh dental follicle tissues and direct cryopreservation of dental follicle tissues can preserve the biological properties of the cells. As a consequence, cryopreservation technology enables DFSCs to the off-the-shelf products for clinical applications.
Cryopreservation addresses the preservation of DFSCs. Another problem is that it is questionable whether allogeneic stem cells exhibit the same therapeutic effects as autologous stem cells. A previous study compared the therapeutic effects of allogeneic and autologous PDLSCs on periodontal tissue regeneration in a miniature pig model of periodontitis. Significant periodontal tissue regeneration was achieved in both transplanted groups without significant difference due to low immunogenicity and marked immunosuppression of T-cell antigen via PGE2[110]. Furthermore, allogeneic DSCs likely did not affect the therapeutic effects because of their inherent characteristics. However, more experiments should be performed to compare the outcomes of allogeneic and autologous DFSCs in different diseases.
In addition to cell cryopreservation, some biological materials can be cryopreserved. For instance, after being cryopreserved in liquid nitrogen with cryoprotectant for several months, the cryopreserved TDM exhibited superior mechanical properties, more dentin-related proteins, and a larger pore diameter than the fresh TDM. The cryopreserved TDM was also able to induce dental follicle cells to regenerate new dentin-pulp-like tissues[111], suggesting that the cryopreservation techniques address the preservation of biological materials and also enable them to be used as off-the-shelf scaffold for tissue engineering.
In this review, it was found that among MSCs, stem cells derived from dental tissue originating from the craniofacial neural crest exhibit dental-like tissue differentiation potential and neuro-ectodermal features, which makes them a promising alternative for the treatment of oral and neurological diseases (Figure 2). Moreover, in contrast to other DSCs, those from the early-developing tissues exhibit several superior properties, including larger tissue volume, higher cell proliferation rate and colony-formation capacity, more similar biological profiles to progenitor cells of origin, and better anti-inflammatory effects. These advantages are part of the critical mechanism by which MSCs exert therapeutic effects and are relevant for large-scale scaling and industrial generation for clinical applications. Cryopreservation preserves the biological properties of DFSCs and enables them to be used as off-shelf products for clinical applications. Therefore, DFSCs could have great clinical prospects and translational value in oral and neurological diseases with natural advantages.
Currently, SHEDs, DPSCs, PDLSCs, and GMSCs have been used in clinics[54,65,112,113], and clinical trials of DFSCs are also forthcoming. For future clinical applications of DFSCs, several key points need further investigation. First, as one of the three essential elements in tissue engineering, the selection of stem cells is vital. Therefore, potency assessment and screening criteria should be established, including donor screening and culture system optimization. In addition to sterility, safety, activity, homogeneity, purity, and stability, the levels of released cytokines or markers associated with immunomodulation (e.g., IL-4 and IL-10), dental tissue (e.g., VEGF, dentin sialophosphoprotein), or neural regeneration (e.g., Nestin, GDNF, and brain-derived neurotrophic factor, nerve growth factor) should be detected[17]. Furthermore, the potency evaluation system should contain the inhibition of proinflammatory immune cells (e.g., Th1, Th17, and macrophage M1) and cytokines (e.g., TNF-α, interferon-γ, IL-1, and IL-6), the promotion of anti-inflammatory immune cells (e.g., Tregs, macrophage M2) and cytokines (e.g., IL-4 and IL-10) after PBMCs co-culture with DFSCs in vitro, and the promotion of neuron and oligodendrocyte or multi-differentiation capacity that includes neural/osteogenic differentiation[79,103,105,114-120]. Then, in vivo experiments should be used to verify the correctness of the potency assessment system. Second, in the field of tissue engineering, appropriate scaffold materials have a synergetic effect on the promotion of regeneration with stem cells. Some of these materials include beta-tricalcium phosphate, collagen sponge, xenogeneic bone substitute, and TDM, some of which have been used in the clinic[54,55,74,113,121,122]. Without a doubt, the quest for more suitable scaffold materials remains a long-term process. Third, a wide range of bioactive factors and RNAs, including proteins, cytokines, proinflammatory components, extracts from biological materials, long non-coding RNAs, and microRNAs, have been used to enhance differentiation and immunomodulation capacities[53,123-127], and these strategies may further improve the therapeutic potential of DFSCs in clinical applications in the future. Collectively, with the development of materials and preconditioning strategies, and in combination with the natural superiority exhibited by DFSCs in terms of medicinal properties, DFSC-based therapeutics are a promising strategy for the future treatment of oral and neurological diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng YZ, China; Rotondo JC, Italy S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 2. | Brianna, Ling APK, Wong YP. Applying stem cell therapy in intractable diseases: a narrative review of decades of progress and challenges. Stem Cell Investig. 2022;9:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hussain A, Tebyaniyan H, Khayatan D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022;2022:5304860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Soudi A, Yazdanian M, Ranjbar R, Tebyanian H, Yazdanian A, Tahmasebi E, Keshvad A, Seifalian A. Role and application of stem cells in dental regeneration: A comprehensive overview. EXCLI J. 2021;20:454-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 5. | Aydin S, Şahin F. Stem Cells Derived from Dental Tissues. Adv Exp Med Biol. 2019;1144:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Paz AG, Maghaireh H, Mangano FG. Stem Cells in Dentistry: Types of Intra- and Extraoral Tissue-Derived Stem Cells and Clinical Applications. Stem Cells Int. 2018;2018:4313610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 584] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 8. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18178] [Article Influence: 956.7] [Reference Citation Analysis (0)] |
| 9. | Jung Y, Bauer G, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1035] [Cited by in RCA: 948] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 11. | Rouhani FJ, Zou X, Danecek P, Badja C, Amarante TD, Koh G, Wu Q, Memari Y, Durbin R, Martincorena I, Bassett AR, Gaffney D, Nik-Zainal S. Substantial somatic genomic variation and selection for BCOR mutations in human induced pluripotent stem cells. Nat Genet. 2022;54:1406-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 12. | Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33:890-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 13. | Han L, He H, Yang Y, Meng Q, Ye F, Chen G, Zhang J. Distinctive Clinical and Pathologic Features of Immature Teratomas Arising from Induced Pluripotent Stem Cell-Derived Beta Cell Injection in a Diabetes Patient. Stem Cells Dev. 2022;31:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 14. | Cui J, Jin L, Ding M, He J, Yang L, Cui S, Wang X, Ma J, Liu A. Efficacy and safety of mesenchymal stem cells in the treatment of systemic sclerosis: a systematic review and meta-analysis. Stem Cell Res Ther. 2022;13:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Qu W, Wang Z, Engelberg-Cook E, Yan D, Siddik AB, Bu G, Allickson JG, Kubrova E, Caplan AI, Hare JM, Ricordi C, Pepine CJ, Kurtzberg J, Pascual JM, Mallea JM, Rodriguez RL, Nayfeh T, Saadi S, Durvasula RV, Richards EM, March K, Sanfilippo FP. Efficacy and Safety of MSC Cell Therapies for Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Stem Cells Transl Med. 2022;11:688-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 17. | Yang C, Wu M, You M, Chen Y, Luo M, Chen Q. The therapeutic applications of mesenchymal stromal cells from human perinatal tissues in autoimmune diseases. Stem Cell Res Ther. 2021;12:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Lightner AL, Ream J, Nachand D, Fulmer C, Regueiro M, Steele SR. Remestemcel-L allogeneic bone marrow-derived mesenchymal stem cell product to treat medically refractory Crohn’s colitis: preliminary phase IB/IIA study. Br J Surg. 2022;109:653-655. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Lian XF, Lu DH, Liu HL, Liu YJ, Han XQ, Yang Y, Lin Y, Zeng QX, Huang ZJ, Xie F, Huang CH, Wu HM, Long AM, Deng LP, Zhang F. Effectiveness and safety of human umbilical cord-mesenchymal stem cells for treating type 2 diabetes mellitus. World J Diabetes. 2022;13:877-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Kim HJ, Cho KR, Jang H, Lee NK, Jung YH, Kim JP, Lee JI, Chang JW, Park S, Kim ST, Moon SW, Seo SW, Choi SJ, Na DL. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimers Res Ther. 2021;13:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 21. | Aghayan HR, Salimian F, Abedini A, Fattah Ghazi S, Yunesian M, Alavi-Moghadam S, Makarem J, Majidzadeh-A K, Hatamkhani A, Moghri M, Danesh A, Haddad-Marandi MR, Sanati H, Abbasvandi F, Arjmand B, Azimi P, Ghavamzadeh A, Sarrami-Forooshani R. Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): safety profile assessment. Stem Cell Res Ther. 2022;13:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 22. | Bajek A, Olkowska J, Walentowicz-Sadłecka M, Walentowicz P, Sadłecki P, Grabiec M, Bodnar M, Marszałek A, Dębski R, Porowińska D, Czarnecka J, Kaźmierski Ł, Drewa T. High Quality Independent From a Donor: Human Amniotic Fluid Derived Stem Cells-A Practical Analysis Based on 165 Clinical Cases. J Cell Biochem. 2017;118:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Li S, Li H, Zhangdi H, Xu R, Zhang X, Liu J, Hu Y, Ning D, Jin S. Hair follicle-MSC-derived small extracellular vesicles as a novel remedy for acute pancreatitis. J Control Release. 2022;352:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 24. | Zhou L, Wang H, Yao S, Li L, Kuang X. Efficacy of Human Adipose Derived Mesenchymal Stem Cells in Promoting Skin Wound Healing. J Healthc Eng. 2022;2022:6590025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Suda S, Nito C, Ihara M, Iguchi Y, Urabe T, Matsumaru Y, Sakai N, Kimura K; J- REPAIR trial group. Randomised placebo-controlled multicentre trial to evaluate the efficacy and safety of JTR-161, allogeneic human dental pulp stem cells, in patients with Acute Ischaemic stRoke (J-REPAIR). BMJ Open. 2022;12:e054269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Poliwoda S, Noor N, Downs E, Schaaf A, Cantwell A, Ganti L, Kaye AD, Mosel LI, Carroll CB, Viswanath O, Urits I. Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthop Rev (Pavia). 2022;14:37498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 27. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3363] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 28. | Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2019;13:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Li B, Ouchi T, Cao Y, Zhao Z, Men Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front Cell Dev Biol. 2021;9:654559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Saito MT, Silvério KG, Casati MZ, Sallum EA, Nociti FH Jr. Tooth-derived stem cells: Update and perspectives. World J Stem Cells. 2015;7:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 31. | Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 897] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 32. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 1983] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 33. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2371] [Cited by in RCA: 2502] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 34. | Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787-7798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 569] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 35. | Handa K, Saito M, Yamauchi M, Kiyono T, Sato S, Teranaka T, Sampath Narayanan A. Cementum matrix formation in vivo by cultured dental follicle cells. Bone. 2002;31:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Bi R, Lyu P, Song Y, Li P, Song D, Cui C, Fan Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Ten Cate AR. The development of the periodontium--a largely ectomesenchymally derived unit. Periodontol 2000. 1997;13:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Shoi K, Aoki K, Ohya K, Takagi Y, Shimokawa H. Characterization of pulp and follicle stem cells from impacted supernumerary maxillary incisors. Pediatr Dent. 2014;36:79-84. [PubMed] |
| 39. | Chen G, Sun Q, Xie L, Jiang Z, Feng L, Yu M, Guo W, Tian W. Comparison of the Odontogenic Differentiation Potential of Dental Follicle, Dental Papilla, and Cranial Neural Crest Cells. J Endod. 2015;41:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Yildirim S, Zibandeh N, Genc D, Ozcan EM, Goker K, Akkoc T. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016;2016:4682875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Nuñez J, Vignoletti F, Caffesse RG, Sanz M. Cellular therapy in periodontal regeneration. Periodontol 2000. 2019;79:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 42. | Liu J, Ruan J, Weir MD, Ren K, Schneider A, Wang P, Oates TW, Chang X, Xu HHK. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 43. | Novello S, Debouche A, Philippe M, Naudet F, Jeanne S. Clinical application of mesenchymal stem cells in periodontal regeneration: A systematic review and meta-analysis. J Periodontal Res. 2020;55:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Yan XZ, Yang F, Jansen JA, de Vries RB, van den Beucken JJ. Cell-Based Approaches in Periodontal Regeneration: A Systematic Review and Meta-Analysis of Periodontal Defect Models in Animal Experimental Work. Tissue Eng Part B Rev. 2015;21:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Li Q, Yang G, Li J, Ding M, Zhou N, Dong H, Mou Y. Stem cell therapies for periodontal tissue regeneration: a network meta-analysis of preclinical studies. Stem Cell Res Ther. 2020;11:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 46. | Flores MG, Hasegawa M, Yamato M, Takagi R, Okano T, Ishikawa I. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J Periodontal Res. 2008;43:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Bou-Ghannam S, Kim K, Grainger DW, Okano T. 3D cell sheet structure augments mesenchymal stem cell cytokine production. Sci Rep. 2021;11:8170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Guo S, Guo W, Ding Y, Gong J, Zou Q, Xie D, Chen Y, Wu Y, Tian W. Comparative study of human dental follicle cell sheets and periodontal ligament cell sheets for periodontal tissue regeneration. Cell Transplant. 2013;22:1061-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Zeichner-David M, Oishi K, Su Z, Zakartchenko V, Chen LS, Arzate H, Bringas P Jr. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev Dyn. 2003;228:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Bai Y, Bai Y, Matsuzaka K, Hashimoto S, Fukuyama T, Wu L, Miwa T, Liu X, Wang X, Inoue T. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011;48:1417-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Guo S, Kang J, Ji B, Guo W, Ding Y, Wu Y, Tian W. Periodontal-Derived Mesenchymal Cell Sheets Promote Periodontal Regeneration in Inflammatory Microenvironment. Tissue Eng Part A. 2017;23:585-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Shi W, Guo S, Liu L, Liu Q, Huo F, Ding Y, Tian W. Small Extracellular Vesicles from Lipopolysaccharide-Preconditioned Dental Follicle Cells Promote Periodontal Regeneration in an Inflammatory Microenvironment. ACS Biomater Sci Eng. 2020;6:5797-5810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Huang Y, Liu Q, Liu L, Huo F, Guo S, Tian W. Lipopolysaccharide-Preconditioned Dental Follicle Stem Cells Derived Small Extracellular Vesicles Treating Periodontitis via Reactive Oxygen Species/Mitogen-Activated Protein Kinase Signaling-Mediated Antioxidant Effect. Int J Nanomedicine. 2022;17:799-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 54. | Sánchez N, Fierravanti L, Núñez J, Vignoletti F, González-Zamora M, Santamaría S, Suárez-Sancho S, Fernández-Santos ME, Figuero E, Herrera D, García-Sanz JA, Sanz M. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: A 12-month quasi-randomized controlled pilot clinical trial. J Clin Periodontol. 2020;47:1391-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, Lu H, Chu Q, Xu J, Yu Y, Wu RX, Yin Y, Shi S, Jin Y. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 56. | Tian Y, Bai D, Guo W, Li J, Zeng J, Yang L, Jiang Z, Feng L, Yu M, Tian W. Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen Med. 2015;10:461-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Hong H, Chen X, Li K, Wang N, Li M, Yang B, Yu X, Wei X. Dental follicle stem cells rescue the regenerative capacity of inflamed rat dental pulp through a paracrine pathway. Stem Cell Res Ther. 2020;11:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Ricucci D, Siqueira JF Jr, Abdelsayed RA, Lio SG, Rôças IN. Bacterial Invasion of Pulp Blood Vessels in Teeth with Symptomatic Irreversible Pulpitis. J Endod. 2021;47:1854-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Sugiaman VK, Djuanda R, Pranata N, Naliani S, Demolsky WL, Jeffrey. Tissue Engineering with Stem Cell from Human Exfoliated Deciduous Teeth (SHED) and Collagen Matrix, Regulated by Growth Factor in Regenerating the Dental Pulp. Polymers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 60. | Guan X, Zhou Y, Yang Q, Zhu T, Chen X, Deng S, Zhang D. Vital Pulp Therapy in Permanent Teeth with Irreversible Pulpitis Caused by Caries: A Prospective Cohort Study. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, John AS, George A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 62. | Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 63. | Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, Utunomiya S, Nakamura H, Matsushita K, Nakashima M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med. 2013;2:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 64. | Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 2017;8:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 65. | Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 66. | Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, Li Y, Zou Q, Xie D, An X, Chen Y, Tian W. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32:4525-4538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, Liu Y, Li Y, Zhou Y, Zhou J, Zhang M, Deng Z, Jin Y. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30:6708-6723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 68. | Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, Wang H, Zheng X, Long J, Tang W, Guo W, Tian W. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix - based scaffold. Biomaterials. 2012;33:2449-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 69. | Chen G, Chen J, Yang B, Li L, Luo X, Zhang X, Feng L, Jiang Z, Yu M, Guo W, Tian W. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials. 2015;52:56-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 70. | Furquim CP, Kumagai RY, Bustillos-Torrez W, Meza-Mauricio J, Tanaka CJ, Santana V, Retamal-Valdes B, Shibli JA. Whole Tooth Regeneration: Can Animal Studies be Translated into Clinical Application? Tissue Eng Part C Methods. 2022;28:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 71. | Kuo TF, Huang AT, Chang HH, Lin FH, Chen ST, Chen RS, Chou CH, Lin HC, Chiang H, Chen MH. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. J Biomed Mater Res A. 2008;86:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC. Bioengineered dental tissues grown in the rat jaw. J Dent Res. 2008;87:745-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Wang F, Wu Z, Fan Z, Wu T, Wang J, Zhang C, Wang S. The cell re-association-based whole-tooth regeneration strategies in large animal, Sus scrofa. Cell Prolif. 2018;51:e12479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Luo X, Yang B, Sheng L, Chen J, Li H, Xie L, Chen G, Yu M, Guo W, Tian W. CAD based design sensitivity analysis and shape optimization of scaffolds for bio-root regeneration in swine. Biomaterials. 2015;57:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Yang B, Yang X, Luo X, Chen G, Chen J, Huo F, Zhu Z, Tian Y, Guo W, Tian W. Dfcs/tdm based artificial bio-root to obtain long-term functional root regeneration in non-human primate. Chem Eng J. 2023;451:1-22. [DOI] [Full Text] |
| 76. | Völlner F, Ernst W, Driemel O, Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009;77:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Yang C, Li X, Sun L, Guo W, Tian W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J Neural Eng. 2017;14:026005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Yang C, Sun L, Li X, Xie L, Yu M, Feng L, Jiang Z, Guo W, Tian W. The potential of dental stem cells differentiating into neurogenic cell lineage after cultivation in different modes in vitro. Cell Reprogram. 2014;16:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Morsczeck C, Völlner F, Saugspier M, Brandl C, Reichert TE, Driemel O, Schmalz G. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin Oral Investig. 2010;14:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Al-Maswary AA, O’Reilly M, Holmes AP, Walmsley AD, Cooper PR, Scheven BA. Exploring the neurogenic differentiation of human dental pulp stem cells. PLoS One. 2022;17:e0277134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 81. | McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 801] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 82. | Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 83. | Yang R, Pan J, Wang Y, Xia P, Tai M, Jiang Z, Chen G. Application and prospects of somatic cell reprogramming technology for spinal cord injury treatment. Front Cell Neurosci. 2022;16:1005399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Yasuda A, Tsuji O, Shibata S, Nori S, Takano M, Kobayashi Y, Takahashi Y, Fujiyoshi K, Hara CM, Miyawaki A, Okano HJ, Toyama Y, Nakamura M, Okano H. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011;29:1983-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 85. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 86. | Li X, Yang C, Li L, Xiong J, Xie L, Yang B, Yu M, Feng L, Jiang Z, Guo W, Tian W. A therapeutic strategy for spinal cord defect: human dental follicle cells combined with aligned PCL/PLGA electrospun material. Biomed Res Int. 2015;2015:197183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Hayes MT. Parkinson’s Disease and Parkinsonism. Am J Med. 2019;132:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 441] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 88. | Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3139] [Cited by in RCA: 3159] [Article Influence: 185.8] [Reference Citation Analysis (1)] |
| 89. | Lin G, Wang L, Marcogliese PC, Bellen HJ. Sphingolipids in the Pathogenesis of Parkinson’s Disease and Parkinsonism. Trends Endocrinol Metab. 2019;30:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 90. | Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1749] [Cited by in RCA: 1569] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 91. | Mursaleen LR, Stamford JA. Drugs of abuse and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Parkes JD, Baxter RC, Marsden CD, Rees JE. Comparative trial of benzhexol, amantadine, and levodopa in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1974;37:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal Stem Cells for Neurological Disorders. Adv Sci (Weinh). 2021;8:2002944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 94. | Staff NP, Jones DT, Singer W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin Proc. 2019;94:892-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 95. | Chi K, Fu RH, Huang YC, Chen SY, Hsu CJ, Lin SZ, Tu CT, Chang LH, Wu PA, Liu SP. Adipose-derived Stem Cells Stimulated with n-Butylidenephthalide Exhibit Therapeutic Effects in a Mouse Model of Parkinson’s Disease. Cell Transplant. 2018;27:456-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Chen D, Fu W, Zhuang W, Lv C, Li F, Wang X. Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson’s disease. J Neurosci Res. 2017;95:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 97. | Mathieu P, Roca V, Gamba C, Del Pozo A, Pitossi F. Neuroprotective effects of human umbilical cord mesenchymal stromal cells in an immunocompetent animal model of Parkinson’s disease. J Neuroimmunol. 2012;246:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Zhang N, Lu X, Wu S, Li X, Duan J, Chen C, Wang W, Song H, Tong J, Li S, Liu Y, Kang X, Wang X, Han F. Intrastriatal transplantation of stem cells from human exfoliated deciduous teeth reduces motor defects in Parkinsonian rats. Cytotherapy. 2018;20:670-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Bi F, Xiong J, Han X, Yang C, Li X, Chen G, Guo W, Tian W. Dental follicle cells show potential for treating Parkinson’s disease through dopaminergic-neuronogenic differentiation. Hum Cell. 2022;35:1708-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 100. | Wang D, Wang Y, Tian W, Pan J. Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif. 2019;52:e12572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 101. | Nagpal A, Kremer KL, Hamilton-Bruce MA, Kaidonis X, Milton AG, Levi C, Shi S, Carey L, Hillier S, Rose M, Zacest A, Takhar P, Koblar SA. TOOTH (The Open study Of dental pulp stem cell Therapy in Humans): Study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int J Stroke. 2016;11:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 808] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 103. | Chen X, Yang B, Tian J, Hong H, Du Y, Li K, Li X, Wang N, Yu X, Wei X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol Biochem. 2018;51:2290-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 104. | Zibandeh N, Genç D, İnanç GN, Direskeneli RH, Akkoç T. IFN-γ stimulated dental follicle mesenchymal stem cells regulate activated lymphocyte response in rheumatoid arthritis patients in vitro. Turk J Med Sci. 2019;49:1779-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 105. | Zibandeh N, Genc D, Duran Y, Banzragch M, Sokwala S, Goker K, Atug O, Akkoç T. Human dental follicle mesenchymal stem cells alleviate T cell response in inflamed tissue of Crohn’s patients. Turk J Gastroenterol. 2020;31:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Arora V, Arora P, Munshi AK. Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent. 2009;33:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 107. | Park BW, Jang SJ, Byun JH, Kang YH, Choi MJ, Park WU, Lee WJ, Rho GJ. Cryopreservation of human dental follicle tissue for use as a resource of autologous mesenchymal stem cells. J Tissue Eng Regen Med. 2017;11:489-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 108. | Kang YH, Lee HJ, Jang SJ, Byun JH, Lee JS, Lee HC, Park WU, Lee JH, Rho GJ, Park BW. Immunomodulatory properties and in vivo osteogenesis of human dental stem cells from fresh and cryopreserved dental follicles. Differentiation. 2015;90:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Yang H, Li J, Sun J, Guo W, Li H, Chen J, Hu Y, Tian W, Li S. Cells isolated from cryopreserved dental follicle display similar characteristics to cryopreserved dental follicle cells. Cryobiology. 2017;78:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829-1838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 111. | Jiao L, Xie L, Yang B, Yu M, Jiang Z, Feng L, Guo W, Tian W. Cryopreserved dentin matrix as a scaffold material for dentin-pulp tissue regeneration. Biomaterials. 2014;35:4929-4939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 112. | Yamada Y, Nakamura-Yamada S, Konoki R, Baba S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem Cell Res Ther. 2020;11:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 113. | Ferrarotti F, Romano F, Gamba MN, Quirico A, Giraudi M, Audagna M, Aimetti M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J Clin Periodontol. 2018;45:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 114. | Yang C, Chen Y, Zhong L, You M, Yan Z, Luo M, Zhang B, Yang B, Chen Q. Homogeneity and heterogeneity of biological characteristics in mesenchymal stem cells from human umbilical cords and exfoliated deciduous teeth. Biochem Cell Biol. 2020;98:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 115. | Kanao S, Ogura N, Takahashi K, Ito K, Suemitsu M, Kuyama K, Kondoh T. Capacity of Human Dental Follicle Cells to Differentiate into Neural Cells In vitro. Stem Cells Int. 2017;2017:8371326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Heng BC, Lim LW, Wu W, Zhang C. An Overview of Protocols for the Neural Induction of Dental and Oral Stem Cells In vitro. Tissue Eng Part B Rev. 2016;22:220-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 117. | Zhang J, Ding H, Liu X, Sheng Y, Jiang C. Dental Follicle Stem Cells: Tissue Engineering and Immunomodulation. Stem Cells Dev. 2019;28:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 118. | Genç D, Zibandeh N, Nain E, Gökalp M, Özen AO, Göker MK, Akkoç T. Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin Exp Allergy. 2018;48:663-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 119. | Mori G, Ballini A, Carbone C, Oranger A, Brunetti G, Di Benedetto A, Rapone B, Cantore S, Di Comite M, Colucci S, Grano M, Grassi FR. Osteogenic differentiation of dental follicle stem cells. Int J Med Sci. 2012;9:480-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 120. | Pan K, Sun Q, Zhang J, Ge S, Li S, Zhao Y, Yang P. Multilineage differentiation of dental follicle cells and the roles of Runx2 over-expression in enhancing osteoblast/cementoblast-related gene expression in dental follicle cells. Cell Prolif. 2010;43:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Abdal-Wahab M, Abdel Ghaffar KA, Ezzatt OM, Hassan AAA, El Ansary MMS, Gamal AY. Regenerative potential of cultured gingival fibroblasts in treatment of periodontal intrabony defects (randomized clinical and biochemical trial). J Periodontal Res. 2020;55:441-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 122. | Apatzidou DA, Bakopoulou AA, Kouzi-Koliakou K, Karagiannis V, Konstantinidis A. A tissue-engineered biocomplex for periodontal reconstruction. A proof-of-principle randomized clinical study. J Clin Periodontol. 2021;48:1111-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 123. | Wu J, Jin F, Tang L, Yu J, Xu L, Yang Z, Wu G, Duan Y, Jin Y. Dentin non-collagenous proteins (dNCPs) can stimulate dental follicle cells to differentiate into cementoblast lineages. Biol Cell. 2008;100:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Felthaus O, Ernst W, Driemel O, Reichert TE, Schmalz G, Morsczeck C. TGF-beta stimulates glial-like differentiation in murine dental follicle precursor cells (mDFPCs). Neurosci Lett. 2010;471:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Meng M, Chen Y, Chen X, Zhang Q, Guo W, Zhou X, Zou J. IL-1α Regulates Osteogenesis and Osteoclastic Activity of Dental Follicle Cells Through JNK and p38 MAPK Pathways. Stem Cells Dev. 2020;29:1552-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Yang H, Li J, Hu Y, Sun J, Guo W, Li H, Chen J, Huo F, Tian W, Li S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent Mater. 2019;35:1238-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 127. | Lanzillotti C, De Mattei M, Mazziotta C, Taraballi F, Rotondo JC, Tognon M, Martini F. Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Front Cell Dev Biol. 2021;9:646032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |