Published online Mar 26, 2023. doi: 10.4252/wjsc.v15.i3.83
Peer-review started: December 26, 2022
First decision: January 3, 2023
Revised: January 17, 2023
Accepted: March 16, 2023
Article in press: March 16, 2023
Published online: March 26, 2023
Processing time: 87 Days and 1.5 Hours
Osteoporosis is a systemic bone disease, which leads to decreased bone mass and an increased risk of fragility fractures. Currently, there are many anti-resorption drugs and osteosynthesis drugs, which are effective in the treatment of osteoporosis, but their usage is limited due to their contraindications and side effects. In regenerative medicine, the unique repair ability of mesenchymal stem cells (MSCs) has been favored by researchers. The exosomes secreted by MSCs have signal transduction and molecular delivery mechanisms, which may have therapeutic effects. In this review, we describe the regulatory effects of MSCs-derived exosomes on osteoclasts, osteoblasts, and bone immunity. We aim to summarize the preclinical studies of exosome therapy in osteoporosis. Furth
Core Tip: Osteoporosis is one of the major diseases endangering bone health in the elderly. The existing treatment drugs have problems such as long-term administration and side effects; thus, it is fundamentally difficult to cure osteoporosis. Exosomes derived from mesenchymal stem cells (MSCs) are vesicles that deliver signals and molecules between cells and have shown substantial positive effects in pre-clinical trials. In this review, we summarize the latest progress of MSCs-derived exosomes in the regulation of bone metabolism.
- Citation: Huo KL, Yang TY, Zhang WW, Shao J. Mesenchymal stem/stromal cells-derived exosomes for osteoporosis treatment. World J Stem Cells 2023; 15(3): 83-89
- URL: https://www.wjgnet.com/1948-0210/full/v15/i3/83.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i3.83
Osteoporosis (OP) is a chronic systemic bone disease. It is characterized by decreased bone mass and destruction of bone microstructures, resulting in decreased bone mineral density (BMD) and ultimately an increased risk of fragility fractures[1]. The dynamic balance between bone formation and bone resorption is an important way to maintain normal bone metabolism. Osteoblasts promote bone formation by mineralizing the matrix, while osteoclasts degrade the bone matrix by secreting H+ and releasing cathepsin K, accelerating bone dissolution[2].
At present, the prevention and treatment of OP can be divided into adjuvant therapy and drug therapy. The therapeutic effects of drugs are mainly divided into two aspects: Inhibition of osteoclasts (inhibition of bone resorption) and stimulation of osteoblasts (promotion of bone formation). Among them, the drugs that inhibit bone resorption are denosumab, bisphosphonates and selective estrogen receptor modulators; and drugs that promote bone formation are teriparatide and abaloparatide. Romosozumab, a monoclonal antibody directed against sclerostin, has a dual regulatory effect, inhibiting bone resorption and promoting bone formation at the same time[3].
Although a variety of therapeutic drugs for OP have emerged, all the drugs mentioned above have side effects[4], and more novel and effective drugs and therapies are needed. There are new advances in the research on stem cells and their exosomes in tissue repair and treatment. Therefore, the new scheme of MSCs-derived exosomes for the treatment of OP has gradually become a therapeutic option[5].
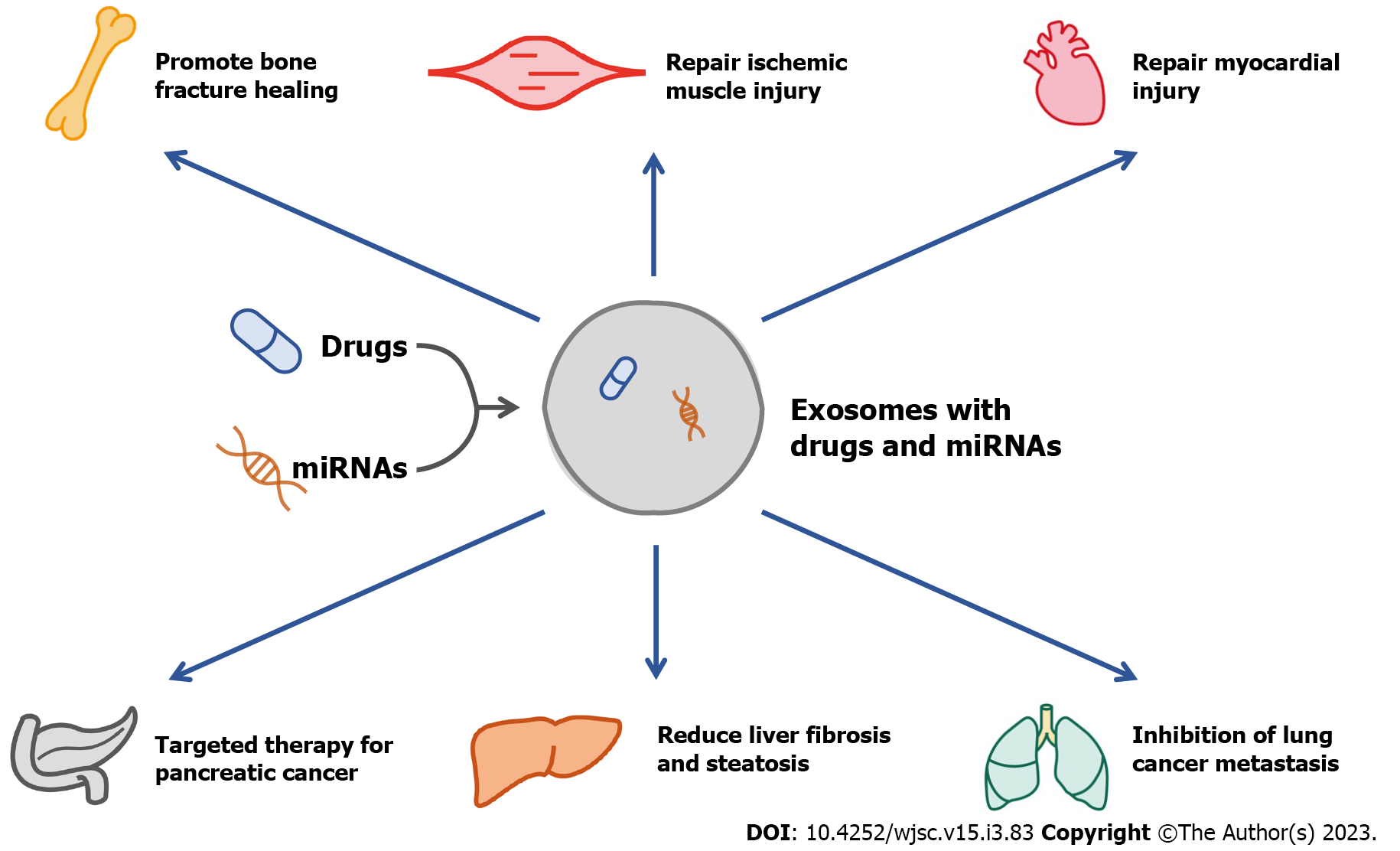
Exosomes are small vesicles secreted by cells with a diameter of 40 to 160 nm, which carry signals and molecules among cells. In addition, the exosomes secreted by the cells in diseased states contain specific microRNAs (miRNAs), which is helpful for the diagnosis of diseases[6]. As a carrier of signal transduction and drug delivery, exosomes also have good potential in the treatment of diseases, and their effectiveness has been confirmed by numerous animal studies[7-13] (Figure 1). In 1974, Friedenstein et al[14] first discovered that mesenchymal stem cells (MSCs) in bone marrow have the potential for osteogenic differentiation. Later, researchers continued to explore the function of MSCs and found that MSCs are pluripotent stem cells, which have the ability to differentiate into osteoblasts, adipocytes, chondrocytes, cardiac muscle cells and skeletal muscle cells[15]. Previous studies have found that therapy with MSCs can accelerate bone tissue repair and regeneration and maintain bone mass in OP[16,17].
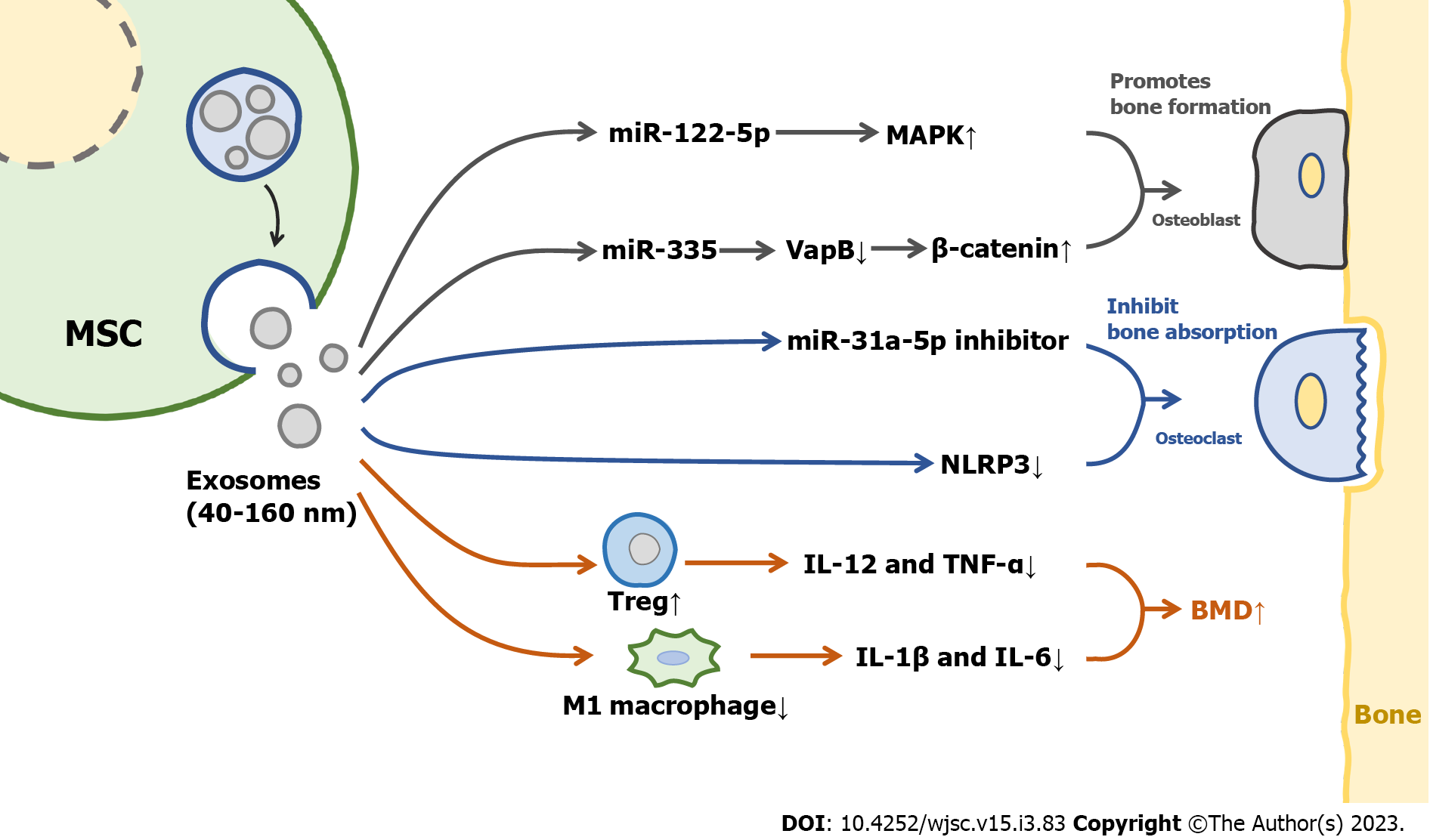
MSCs-derived exosomes are likely to play a major role in mediating the therapeutic effect of MSCs[18,19]. A schematic diagram illustrating the mechanisms of MSCs-derived exosomes in the treatment of OP is shown in Figure 2. Therefore, MSCs-derived exosomes are expected to be a new biological agent in the future.
Exosomes have the characteristics of good targeting ability, high permeability and low toxicity. Therefore, exosomes therapy has become one of the hot spots in the study of OP treatment. Bone regeneration is one of the main modalities in the treatment of OP and bone fracture. MSCs-derived exosomes can promote the formation of new bone with vasculature, biomechanics, and histology[20]. MSCs-derived exosomes regulate bone metabolism and treat OP through signaling pathways, such as stimulating osteoblast differentiation from bone marrow stem cells, promoting osteoblast proliferation, inducing angiogenesis, and immunomodulation[21]. During treatment, MSCs-derived exosomes achieve the purpose of bone regeneration and treatment of OP by carrying and transporting proteins, miRNAs, and artificial synthetic drugs[22-24]. According to the pathogenesis of OP, exosomes are mainly involved in regulating the effects of osteoblasts, osteoclasts and bone immunity (Table 1).
| Ref. | Exosome source | Cell type | Consequence |
| Zuo et al[24], 2019 | BMSCs | BMSCs (after irradiation) | Alleviating radiation-induced bone loss by restoring Wnt/β-Catenin signaling pathway |
| Zhao et al[25], 2018 | BMSCs | Osteoblasts (hFOB 1.19) | Promoting proliferation of osteoblasts via MAPK pathway |
| Liao et al[26], 2019 | BMSCs | Osteoblasts | Promoting proliferation of osteoblasts by carrying miR-122-5p |
| Qi et al[27], 2016 | hiPSC-MSCs | Osteoblasts | Promoting osteoblast proliferation, differentiation and bone formation |
| Hu et al[28], 2021 | BMSCs | Osteoblasts | Promoting differentiation of osteoblasts by carrying miR-335 |
| Zhang et al[31], 2021 | AD-MSCs | Osteoclasts | Inhibiting NLRP3 inflammasome activation in osteoclasts |
| Nakao et al[32], 2021 | GMSCs | Osteoclasts | Inhibiting RANKL and reducing osteoclast formation by carrying miR-1260b via Wnt5a/JNK signal pathway |
| Xu et al[33], 2018 | BMSCs | Osteoclasts | Increasing the number of osteoclasts by carrying miR-31a-5p via RhoA pathway |
| Wei et al[40], 2019 | BMSCs | Osteoblasts | Promoting osteoblast differentiation by inhibiting macrophage polarization and reducing the levels of inflammatory factors |
Osteoblasts are derived from pluripotent MSCs, which are major functional cells in bone matrix synthesis, secretion, and mineralization. Exosomes can directly regulate the activity of osteoblasts and then affect OP. MSCs-derived exosomes promoted the cell cycle of hFOB 1.19 cells, a type of osteoblast, and activated their proliferative activity through the MAPK pathway[25]. It was also found that overexpression of miR-122-5p in MSCs-derived exosomes of bone marrow can increase the BMD of the femoral head through the MAPK pathway[26]. Co-culture of MSCs-derived exosomes with bone marrow MSCs from osteoporotic rats resulted in increased levels of osteogenesis-related proteins and mineral deposition; in addition, the use of MSCs-derived exosomes promoted bone regeneration in a rat model of calvarial defects[27]. One study showed that the infusion of bone marrow MSCs-derived exosomes carrying miR-335 into mice with fractures significantly accelerated fracture healing, as miR-335 in exosomes was able to inhibit VapB, activate the Wnt/β-catenin pathway, and promote osteoblast differentiation[28]. Notably, exosomal miRNAs derived from MSCs are critical in regulating the function of osteoblasts. These miRNAs participate in and regulate some key signaling pathways and alter protein expression in osteoblasts. The positive roles of exosomes in osteoblast proliferation and differentiation, and the excellent therapeutic performance of exosomes in bone repair and bone mass recovery, indicate the potential of MSCs-derived exosomes in the treatment of OP.
Osteoclasts originate from hematopoietic progenitor cells in the bone marrow, and play an osteolytic role by secreting H+ and cathepsin K[29,30]. Adipose MSCs-derived exosomes inhibited NLRP3 in the osteoclasts of diabetic OP rat models and increased BMD[31]. RANKL is a major factor in promoting the differentiation of preosteoclasts into osteoclasts, gingiva MSCs-derived exosomes can target Wnt5a in periodontal osteoclasts and inhibit the expression of RANKL, thus reducing the differentiation of osteoclasts[32]. Of course, as a carrier of miRNA, exosomes will also have an effect on osteoclasts by carrying miRNA. Bone marrow MSCs-derived exosomes delivered miR-31a-5p to osteoclasts, which increased osteoclast number and bone resorption. In addition, BMD significantly increased in a rat model treated with miR-31a-5p inhibitor[33]. These findings suggested that MSCs-derived exosomes can regulate osteoclast differentiation, increase bone density, and inhibit OP. Notably, bone marrow stromal cell-derived exosomes do not have any effect on osteoclasts at the surface of trabecular bone, and therefore do not play a role in preventing bone resorption[34]. There are few studies on the regulation of osteoclast differentiation by MSCs-derived exosomes. Therefore, we need to further explore the potential of MSCs-derived exosomes in the treatment of OP by regulating osteoclast differentiation.
There is an intricate relationship between the immune system and the skeletal system. Activated immune cells release inflammatory mediators and cytokines that upset the balance of bone remodeling and activate osteoclasts, leading to bone loss and OP[35,36]. MSCs-derived exosomes can regulate the immune system[37]. After treatment with adipose MSCs-derived exosomes in mice with colitis, regulatory T cells returned to normal baseline level, and the levels of inflammatory factors such as interleukin (IL)-12 and tumor necrosis factor-α reduced[38]. Exosomes derived from adipose and bone marrow MSCs can significantly increase the level of type II collagen in articular cartilage, promote articular cartilage formation and accelerate the recovery of osteoarthritis in mice[39]. MSCs-derived exosomes promote the differentiation of bone marrow MSCs into osteoblasts by inhibiting the polarization of M1 macrophages and reducing the levels of inflammatory cytokines such as Il-1β and IL-6[40]. MSCs-derived exosomes can regulate immunity and inflammation in vivo. Unfortunately, little is known about their role in bone immunity. However, available findings suggest the potential of MSCs-derived exosomes in the treatment of OP by modulating bone immunity.
At present, drug treatment of OP still focuses on inhibiting bone resorption and promoting bone formation. Drug therapy has a good effect, but long-term use of drugs can cause serious side effects. In the field of bone regeneration, stem cell therapy has significant efficacy, and exosomes are one of the important carriers of cell information and factors. Therefore, MSCs-derived exosomes may be a promising biological agent in the treatment of OP. This review focuses on the mechanism of MSCs-derived exosomes in the treatment of OP, by delivering miRNA, regulating related targets, and regulating bone immunity. Therefore, the potential of MSCs-derived exosomes in the treatment of OP is anticipated, but further preclinical studies are needed to prove the safety and reliability of exosome therapy and provide strong evidence for the clinical conversion of exosome therapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gallone A, Italy; Jeyaraman M, India; Shalaby MN, Egypt; Ventura C, Italy S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 1469] [Article Influence: 244.8] [Reference Citation Analysis (0)] |
| 2. | Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 628] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 872] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 4. | Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 536] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 5. | Xie X, Xiong Y, Panayi AC, Hu L, Zhou W, Xue H, Lin Z, Chen L, Yan C, Mi B, Liu G. Exosomes as a Novel Approach to Reverse Osteoporosis: A Review of the Literature. Front Bioeng Biotechnol. 2020;8:594247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Kong D, Chen T, Zheng X, Yang T, Zhang Y, Shao J. Comparative profile of exosomal microRNAs in postmenopausal women with various bone mineral densities by small RNA sequencing. Genomics. 2021;113:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6542] [Article Influence: 1308.4] [Reference Citation Analysis (0)] |
| 8. | Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 9. | Yan B, Zhang Y, Liang C, Liu B, Ding F, Wang Y, Zhu B, Zhao R, Yu XY, Li Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics. 2020;10:6728-6742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Liu S, Chen X, Bao L, Liu T, Yuan P, Yang X, Qiu X, Gooding JJ, Bai Y, Xiao J, Pu F, Jin Y. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng. 2020;4:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 11. | Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1167] [Cited by in RCA: 1899] [Article Influence: 237.4] [Reference Citation Analysis (1)] |
| 12. | Gao H, Jin Z, Bandyopadhyay G, Cunha E Rocha K, Liu X, Zhao H, Zhang D, Jouihan H, Pourshahian S, Kisseleva T, Brenner DA, Ying W, Olefsky JM. MiR-690 treatment causes decreased fibrosis and steatosis and restores specific Kupffer cell functions in NASH. Cell Metab. 2022;34:978-990.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 13. | Tian W, Yang X, Yang H, Lv M, Sun X, Zhou B. Exosomal miR-338-3p suppresses non-small-cell lung cancer cells metastasis by inhibiting CHL1 through the MAPK signaling pathway. Cell Death Dis. 2021;12:1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 948] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2698] [Article Influence: 168.6] [Reference Citation Analysis (0)] |
| 16. | Chen T, Yang T, Zhang W, Shao J. The therapeutic potential of mesenchymal stem cells in treating osteoporosis. Biol Res. 2021;54:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Xu GP, Zhang XF, Sun L, Chen EM. Current and future uses of skeletal stem cells for bone regeneration. World J Stem Cells. 2020;12:339-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 1819] [Article Influence: 363.8] [Reference Citation Analysis (0)] |
| 19. | Álvarez-Viejo M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J Stem Cells. 2020;12:100-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Tan SHS, Wong JRY, Sim SJY, Tjio CKE, Wong KL, Chew JRJ, Hui JHP, Toh WS. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio. 2020;7:100067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Rudiansyah M, El-Sehrawy AA, Ahmad I, Terefe EM, Abdelbasset WK, Bokov DO, Salazar A, Rizaev JA, Muthanna FMS, Shalaby MN. Osteoporosis treatment by mesenchymal stromal/stem cells and their exosomes: Emphasis on signaling pathways and mechanisms. Life Sci. 2022;306:120717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Chen L, Mou S, Li F, Zeng Y, Sun Y, Horch RE, Wei W, Wang Z, Sun J. Self-Assembled Human Adipose-Derived Stem Cell-Derived Extracellular Vesicle-Functionalized Biotin-Doped Polypyrrole Titanium with Long-Term Stability and Potential Osteoinductive Ability. ACS Appl Mater Interfaces. 2019;11:46183-46196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Li QC, Li C, Zhang W, Pi W, Han N. Potential Effects of Exosomes and their MicroRNA Carrier on Osteoporosis. Curr Pharm Des. 2022;28:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Zuo R, Liu M, Wang Y, Li J, Wang W, Wu J, Sun C, Li B, Wang Z, Lan W, Zhang C, Shi C, Zhou Y. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res Ther. 2019;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 25. | Zhao P, Xiao L, Peng J, Qian YQ, Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22:3962-3970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 26. | Liao W, Ning Y, Xu HJ, Zou WZ, Hu J, Liu XZ, Yang Y, Li ZH. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci (Lond). 2019;133:1955-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 27. | Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int J Biol Sci. 2016;12:836-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 408] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 28. | Hu H, Wang D, Li L, Yin H, He G, Zhang Y. Role of microRNA-335 carried by bone marrow mesenchymal stem cells-derived extracellular vesicles in bone fracture recovery. Cell Death Dis. 2021;12:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5206] [Cited by in RCA: 4833] [Article Influence: 219.7] [Reference Citation Analysis (0)] |
| 30. | Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Lüth A, Koskivirta I, Kleuser B, Vacher J, Vuorio E, Horne WC, Baron R. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013;123:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Zhang L, Wang Q, Su H, Cheng J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J Biosci Bioeng. 2021;131:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 32. | Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C, Yamato H, Yotsumoto K, Tanaka U, Taketomi T, Uchiumi T, Le AD, Shi S, Nishimura F. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 309] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 33. | Xu R, Shen X, Si Y, Fu Y, Zhu W, Xiao T, Fu Z, Zhang P, Cheng J, Jiang H. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17:e12794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 34. | Luo ZW, Li FX, Liu YW, Rao SS, Yin H, Huang J, Chen CY, Hu Y, Zhang Y, Tan YJ, Yuan LQ, Chen TH, Liu HM, Cao J, Liu ZZ, Wang ZX, Xie H. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11:20884-20892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 35. | Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 549] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 36. | Srivastava RK, Dar HY, Mishra PK. Immunoporosis: Immunology of Osteoporosis-Role of T Cells. Front Immunol. 2018;9:657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 37. | Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, Jung JY, Choi H, Lee JH, Sung S, Yi YW, Cho BS. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 38. | Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, Hashemi SM. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236:5906-5920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 39. | Fazaeli H, Kalhor N, Naserpour L, Davoodi F, Sheykhhasan M, Hosseini SKE, Rabiei M, Sheikholeslami A. A Comparative Study on the Effect of Exosomes Secreted by Mesenchymal Stem Cells Derived from Adipose and Bone Marrow Tissues in the Treatment of Osteoarthritis-Induced Mouse Model. Biomed Res Int. 2021;2021:9688138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Wei F, Li Z, Crawford R, Xiao Y, Zhou Y. Immunoregulatory role of exosomes derived from differentiating mesenchymal stromal cells on inflammation and osteogenesis. J Tissue Eng Regen Med. 2019;13:1978-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |