Published online Mar 26, 2023. doi: 10.4252/wjsc.v15.i3.90
Peer-review started: June 30, 2022
First decision: December 30, 2022
Revised: January 6, 2023
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: March 26, 2023
Processing time: 265 Days and 18.4 Hours
The low survival rate of mesenchymal stem cells (MSCs) caused by anoikis, a form of apoptosis, limits the therapeutic efficacy of MSCs. As a proapoptotic molecule, mammalian Ste20-like kinase 1 (Mst1) can increase the production of reactive oxygen species (ROS), thereby promoting anoikis. Recently, we found that Mst1 inhibition could protect mouse bone marrow MSCs (mBMSCs) from H2O2-induced cell apoptosis by inducing autophagy and reducing ROS production. However, the influence of Mst1 inhibition on anoikis in mBMSCs remains unclear.
To investigate the mechanisms by which Mst1 inhibition acts on anoikis in isolated mBMSCs.
Poly-2-hydroxyethyl methacrylate-induced anoikis was used following the silencing of Mst1 expression by short hairpin RNA (shRNA) adenovirus transfection. Integrin (ITGs) were tested by flow cytometry. Autophagy and ITGα5β1 were inhibited using 3-methyladenine and small interfering RNA, respe
In isolated mBMSCs, Mst1 expression was upregulated, and Mst1 inhibition significantly reduced cell apoptosis, induced autophagy and decreased ROS levels. Mechanistically, we found that Mst1 inhibition could upregulate ITGα5 and ITGβ1 expression but not ITGα4, ITGαv, or ITGβ3 expression. Moreover, autophagy induced by upregulated ITGα5β1 expression following Mst1 inhibition played an essential role in the protective efficacy of Mst1 inhibition in averting anoikis.
Mst1 inhibition ameliorated autophagy formation, increased ITGα5β1 expression, and decreased the excessive production of ROS, thereby reducing cell apoptosis in isolated mBMSCs. Based on these results, Mst1 inhibition may provide a promising strategy to overcome anoikis of implanted MSCs.
Core Tip: In isolated mouse bone marrow mesenchymal stem cell (mBMSCs), Mammalian sterile 20-like kinase 1 (Mst1) inhibition could ameliorate not only autophagy formation but also upregulate integrin (ITG) α5β1 expression (but not ITGα4, ITGαv, or ITGβ3). In addition, Mst1 inhibition-induced autophagy could scavenge the excessive production of ITGα5β1-triggered ROS. Therefore, Mst1 inhibition-based infusion may improve the survival of MSCs, thereby serving as an ideal candidate for clinical trans
- Citation: Zhang T, Zhang Q, Yu WC. Mammalian Ste20-like kinase 1 inhibition as a cellular mediator of anoikis in mouse bone marrow mesenchymal stem cells. World J Stem Cells 2023; 15(3): 90-104
- URL: https://www.wjgnet.com/1948-0210/full/v15/i3/90.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i3.90
Mesenchymal stem cell (MSC) therapy is characterized by anti-inflammatory, immunomodulatory, and regenerative properties, providing an attractive therapeutic approach for pulmonary arterial hypertension (PAH)[1]. Despite the therapeutic potential of MSCs for improving the outcomes of PAH patients[2,3], no more than 5% of cells survive after transplant[4]. Thus, the low survival rate of the grafted cells is widely perceived as the major hindrance for an MSC-based therapy for PAH.
Anoikis occurs when cells detach from the extracellular matrix and subsequently undergo apoptosis, and potentially acts as a major enabling factor for the apoptosis of transplanted cells[5,6]. Indeed, after isolation from the extracellular matrix (ECM) and injection into the circulatory system for transplantation, MSCs will undergo anoikis, also referred to as cell isolation-induced apoptosis, leading to a series of alterations in anoikis signalling pathways[3,7,8]. Anoikis can be induced by destruction of integrin (ITGs) signalling or deletion of ITGs genes[9]. After isolation, focal adhesion kinase (FAK), a key downstream target of ITGs, is recruited to focal adhesion sites, consequently activating cell survival signals, such as blocking caspase 3 expression[10]. However, it remains unknown whether ITGs signalling is involved in the process of anoikis in MSCs.
Autophagy is a dynamic process that maintains homeostasis by preventing the accumulation of excessive biomolecules and impaired cells and organelles. There is accumulating evidence of a link between autophagy and anoikis[11]. Previously, we demonstrated that mammalian Ste20-like kinase 1 (Mst1) inhibition could reduce H2O2-induced apoptosis of mBMSCs by inducing autophagy formation[12]. Mst1 is a serine/threonine kinase, known as a key mediator in cellular processes, including mediating the apoptosis[13]. However, the molecular mechanism by which Mst1 inhibition mediates autophagy and anoikis in isolated mBMSCs remains to be clarified.
In this study, we investigated the potential regulatory effect of Mst1 inhibition on ITGs signalling, autophagy and anoikis in isolated mBMSCs.
The mBMSCs were obtained as previously described[12]. Cultured mBMSCs between passages 3 and 5 were selected for subsequent experiments.
Adenovirus harbouring Mst1 short hair RNA (Ad-sh-Mst1) and the control vector for Mst1 shRNA (Ad-NC-Mst1) were purchased from WZ Biosciences (China). Vector details have been previously described[12]. The shRNA sequence targeting Mst1 in mice was GCCCTCACGTA GTCAAGTATT.
The small interfering RNAs (siRNAs) were obtained from GenePharma (China). The sense and antisense strand sequences of siRNA are as follows: Mouse siRNA-ITGα5, 5'-GCAGGGAGA
Petri dishes coated with polyhydroxyethyl methacrylate [Poly-HEMA, 529257, Sigma, United States of America (USA)] were used to prevent cells from adhering to the tissue culture plates. Briefly, poly-HEMA stock material was dissolved in 95% ethanol at a concentration of 12 mg/mL, and 1 mL of 12 mg/mL poly-HEMA was added to each well of a 6-well plate and then dried overnight on a clean bench. Cells were transfected as previously described. Cells (5 × 105) were coated with 12.5 mg/mL poly-HEMA in each well for a certain period of time.
To inhibit autophagy, cells were pretreated with 5 mmol/L 3-MA (189490, Selleck, USA) for 1 h and then cultured in poly-HEMA-precoated plates for a certain period of time.
As mentioned above, cellular ROS were assessed using the ROS probe 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA), S0033, Beyotime Biotechnology, China)[12]. The mean fluorescence intensity was detected via flow cytometry.
Anoikis was analysed using an in situ Direct DNA Fragmentation Terminal-deoxynucleoitidyl Transferase Mediated Nick End Labeling (TUNEL) Assay Kit [ab66108, Abcam, The United Kingdom of Great Britain and Northern Ireland (UK)]. After incubation in poly-HEMA-coated plates, the cells were collected and added to 70% ice ethanol for 30 min. Ethanol was then removed, and the cells were resuspended in washing buffer and then stained with a staining solution for 60 min. Prior to the addition of the PI/RNase A solution, the cells were washed twice with rinse buffer. Quantification analysis was performed by BectonDickinson Fluorescence Activating Cell Sorter (BD FACSDiva) software, [Ex/Em = 488/520 nm for fluoresceine isothiocyanate and 488/623 nm for propidium iodide)].
Anoikis was also detected by a CytoSelect™ 24-Well Anoikis Assay (XY-CBA-080, Cell Biolabs, USA) according to the manufacturer's instructions. Briefly, cells (1 × 106 cells/well) were cultured in each well of 24-well plate for 36 h before staining with ethidium homodimer (EthD-1) at 37°C for 1 h. The presence of red EthD-1 fluorescence in dead cells was observed by a fluorescence microscope, and cell viability was determined using a thiazolyl blue tetrazolium bromide (MTT) assay.
Cells were incubated in poly-HEMA-coated petri dishes for 36 h, centrifuged at 300 × g for 5 min and cultured in antibodies (ITGα4 [1/500 dilution, 553157, BD], ITGα5 [1/500 dilution, 557447, BD], ITGαv [1/300 dilution, 740946, BD], ITGβ1 [1/500 dilution, 561796, BD], ITGβ3 [1/100 dilution, 740677, BD]) for 1 h according to the operation manual.
After culture in poly-HEMA-coated petri dishes, the collected cells were resuspended in complete α-MEM and then plated in triplicate (5× 104 cells/well) onto wells coated with fibronectin (10 g/mL), which was previously blocked with 1% BSA for 1 h. After 6 h, the cells were washed with phosphate belanced solution (PBS) and stained with crystal violet. Unbound dye was removed with PBS before adding a 10% acetic acid solution. The absorbance was read at 630 nm using a Multiskan MK3 microplate reader. The experiment was repeated three times. Cell adhesion was calculated according to the proportion of adhered cells in the control group.
The supernatants in each group were collected after culture in poly-HEMA-coated petri dishes for 36 h. The levels of anti-inflammatory cytokines were measured using a BD™ Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 Cytokine Kit (561665, BD, USA) in accordance with the instruction manual. The levels of interleukin (IL)-4 (IL-4), IL-10, IL-17A and IL-6 in cell supernatants were measured using flow cytometry. Data analysis was performed as previously described[14].
All animal procedures were approved by the Animal Care and Use Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University (IACUC protocol, Approval No. 2020-333). A total of 10 female nude mice (4 wk old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. and raised in a specific pathogen-free environment. Mice were placed at a standard room temperature at a normal day-night cycle with free access to standard diet and water. Afterwards, 5.0 × 106 mBMSCs (n = 3), mBMSC/NC-Mst1 (n = 3), and mBMSC/sh-Mst1 (n = 4) were injected into the right flank near the hind legs of each nude mouse. The tumours were measured with a Vernier calliper every 4 d. Sixty days after cell inoculation, all mice were anaesthetized with ether, and tissues were collected.
qPCR was performed as previously reported[12]. mBMSCs were differentiated via 21-d exposure to osteogenic or adipogenic conditions, and total mRNA from mBMSCs subjected to these conditions and siRNA-transfected cells was isolated using TRIzol Reagent (15596026, Thermo Fisher Scientific, USA). The RNA was subsequently reverse transcribed into cDNA and amplified using the SYBR® Premix Ex TaqTM II kit (RR420, Takara, JPN) and d ABI 7500 real-time PCR system (Applied Biosystems). Each experiment was repeated three times. Data were normalized through the 2-ΔΔCT method using the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. The primer sequences are shown in Supporting Information Supplementary Table 1.
To determine protein expression, Western blot analysis was performed. After culture in poly-HEMA-coated plates, whole-cell protein extracts were prepared in radio-immunoprecipitation assay lysis buffer, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride membranes. The membranes were then blocked with 5% skimmed milk or Bovine serum albumin in Tris-Buffered Saline Tween-20 for 1 h and incubated overnight at 4 ℃ with the following primary antibodies (diluted by Western Primary Antibody Buffer, P0023A, Beyotime): Mst1 (1:1000, ab51134, Abcam), ITGα5 (1:1000, ab150361, Abcam), ITGβ1 (1:1000, ab179471, Abcam), phospho-FAK (Tyr397) [1:500, 3283S, Cell Signaling Technology (CST)], FAK (1:1,000, 3285S, CST), activated caspase 3 (1:1000, ab214430, Abcam), and caspase 3 (1:1000, ab18297, Abcam). GAPDH (1:1000, 5174S, CST) served as the loading control. Anti-rabbit IgG and HRP-linked antibodies (1:1000, 7074S, CST) were used. The relative protein expression levels were compared with GAPDH using ImageJ software.
All results are expressed as the mean ± SD. One-way Analysis of Variance was used for data analysis. P < 0.05 was considered statistically significant.
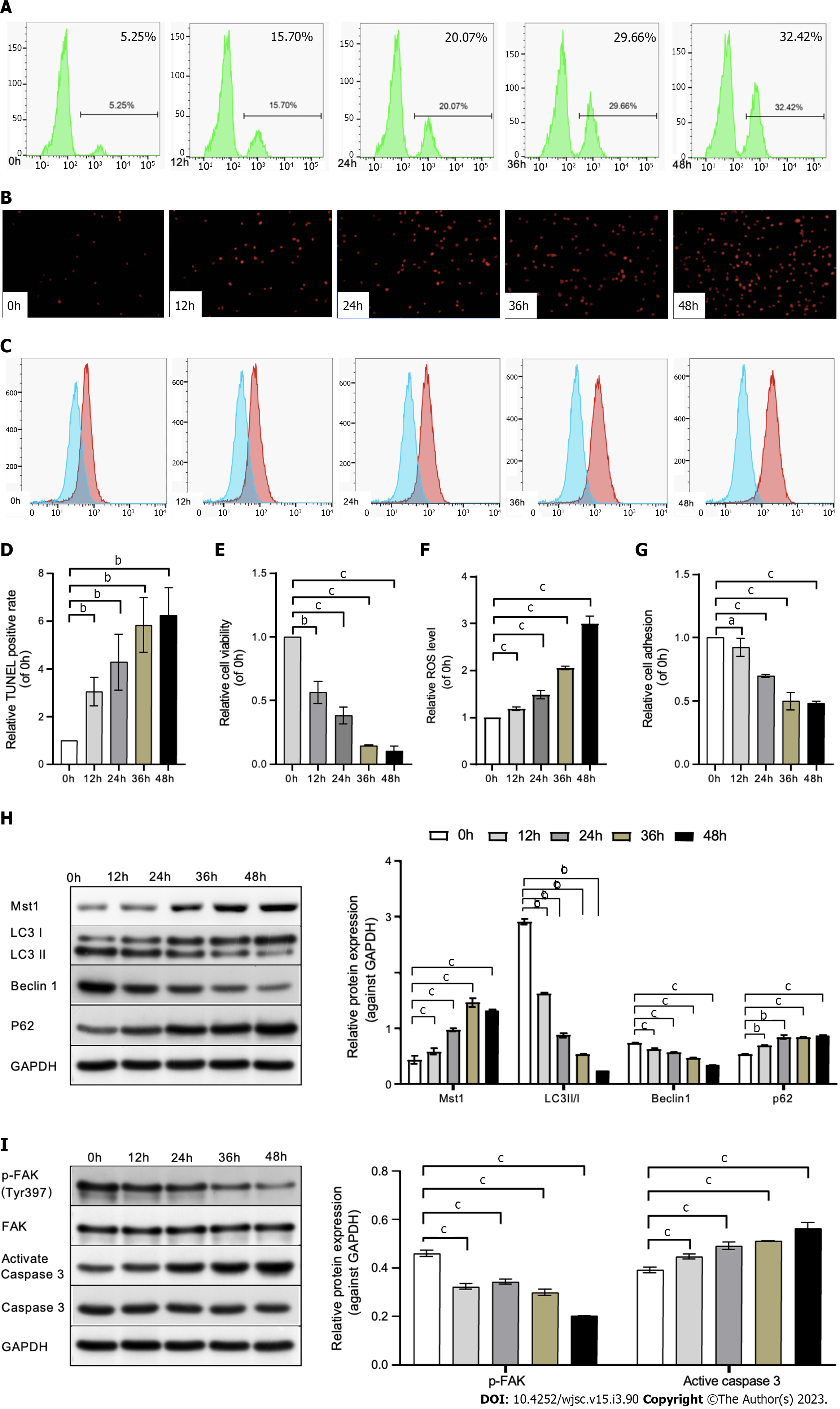
As the ability to reduce cell adhesivity to culture plates, Poly-HEMA was used to simulate an anchorage-independent culture condition. In present study, the sensitivity of mBMSCs to anoikis in Poly-HEMA-pre-coated condition were tested.
Using the TUNEL and Anoikis Assay Kit, the results showed an increased rate of mBMSC apoptosis in a time-dependent manner under poly-HEMA-induced isolated conditions (Figures 1A, B, D and E), suggesting that anoikis of mBMSCs could be induced in precoated poly-HEMA plates. In addition, the cell adhesion decreased at 24 h, 36 h, and 48 h compared with that at 0 h (Figure 1G).
Moreover, staining of intracellular ROS with the ROS probe DCFH-DA showed increased ROS levels at 24, 36, and 48 h compared with 0 h (Figures 1C and F), demonstrating the production of ROS in poly-HEMA-induced isolated mBMSCs.
To determine the alterations in Mst1 expression, autophagy and the FAK/Caspase 3 pathway in mBMSCs under isolated conditions, the protein level of Mst1, autophagy-related proteins (LC3 II/I, Beclin1, p62), p-FAK, and activated caspase 3 was detected by Western blot analysis. The data suggested that Mst1 was upregulated in isolated mBMSCs (Figure 1H). Moreover, the expression of p-FAK decreased, and the activation of caspase 3 increased in a time-dependent manner (Figure 1I). Similarly, LC3 II/I and Beclin1 expression was downregulated, and p62 expression was upregulated in a time-dependent manner (Figure 1H).
The mBMSCs were infected with adenovirus containing Mst1 shRNA. The effect of shRNA on inhibiting Mst1 expression were measured by qPCR and Western blot (Supplemen
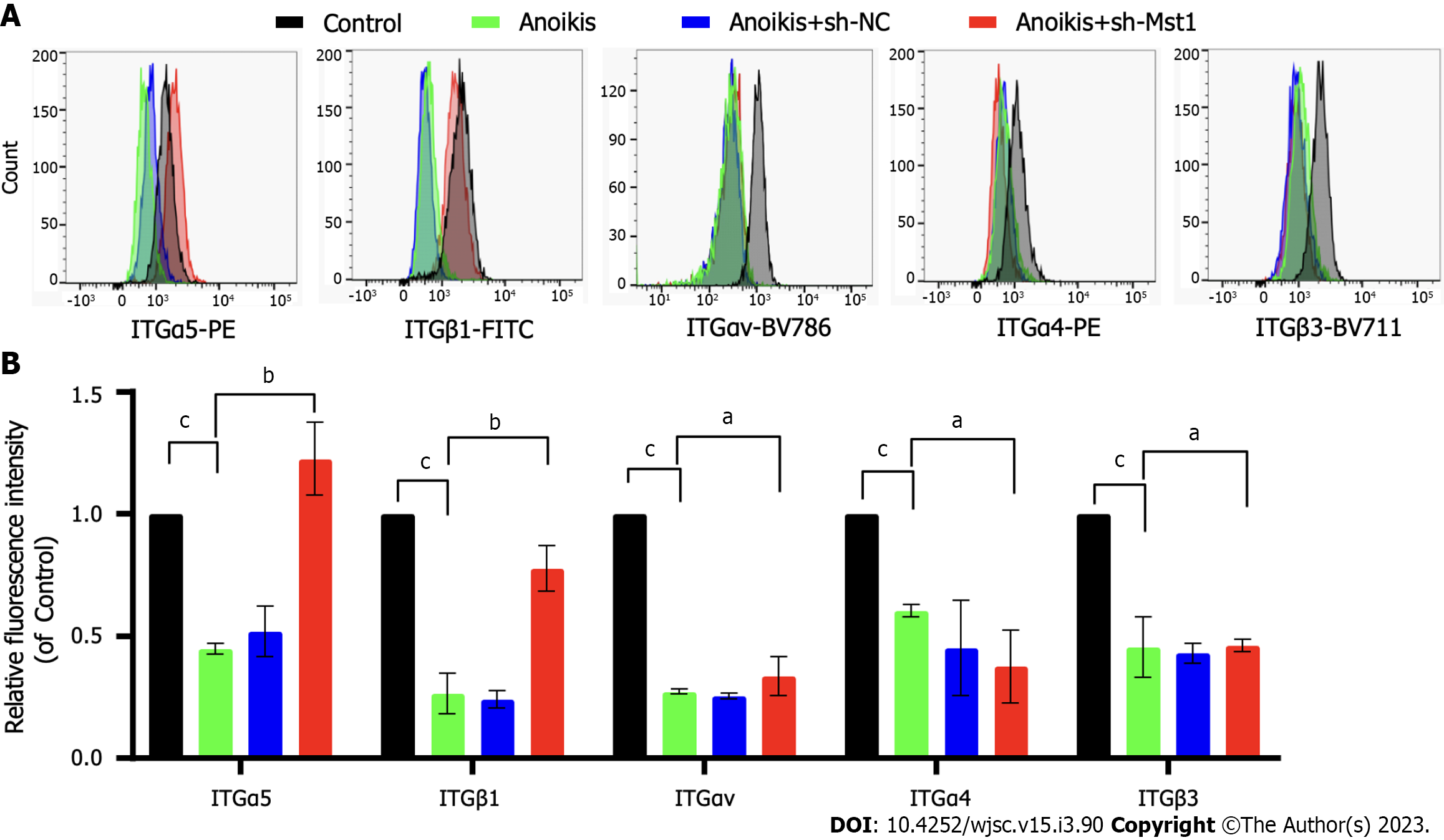
Evidence has shown that ITGs, the heterodimeric cell surface adhesion receptors, mediates anoikis. In this study, the alterations of ITGs in isolated mBMSCs/sh-Mst1 were tested.
The expression profiles of ITGα5, ITGαv, ITGα4, ITGβ1, and ITGβ3 in poly-HEMA-treated mBMSCs were compared by flow cytometry. Compared with the control mBMSC levels, the poly-HEMA-treated isolated mBMSC levels of ITGα5, ITGαv, ITGα4, ITGβ1, and ITGβ3 were significantly decreased (Figure 2). Compared with isolated mBMSCs, isolated mBMSCs/sh-Mst1 show an upwards trend in ITGα5 and ITGβ1 expression. However, there was no difference of the expression profiles of ITGαv, ITGα4, and ITGβ3 between isolated mBMSCs and isolated mBMSCs/sh-Mst1 (Figure 2). This study suggested that the inhibition of Mst1 could reactivate the expression of ITGα5 and ITGβ1.
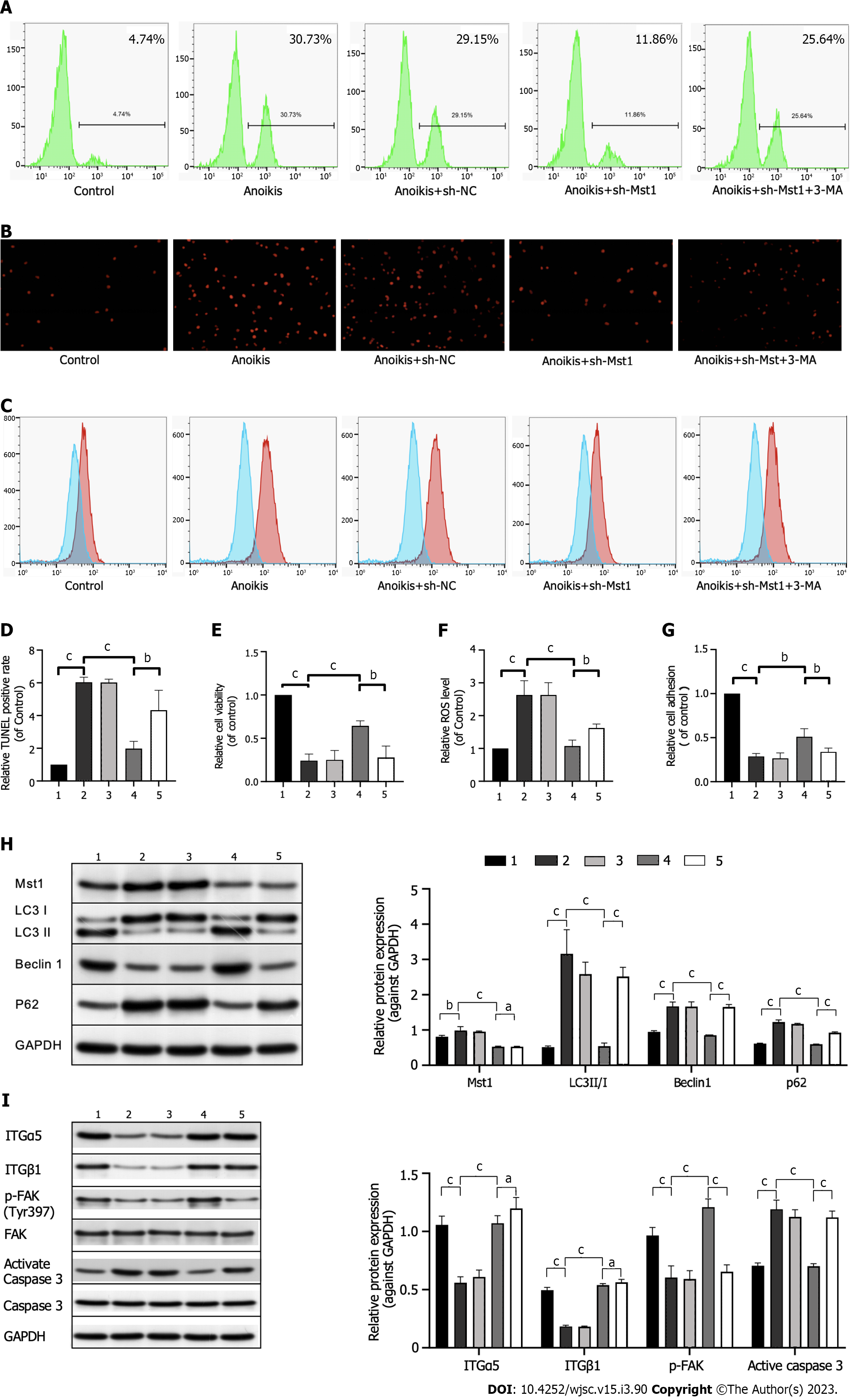
mBMSCs were cultured in precoated poly-HEMA plates for 36 h. A significant decrease in cell apoptosis was observed in mBMSCs/sh-Mst1 (Figures 3A, B, D and E). Similar to the above results, cell adhesion was ameliorated by silencing Mst1 expression (Figure 3G). These results indicated that Mst1 inhibition suppressed ECM-isolatedinduced anoikis in mBMSCs.
In addition, flow cytometric analysis confirmed decreased ROS levels in isolated mBMSCs/sh-Mst1 compared with those of isolated mBMSCs, whereas ROS levels were re-elevated by the autophagy inhibitor 3-MA (Figures 3C and F).
Western blot assay further suggested the above conception. FAK, has been recognised as the key mediator of cell–substrate adhesion. Western blot analysis results showed that mBMSC/sh-Mst1 exhibited robust FAK activation (Figure 3I). Similar to apoptosis, the activation of caspase can induce anoikis. Thus, we tested effect of Mst1 inhibition on the activation of caspase 3 by Western blotting. In Figure 3I, silencing Mst1 expression significantly inhibited caspase 3 activation in suspension-grown mBMSCs. This study indicated that silencing Mst1 expression could reactivate the FAK/Caspase3 pathway in anchorage-independent mBMSCs. However, 3-MA, an autophagy inhibitor, had no effect on the expression of ITGα5 and ITGβ1 or on cell adhesion (Figure 3I).
Consistent with the previous results, Mst1 inhibition reactivated autophagy in mBMSCs under isolated conditions, which can be demonstrated by the upregulated expression of LC3 II/I and Beclin1 and downregulated expression of p62 (Figure 3H). Furthermore, the number of mBMSCs/sh-Mst1 undergoing anoikis was increased after pretreatment with 3-MA (Figure 3H). In conclusion, the protective effect of Mst1 knockdown on anoikis in mBMSCs is associated with autophagy.
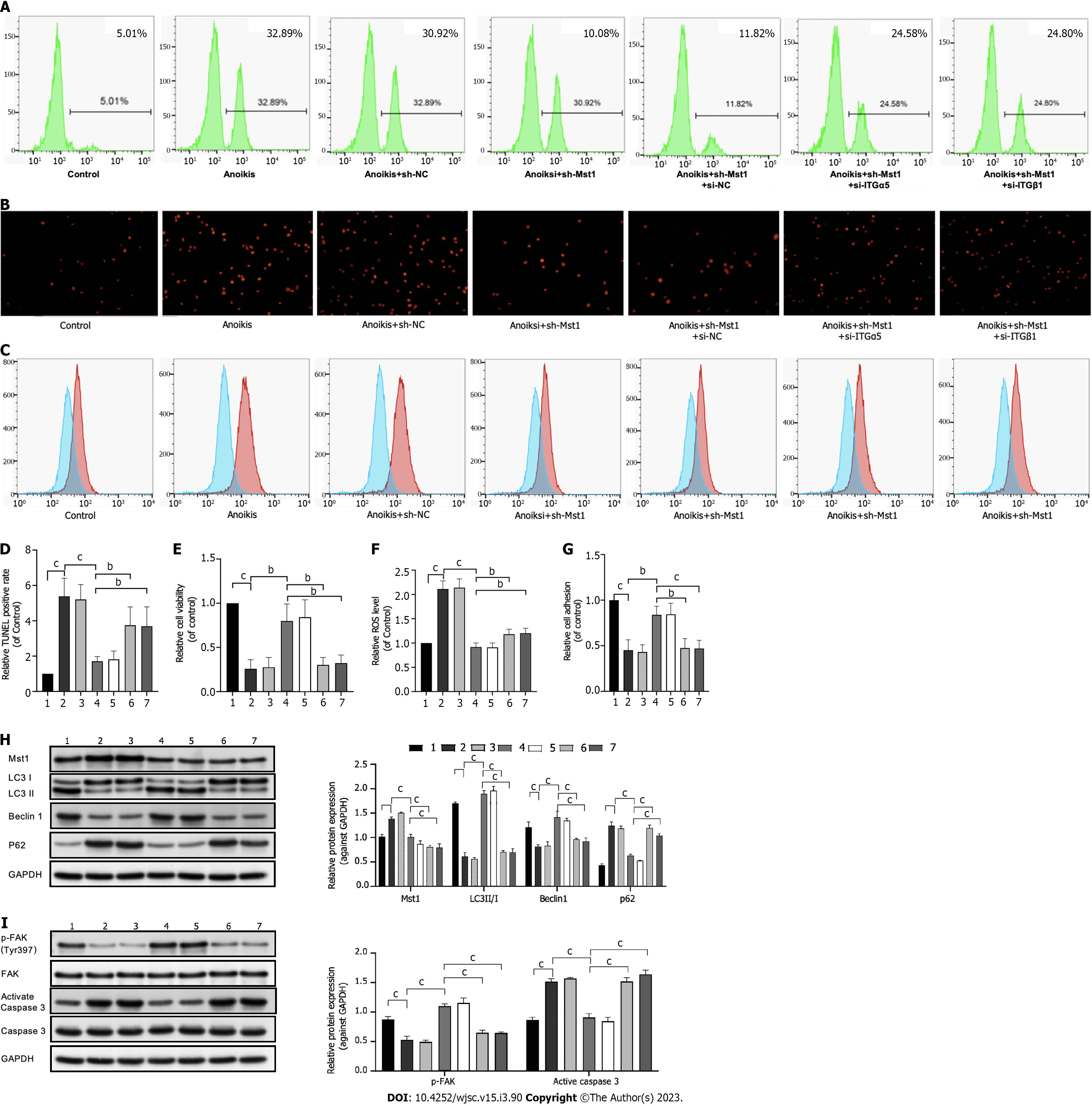
To determine whether ITGα5 or ITGβ1 contributes to anoikis resistance in mBMSC/sh-Mst1 cells, siRNA was used to knock down ITGα5 or ITGβ1 expression, respectively (Supplementary Figure 2).
In isolated mBMSC/sh-Mst1, cell apoptosis was increased, and cell adhesion was blocked by siRNA-mediated ablation of ITGα5 or ITGβ1 (Figures 4A, B, D, E and G). Similarly, p-FAK expression was downregulated and caspase3 activation was upregulated using ITGα5 or ITGβ1 siRNA (Figure 4I). In addition, LC3 II/I, Beclin1 and p62 expression was also reversed by ITGα5 or ITGβ1 siRNA (Figure 4H). In addition, the results in Figures 4C and F suggested that the ROS level was reduced by ITGα5 or ITGβ1 siRNA.
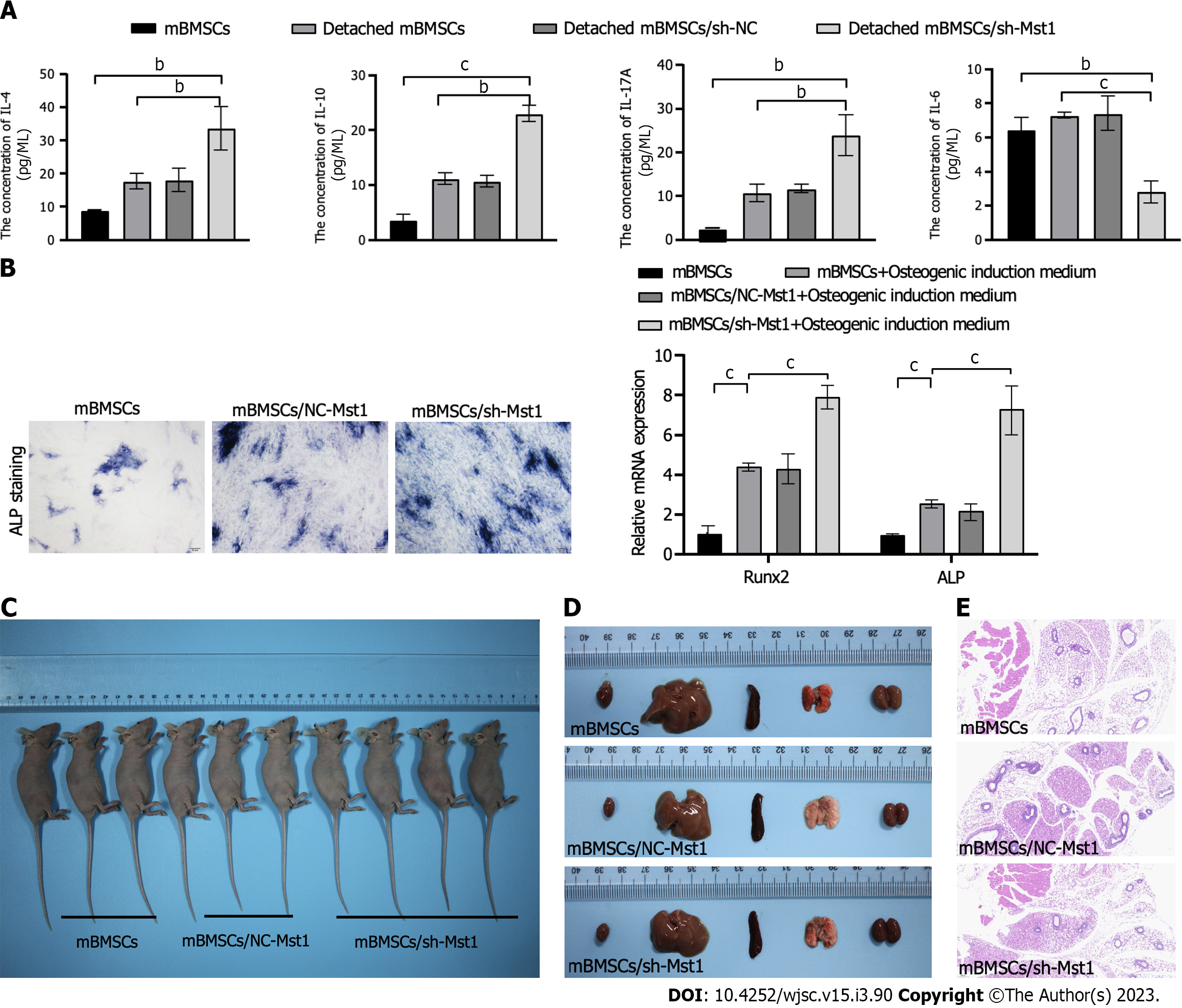
In isolated conditions, the levels of anti-inflammatory cytokines IL-4, IL-10 and IL-17A increased, while the level of pro-inflammatory cytokine IL-6 decreased in mBMSCs/sh-Mst1 compared with those of other mBMSCs (Figure 5A).
We assessed the effect of silencing Mst1 expression on the osteogenic differentiation of mBMSCs. In Figure 5B, Mst1 inhibition was correlated with increased osteogenic differentiation of mBMSCs. Subsequently, qPCR was performed to accurately determine the role of Mst1 inhibition on osteogenic differentiation in mBMSCs. As known as the markers of osteoblast differentiation, we tested the mRNA levels of runt-related transcription factor 2 (Runx2) and alkaline phosphatase (ALP). We found that the expression of Runx2 and ALP were both increased in mBMSC/sh-Mst1 (Figure 5B)[15].
There was no tumour-like mass in animals injected with mBMSC/sh-Mst1 after 60 d post-injection. After 60 d post-injection, we collected the subcutaneous tissue and the lung, liver, kidney and heart. There were no difference of the weights of the lung, liver, kidney and heart among each group (Figures 5C and Supplementary Table 2). It also showed that no stromal structures appeared in subcutaneous tissue of mBMSCs/sh-Mst1 groups (Figure 5C).
Convincing suggestion has confirmed that anoikis limits the therapeutic efficacy of MSC transplantation for tissue repair[16]. Herein, this study has proven that mBMSC/sh-Mst1 could survive after isolation from the ECM, and this response was mediated by the effect of Mst1 inhibition-induced autophagy on ITGα5β1-modulated production of ROS.
Corresponding alterations in cell-ECM isolation and autophagy also exist[17]. As a special type of apoptotic cell death, anoikis contributes to the loss of cell attachment to the ECM[18,19]. In the present study, we observed increased cell apoptosis and inhibited autophagy, as well as upregulated Mst1 expression in isolated mBMSCs. One hypothesis derived from a combination of previous studies is that Mst1 inhibition can not only overcome anoikis but also induce autophagy in isolated mBMSCs. In this study, we confirmed that mBMSCs averted anoikis by Mst1 inhibition-induced autophagy. Autophagy promotes cell survival or apoptosis in a stimulus-dependent manner. A series of experiments have elucidated the role of autophagy in promoting cell survival during anoikis[20]. Accordingly, our results established Mst1 inhibition-induced autophagy as a survival mechanism in isolated mBMSCs.
ITGs are transmembrane αβ heterodimers, with at least 18 well-known α and 8 β subunits. An increasing amount of experimental data has demonstrated that cells can overcome anoikis by changing ITGs expression[21]. In addition, ITGs-mediated cell adhesion to ECM is critical for maintaining appropriate cellular function and survival[22]. Therefore, the upregulation of ITGs allows cells to survive during anoikis[9,22,23]. This study has proved that the expression of ITGα5 and ITGβ1 were increased in cultured mBMSCs/sh-Mst1 under cell isolation conditions. Furthermore, upregulated ITGα5 and ITGβ1 expression may be the underlying mechanism of anoikis resistance in mBMSCs/sh-Mst1. These results suggested the role of ITGα5β1 downstream of Mst1, as well as a collaboration between ITGα5 and ITGβ1, in anoikis-resistant mBMSCs/sh-Mst1.
ITGs relay signals from the ECM to initiate intracellular signalling through intracellular ROS production[24], by which p-FAK expression is mediated[25]. Moreover, a recent study confirmed that excessive or persistent increases in ROS levels might promote the process of anoikis[26]. However, high ROS levels may also promote the formation of autophagy, which could contribute to reducing ROS accumulation[27]. Despite the essential role of increased ROS levels in anoikis resistance reported in several studies[28], we still hypothesized the necessity of appropriate cellular regulation of ROS levels for anoikis inhibition. As a result, we speculated that a negative-feedback loop was formed among Mst1 inhibition-induced autophagy, Mst1 inhibition-triggered ITGα5β1 and ROS levels. Mst1 inhibition increased ITGα5β1 expression, thereby facilitating cell adhesion. In addition, Mst1 inhibition-induced autophagy reduced the level of ITGα5β1-triggered ROS in isolated mBMSCs, which contributed to the evasion of anoikis, elucidating why 3-MA did not affect the expression of ITGα5 or ITGβ1.
Mst1 has been known to play a key role in the signalling pathway that controls manifold cellular processes[29]. In the present study, silencing Mst1 expression was found to ameliorate the anti-inflammatory cytokine production, osteogenic differentiation capability and cell proliferation of mBMSCs, thereby making mBMSCs/sh-Mst1 an attractive target for anti-inflammatory, immunomodulatory, and regenerative therapies and potentially improving the curative efficacy of mBMSCs in PAH[1-3].
Regardless of the extraordinary safety profile of MSC therapy verified in clinical trial data, several scholarly reviews have proposed that MSCs play a role in tumorigenesis and progression[18,30,31]. Therefore, the enhancement of the anti-anoikis ability of MSCs may promote tumorigenesis. However, in the present study, tumorigenic experiments in nude mice demonstrated the safety profile of mBMSC/sh-Mst1 administration.
In summary, the mechanism by which Mst1 inhibition acts on anoikis in mBMSCs was expounded in this study. First, Mst1 inhibition was demonstrated to ameliorate not only autophagy formation but also ITGα5β1 expression. Second, Mst1 inhibition-induced autophagy could scavenge the excessive production of ITGα5β1-triggered ROS. Third, silencing Mst1 expression not only ameliorated the pluripotency of mBMSCs but also retained the safety profile of mBMSCs. Overall, Mst1 inhibition-based infusion may improve the therapeutic efficacy of MSCs, thereby serving as the ideal candidate for clinical transplant therapy in PAH.
Anoikis plays a limiting role in the therapeutic efficacy of mesenchymal stem cells (MSCs). As a proapoptotic molecule, mammalian Ste20-like kinase 1 (Mst1) can increase the production of reactive oxygen species (ROS), thereby promoting anoikis. Recently, Mst1 inhibition was found to protect mouse bone marrow MSCs (mBMSCs) from H2O2-induced cell apoptosis by inducing autophagy and reducing ROS production. However, the influence of Mst1 inhibition on anoikis in mBMSCs remains unclear.
To investigate whether Mst1 inhibition could reduce anoikis in isolated mBMSCs.
To investigate the mechanisms by which Mst1 inhibition acts on anoikis in isolated mBMSCs.
Poly-2-hydroxyethyl methacrylate-induced anoikis was used following Mst1 inhibition in mBMSCs. Integrin (ITGs) levels were tested by flow cytometry. Autophagy and ITGα5β1 were inhibited using 3-methyladenine and small interfering RNA, respectively. The alterations in anoikis were evaluated by Terminal-deoxynucleoitidyl Transferase Mediated Nick End Labeling and anoikis assays. The levels of the anoikis-related proteins ITGα5, ITGβ1, and phospho-focal adhesion kinase, which activate caspase 3, and the autophagy-related proteins microtubules associated protein 1 light chain 3 II/I, Beclin1 and p62 were detected by Western blotting.
In isolated mBMSCs, Mst1 expression was upregulated, and Mst1 inhibition significantly reduced cell apoptosis, induced autophagy and decreased ROS levels. Mechanistically, we found that Mst1 inhibition upregulated ITGα5 and ITGβ1 expression but not ITGα4, ITGαv, or ITGβ3 expression. Moreover, ITGα5β1 upregulation and autophagy induction by Mst1 inhibition played an essential role in terms of the protective efficacy of Mst1 inhibition on averting anoikis.
Mst1 inhibition ameliorated autophagy formation, increased ITGα5β1 expression, and decreased the excessive production of ROS, thereby reducing cell apoptosis in isolated mBMSCs. On this basis, Mst1 inhibition may provide a promising strategy to overcome the anoikis of transplanted MSCs.
In isolated mBMSCs, Mst1 inhibition ameliorated not only autophagy formation but also ITGα5β1 expression (not ITGα4, ITGαv, or ITGβ3). Mst1 inhibition-induced autophagy scavenged excessive ITGα5β1-triggered ROS. Consequently, Mst1 inhibition-based infusion may improve the therapeutic efficacy of MSCs, thereby serving as an ideal candidate for clinical transplantation in pulmonary arterial hypertension.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SC, United States; Begum S, Pakistan; Gallone A, Italy; Tanabe S, Japan S-Editor: Liu GL L-Editor: A P-Editor: Zhang XD
| 1. | Bari E, Ferrarotti I, Torre ML, Corsico AG, Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through "pharmaceuticalization" for the best formulation. J Control Release. 2019;309:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med. 2020;14:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 3. | Muhammad SA, Abbas AY, Saidu Y, Fakurazi S, Bilbis LS. Therapeutic efficacy of mesenchymal stromal cells and secretome in pulmonary arterial hypertension: A systematic review and meta-analysis. Biochimie. 2020;168:156-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Gomberg-Maitland M, Bull TM, Saggar R, Barst RJ, Elgazayerly A, Fleming TR, Grimminger F, Rainisio M, Stewart DJ, Stockbridge N, Ventura C, Ghofrani AH, Rubin LJ. New trial designs and potential therapies for pulmonary artery hypertension. J Am Coll Cardiol. 2013;62:D82-D91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Hernanda PY, Pedroza-Gonzalez A, van der Laan LJ, Bröker ME, Hoogduijn MJ, Ijzermans JN, Bruno MJ, Janssen HL, Peppelenbosch MP, Pan Q. Tumor promotion through the mesenchymal stem cell compartment in human hepatocellular carcinoma. Carcinogenesis. 2013;34:2330-2340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Yu X, Cohen DM, Chen CS. miR-125b Is an adhesion-regulated microRNA that protects mesenchymal stem cells from anoikis. Stem Cells. 2012;30:956-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev. 2015;2015:632902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 9. | Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 817] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 10. | Terasaki M, Iida T, Kikuchi F, Tamura K, Endo T, Kuramitsu Y, Tanaka T, Maeda H, Miyashita K, Mutoh M. Fucoxanthin potentiates anoikis in colon mucosa and prevents carcinogenesis in AOM/DSS model mice. J Nutr Biochem. 2019;64:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Yang J, Zheng Z, Yan X, Li X, Liu Z, Ma Z. Integration of autophagy and anoikis resistance in solid tumors. Anat Rec (Hoboken). 2013;296:1501-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Zhang Q, Cheng X, Zhang H, Zhang T, Wang Z, Zhang W, Yu W. Dissecting molecular mechanisms underlying H(2)O(2)-induced apoptosis of mouse bone marrow mesenchymal stem cell: role of Mst1 inhibition. Stem Cell Res Ther. 2020;11:526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Avruch J, Zhou D, Fitamant J, Bardeesy N, Mou F, Barrufet LR. Protein kinases of the Hippo pathway: regulation and substrates. Semin Cell Dev Biol. 2012;23:770-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Yu W, Chen H, Yang H, Ding J, Xia P, Mei X, Wang L, Chen S, Zou C, Wang LX. Dissecting Molecular Mechanisms Underlying Pulmonary Vascular Smooth Muscle Cell Dedifferentiation in Pulmonary Hypertension: Role of Mutated Caveolin-1 (Cav1(F92A))-Bone Marrow Mesenchymal Stem Cells. Heart Lung Circ. 2019;28:1587-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Chou LY, Chen CH, Chuang SC, Cheng TL, Lin YH, Chou HC, Fu YC, Wang YH, Wang CZ. Discoidin Domain Receptor 1 Regulates Runx2 during Osteogenesis of Osteoblasts and Promotes Bone Ossification via Phosphorylation of p38. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Song H, Cha MJ, Song BW, Kim IK, Chang W, Lim S, Choi EJ, Ham O, Lee SY, Chung N, Jang Y, Hwang KC. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Debnath J. Detachment-induced autophagy during anoikis and lumen formation in epithelial acini. Autophagy. 2008;4:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Tang Q, Chen Q, Lai X, Liu S, Chen Y, Zheng Z, Xie Q, Maldonado M, Cai Z, Qin S, Ho G, Ma L. Malignant transformation potentials of human umbilical cord mesenchymal stem cells both spontaneously and via 3-methycholanthrene induction. PLoS One. 2013;8:e81844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Gilmore AP. Anoikis. Cell Death Differ. 2005;12 Suppl 2:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 493] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 20. | Tuloup-Minguez V, Greffard A, Codogno P, Botti J. Regulation of autophagy by extracellular matrix glycoproteins in HeLa cells. Autophagy. 2011;7:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2554] [Cited by in RCA: 2446] [Article Influence: 152.9] [Reference Citation Analysis (0)] |
| 22. | Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 843] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 23. | Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3412] [Cited by in RCA: 3285] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 24. | Huang H, Du W, Brekken RA. Extracellular Matrix Induction of Intracellular Reactive Oxygen Species. Antioxid Redox Signal. 2017;27:774-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Li K, Zhao G, Ao J, Gong D, Zhang J, Chen Y, Li J, Huang L, Xiang R, Hu J, Lin P, Wei Y. ZNF32 induces anoikis resistance through maintaining redox homeostasis and activating Src/FAK signaling in hepatocellular carcinoma. Cancer Lett. 2019;442:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Mao Y, Qiao JD, Chen S, Zhou X, Wang Z, Cai S, Li L, Luo Y. Kallistatin Inhibits Anoikis Resistance and Metastasis of Ectopic Endometrium Cells by Modulating MnSOD and Caspase 3 Signaling. Reprod Sci. 2021;28:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell Mol Neurobiol. 2015;35:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 659] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 28. | Idelchik MDPS, Begley U, Begley TJ, Melendez JA. Mitochondrial ROS control of cancer. Semin Cancer Biol. 2017;47:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 29. | Wang S, Zhou L, Ling L, Meng X, Chu F, Zhang S, Zhou F. The Crosstalk Between Hippo-YAP Pathway and Innate Immunity. Front Immunol. 2020;11:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 30. | Galland S, Stamenkovic I. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J Pathol. 2020;250:555-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 31. | Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020;60:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |