Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.120
Peer-review started: November 28, 2022
First decision: January 23, 2023
Revised: January 28, 2023
Accepted: March 16, 2023
Article in press: March 16, 2023
Published online: April 26, 2023
Processing time: 148 Days and 23.7 Hours
Obesity, the global pandemic since industrialization, is the number one lifestyle-related risk factor for premature death, which increases the incidence and mortality of various diseases and conditions, including cancer. In recent years, the theory of cancer stem cells (CSCs), which have the capacity for self-renewal, metastasis and treatment resistance, has been bolstered by increasing evidence. However, research on how obesity affects CSCs to facilitate cancer initiation, progression and therapy resistance is still in its infancy, although evidence has already begun to accumulate. Regarding the ever-increasing burden of obesity and obesity-related cancer, it is pertinent to summarize evidence about the effects of obesity on CSCs, as elucidating these effects will contribute to the improvement in the management of obesity-related cancers. In this review, we discuss the association between obesity and CSCs, with a particular focus on how obesity promotes cancer initiation, progression and therapy resistance through CSCs and the mechanisms underlying these effects. In addition, the prospect of preventing cancer and targeting the mechanisms linking obesity and CSCs to reduce cancer risk or to improve the survival of patients with cancer is considered.
Core Tip: Obesity increases the incidence and mortality of various cancers; however, research on how obesity affects cancer stem cells (CSCs) is still in its infancy. In this review, we discuss the association between obesity and CSCs, with a particular focus on how obesity promotes cancer initiation, progression and therapy resistance through CSCs and the mechanisms underlying these effects. In addition, the prospect of preventing cancer and targeting the mechanisms linking obesity and CSCs to reduce cancer risk or to improve the survival of patients with cancer is considered.
- Citation: Xie WJ, Li J. Obesity and cancer stem cells: Roles in cancer initiation, progression and therapy resistance. World J Stem Cells 2023; 15(4): 120-135
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/120.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.120
For millions of years, humans and their predecessors have evolved under the pressure of undernutrition, which selects a genotype that enables overeating, low energy expenditure, a high degree of calorie absorption and efficient energy storage in adipose tissue[1]. Therefore, with the development of the social economy in the past few decades, overnutrition and an increasingly sedentary lifestyle tip the balance from a few calories consumed but more expended to more calories consumed but little expended, leading to the pandemic of excess body weight, which is mainly measured by body mass index (BMI). Over the past four decades, the prevalence of overweight and obesity has nearly tripled globally. Between 1975 and 2016, the worldwide prevalence of obesity increased from less than 1% to 6%-8% among children, from 3% to more than 11% among men and from 6% to 15% among women[2]. Based on data from the Global Burden of Disease (GBD) 2015, overweight or obesity affects over 2.1 billion people, or nearly 30% of the global population[3]. Obesity was estimated to increase the economic burden by approximately 2 trillion United States dollars, or 2.8% of the global gross domestic product, and to lead to the loss of an estimated 5-20 years of life expectancy, representing one of the most serious unmet public health challenges of the 21st century[4-6].
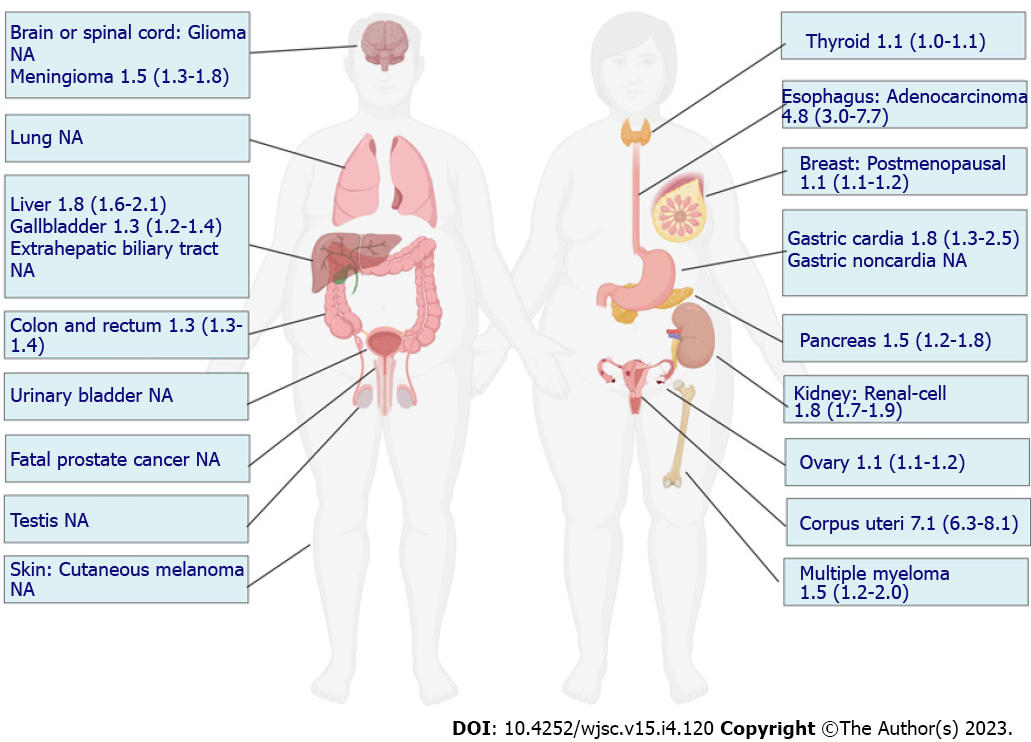
Malignancy, a set of diseases caused by the interplay between genetic and environmental or behavioral factors, ranks as the third leading cause of premature death and disability attributable to excess body weight worldwide following cardiovascular disease and type 2 diabetes mellitus[7]. Recent studies have demonstrated that excess body weight is associated with higher risks of several types of cancer, including esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney[8]. In 2019, the estimated number of high BMI-related cancer cases accounted for 4.59% and 4.45% of all cancer-cause deaths and disability-adjusted life years, respectively[9]. Obesity can not only increase the risk of tumorigenesis but also promote the progression and metastasis of developed cancer and can affect the therapeutic efficacy and survival of patients with cancer[10].
Regarding the altered biological processes that occur in the context of obesity that contribute to cancer, the majority of studies have focused on common themes, including inflammation, hypoxia, angiogenesis and altered energy metabolism, which influence the proliferation and survival of cancer cells[10]. However, in recent years, emerging challenges in cancer management have promoted the proposal of many theories to explain the initiation and progression of cancer; one of them is the hypothesis of cancer stem cells (CSCs), which has been bolstered by an accumulating body of evidence[11]. CSCs, also referred to as treatment-refractory, tumor-initiating cells, constitute a small subpopulation of cancer cells within tumors capable of self-renewal, which can divide and differentiate into various tumor cell types (intratumoral heterogeneity). They can secrete antiapoptotic factors, undergo epithelial-to-mesenchymal transition (EMT), and display a higher performance of drug efflux pumps. Therefore, CSCs are preferentially aggressive and pose a high risk of therapy resistance and disease relapse[11]. With the rapidly increasing incidence of cancer attributable to obesity, a better under
In the context of an increase in the global prevalence of obesity, large-scale epidemiological studies have demonstrated a compelling increased risk of tumorigenesis in individuals with obesity, and several landmark studies have summarized this evidence. To evaluate the strength and validity of the evidence for the association between adiposity and the risk of developing or dying from cancer, an umbrella review of the literature comprising 204 meta-analyses of large studies with limited heterogeneity or evidence of bias was published in 2017, which concluded that the associations for 11 cancers were supported by strong evidence, while others could be genuine, but substantial uncertainty remains[8]. In 2016, data from a meta-analysis reported by the International Agency for Research on Cancer supported relative risks of 1.5 to 1.8 in obesity for these tumor sites[12]. In GBD 2019, 13 cancer types were also found to be affected by a high BMI[9]. Although some inconsistencies in cancer types contributing to obesity were reported across these studies, a consistent and compelling association has been demonstrated in many cancer types, including esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney (Figure 1). In the majority of these cancers, the CSC theory has been established in tumorigenesis. For example, in the intestine, inactivation of the adenomatous polyposis coli (APC) gene can lead to the rapid and lethal generation of adenomas in intestinal stem cells (ISCs) but not in non-stem cells[13]. Breast cancer was found to originate from a rare population of mammary gland progenitor cells, the depletion of which significantly impaired tumor growth[14]. Therefore, to increase the risk of these cancers, obesity may disturb the normal biology of stem/progenitor cells residing in these tissues, which is conducive to their transformation.
Except for compelling epidemiological evidence, no attempts have been made to investigate the biology of cancer-related adult stem cells in populations with obesity. Multiple animal models have been developed to recapitulate the effects of obesity or a pro-obesity diet on the initiation of cancer and have suggested that a high-fat diet (HFD) can promote tumorigenesis in the colorectum, prostate and liver[15-17]. However, the cellular origin of cancer was not defined in these studies. In recent years, research revealing the links between obesity or HFD and adult stem cells has increased (Table 1)[18-28]. Although discrepancies exist, the majority of these studies reported one or more of the following findings: Obesity or a HFD increases the depth or number of crypts in the intestine; non-stem cell progenitors in a HFD setting acquire stem cell attributes; the number and the capacity to form organoids of stem cells or progenitor cells are increased by a HFD; stem cells undergo autonomous changes in response to a HFD that poise them for niche-independent growth. Although studies exploring the initiation of carcinogenesis from these stem cells are very limited, the alterations in stem cells reported in these studies can predispose them to transformation. First, obesity or a HFD expands the pool of cells - both stem cells and progenitor cells - that can serve as the cellular origin of nascent cancers. Second, stem cells from mice on a HFD become functionally uncoupled from their niche in the organoid assay and in vivo, consistent with the hallmarks of cancer cells. Third, several studies have shown the possible links between these perturbations and tumorigenesis. For instance, when injected with azoxymethane, aberrant crypt foci (ACF), an early-appearing lesion of colon carcinogenesis, were increased in male mice fed a HFD[21]. In another study, more spontaneous intestinal low-grade adenomas and carcinomas were observed in HFD-fed mice than in standard diet-fed mice[19].
| Tissue | Findings | Ref. |
| Intestine | Increased crypt depth and villus height; increased number of Olmf4-positive ISCs; increased size of the enterospheres that developed from ISCs | [18] |
| Intestine | Increase in crypt depth; non-stem progenitor intestinal cells gain more stemness features and self-renewal; 50% increase in the number of Olfm4+ ISCs; 23% decrease in the number of Paneth cells; more likely to initiate mini-intestines; organoids had higher frequencies of Lgr5+ ISCs; ISCs by themselves had an increased capacity to initiate organoids | [19] |
| Intestine | Combined with Pten inactivation, obesity is insufficient to drive Lgr5+ ISC-derived tumorigenesis | [20] |
| Intestine | Increased aberrant crypt and crypt foci; increased proliferation of colonocytes per mouse | [21] |
| Intestine | Higher number of Lgr5+ stem cells per crypt | [22] |
| Intestine | Increased number of ISCs and progenitor cells; crypts are further likely to form mini-intestine organoids in a 3D culture | [23] |
| Intestine | Increased intestinal epithelial cell proliferation | [24] |
| Intestine | Reprograms Bmi1+ cells to function and persist as stem-like cells in mucosal homeostasis and tumor development | [25] |
| Intestine | Increased number of crypts; increased total numbers of ISCs and percentage of ISCs in S-phase; reduced numbers of Paneth and goblet cells | [26] |
| Lung | Increased number of AT2 cells; higher stem cell colony forming efficiency | [27] |
| Esophagus | Increased numbers of epithelial progenitors in Barrett’s esophagus | [28] |
At present, elucidating how obesity and a pro-obesity diet contribute to the cellular origin of cancer in the intestine is the central focus of research, and data on stem cells in other tissues are very limited. Several reasons can explain such a tissue preference for studying the impact of obesity and HFD physiology on the initiation of cancer. First, robust epidemiological evidence has been accumulated for the increased risk of colon cancer in obese populations, and a better understanding of the altered biology of ISCs that occurs in the context of obesity will provide immeasurable public health benefits[10]. Second, the stem cell theory advanced most rapidly in ISCs, from which the histological architecture of the intestine has been well established[29]. Third, ISCs reside in the base of the intestinal crypt and directly interact with luminal nutrients, bacteria, and other intraepithelial and subepithelial cells, making the intestine an ideal system for studying the pathophysiological changes on a HFD[29]. Finally, the natural orifice of the intestine makes in situ manipulations for tumor induction or diagnostic tests easier than other in vivo cancer models[30]. Despite all of this, as consistent and compelling associations have been demonstrated between obesity and more than 10 cancer types, elucidating how stem cells in tissues other than the intestine are perturbed by obesity or HFD holds the same importance. Currently, the majority of obesity models are induced by HFD; however, other dietary patterns, such as a high-sugar diet and Western-styled diet, have also been shown to be obesogenic, and the effects of these dietary patterns on the initiation of cancer warrant further studies. Furthermore, the alterations in stem cell biology in tissues with increased cancer incidence warrant further investigation in obese human beings, not just in animals.
In addition to promoting tumorigenesis, obesity might also promote the progression of established cancers, affect the efficacy of present forefront antitumor therapies and shorten the survival of patients with cancer. For instance, a meta-analysis including 86490 patients treated for clinically localized prostate cancer showed a moderate and consistent relationship between obesity and biochemical recurrence, and there was a 10% increase in biochemical recurrence per 5 kg/m2 increase in BMI[31]. In the Carolina Breast Cancer Study phase 3, a high waist-to-hip ratio was found to be associated with a high risk of metastasis[32]. Poor survival was also reported in overweight or obese patients with colorectal, endometrial and breast cancer[33-35]. In addition to the increased likelihood of recurrence, the poor prognosis of obese patients with cancer also results from the reduction in the efficacy of antitumor therapies[36]. Mechanistically, the link between obesity and increased recurrence, therapy resistance and poor survival is likely multifactorial, with some differences related to more advanced stages being attributed to reduced participation in routine screening or the systemic effects of obesity on drug pharmacokinetics and metabolism[37,38]. In addition to these explanations, emerging evidence has shown that the activation of stem cell programs in cancers can lead to progression, metastatic growth and therapy resistance[11].
The key roles of obesity in the activation of stem cell programs have attracted much attention in recent years; however, as in studies on the effects of obesity on cancer-initiating cells, the promotion effects of obesity on cancer through CSCs are also mainly limited to animal models, which are utilized to investigate how specific obesity-related factors induce the stemness of cancer cells. Knowledge about CSCs in obese patients with cancer is still not clear. For example, obesity increases inflammation in the tumor microenvironment (TME) through local and systemic adipokines, proinflammatory cytokines or hormones, which modulate the stemness of cancer cells[39]. In a mouse model of hepatocellular carcinoma, diet-induced obesity increased inflammatory signaling via STAT3, and this finding was associated with larger tumors with a cancer-stem-cell-like phenotype[40]. Prolonged culture of breast cancer cells, which developed from a fat-rich environment, with adipocytes increased the proportion of cells expressing stem-like markers in vitro and the abundance of cancer cells with metastatic potential in vivo[41]. Regarding the involvement of CSCs in therapy resistance, leptin was found to interfere with the efficacy of 5-fluorouracil (5-FU) in colon tumor stem cells by increasing cell viability and reducing 5-FU-induced DNA damage[42].
EMT is a reversible cellular process during which epithelial cells transiently acquire mesenchymal phenotypes, such as an elongated, fibroblast-like morphology as well as an increased capacity for migration and invasion[43]. It is now widely accepted that EMT has well-established roles in cancer metastasis[43]. In the majority of carcinomas, only CSCs exhibit aspects of EMT-program activation[44]. Various extracellular stimuli, including obesity-related factors, have been implicated in the induction of EMT programs. For instance, esophageal cancer cells cocultured with visceral adipose tissue taken from obese patients resulted in the induced expression of genes involved in EMT, which was also noted in tumor biopsies from obese patients[45]. Cytokines and growth factors released by adipose stem cells (ASCs) can induce EMT-like changes in various cancer cells[10]. The adipokine leptin has also been found to activate EMT programs to enhance the proliferation and metastasis of breast cancer cells[46]. Therefore, obesity can propel primary tumor cells toward EMT events, leading to malignant progression.
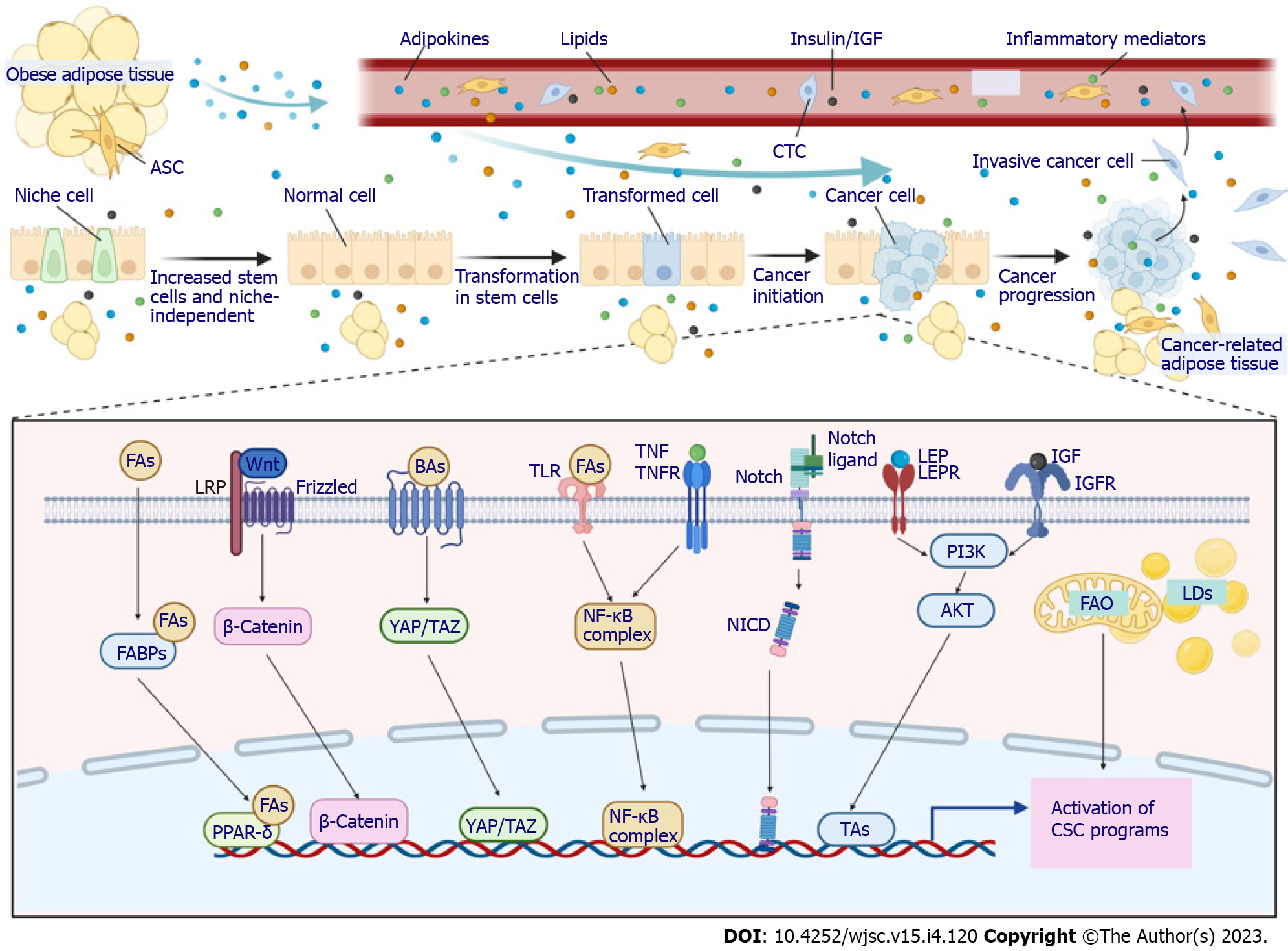
As discussed above, CSCs participate in every step of tumorigenesis promoted by obesity. Understanding the key links between obesity and CSCs, therefore, offers important potential to decrease the incidence and improve the outcomes of obese patients with cancer. Several main factors are considered to connect obesity and cancer: Components of pro-obesity diets, metabolic and hormonal alterations associated with obesity, dysfunctional adipose tissue in the TME, low-grade obesity-related inflammation, self-renewal and stemness pathways, and microbiome dysbiosis. Each of these factors is intimately linked to and cross-talks with each other. For example, fatty acids in pro-obesity diets accumulate in adipocytes, leading to expansion and dysfunction of adipose tissue, which is intimately linked to endocrine and paracrine dysregulation, such as increased circulating insulin, insulin-like growth factor-1 (IGF-1) and leptin. All of these alterations activate and maintain a prolonged low-grade inflammatory state, predisposing individuals with obesity to an increased cancer risk and poor outcomes[47-49]. Although these factors were mainly investigated in nonspecific conditions, their involvement in CSC biology is also beginning to accumulate evidence (Figure 2).
Although the mechanistic links are not completely understood, nutrient-sensing signaling activated by components of a pro-obesity diet has been shown to influence stem cell behavior and tumorigenesis. This was also indirectly suggested in a leptin-receptor-deficient (db/db) mouse model, which becomes obese on control diets and does not rely on a HFD. In db/db mice, the number of ISCs was reduced, while ISC function was not affected, highlighting that components of a pro-obesity diet can regulate stem cells independently of obesity[19]. As early as 1989, Blakeborough et al[50] reported that diets high in fat support a Bacteroides-dominated colonic microflora and increase the excretion of secondary bile acids to augment free radical production, which may overcome the antioxidant defense mechanisms of stem cells, causing DNA damage, tumorigenesis and proliferation of transformed stem cells. Since then, numerous studies have suggested for decades that a pro-obesity diet engages many diverse pathways in stem cells in various tissues that collectively contribute to tumorigenesis. For instance, a HFD increases leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) expression and promotes tumor growth in a xenograft model independent of obesity. Mechanistically, dietary fats stimulate vitamin A-bound serum retinol-binding protein 4 and retinoic acid 6, which are implicated in colon stem cell self-renewal, to activate the JAK-STAT3 pathway and boost Lgr5 expression and the tumorigenicity of ISCs[51].
The representative components of a pro-obesity diet, that is, fatty acids such as palmitic acid or oleic acid, were found to enhance the number and self-renewal potential of ISCs and to permit organoid body formation without supporting signaling from their niche cells in a well-designed study[19]. The molecular mechanism by which fatty acids deregulate ISCs to promote tumorigenesis was also delineated in this study. It was proposed that fatty acids can be transported to the nucleus by fatty acid-binding proteins or can be produced directly by lipid metabolism in the nucleus, where they increase the number and self-renewal of ISCs via peroxisome proliferator-activated receptor δ (PPAR-δ), a nuclear receptor that senses fatty acid derivatives, the synthetic activation of which mimics both the in vivo and in vitro impact of a HFD and fatty acid treatment[19]. In contrast, loss of PPAR-δ completely abrogated the effects of fatty acids on Lgr5+ ISC function with respect to organoid-initiating capacity[19]. The downstream signaling mediating the effects of PPAR-δ activation was attributed to WNT/β-catenin, as demonstrated by increased β-catenin staining and upregulation of its target genes (Jag1, Jag 2 and Bmp4) in ISCs and progenitors from HFD- and PPAR-δ agonist-treated mice[19]. Free fatty acids produced by obese fat lipolysis also serve as ligands for Toll-like receptor 4 (TLR4) on cancer cells to activate nuclear factor-kappaB (NF-κB), leading to an increase in CSCs[52]. Another critical element in a pro-obesity diet that is significantly elevated in some obese individuals, cholesterol, was also demonstrated to affect stem cell function, thus promoting tumorigenesis. In Drosophila, dietary cholesterol modulated the differentiation of ISCs by stabilizing the Delta ligand and Notch extracellular domain and altering their trafficking in endosomal vesicles, while a low-sterol diet slowed the proliferation of enteroendocrine tumors initiated by Notch pathway disruption[53]. In a rodent animal model, evidence also showed that dietary cholesterol acts as a mitogen for ISCs, while disruption of cholesterol homeostasis dramatically enhances tumor formation in APCmin mice[54].
Although bile acids are not elements in a pro-obesity diet, their excretion is essential for the digestion and absorption of dietary fat and is increased when a HFD is consumed. Bile acids are endogenous agonists of the G protein-coupled bile acid receptor, the activation of which augments Yes-associated protein 1 (YAP1) signaling, leading to increased stem cell number and proliferation and enhanced organoid-forming capability of ISCs[55]. In addition, bile acids, such as tauro-β-muricholic acid and deoxycholic acid, can antagonize intestinal farnesoid X receptor (FXR), a master regulator of bile acid homeostasis. Antagonizing FXR in the intestinal lumen enhances the proliferation and DNA damage of stem cells, initiating the transformation of ISCs to a malignant phenotype and promoting adenoma-to-adenocarcinoma progression[56]. Conversely, selective activation of intestinal FXR by its agonist can restrict abnormal ISC growth and skew differentiation toward goblet cells, thus curtailing HFD-induced intestinal cancer progression[56].
Aerobic glycolysis has long been viewed as the main metabolic characteristic of cancer cells. However, in recent years, CSCs have been found to be intimately dependent on lipid metabolism to maintain their self-renewal capability. Therefore, in addition to the abovementioned studies that investigated the regulation of CSC function by lipids as elements of a pro-obesity diet, numerous studies have explored the effects of lipids on CSCs at the cellular metabolism level. Metabolism of fatty acids and cholesterol, including de novo biosynthesis, storage and fatty acid oxidation (FAO), supports the stemness, proliferation and chemotherapy resistance of CSCs[57]. Metabolic analysis demonstrated that lipid synthesis, including de novo lipid biosynthesis, lipid desaturation, and cholesterol synthesis, displays high activity in CSCs, indicating that lipid synthesis plays critical roles in stemness maintenance[58]. Human breast cancer-derived data suggest that FAO promotes cancer cell stemness and chemoresistance. Blocking FAO resensitizes them to chemotherapy and inhibits CSCs in mouse breast tumors in vivo[59]. Furthermore, cytarabine-resistant acute myeloid leukemia cells, which are enriched in leukemic stem cells, exhibited increased FAO[60]. FAO is also responsible for the stemness and chemotherapy resistance in gastric cancer induced by mesenchymal stem cells (MSCs)[61]. To meet the critical functions of lipids in CSCs, lipid droplets, organelles that store neutral lipids, accumulate and are more abundant in CSCs in numerous types of cancer[57]. Although evidence connecting lipid metabolism and CSCs is increasingly accumulating, whether obesity can augment the lipid metabolic alteration in CSCs is not clear because studies on the metabolic adaptations of CSCs in obese environments are limited. However, the incidence of hyperlipidemia is higher in obese populations, and in obese individuals, CSCs more readily reside in a fat-rich TME, which may provide more lipids to CSCs. Therefore, theoretically, lipid metabolic alterations in obesity support the stemness of cancer cells, although further studies are warranted to validate such effects.
Another common metabolic alteration of obese patients is insulin resistance, leading to increased levels of circulating insulin and IGF-1, which contribute to the increased risk and mortality of several cancers in obese individuals. Mice with diet-induced obesity exhibited increased concentrations of plasma glucose, insulin, and IGF-1, which were significantly correlated with increased proliferation and self-renewal of ISCs, as well as decreased Paneth cell numbers[26]. In addition, insulin significantly increased the capacity of organoid formation in vitro[26]. Reports have suggested that the PI3K/AKT pathway is the major contributor to the abnormal renewal of ISCs endowed by insulin/IGF-1[62]. Therefore, insulin/IGF-1 signaling was suggested to mediate the effects of obesity on the function of stem cells, which is conducive to their transformation. Even insulin/IGF-1 levels in newborns are associated with the risk of future breast cancer, possibly resulting from an increased total number of stem cells[63]. Parallel to their function in normal stem cells, evidence suggests the roles of insulin/IGF-1 in cancer progenitor/stem cells from solid and hematopoietic malignancies. Insulin/IGF-1 and their receptors are overexpressed or overactivated in human thyroid, hepatic and breast CSCs and participate in the self-renewal, EMT and chemoresistance of cancer cells[64]. These emerging discoveries will undoubtedly promote renewed efforts aimed at targeting the insulin/IGF-1 system that contribute to CSC biology.
Adipose tissue has long been viewed as an energy reservoir; however, this perspective has changed in recent years, as numerous bioactive adipokines, including more than 50 different metabolic and hormonal factors, cytokines and chemokines, were reported to be released by adipose tissue[65]. Two of the major adipose tissue-derived hormones are leptin and adiponectin, which have opposite effects. In contrast to lean adipose tissue, which mainly secretes the antimitogenic adipokine adiponectin in obesity, increased preadipocytes yield high levels of leptin, which has proangiogenic and promitogenic effects[66,67].
Leptin acts as a growth factor for many tissues, such as the mammary gland, lung, liver, and colonic epithelium[68]. The links between leptin and CSCs have been comprehensively studied in breast cancer[69]. In a diet-induced obese mouse model, mammary epithelial polarity was disrupted, which can contribute to overactivation of the PI3K/AKT pathway downstream of the paracrine effect of leptin expressed by neighboring adipocytes. Leptin expands the pool of stem/progenitor cells in the breast epithelium and causes mitotic spindle misalignment, which is an early step in tumor initiation[70]. The leptin receptor was found to be expressed on breast CSCs, and in orthotopically transplanted breast cancer, leptin can promote CSC enrichment[71,72]. Inactivation of the leptin receptor attenuated the expression of CSC transcription factors and reduced the self-renewal of cancer cells in tumor sphere assays[71]. Leptin-mediated cancer initiation, progression and therapy resistance through CSCs in other cancers have also been extensively investigated[69]. For instance, leptin was found to initiate the early transformation of colon cancer. ACF multiplicity, as early-appearing lesions of tumorigenesis, was increased by a HFD in ob/ob mice or in a genetic mouse (db/db) model with leptin receptor deletion[73,74]. However, in db/db mice fed a control diet, the function of ISCs and the activity of PPAR-δ and Wnt/β-catenin signaling were not changed, indicating that obesity and elements in a pro-obesity diet may cause different alterations in the function of stem cells[19]. In addition, no leptin receptor was found on colonic stem cells, and leptin did not increase the pool of Lgr5+ stem cells, suggesting that leptin may be dispensable in the early stages of colon carcinogenesis[22]. Collectively, these findings indicate that although the crucial role of leptin in CSCs may be affected by the cellular origin of cancer, the potential of leptin pathways in cancer initiation and progression will lead to future areas of therapeutic management.
Although data are scarce, other adipokines with altered secretion in obese adipose tissue were also shown to affect the function of CSCs. For example, DeClercq et al[22] specifically investigated the effect of a HFD on colonic stem cell maintenance during cancer initiation and found that the number of stem cells and their proliferation capacity were significantly increased, while the incidence of apoptosis was decreased. The authors proposed that these effects are the result of decreased adiponectin signaling based on the findings that the reduction in stem cell number and increase in apoptosis were diminished in organoid cultures from obese mice treated with an adiponectin receptor agonist[22]. In addition, following a decrease in adiponectin signaling, obesity can increase tumorigenesis in the intestine[22]. Resistin, another adipokine, was highly associated with the transcription of genes related to CSCs in low malignant breast cancer cells and noncarcinogenic breast epithelial cells[75]. These adipokines with different effects on CSCs and their therapeutic translational potential need further research.
Despite the systemic effects of adipose tissue on CSCs through the secretion of circulating metabolic and hormonal factors, adipose tissue also constitutes an important part of the microenvironment of several cancers, and its dysfunction resulting from obesity is considered a critical determinant of cancer progression[76]. For example, cancer-associated adipose tissue obtained from obese patients with breast cancer was found to increase inflammatory breast cancer aggressiveness via the regulation of CSC markers[77]. Coculture of breast cancer cells with human-derived adipocytes increased the abundance of mammosphere-forming cells and stem-like cancer cells in vitro and increased tumor-initiating cells and metastasis in mouse models[41]. Mechanistic investigations demonstrated that immature adipocyte contact activates Src, thus promoting embryonic stem cell transcription factor upregulation, including Sox2, c-Myc, and Nanog, to mediate CSC expansion[41]. Moreover, Sox2-dependent induction of miR-302b further stimulated c-Myc and Sox2 expression and potentiated cytokine-induced CSC-like properties[41]. However, adipose tissue from different anatomical sites may have different effects on CSCs. For example, serial transplantation of pluripotent stem cells cultured in conditioned medium of breast cancer cells into mammary fat pads evoked the same features of breast cancer, while this result was perturbed following subcutaneous transplantation, indicating that mammary adipose tissue can synergize with secretory factors produced by cancer cells to transform normal cells into CSCs, while subcutaneous adipose tissue cannot[78]. Such performance differences may be caused by the various metabolic characteristics of adipose tissue from different anatomical sites determined by sex steroid hormones[79].
Among various adipose tissue cell types, ASCs are key players in adipose tissue. Under obese conditions, the biology of ASCs is dramatically altered, and ASCs can be recruited to sites of inflammation, including tumors[80]. ASCs are able to produce a large variety of circulating growth factors, cytokines and adipokines, which play important roles in CSC function. In addition to their systemic effects, ASCs represent an important cellular component in the TME. Therefore, in a breast cancer patient-derived xenograft model, cancers grown in the presence of ASCs had increased numbers of CD44+CD24− CSCs in the peripheral blood and had a higher tendency to form metastases[81]. This effect may be mediated by leptin, as the stable knockdown of leptin in obese ASCs led to a significant reduction in circulating CSCs[81]. In addition, ASCs reshape the TME and support the generation of CSCs, which are associated with radioresistance and chemotherapy resistance[82].
In recent decades, the contribution of inflammation to cancer initiation, progression and therapy resistance has regained enormous interest, and the association between inflammation and CSCs has also been explored extensively[83]. Obesity has long been considered a facilitator of mild, chronic, systemic inflammation. Along with the expansion of adipose tissue in obesity, hypoxia causes adipocyte stress and malfunction, recruiting different types of immune cells[84,85]. Both adipocytes and immune cells release numerous adipokines, cytokines, chemokines and hormones, which perpetuate the inflammatory state[84,85]. Therefore, it is reasonable to speculate that obesity can affect CSCs through low-grade inflammation. Although chronic inflammation is not induced consistently in obese mouse models, which may be related to the differences in feeding patterns and other combined interventions, current evidence indicates that inflammation might have a role in alterations in stem cells leading to tumorigenesis. Inflammatory mediators were found to be increased in the intestinal mucosa in mice with obesity or those fed a HFD, resulting from cytokine release by myofibroblasts and immune cells[86]. Local inflammation has been demonstrated to expand colon cell progenitors or stem cells and to induce their proliferation in the intestine. For example, HFD-induced obesity elevated both the colonic proliferative zone and stem cell zone in a pig model, and the proliferative zone was associated with an increase in the innate inflammatory markers TLR4, NF-κB, IL-6, and lipocalin-2[87]. In addition, activation of NF-κB, the central pathway downstream of the majority of proinflammatory cytokines, following local inflammation enhanced the reprogramming of non-stem enterocytes to acquire stem-cell-like properties, which expanded the pool of stem cells and generated tumor-initiating cells[88]. HFD-induced obesity promoted the phosphorylation of GSK3β and then increased the nuclear translocation of β-catenin, thereby activating the expression of WNT signaling target genes[89]. These effects were diminished by the deletion of tumor necrosis factor-alpha (TNF-α), indicating the role of inflammation induced by TNF-α in colon tumorigenesis associated with obesity[89]. Another inflammatory mediator, prostaglandin E2, was also found to be elevated by HFD in the circulation or in local tissues, leading to an increased number and division rate of stem cells[22].
Other proinflammatory mediators have also been demonstrated to facilitate CSC expansion. For example, IL-6 can induce malignant features in human ductal breast carcinoma stem/progenitor cells[90]. IL-8 treatment leads to breast cancer cells partially acquiring some stem-like cell attributes, thereby increasing their aggressiveness[91]. Chemokine (C-C motif) ligand 2, derived from cancer-associated fibroblasts, stimulates the stem cell-specific, sphere-forming phenotype in breast cancer cells and CSC self-renewal[92]. Although these studies were not carried out under obese conditions, these proinflammatory mediators are consistently elevated in obese individuals and are upregulated upon contact with cancer cells. The enrichment of CSCs may be partially attributed to these cytokines, as a few studies indeed found that proinflammatory cytokines, including IL-6, IL-8 and monocyte chemoattractant protein 1, are overexpressed in cancer-associated adipose tissue from obese patients and induce the stemness of cancer cells, while such effects were not found in nonobese patients[77]. Considering the importance of localized and systemic inflammation in the induction and maintenance of stemness in cancer cells and the definite association between inflammation and obesity, elucidating how these inflammatory pathways increase the risk of cancer incidence, progression and therapy resistance via CSCs holds great promise to decrease the burden of cancer in obesity.
Stem cells are proposed to reside in a distinctive microenvironment, that is, the stem cell niche, which induces and maintains the self-renewal and differentiation of stem cells. In the TME, niche signals also play critical roles in cancer cells acquiring more stemness. Although the pathways responsible for establishing a CSC phenotype are diverse and differ among cancer entities, developmental signaling pathways, including the Notch, WNT, Hedgehog and Hippo pathways, are commonly altered in CSCs and interact with each other and with other common oncogenic signaling pathways and have key regulatory functions that support the maintenance and survival of these cells, making them prime targets for anti-CSC therapy[82]. Obesity, HFD and abnormal adipocytes may engage in these self-renewal and stemness pathways directly or indirectly through increased local and systemic levels of many cytokines and adipokines. For example, in ISCs and progenitors from mice fed a HFD, the expression of Jag1 and Jag2, which are ligands for the Notch pathway and are normally expressed by neighboring niche cells, was increased by the activation of WNT/β-catenin, indicating that a HFD drives ISCs to niche independence[19]. Within the ISC niche, MSCs were expanded and secreted predominant levels of Wnt2b in the colon of HFD-fed mice, which promoted the growth of tumorigenic properties and accelerated the expression of CSC-related markers in colon organoids[93]. CSCs isolated from obese mice also exhibited enhanced Notch2 expression[94]. However, such direct evidence supporting the association between obesity and alterations in stemness pathways is scarce, and more studies are needed to test this model. Nonetheless, some emerging data demonstrate alterations in these stemness pathways in obesity-induced cancers. For instance, HFD consumption could upregulate the expression of β-catenin proteins in a mouse xenograft tumor model[95]. Notch signaling activity was increased in breast cancer cells following coculture with obesity-altered ASCs[96]. In addition, YAP, the major player in the Hippo pathway, dictates mitochondrial redox homeostasis to facilitate obesity-associated breast cancer progression[97]. Although the contributions of CSCs were not examined in these studies, regarding the definitive effects of these signaling pathways on CSCs, it is reasonable to speculate that the upregulated activity of these pathways in obesity may promote the progression of cancer through CSCs and that targeting these pathways may be more promising in obesity-induced cancers.
The epithelial barrier surfaces of our body host a diverse microbial community, or microbiota, that is composed of a variety of microorganisms, such as bacteria, fungi, and viruses[98]. Substantial studies have reported that obesity or HFD markedly affects the composition of the commercial microbiota, especially in the intestine[48]. Obesity-induced perturbation of the gut microbiota has been demonstrated to influence stem cell phenotypes. For example, structural changes in the microbiota were associated with HFD-induced myeloid progenitor skewing of the differentiation capacity of hematopoietic stem cells[99]. The intestinal tract bacteria Lactobacillus induces the release of adiponectin by niche cells through NF-κB activation[100]. In pigs with HFD-induced obesity, the elevation of the proliferative zone and stem cell zone was associated with increased abundance of the gut bacterial phyla Proteobacteria and Firmicutes[87]. In addition to these initial data suggesting the association between obesity-related microbiome dysbiosis and the possible development of CSCs, evidence linking the microbiome in obesity to CSC function is lacking. Whether obesity-induced alterations in the composition of the host microbiota affect initiation, progression and therapy resistance and the underlying mechanisms need more investigation with the hope of providing more strategies for cancer prevention and treatment.
If the above-discussed links between obesity and CSCs are founded on convincing evidence, an obvious question is whether targeting both can prevent or improve the outcomes of cancer. At present, targeting both obesity and CSCs has great challenges; however, progress is gradually being made. For example, bariatric surgery has been popularized globally, leading to more weight loss and longer maintenance than diet and lifestyle changes[101]. Intriguingly, in these patients with obesity who received bariatric surgery, a decrease in overall cancer diagnoses was observed[102,103]. However, surgical intervention does not guarantee the recovery of obese patients to a normal state and is typically a harmful method. Theoretically, obesity prevention represents the most promising and scientific solution, which requires joint efforts and cooperation from throughout the whole world[104]. However, under obesity pandemic conditions, exploring strategies to lower the incidence of obesity-related cancer represents the primary goal. Unfortunately, no experience has been gained. As low-grade inflammation plays a central role in linking obesity and cancer, anti-inflammatory therapy may be a promising direction, which has been validated in the prevention of colorectal cancer[105]. For obese patients with established malignancies, there is an urgent need to improve therapeutic efficacy and long-term survival. As mentioned above, various mechanisms have been proposed to link obesity and CSCs; thus, whether blocking these connections can prevent or delay the initiation and progression of cancer needs further study. Encouragingly, such strategies have been explored extensively, and some of them have already advanced into clinical use. For example, the clinical development of therapeutics targeting CSC-associated developmental signaling pathways has resulted in improved patient outcomes[82]. Some inflammatory factor-targeting therapies also show promising results in improving outcomes in patients with cancer[106]. Lifestyle interventions, such as reduced dietary intake and increased physical activity, were demonstrated to cause weight loss, leading to altered expression of inflammatory factors and maintenance of stem cell homeostasis[107,108]. Therefore, what awaits us next is to validate their efficacy in obese people with cancer.
As stated above, notwithstanding the clear and compelling link between obesity and CSCs, as well as an understanding of the mechanisms connecting them, this research area is still in its infancy. Most scientific research exploring the association of CSCs and obesity originates from mouse models or was inferred indirectly from the conclusions of different research fields. Therefore, there are still many major questions waiting for answers. For example, how can the profound differences in cancer incidence across different anatomical sites influenced by obesity be explained? Do the differences in the microenvironment across adult stem cell niches contribute to these variations? Regarding alterations in stem cell biology, how much overlap is there among animal models and obese patients? What role does the microbiome play in mediating the induction, maintenance and therapy response of CSCs? Does obesity differentially regulate normal and malignant stem cells, and how does it do so? How does obesity influence the crosstalk between CSCs and immunoediting? To what extent are stem cells conditioned in obesity reversed when obesity is improved? Does obesity enhance the establishment of premetastatic niches? Can strategies aimed at targeting the mechanisms linking obesity and CSCs prevent the initiation and delay the progression of cancer?
Despite a wealth of unknowns, it is clear that obesity increases the stem cell pool and induces biological modulation in these cells, which predisposes these stem cells to transformation. Regarding the increased prevalence of obesity and its convincing association with cancer, programs are urgently needed to decrease the incidence of obesity. At this time, primary obesity prevention through public health policies, including dietary and lifestyle changes, represents a compelling approach toward a reduction in the incidence of obesity and its associated cancer. Identifying obese patients with increased cancer risk and developing appropriate management of obesity or applying cancer prevention methods such as anti-inflammatory agents in these populations represents another compelling approach toward a reduction in the burden of cancer in obesity. Several mechanisms have been proposed to explain the association between obesity and CSCs, and large amounts of agents targeting these pathways have been developed. Testing them in patients with obesity and comparing their efficacy with that in nonobese individuals are important components of future translational research and clinical trials.
The author thanks the Health Commission of Mianyang City and the Science and Education Department of the Third Hospital of Mianyang for their support. The space limitations of this review have unfortunately meant that I have not been able to separately cite many of the original publications that have contributed substantially to the literature. I sincerely apologize to the authors of these publications. All figures in this review were created with BioRender.com.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hejazi J, Iran; Papadopoulos K, Thailand; Li SC, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 2969] [Article Influence: 494.8] [Reference Citation Analysis (0)] |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4666] [Article Influence: 583.3] [Reference Citation Analysis (2)] |
| 3. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5066] [Article Influence: 633.3] [Reference Citation Analysis (2)] |
| 4. | Malik VS, Hu FB. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat Rev Endocrinol. 2022;18:205-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 414] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 5. | Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1546] [Cited by in RCA: 1436] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 6. | Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3582] [Cited by in RCA: 3261] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 7. | Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020;17:e1003198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 522] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 8. | Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 9. | Zhi X, Kuang XH, Liu K, Li J. The global burden and temporal trend of cancer attributable to high body mass index: Estimates from the Global Burden of Disease Study 2019. Front Nutr. 2022;9:918330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | O'Sullivan J, Lysaght J, Donohoe CL, Reynolds JV. Obesity and gastrointestinal cancer: the interrelationship of adipose and tumour microenvironments. Nat Rev Gastroenterol Hepatol. 2018;15:699-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 12. | Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2163] [Cited by in RCA: 2421] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 13. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1668] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 14. | Blaas L, Pucci F, Messal HA, Andersson AB, Josue Ruiz E, Gerling M, Douagi I, Spencer-Dene B, Musch A, Mitter R, Bhaw L, Stone R, Bornhorst D, Sesay AK, Jonkers J, Stamp G, Malanchi I, Toftgård R, Behrens A. Lgr6 Labels a rare population of mammary gland progenitor cells that are able to originate luminal mammary tumours. Nat Cell Biol. 2016;18:1346-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Yang J, Wei H, Zhou Y, Szeto CH, Li C, Lin Y, Coker OO, Lau HCH, Chan AWH, Sung JJY, Yu J. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology. 2022;162:135-149.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 292] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 16. | Hayashi T, Fujita K, Matsushita M, Hayashi Y, Uemura M, Nonomura N. Metformin inhibits prostate cancer growth induced by a high-fat diet in Pten-deficient model mice. Int J Urol. 2019;26:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, Chan AWH, Wei H, Yang X, Sung JJY, Yu J. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 542] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 18. | Zhou W, Davis EA, Dailey MJ. Obesity, independent of diet, drives lasting effects on intestinal epithelial stem cell proliferation in mice. Exp Biol Med (Maywood). 2018;243:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz ÖH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 613] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 20. | Tabrizian T, Wang D, Guan F, Hu Z, Beck AP, Delahaye F, Huffman DM. Apc inactivation, but not obesity, synergizes with Pten deficiency to drive intestinal stem cell-derived tumorigenesis. Endocr Relat Cancer. 2017;24:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Sikalidis AK, Fitch MD, Fleming SE. Diet induced obesity increases the risk of colonic tumorigenesis in mice. Pathol Oncol Res. 2013;19:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | DeClercq V, McMurray DN, Chapkin RS. Obesity promotes colonic stem cell expansion during cancer initiation. Cancer Lett. 2015;369:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Xie Y, Ding F, Di W, Lv Y, Xia F, Sheng Y, Yu J, Ding G. Impact of a highfat diet on intestinal stem cells and epithelial barrier function in middleaged female mice. Mol Med Rep. 2020;21:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Mao J, Hu X, Xiao Y, Yang C, Ding Y, Hou N, Wang J, Cheng H, Zhang X. Overnutrition stimulates intestinal epithelium proliferation through β-catenin signaling in obese mice. Diabetes. 2013;62:3736-3746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Li W, Zimmerman SE, Peregrina K, Houston M, Mayoral J, Zhang J, Maqbool S, Zhang Z, Cai Y, Ye K, Augenlicht LH. The nutritional environment determines which and how intestinal stem cells contribute to homeostasis and tumorigenesis. Carcinogenesis. 2019;40:937-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Mah AT, Van Landeghem L, Gavin HE, Magness ST, Lund PK. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology. 2014;155:3302-3314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 27. | Hegab AE, Ozaki M, Meligy FY, Kagawa S, Ishii M, Betsuyaku T. High fat diet activates adult mouse lung stem cells and accelerates several aging-induced effects. Stem Cell Res. 2018;33:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Münch NS, Fang HY, Ingermann J, Maurer HC, Anand A, Kellner V, Sahm V, Wiethaler M, Baumeister T, Wein F, Einwächter H, Bolze F, Klingenspor M, Haller D, Kavanagh M, Lysaght J, Friedman R, Dannenberg AJ, Pollak M, Holt PR, Muthupalani S, Fox JG, Whary MT, Lee Y, Ren TY, Elliot R, Fitzgerald R, Steiger K, Schmid RM, Wang TC, Quante M. High-Fat Diet Accelerates Carcinogenesis in a Mouse Model of Barrett's Esophagus via Interleukin 8 and Alterations to the Gut Microbiome. Gastroenterology. 2019;157:492-506.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 653] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 30. | Roper J, Tammela T, Akkad A, Almeqdadi M, Santos SB, Jacks T, Yilmaz ÖH. Colonoscopy-based colorectal cancer modeling in mice with CRISPR-Cas9 genome editing and organoid transplantation. Nat Protoc. 2018;13:217-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Rivera-Izquierdo M, Pérez de Rojas J, Martínez-Ruiz V, Arrabal-Polo MÁ, Pérez-Gómez B, Jiménez-Moleón JJ. Obesity and biochemical recurrence in clinically localised prostate cancer: a systematic review and meta-analysis of 86,490 patients. Prostate Cancer Prostatic Dis. 2022;25:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Olsson LT, Walens A, Hamilton AM, Benefield HC, Fleming JM, Carey LA, Hursting SD, Williams KP, Troester MA. Obesity and Breast Cancer Metastasis across Genomic Subtypes. Cancer Epidemiol Biomarkers Prev. 2022;31:1944-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Dannenberg AJ, Kwan ML, Xiao J, Quesenberry C, Weltzien EK, Castillo AL, Caan BJ. Metabolic Dysfunction, Obesity, and Survival Among Patients With Early-Stage Colorectal Cancer. J Clin Oncol. 2016;34:3664-3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Kokts-Porietis RL, McNeil J, Morielli AR, Cook LS, Courneya KS, Friedenreich CM. Prospective Cohort Study of Pre- and Postdiagnosis Obesity and Endometrial Cancer Survival. J Natl Cancer Inst. 2022;114:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 586] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 36. | Hoy AJ, Nagarajan SR, Butler LM. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer. 2021;21:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 227] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 37. | Maruthur NM, Bolen S, Gudzune K, Brancati FL, Clark JM. Body mass index and colon cancer screening: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:737-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Horowitz NS, Wright AA. Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol Oncol. 2015;138:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Olson OC, Quail DF, Joyce JA. Obesity and the tumor microenvironment. Science. 2017;358:1130-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Uthaya Kumar DB, Chen CL, Liu JC, Feldman DE, Sher LS, French S, DiNorcia J, French SW, Naini BV, Junrungsee S, Agopian VG, Zarrinpar A, Machida K. TLR4 Signaling via NANOG Cooperates With STAT3 to Activate Twist1 and Promote Formation of Tumor-Initiating Stem-Like Cells in Livers of Mice. Gastroenterology. 2016;150:707-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D, Morata-Tarifa C, Kim M, Ince TA, Azzam DJ, Wander SA, Wang B, Ergonul B, Datar RH, Cote RJ, Howard GA, El-Ashry D, Torné-Poyatos P, Marchal JA, Slingerland JM. Interactions between Adipocytes and Breast Cancer Cells Stimulate Cytokine Production and Drive Src/Sox2/miR-302b-Mediated Malignant Progression. Cancer Res. 2016;76:491-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 42. | Bartucci M, Svensson S, Ricci-Vitiani L, Dattilo R, Biffoni M, Signore M, Ferla R, De Maria R, Surmacz E. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010;17:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis AK, Huang RYJ, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massagué J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G; EMT International Association (TEMTIA). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 1387] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 44. | Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1905] [Article Influence: 238.1] [Reference Citation Analysis (0)] |
| 45. | Allott EH, Morine MJ, Lysaght J, McGarrigle SA, Donohoe CL, Reynolds JV, Roche HM, Pidgeon GP. Elevated Tumor Expression of PAI-1 and SNAI2 in Obese Esophageal Adenocarcinoma Patients and Impact on Prognosis. Clin Transl Gastroenterol. 2012;3:e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S, Gimble JM, Burow ME, Bunnell BA. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 47. | Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 993] [Article Influence: 331.0] [Reference Citation Analysis (0)] |
| 48. | Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018;15:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 49. | Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016;34:4270-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 660] [Article Influence: 73.3] [Reference Citation Analysis (1)] |
| 50. | Blakeborough MH, Owen RW, Bilton RF. Free radical generating mechanisms in the colon: their role in the induction and promotion of colorectal cancer? Free Radic Res Commun. 1989;6:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Karunanithi S, Levi L, DeVecchio J, Karagkounis G, Reizes O, Lathia JD, Kalady MF, Noy N. RBP4-STRA6 Pathway Drives Cancer Stem Cell Maintenance and Mediates High-Fat Diet-Induced Colon Carcinogenesis. Stem Cell Reports. 2017;9:438-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 52. | Rinkenbaugh AL, Baldwin AS. The NF-κB Pathway and Cancer Stem Cells. Cells. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 53. | Obniski R, Sieber M, Spradling AC. Dietary Lipids Modulate Notch Signaling and Influence Adult Intestinal Development and Metabolism in Drosophila. Dev Cell. 2018;47:98-111.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 54. | Wang B, Rong X, Palladino END, Wang J, Fogelman AM, Martín MG, Alrefai WA, Ford DA, Tontonoz P. Phospholipid Remodeling and Cholesterol Availability Regulate Intestinal Stemness and Tumorigenesis. Cell Stem Cell. 2018;22:206-220.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 55. | Sorrentino G, Perino A, Yildiz E, El Alam G, Bou Sleiman M, Gioiello A, Pellicciari R, Schoonjans K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology. 2020;159:956-968.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 56. | Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, Zhu Q, Zhang T, Leblanc M, Liu S, He M, Waizenegger W, Gasser E, Schnabl B, Atkins AR, Yu RT, Knight R, Liddle C, Downes M, Evans RM. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell. 2019;176:1098-1112.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 324] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 57. | Wang SY, Hu QC, Wu T, Xia J, Tao XA, Cheng B. Abnormal lipid synthesis as a therapeutic target for cancer stem cells. World J Stem Cells. 2022;14:146-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (3)] |
| 58. | Yi M, Li J, Chen S, Cai J, Ban Y, Peng Q, Zhou Y, Zeng Z, Peng S, Li X, Xiong W, Li G, Xiang B. Emerging role of lipid metabolism alterations in Cancer stem cells. J Exp Clin Cancer Res. 2018;37:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 59. | Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27:136-150.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 551] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 60. | Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R, Bosc C, Sugita M, Stuani L, Fraisse M, Scotland S, Larrue C, Boutzen H, Féliu V, Nicolau-Travers ML, Cassant-Sourdy S, Broin N, David M, Serhan N, Sarry A, Tavitian S, Kaoma T, Vallar L, Iacovoni J, Linares LK, Montersino C, Castellano R, Griessinger E, Collette Y, Duchamp O, Barreira Y, Hirsch P, Palama T, Gales L, Delhommeau F, Garmy-Susini BH, Portais JC, Vergez F, Selak M, Danet-Desnoyers G, Carroll M, Récher C, Sarry JE. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017;7:716-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 642] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 61. | He W, Liang B, Wang C, Li S, Zhao Y, Huang Q, Liu Z, Yao Z, Wu Q, Liao W, Zhang S, Liu Y, Xiang Y, Liu J, Shi M. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38:4637-4654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 62. | Zhou W, Rowitz BM, Dailey MJ. Insulin/IGF-1 enhances intestinal epithelial crypt proliferation through PI3K/Akt, and not ERK signaling in obese humans. Exp Biol Med (Maywood). 2018;243:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Savarese TM, Strohsnitter WC, Low HP, Liu Q, Baik I, Okulicz W, Chelmow DP, Lagiou P, Quesenberry PJ, Noller KL, Hsieh CC. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol (Lausanne). 2014;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 65. | Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 66. | McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne). 2013;4:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 358] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 67. | Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2065] [Cited by in RCA: 2251] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 68. | Pourvali K, Monji H. Obesity and intestinal stem cell susceptibility to carcinogenesis. Nutr Metab (Lond). 2021;18:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 69. | Crean-Tate KK, Reizes O. Leptin Regulation of Cancer Stem Cells in Breast and Gynecologic Cancer. Endocrinology. 2018;159:3069-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Tenvooren I, Jenks MZ, Rashid H, Cook KL, Muhlemann JK, Sistrunk C, Holmes J, Wang K, Bonin K, Hodges K, Lo HW, Shaikh A, Camarillo IG, Lelièvre SA, Seewaldt V, Vidi PA. Elevated leptin disrupts epithelial polarity and promotes premalignant alterations in the mammary gland. Oncogene. 2019;38:3855-3870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, Lathia JD, Reizes O. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 73. | Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H, Nakajima A. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | Higurashi T, Endo H, Uchiyama T, Uchiyama S, Yamada E, Ohkubo H, Sakai E, Takahashi H, Maeda S, Wada K, Natsumeda Y, Hippo Y, Nakajima A, Nakagama H. Conditional knockout of the leptin receptor in the colonic epithelium revealed the local effects of leptin receptor signaling in the progression of colonic tumors in mice. Carcinogenesis. 2014;35:2134-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Avtanski D, Garcia A, Caraballo B, Thangeswaran P, Marin S, Bianco J, Lavi A, Poretsky L. Resistin induces breast cancer cells epithelial to mesenchymal transition (EMT) and stemness through both adenylyl cyclase-associated protein 1 (CAP1)-dependent and CAP1-independent mechanisms. Cytokine. 2019;120:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 76. | Wu Q, Li B, Li Z, Li J, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 298] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 77. | Ibrahim AS, El-Shinawi M, Sabet S, Ibrahim SA, Mohamed MM. Role of adipose tissue-derived cytokines in the progression of inflammatory breast cancer in patients with obesity. Lipids Health Dis. 2022;21:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 78. | Abu Quora HA, Zahra MH, El-Ghlban S, Nair N, Afify SM, Hassan G, Nawara HM, Sheta M, Monzur S, Fu X, Osman A, Seno A, Seno M. Microenvironment of mammary fat pads affected the characteristics of the tumors derived from the induced cancer stem cells. Am J Cancer Res. 2021;11:3475-3495. [PubMed] |
| 79. | Rebuffé-Scrive M, Eldh J, Hafström LO, Björntorp P. Metabolism of mammary, abdominal, and femoral adipocytes in women before and after menopause. Metabolism. 1986;35:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2443] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 81. | Sabol RA, Bowles AC, Côté A, Wise R, O'Donnell B, Matossian MD, Hossain FM, Burks HE, Del Valle L, Miele L, Collins-Burow BM, Burow ME, Bunnell BA. Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Res. 2019;21:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. 2020;17:204-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 504] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 83. | Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 2427] [Article Influence: 404.5] [Reference Citation Analysis (0)] |
| 84. | Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20:242-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 947] [Article Influence: 157.8] [Reference Citation Analysis (0)] |
| 85. | Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320:C375-C391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 982] [Article Influence: 196.4] [Reference Citation Analysis (0)] |
| 86. | Kizil C, Kyritsis N, Brand M. Effects of inflammation on stem cells: together they strive? EMBO Rep. 2015;16:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 87. | Charepalli V, Reddivari L, Radhakrishnan S, Eriksson E, Xiao X, Kim SW, Shen F, Vijay-Kumar M, Li Q, Bhat VB, Knight R, Vanamala JKP. Pigs, Unlike Mice, Have Two Distinct Colonic Stem Cell Populations Similar to Humans That Respond to High-Calorie Diet prior to Insulin Resistance. Cancer Prev Res (Phila). 2017;10:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 832] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 89. | Deng H, Gerencser AA, Jasper H. Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature. 2015;528:212-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 90. | Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafè M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988-4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 618] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 91. | Ospina-Muñoz N, Vernot JP. Partial acquisition of stemness properties in tumorspheres obtained from interleukin-8-treated MCF-7 cells. Tumour Biol. 2020;42:1010428320979438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li AX, Wu X, Ye W, Chen S, Zhou W, Yu Y, Wang YZ, Ren X, Li H, Scherle P, Kuroki Y, Wang SE. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 93. | Kim TY, Kim S, Kim Y, Lee YS, Lee S, Lee SH, Kweon MN. A High-Fat Diet Activates the BAs-FXR Axis and Triggers Cancer-Associated Fibroblast Properties in the Colon. Cell Mol Gastroenterol Hepatol. 2022;13:1141-1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Hillers-Ziemer LE, McMahon RQ, Hietpas M, Paderta G, LeBeau J, McCready J, Arendt LM. Obesity Promotes Cooperation of Cancer Stem-Like Cells and Macrophages to Enhance Mammary Tumor Angiogenesis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Tang FY, Pai MH, Chiang EP. Consumption of high-fat diet induces tumor progression and epithelial-mesenchymal transition of colorectal cancer in a mouse xenograft model. J Nutr Biochem. 2012;23:1302-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Sabol RA, Villela VA, Denys A, Freeman BT, Hartono AB, Wise RM, Harrison MAA, Sandler MB, Hossain F, Miele L, Bunnell BA. Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 97. | Dai JZ, Wang YJ, Chen CH, Tsai IL, Chao YC, Lin CW. YAP Dictates Mitochondrial Redox Homeostasis to Facilitate Obesity-Associated Breast Cancer Progression. Adv Sci (Weinh). 2022;9:e2103687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 99. | Luo Y, Chen GL, Hannemann N, Ipseiz N, Krönke G, Bäuerle T, Munos L, Wirtz S, Schett G, Bozec A. Microbiota from Obese Mice Regulate Hematopoietic Stem Cell Differentiation by Altering the Bone Niche. Cell Metab. 2015;22:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 100. | Su X, Yan H, Huang Y, Yun H, Zeng B, Wang E, Liu Y, Zhang Y, Liu F, Che Y, Zhang Z, Yang R. Expression of FABP4, adipsin and adiponectin in Paneth cells is modulated by gut Lactobacillus. Sci Rep. 2015;5:18588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, Rasmussen-Torvik LJ, Balicer RD. Association of Bariatric Surgery Using Laparoscopic Banding, Roux-en-Y Gastric Bypass, or Laparoscopic Sleeve Gastrectomy vs Usual Care Obesity Management With All-Cause Mortality. JAMA. 2018;319:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 102. | Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Carlsson LM; Swedish Obese Subjects Study. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 510] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 103. | Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, Halverson RC, Simper SC, Hopkins PN, Hunt SC. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring). 2009;17:796-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 104. | Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy Weight and Obesity Prevention: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1506-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 318] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 105. | Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18:261-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 106. | Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 559] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 107. | Campbell KL, Landells CE, Fan J, Brenner DR. A Systematic Review of the Effect of Lifestyle Interventions on Adipose Tissue Gene Expression: Implications for Carcinogenesis. Obesity (Silver Spring). 2017;25 Suppl 2:S40-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Calibasi-Kocal G, Mashinchian O, Basbinar Y, Ellidokuz E, Cheng CW, Yilmaz ÖH. Nutritional Control of Intestinal Stem Cells in Homeostasis and Tumorigenesis. Trends Endocrinol Metab. 2021;32:20-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |