Published online Aug 26, 2022. doi: 10.4252/wjsc.v14.i8.616
Peer-review started: March 5, 2022
First decision: April 19, 2022
Revised: May 2, 2022
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: August 26, 2022
Processing time: 174 Days and 3.5 Hours
The therapeutic potential of mesenchymal stem cells (MSCs) in the form of three-dimensional spheroids has been extensively demonstrated. The underlying mechanisms for the altered cellular behavior of spheroids have also been investigated. Cell membrane fluidity is a critically important physical property for the regulation of cell behavior, but it has not been studied for the spheroid-forming cells to date.
To explore the association between cell membrane fluidity and the morphological changes of MSC spheroids on the surface of biomaterials to elucidate the role of membrane fluidity during the spheroid-forming process of MSCs.
We generated three-dimensional (3D) MSC spheroids on the surface of various culture substrates including chitosan (CS), CS-hyaluronan (CS-HA), and polyvinyl alcohol (PVA) substrates. The cell membrane fluidity and cell morphological change were examined by a time-lapse recording system as well as a high-resolution 3D cellular image explorer. MSCs and normal/cancer cells were pre-stained with fluorescent dyes and co-cultured on the biomaterials to investigate the exchange of cell membrane during the formation of heterogeneous cellular spheroids.
We discovered that vesicle-like bubbles randomly appeared on the outer layer of MSC spheroids cultured on different biomaterial surfaces. The average diameter of the vesicle-like bubbles of MSC spheroids on CS-HA at 37 °C was approximately 10 μm, smaller than that on PVA substrates (approximately 27 μm). Based on time-lapse images, these unique bubbles originated from the dynamic movement of the cell membrane during spheroid formation, which indicated an increment of membrane fluidity for MSCs cultured on these substrates. Moreover, the membrane interaction in two different types of cells with similar membrane fluidity may further induce a higher level of membrane translocation during the formation of heterogeneous spheroids.
Changes in cell membrane fluidity may be a novel path to elucidate the complicated physiological alterations in 3D spheroid-forming cells.
Core Tip: The fluidity of the cell membrane is an important physical property for the regulation of cell behavior. To date, the changes in cell membrane fluidity are not fully understood in spheroid-forming cells. In this study, various substrates were utilized to grow mesenchymal stem cells and to form homotypic or heterotypic cellular spheroids on the substrates. During the spheroid-forming process, vesicle-like bubbles were randomly generated on the cell membrane. The evidence suggests that the physiological alterations of cells after they form cellular spheroids may be associated with changes in membrane fluidity during the spheroid-forming process.
- Citation: Wong CW, Han HW, Hsu SH. Changes of cell membrane fluidity for mesenchymal stem cell spheroids on biomaterial surfaces. World J Stem Cells 2022; 14(8): 616-632
- URL: https://www.wjgnet.com/1948-0210/full/v14/i8/616.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i8.616
Three-dimensional (3D) cell culture systems have been extensively developed in recent years for biomedical research and cell therapy[1,2]. In addition to the biomimetic nature of 3D culture, the cellular contact and microenvironment in a 3D culture system can dramatically alter the physiological properties of certain types of cells. For example, the tumorigenicity of cancer cells and the stemness of stem cells were both enhanced when the monolayer cultured cells assembled themselves into cellular spheroids in vitro[3]. For cancer cells, the gene expression levels of cancer stem cell markers were highly expressed after they formed 3D tumor spheroids. Meanwhile, the spheroidal culture also enhanced the levels of transcription factors associated with the self-renewal of multipotent stem cells[4]. Besides, the osteogenic differentiation capacity of mesenchymal stem cells (MSCs) was promoted when MSCs were cultured as spheroids or in various 3D environments[5,6]. Therefore, the 3D cell culture methods are potential tools for anti-cancer drug screening[7], tissue engineering[8], and regenerative medicine[9].
MSCs are easy to isolate and have multi-differentiation ability, which are attractive cell sources for basic research as well as for cell therapy and tissue engineering. To date, 3D spheroids of MSCs have been generated by many different strategies, including hanging drop[10], ultra-low attachment surface[11], rotating bioreactors[12], suspension culture[13], and biomaterial-based approaches[14]. The cellular spheroids of MSCs can also be established by the techniques of scaffolds such as chitosan (CS) hydrogel[15], hyaluronic acid hydrogel[16], and CS-hyaluronan (CS-HA) electrospun fibers[17]. These mentioned materials have similar properties to natural extracellular matrix, and their 3D scaffolding structures provide more contact space for cell growth. Meanwhile, flat CS and CS-HA membranes as the cell culture substrates are also the well-established scaffold-free technique to drive the self-assembly of cellular spheroids[18].
The proliferation, maintenance of stemness, and ability of differentiation are common properties for evaluation of the physiological behavior of 3D MSC spheroids. For instance, upregulation of stemness genes has been observed in the spheroidal culture of MSCs[19]. Other than the biochemical changes and signaling modulation, the changes of physical properties may also occur as a result of spheroid formation but have not been deliberated extensively. It has been reported that the cell size and volume significantly decreased in spheroid-forming MSCs[20]. Cell volume may be regulated by the cell membrane fluidity[21], but not examined in literature. Cell membrane fluidity can be affected by the composition of saturated and unsaturated fats in cell membrane, while increasing unsaturated fat or cholesterol levels enhances the fluidity of cell membrane[22]. Additionally, cell membrane fluidity is regulated by temperature variation in plant cells, insect cells, and mammalian cells[23,24]. Membrane fluidity is an important factor to influence cell adhesion and migration. Low fluidity of the cell membrane reduces the motility of cancer cells in the process of the epithelial–mesenchymal transition[25]. Meanwhile, signaling transduction is also affected by the variation of membrane fluidity. The translocation of mobile molecules in the membrane of murine embryonic fibroblasts (MEFs) was significantly decreased by the treatment of formaldehyde (FA), and the reduced membrane fluidity in FA-fixed MEFs directly affected the pluripotency of co-cultured induced pluripotent stem cells (iPSCs)[26]. In another report, the fluidity change of the cell membrane after chemical fixation could regulate the differentiation of stem cells[27]. Moreover, changes in the lipid composition of the membrane were linked with the immunomodulatory properties of MSCs[28]. The above evidence reveals that cellular membrane fluidity has strong influence on cell behavior.
As the formation of 3D cellular spheroids starts with cell-cell interaction, the spheroid-forming cells are tightly connected within the spheroids. It is thus possible that the compact environment in the spheroids may result in the alteration of cell membrane fluidity and further alter the cell behavior. In this research, we utilized different kinds of materials as cell culture substrates to create 3D spheroids from MSCs on the surface of each substrate, and we further investigated the linkage between cell membrane fluidity and the morphological change. In addition, we co-cultured MSCs with other types of cells to generate cellular co-spheroids. By these approaches, we tried to unveil the interaction of cells through membrane exchange in the homogeneous MSC spheroids as well as in the heterotypic co-spheroids of MSCs/cancer cells and co-spheroids of MSCs/fibroblasts.
CS [molecular weight (MW): 510 kDa] powder with 77.7 % deacetylation and hyaluronan (HA, MW: 1880 kDa) powder were purchased from Sigma-Aldrich (St. Louis, MO, United States) and SciVision Biotech Inc (Kaohsiung, Taiwan), respectively. CS powder was dissolved in 1% acetic acid to prepare 1 wt% CS solution. The CS solution (1 wt%, 1.5 mL) was coated on each well of the 6-well multi-well tissue culture polystyrene (TCPS) plates. The CS-coated culture plates were placed in a laminar cabinet for 24 h, and the CS membranes were formed after evaporation of solvent. For the CS-HA substrate preparation, the HA powder was dissolved in distilled deionized water to prepare HA solution (3 mg/mL). 1.5 mL of HA solution was added onto CS-coated wells and then air-dried for 24 h. HA-coated CS membranes were crosslinked with ethyl (dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) solution (weight ratio of HA/EDC/NHS adjusted to 1:1.84:0.23) and shaken continuously at 4 °C for 48 h. To prepare polyvinyl alcohol (PVA)-coated culture plates, 1.5 mL of PVA solution (0.5 % in distilled water) was coated onto each well of 6-well TCPS plates and then evaporated in a laminar cabinet for 24 h to obtain the PVA-coated plates.
Human bone marrow MSCs were obtained from the Tulane Center for Preparation and Distribution of Adult Stem Cells[29]. MSCs were cultured in the mixed medium composed of Dulbecco’s modified Eagle medium-low glucose (DMEM-LG,) and Ham's F-12 (Gibco, Waltham, MA, United States). The mixed culture media were supplemented with 10% fetal bovine serum (Gibco), penicillin-streptomycin (Gibco), and 10 mg/L L-glutamine (Invitrogen, Carlsbad, CA, United States). Lung cancer cells (A549) and murine fibroblasts (NIH/3T3, 3T3) were obtained from the American Type Culture Collection (Manassas, VA, United States). The A549 and 3T3 were maintained in the Roswell Park Memorial Institute (Gibco) 1640 and high glucose DMEM (Gibco), respectively. For co-culture of MSC/A549 and MSC/3T3, the respective medium for each cell was mixed with each other in 1:1 volume ratio. All cells were incubated in the cell culture incubator at 37 °C with 5% CO2 under a water-saturated atmosphere.
Cells were seeded into 6-well TCPS culture plates at a density of 2.5 × 104 cells/well. For 3D spheroid cell culture model, 5 × 105 cells were seeded on each well of the CS-, CS-HA-, and PVA-coated plates for spheroid formation. The formation of spheroids and vesicle-like bubbles was directly observed by an inverted microscope (Leica, Wetzlar, Germany). The size of vesicles on each cellular spheroid was measured by ImageJ software. The process of formation for MSC cellular spheroids was recorded by a time-lapse recording system [real-time cell culture monitoring (CCM) system; ASTEC Co., Ltd., Fukuoka, Japan] for 15 h. All tracking pictures were sampled every 20 min to assemble videos by using CCM software.
A total of 5 × 105 MSCs were seeded on a 35 mm glass bottom dish coated with CS-HA (Ibidi, Martinsried, Germany). To acquire high-resolution images of the dynamic morphology for the cell membrane, cells were examined and recorded by the 3D Cell Explorer (Nanolive SA, Tolochenaz, Switzerland). After video collection, pseudo-color was assigned to the cell membrane by the unique refractive index under STEVE software (Nanolive SA).
To observe the exchange of cell membrane in the spheroids, the membrane of cell was respectively stained with PKH67 (green) and PKH26 (red) fluorescence dyes (Sigma) following the manufacturer's instructions before cell seeding. After membrane labeling, equal numbers of red- and green-labeled cells were seeded on the culture plates (a total of 2.5 × 105 and 5 × 105 cells for TCPS control and spheroid-forming groups, respectively). After culturing, the adherent cells on TCPS as well as the cellular spheroids on various culture substrates were respectively detached by trypsin and dissociated by Accumax (Stem cells, Dayton, OH, United States), and then the single cell suspension was subjected to the observation of fluorescent signals on the cell membrane by a fluorescent microscope.
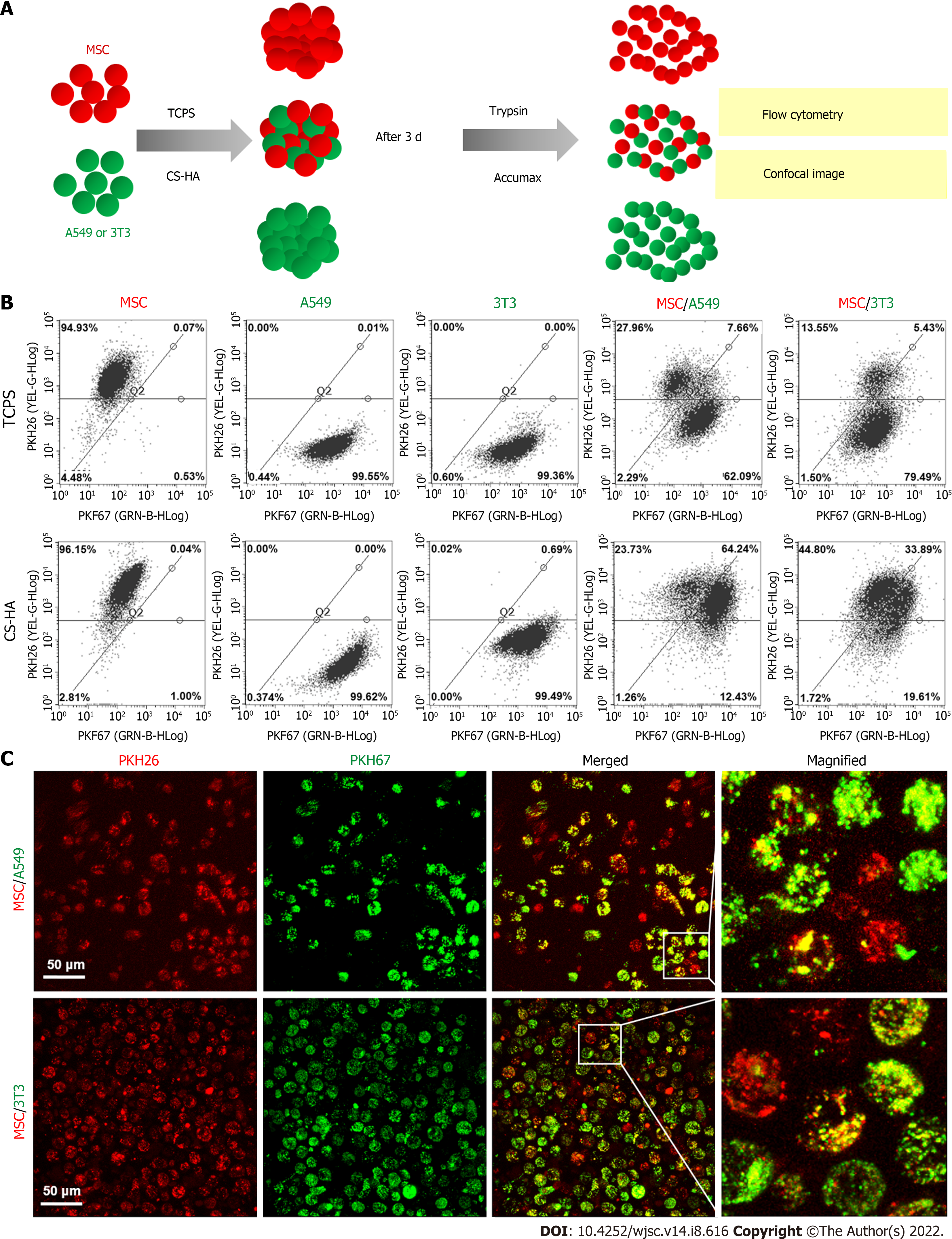
To investigate the cell membrane dynamics of heterogeneous co-spheroids on CS-HA substrates, two types of cells were individually stained with PKH26 and PKH67 dyes as indicated. After staining, two types of cells were co-cultured on CS-HA substrates with equal cell numbers. After 3 d of incubation, the co-spheroids were dissociated by Accumax, and the single cell suspension was subjected to flow cytometry (Guava Easy-Cyte; Millipore, Burlington, MA, United States) to determine the respective ratio of cells labeled with single or double fluorescent dyes. The images of the single cell suspension from dissociated co-spheroids were taken with a TCS SP5 II confocal laser scanning microscope (Leica). Z-stack images of single cells were acquired in 1-µm sections (total stacked approximately 27 images).
The cell viability assay of co-spheroids was carried out using the LIVE/DEAD Cell Vitality Kit (Invitrogen, United States). Two types of cells were co-cultured on CS-HA substrates with equal cell numbers. After 2 d of incubation, the co-spheroids were collected and transferred to the Lab-Tek 4-well chambered coverglass (Nunc plates; Thermo Fisher Scientific, Waltham, MA, United States). After PBS wash, the co-spheroids were treated with the LIVE/DEAD Cells Vitality Kit to label the live and dead cells in the co-spheroids. The images were acquired by confocal laser scanning microscopy (Leica TCS SP5 II, Germany). The total thickness of Z-stack scanning was approximately 75 μm.
All experimental data were repeated independently at least twice to ensure reproducibility, and the quantitative data were presented by mean ± SD using the GraphPad Prism 6.0 software (La Jolla, CA, United States). Multiple comparisons of all experimental data were evaluated by the unpaired t-test or one-way analysis of variance test, and P values with statistically significant were represented by the asterisks: aP < 0.05, bP < 0.01. The abbreviation NS indicates non-significant. The frequency distribution histogram was obtained through the Gauss-amp fitting curve using Origin Pro8.5 software (OriginLab, Northampton, MA, United States).
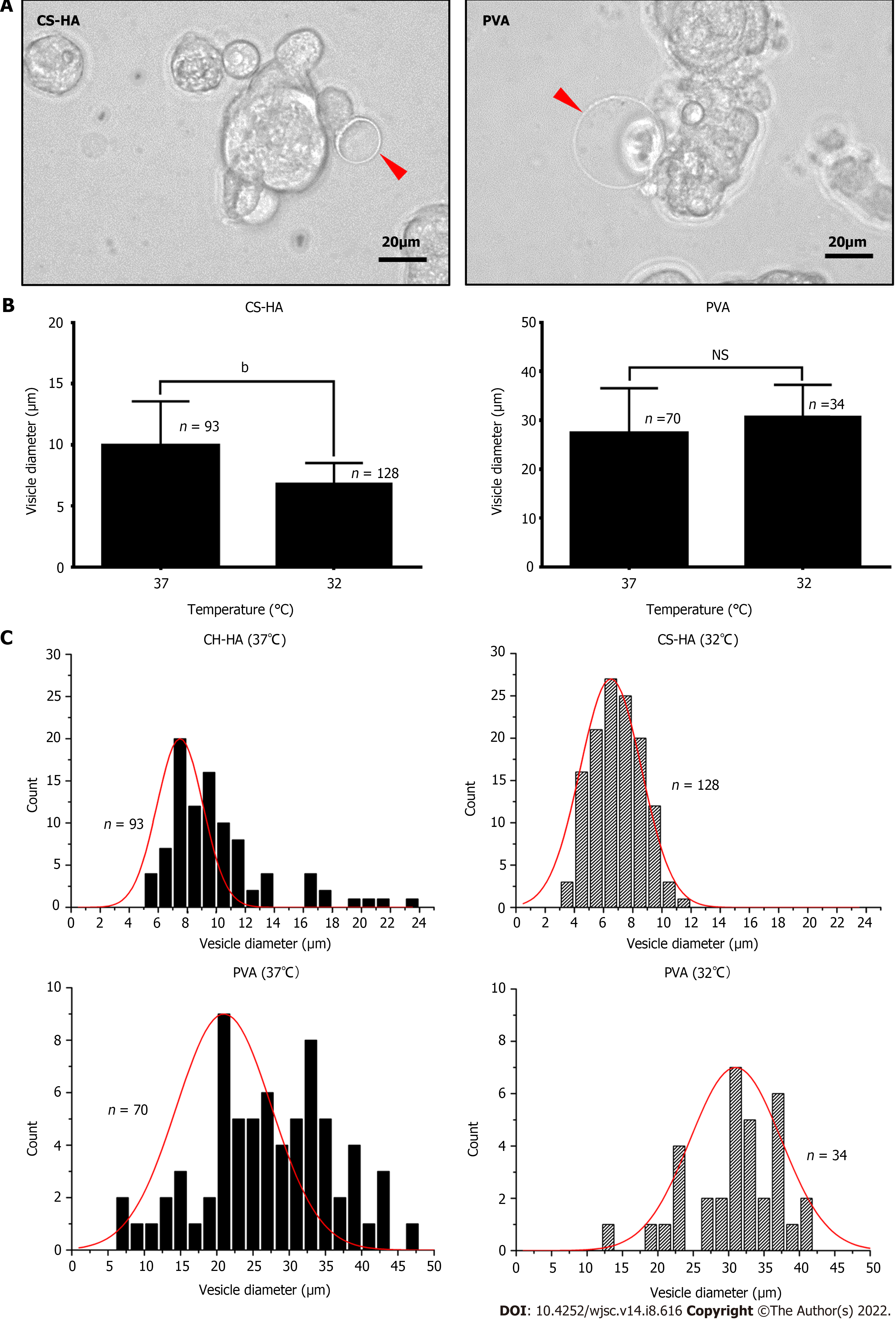
CS and PVA membranes are cell culture substrates commonly used for generation of cell spheroids. Here, we prepared the CS, CS-HA, and PVA membranes, and then seeded human MSCs respectively on these substrates (Supplementary Figure 1). MSCs were self-organized into cell spheroids on each of these substrates. Cell spheroids maintained the floating status on the CS-HA and PVA substrates. Meanwhile, some spheroids seemed to adhere on the CS membranes at 15 h after seeding (Supplementary Figure 1). In particular, several vesicle-like bubbles were observed on the surface of the spheroids formed on CS-HA and PVA membranes (indicated by red arrows in Figure 1A). The statistically significant difference in the vesicle size was observed for MSCs respectively cultured at 37 °C and 32 °C on CS-HA substrates (Figure 1B). The diameter of vesicle-like bubbles for MSC spheroids generated on CS-HA at 37 °C was in the range of 5-23 μm with an average of approximately 10 μm (Figure 1C). By contrast, vesicle-like bubbles for MSC spheroids on CS-HA at 32 °C was in the range of 3-11 μm with an average of approximately 7 μm (Figure 1C). Besides, the sizes of vesicle-like bubbles for MSCs on PVA substrates were 2.7-fold larger than those on CS-HA substrates, according to the size distribution histogram (Figure 1C). The bright-field static images revealed that MSC spheroids on PVA and CS-HA substrates generated vesicle-like bubbles more obviously than those on CS substrates (Supplementary Figure 1). Therefore, the dynamic formation of bubbles for MSC homo-spheroids was further investigated on CS-HA and PVA substrates by time-lapse recording experiments.
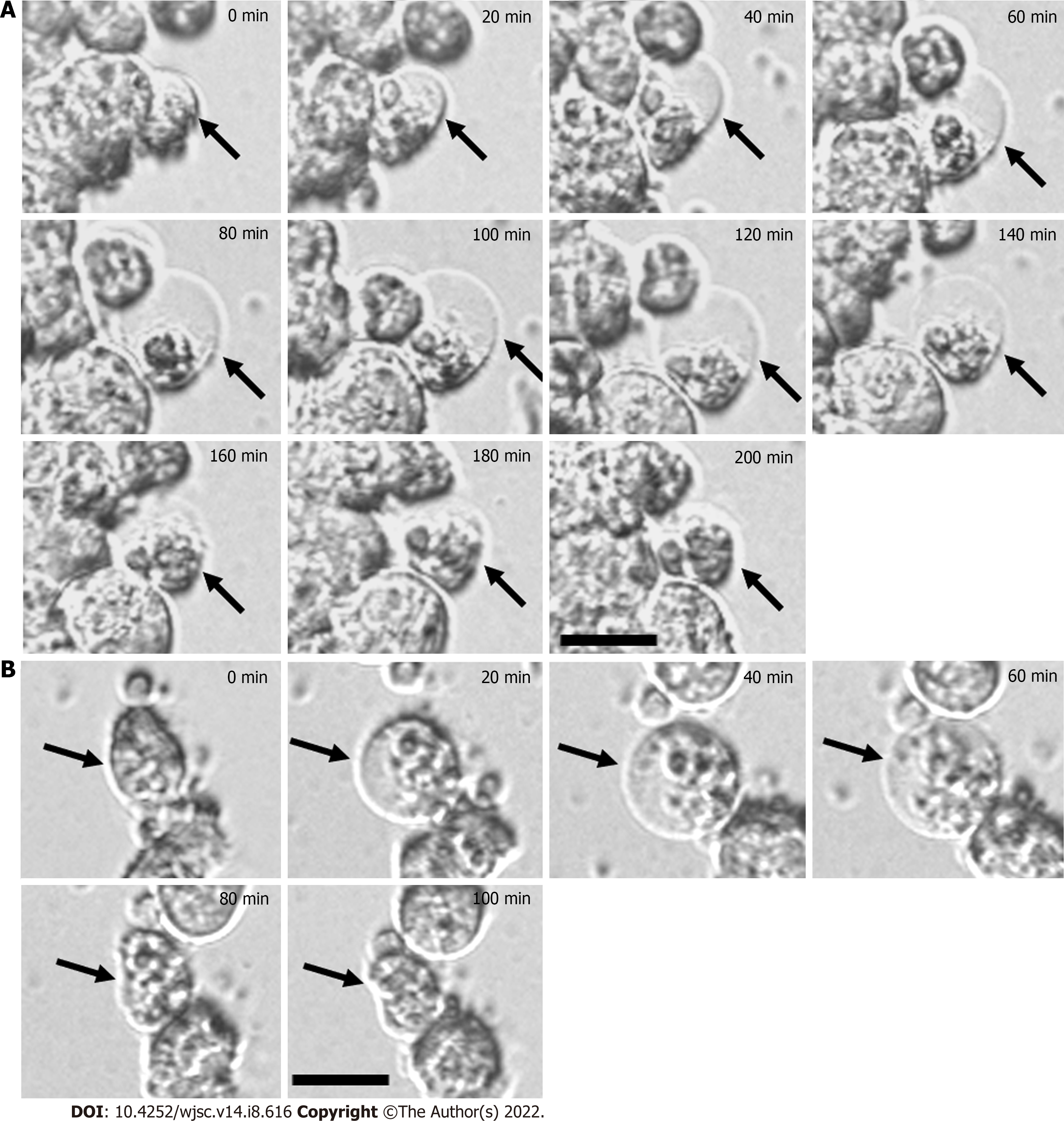
After MSCs were seeded on the CS-HA and PVA substrates for 30 min, the images of cultured MSCs were recorded as time-lapse videos to track the morphological change of MSCs and the dynamic bubble-forming process. Here, the typical sequential images of bubble formation sampled every 20 min are shown in Figure 2. The initial snapshot began from a non-mature bubble (0 min) and the final snapshot displayed the disappearance of the developed bubble. On CS-HA substrates, the bubble-like structure on the surface of MSCs formed randomly (Figure 2A). During the cycle of the membrane bubble formation, the size of the bubble was continuously and rapidly changed every 20 min, and such as morphological feature of MSCs on CS-HA substrates was quite similar to that on PVA substrates during the MSC spheroid formation. On PVA substrates, the bubble on the cell membrane of cultured MSCs appeared in the snapshot at 20 min and remained until 60 min of the recording time (Figure 2B). At 80 min, the bubble disappeared in the snapshot. In this sample, the dynamic alteration of the cell membrane morphology was apparently observed in the indicated cell. These results indicated an alteration of cell membrane fluidity or cell membrane stability in MSCs during the formation of cellular spheroids on the surface of PVA or CS-HA substrates.
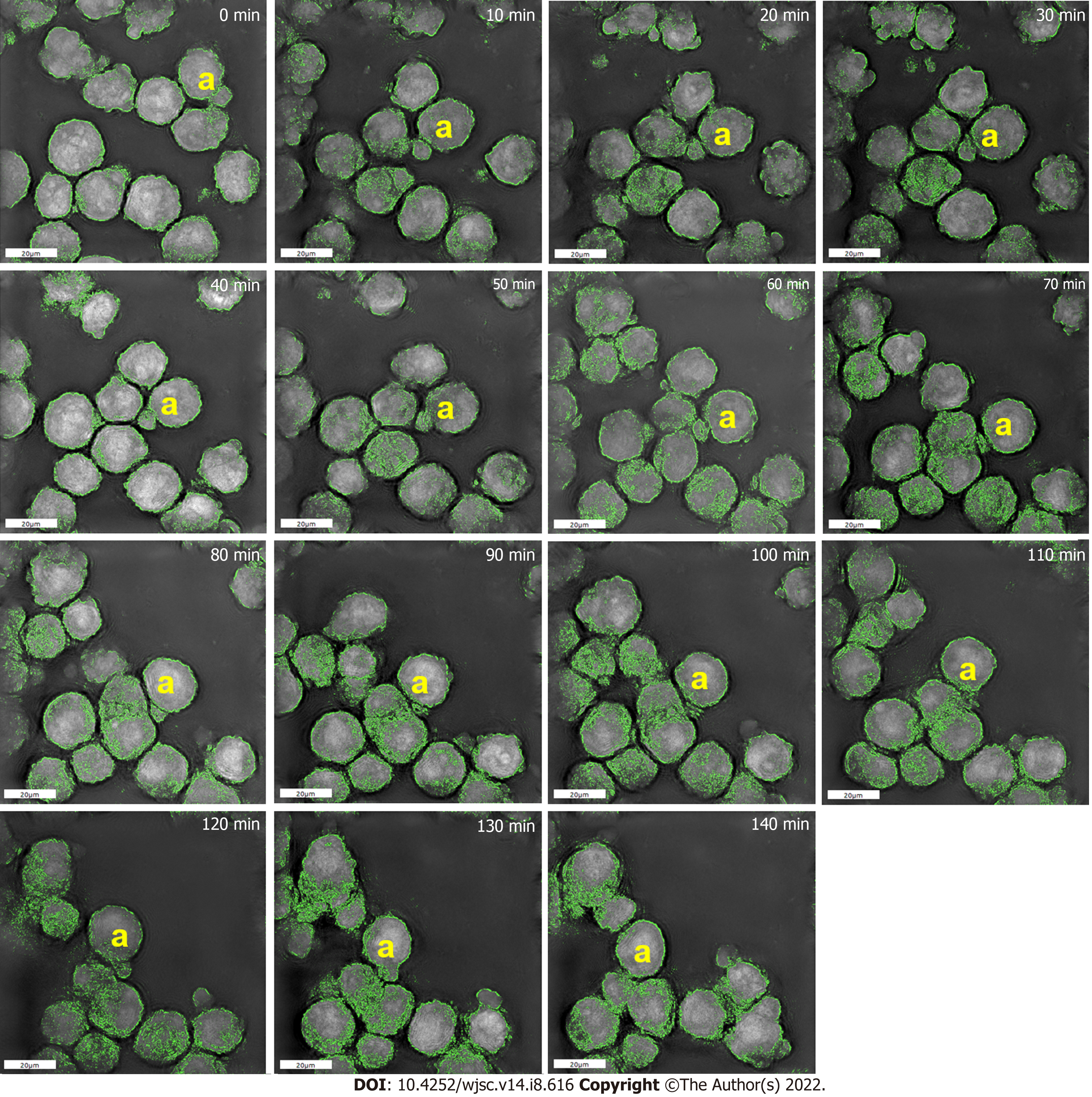
High-resolution 3D microscopy revealed that a single MSC on CS-HA developed a vesicle-like bubble on the cell membrane during the recording period of 15 h. The cell with the bubble was designated by the yellow a-marked cell on all collected images (Figure 3), and the sequential high-resolution images were acquired every 10 min. The bubble continued to exist on the surface of the cell from the beginning until 60 min of the recording time. Interestingly, a part of the membrane of the bubble started to fuse with the membrane of the other cell from 70 min to 110 min. A significant reduction of the volume for the bubble was shown in the period from 80 min to 110 min. After that, the bubble began to fuse back to the original cell at 120 min of recording time. After 130 min, the original bubble on the MSC no long existed, suggesting that the bubble was ready to merge into the original cell. Finally, the bubble disappeared in the image after 140 min of the recording time.
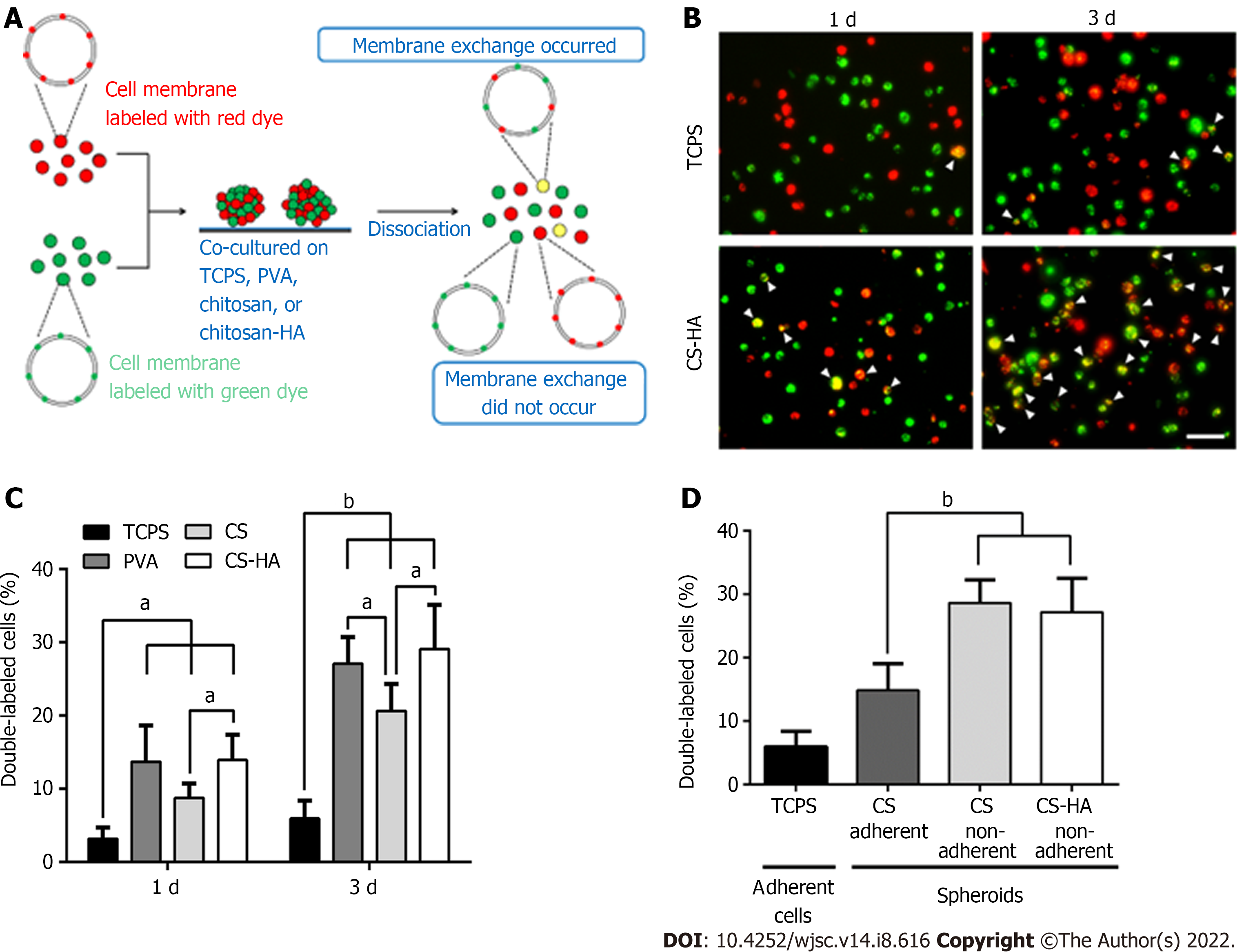
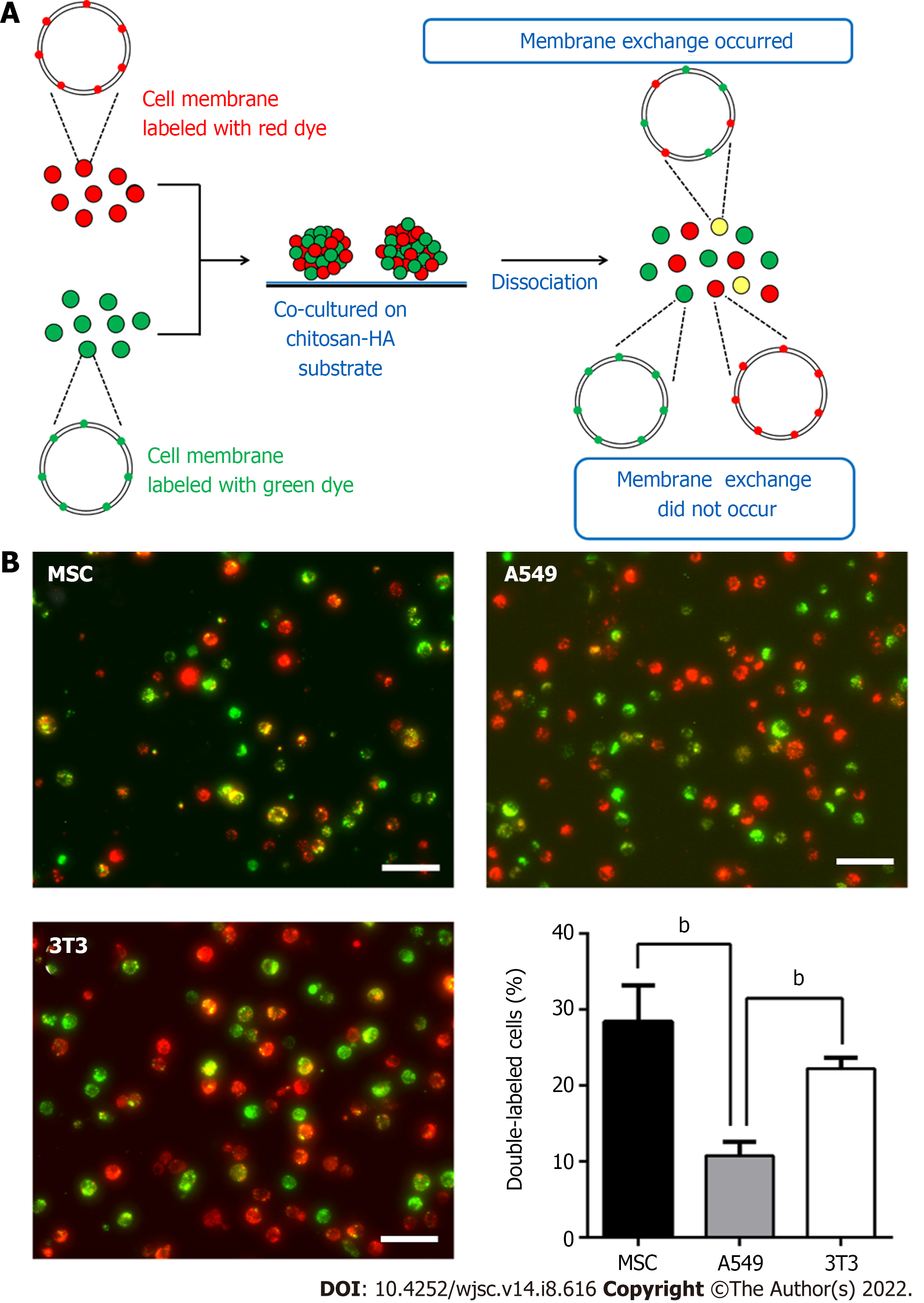
Since the dynamic evolution of vesicle-like bubbles from cell membrane was clearly observed for spheroid-forming MSCs, we used fluorescent dyes to label the cell membrane to further investigate whether the exchange of cell membrane between cells could occur during the spheroid formation of MSCs on various cell culture substrates (Figure 4). The experimental procedures are illustrated in Figure 4A. The double-labeled cells with yellow color indicated a single MSC containing both of the red dye and green dye in the cell membrane (i.e. this cell had exchanged the cell membrane with other cells). By comparing the double-labeled cells on TCPS and on CS-HA substrates, we found that the amount of yellow color cells on CS-HA substrates (approximately 13%) was more than that on TCPS substrates (approximately 3%) after 1 d of incubation (Figure 4B and C). After 3 d of incubation, the population of double-labeled cells on CS-HA substrates significantly increased again (approximately 28%), suggesting that the membrane fluidity of MSCs may have occurred during the formation of MSC spheroids. The population percentages of double-labeled cells on all substrates including CS (approximately 9%), CS-HA (approximately 13%), or PVA (approximately 13%) were greater than that on TCPS (approximately 3%) after 1 d of incubation (Figure 4B and 4C). In addition, the population of double-labeled cells had dramatically increased after 3 d of incubation on PVA, CS, or CS-HA substrates. The MSC spheroids cultured on CS substrates contained both of adherent and non-adherent cells, while the MSC spheroids on PVA and CS-HA substrates were rather non-adherent and suspended. The non-adherent cells collected from CS and CS-HA substrates showed close percentages of population for double-labeled cells (approximately 28% and approximately 27% for CS and CS-HA, respectively) after 3 d of incubation (Figure 4D). Meanwhile, the number of non-adherent cells with yellow color was greater than that of adherent cells on these substrates, indicating that the suspended MSC spheroids had the higher cell membrane fluidity.
The increase in membrane fluidity of mono-cultured MSCs was clearly observed during the formation of suspended MSC spheroids. Next we employed CS-HA substrates to generate non-adherent heterogeneous spheroids and further investigate the membrane fluidity in these spheroids using the strategy described above. First, we used only one type of cell (MSCs, A549, or 3T3) with equal numbers of red- and green-labeling dye to generate cellular spheroids to determine the membrane exchange for each type of cells (Figure 5A). Results are presented in Figure 5B. It was evident that individually cultured MSCs or 3T3 cells had a higher percentage of double-labeled cells than individually cultured A549 cells after 3 d of incubation on CS-HA substrates (approximately 29% for MSCs, approximately 22% for 3T3, and approximately 10% for A549). This finding indicated that the membrane exchange was relatively less frequent in A549 spheroids. By comparing the number of double-labeled cells collected from TCPS and CS-HA substrates, we found that the yellow color A549 or 3T3 cells on CS-HA substrates were more than those on TCPS substrates (approximately 2-fold and approximately 4-fold for A549 and 3T3 cells, respectively) (Supplementary Figure 2).
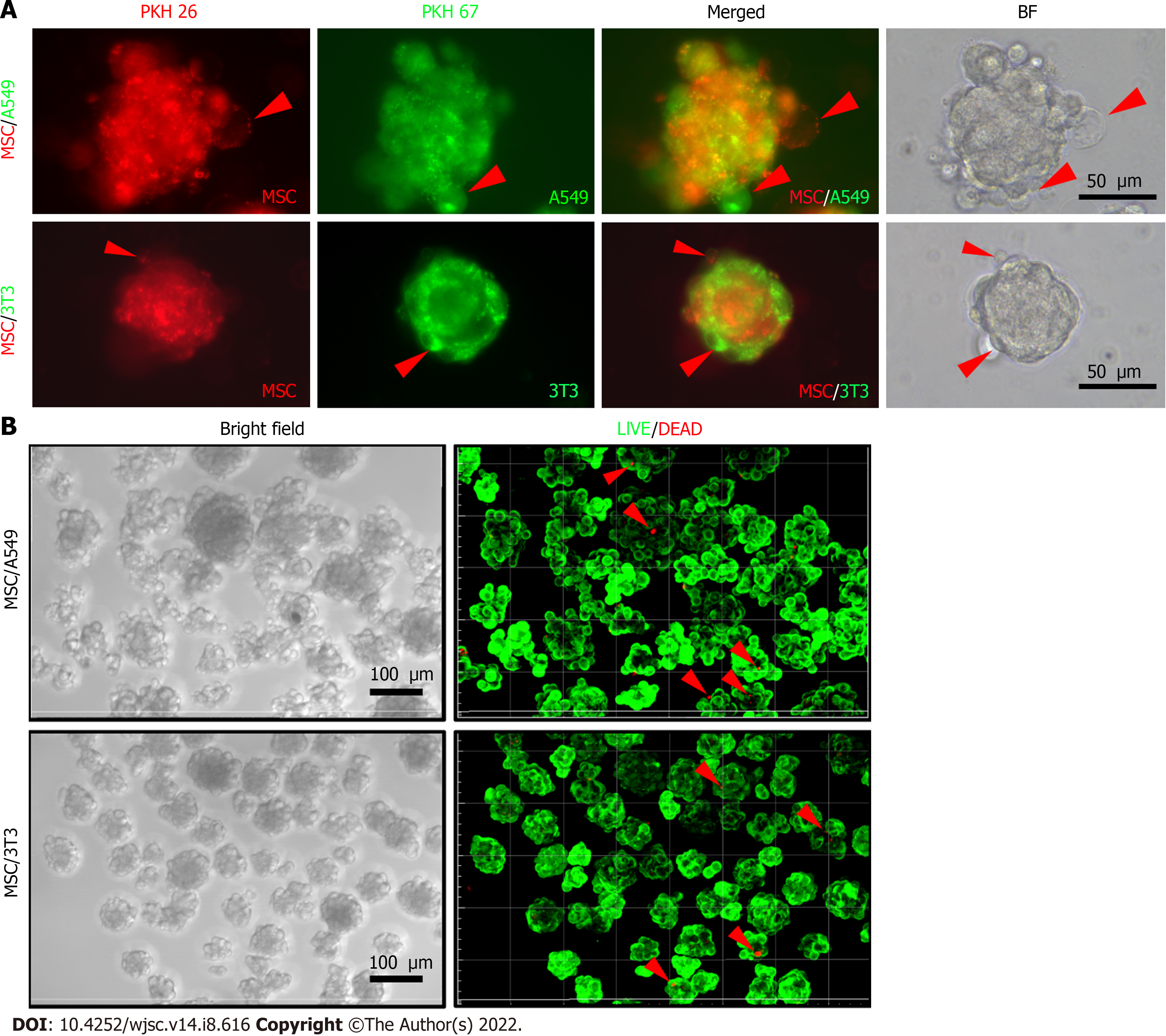
The previous quantitative results supported that the cell membrane fluidity was increased in the 3D-cultured homogeneous cells during the spheroid formation. For heterogeneous cell spheroids, equal numbers of MSCs and A549 (or 3T3) cells were co-cultured as heterotypic co-spheroids on CS-HA substrates. Meanwhile, the vesicle-like bubbles were also observed for the heterogeneous cell spheroids on CS-HA substrates (Figure 6A). To rule out the possibility that spheroids may undergo apoptosis, the cell viability was determined by the LIVE/DEAD staining after spheroid formation. As shown in Figure 6B, overall there were 98% viable cells in the heterogeneous cell spheroids.
We further examined whether the increased membrane fluidity could occur in co-cultured heterogeneous spheroids on TCPS and CS-HA substrates. We analyzed the population of double-labeled cells derived from heterogeneous cell spheroids on CS-HA substrates and used the same staining method and strategy as mentioned above to evaluate the translocation of labeling dyes between the two types of co-cultured cells (Figure 7). Heterogeneous spheroids were dissociated to single cells for analyses of staining intensity by flow cytometry and single cell morphology by confocal imaging (Figure 7A). In the heterogeneous spheroids of MSC/A549, the number of MSCs with the red dye was reduced more than the green colored A549 after 3 d of incubation (Figure 7B and C). In the MSC/A549 co-spheroids, approximately 64% of the cells were double staining positive cells, which were significantly more than those in the MSC/3T3 co-spheroids (approximately 34%) (Figure 7B, the lower panel). Meanwhile, less than 8% of MSC/A549 or MSC/3T3 co-cultured cells in the TCPS group were double-stained cells (Figure 7B, the upper panel). This finding suggested that the cell membrane of MSCs had more fluidity to transfer the labeling dye onto the cell membrane of A549. Besides, the labeling dyes in heterogeneous MSC/3T3 co-spheroids could also be transferred from MSCs to 3T3 cells. By comparing the percentages of double-labeled cells in two different heterogeneous co-spheroids, it was found that A549 in the MSC/A549 co-spheroids had greater ability to transfer the labeled dyes than 3T3 cells in the MSC/3T3 co-spheroids. These results also revealed that cell membrane fluidity may facilitate the opportunity for substance translocation from the cell membrane between two different types of cells.
The signaling pathway and differentiation of cells can be influenced by cell-cell interaction in 3D cell culture systems[10,30]. The crosstalk between MSCs and host cells contributes to self-regeneration via cell-to-cell contact, which is regulated by structural properties of the growth substrates[31]. 2D cultured cells on a flat surface are generally lack of appropriate cell-cell interactions, while 3D cellular spheroids provide proper cell-environment interaction to mimic that of the real tissues. In 3D culture systems, cells are ready to receive cellular responses or stimulus under non-adhesive conditions[32]. In our earlier reports, 3D co-spheroids of MSCs and cancer cells promoted the cancer stemness through direct cell-cell interaction[33]. 3D cellular spheroids allow the cells to interact with each other and thus the cellular behavior is more similar to that in vivo compared to the 2D cell culture system. In this study, we discovered small vesicle-like bubble formation on the membrane of spheroid-forming cells. The viability of cells in the co-spheroids was over 98% (Figure 6B). These findings suggest that the small vesicle-like bubbles may not be the apoptotic bodies. In addition, a significant increase in the fluidity of cell membrane was observed during the process of cellular spheroid formation. The possible change of cell membrane fluidity in spheroid-forming cells is still rarely reported in literature.
Cell membrane fluidity is considered a critical factor for cell migration and adhesion. The characteristics of membrane fluidity also plays an essential role in affecting cell communication. The lipid composition of the cell membrane impacts the fluidity properties. Maintenance of the membrane fluidity relies on the presence of unsaturated lipids and integrated sterols. The inflexible sterols can increase membrane rigidity, while the flexible unsaturated lipid increase the fluidity of cell membrane[34]. In addition, the Rho signaling pathway may regulate the homeostasis of membrane fluidity for cell division and differentiation in eukaryotic cells[35]. Moreover, cell membrane fluidity affects the growth and differentiation of cells via the regulation of intracellular signaling transmission such as phosphoinositide 3-kinase (PI3K)/Akt pathways in adult human MSCs[36]. The dynamic properties of membrane structure are correlated with the differentiation and morphological changes of stem cells[37]. Based on the information, we assumed that the spheroidal culture of MSCs, if affecting the fluidity of cell membrane, may further influence the cell differentiation.
CS substrates are commonly used biomaterials for generation of 3D cellular spheroids. MSC spheroids derived on CS-based culture systems displayed greater differentiation ability[38]. Previously, we have successfully developed the spheroids of MSCs on different substrates including PVA, CS, and CS-HA to enhance the cell differentiation and regeneration capacities[18]. Because HA has the ability to promote the proliferation and migration of cells via CD44 receptor and the PI3K/MAPK signaling pathway[39], the CS-HA substrates provide a suitable microenvironment for altering the behavior of MSCs. The 3D spheroids self-assembled from MSCs cultured on CS-based substrates showed more significant gene regulation than those cultured on PVA[18]. Meanwhile, MSC spheroids on CS-HA revealed the greater expression of stemness marker genes as well as higher differentiation potential than those on CS[18]. In the current study, we found that the cell membrane of MSC homo-spheroids on CS-HA substrates had greater fluidity than that on CS substrates. Therefore, the increase in the stemness of these MSC spheroids may be linked to the increase of cell membrane fluidity.
MSCs offer a promising cell source for regenerative medicine. MSCs have multipotent capacities and can differentiate into a variety of cells to replace damaged tissues. Moreover, various growth factors and anti-inflammatory cytokines released from MSCs can promote tissue repair[40]. Literature has reported that MSCs release biological factors mainly through the secretion of extracellular vehicles (EVs)[41]. The EVs of MSCs exist in two major types, i.e. exosomes and microvesicles. In addition, the EVs usually contain proteins, messenger RNA as well as microRNA (miRNA). During the process of skeletal muscle regeneration, local injection of MSC exosomes that contained miRNAs and growth factors accelerated the muscular regeneration and angiogenesis in a mouse model of muscle injury[42]. The secretion of EVs from MSCs also helped to reduce pulmonary inflammation and vascular dysfunction in trauma-induced or virus-induced lung injury[43]. Meanwhile, literature has also reported that cell membrane fluidity can affect the secretion of EVs in tumor cells[44], where a higher level of membrane fluidity increased the yield of exosomes from the tumor cells. In the present study, the vesicle-like bubbles observed on the cell membrane are morphologically similar to the giant plasma membrane vesicles (GPMVs)[45]. GPMVs are derived from the vesiculation or blebbing of plasma membrane in mammalian cells induced by the treatment of vesiculants[46]. GPMVs are usually 1–10 μm in diameter[47]. Generation of GPMVs mediated the osteogenic differentiation of human MSCs[48], suggesting the possible link between vesicle formation and differentiation potential. Since MSC spheroids on CS-HA had greater differentiation potential than those on CS[18], and they showed more membrane fluidity, it is thus possible that the secretion of exosomes may also increase in these MSC spheroids. We hypothesized that some membrane proteins of MSCs might interact with the hydrophilic substrates during the spheroid formation, and such crosstalk would lead to the generation of vesicle-like bubbles depending on the dynamic remodeling of cell membrane. These sequential events may also explain why the cellular spheroids of MSCs showed high efficacy in the tissue regeneration and bioengineering field.
Time-lapse images revealed that vesicle-like bubbles were rather active and dynamic on the cell membrane of MSCs during the process of spheroid formation. This finding suggested that the increase in membrane fluidity may be triggered by cell-substrate interactions. In our previous study, we discovered that cell-substrate interactions existed between MSCs and the CS-based culture substrates. The MSCs were partially attached to the surface of the CS-based substrates and then detached from the substrates to assemble and merge into 3D cellular spheroids[18]. These observations indicated that cell-substrate interactions may affect cytoskeletal rearrangement to change cell morphology. On the other hand, several studies have demonstrated that the secretion and formation of EVs are associated with cytoskeletal rearrangement and the intracellular calcium concentration[49,50]. Meanwhile, the spheroid formation of MSCs on CS membranes is also modulated by the concentration of calcium[18]. These direct and indirect evidences suggest that cell-substrate interaction may cause a variation of membrane fluidity to generate vesicle bubbles via the regulation of actin cytoskeleton dynamics. Nevertheless, the exact mechanism for the formation of vesicle bubbles requires further investigation.
Cytoskeleton-associated signal transduction is affected by the interaction between cells and culture substrates, which further influences the morphology and migration ability of cells[51]. Therefore, the final shape of cellular spheroids on the surface of cell culture substrates may determine their mobility. In the current study, we cultured stem cells on the hydrophilic substrates to generate 3D spheroids. Stem cells could form a compact cellular spheroids on CS-HA substrates[18], while the spheroidal cancer cells displayed relatively less compacted structure and required a longer period of time to stabilize the spheroidal morphology[52]. Besides, two types of cells were assembled into the co-spheroids with distinct patterns of cell organization on various culture substrates[52]. During the spheroid formation, the hydrophilic materials may interact with some structural proteins anchored on the cell membrane, leading to the cell membrane remodeling. Hence, cell-substrate interactions may change the morphology and compactness of the spheroids as well as the distribution of cells within the co-spheroids. In the present study, the membrane fluidity of stem cells increased more significantly than that of cancer cells when assembled into cellular spheroids. These results suggested that stem cells had more interaction with CS-HA substrates to affect the cell morphology than cancer cells. The different rigidity of cell membrane and alternative expression of surface receptors are both the possible reasons for the difference in cell-substrate interactions. Together with the phenomenon of cytoskeletal rearrangement during the formation of 3D spheroids, we believe that the vesicle-like bubbles were derived from the dynamic remodeling of cell membrane and cytoskeleton in MSCs.
Various types of cells changed the membrane fluidity differently when forming the 3D cellular spheroids (Figure 5B). For each type of cells, a higher level of membrane fluidity was observed in spheroids vs the conventional 2D cultured cells (Figure 4C and Supplementary Figure 2). Compared to 2D cultured cells, the number of double-labeled cells was increased from two-fold to four-fold in homogeneous spheroids for 3D cultured spheroids. For the same reason, the intercellular crosstalk may increase in the co-spheroids as well. Therefore, we further investigated the overall membrane fluidity in heterogeneous cellular co-spheroids. The MSC/A549 co-spheroids showed a slightly higher amount of double-labeled cells than the MSC/3T3 co-spheroid (Figure 7B). Meanwhile, data suggested that the membrane fluidity of MSC spheroids was greater than that of 3T3 spheroids, while the latter was again greater than that of A549 spheroids (Figure 5B). Taken together, these findings suggest that the enhancement of membrane fluidity might occur in different types of cells and on different substrates. Literature has reported that stem cells are educated by co-cultured nurse cells (e.g., maintenance of iPSCs by the co-cultured fibroblasts[26] as well as differentiation of neural stem cells induced by co-cultured endothelial cells[53]). The membrane fluidity and substance exchange may play roles in the aforementioned cell-to-cell educational events. Furthermore, the increased membrane fluidity, the cytoskeleton-associated signal transduction, the spheroid formation, as well as the cell-cell interaction and cell-substrate interaction may be mutually dependent in the biomaterial-based spheroid forming platforms.
The cellular spheroid formation has attracted much research attention, but most of the previous studies only focused on chemical variations such as signaling transduction, gene expression, and differentiation. In the current study, we discovered that the cell membrane fluidity was changed during the process of cellular spheroid formation for cells cultured on the surface of a few biomaterials. The present study has not fully confirmed if the variation of cell membrane fluidity is caused by changes of cell-substrate interaction or by cell-cell interaction, or even by both, during the process of spheroid formation. However, the evidence from our observations suggest that the changes of cell membrane fluidity is an important aspect during cellular spheroid formation and should be considered as an essential part of the physiological changes of the 3D spheroid-forming cells.
We employed various substrates to culture homotypic and heterotypic spheroid-forming cells in this study. The cell membrane of the mono-cultured MSCs displayed obvious vesicle-like bubbles during and after the spheroid formation on the CS-HA based culture system. Using membrane labeling dyes, we observed that the MSC spheroids existed a high level of cell membrane fluidity. Moreover, form
Cell membrane fluidity is a critically important physical property for the regulation of cell behavior, but it has not been studied for the spheroid-forming cells so far.
In present study, we investigated the linkage between cell membrane fluidity and the morphological change from the three-dimensional (3D) spheroids generated by mesenchymal stem cells (MSCs) on various culture substrates.
We tried to unveil the interaction of cells through membrane exchange in the homogeneous MSC spheroids and the heterotypic co-spheroids of MSCs/cancer cells and co-spheroids of MSCs/fibroblasts.
We generated three-dimensional MSC spheroids on the surface of various culture substrates.
We discovered that vesicle-like bubbles randomly appeared on the outer layer of the MSC spheroids.
Current findings provide a novel direction to elucidate the complicated physiological alterations for 3D spheroid-forming cells.
These physiological changes of 3D spheroid-forming cells demonstrated that membrane fluidity may be altered significantly by direct cell-cell interaction on surfaces of biomaterial cell culture substrates. The membrane fluidity and translocation of components provide a new direction to elucidate the role of 3D cellular spheroid formation in the physical/physiological property changes of cells on the material interface.
We are obliged to the College of Bioresources and Agriculture, National Taiwan University, for the kind assistance in confocal microscopy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vynios D, Greece; Wang G, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Cai YX
| 1. | Wong JF, Mohan MD, Young EWK, Simmons CA. Integrated electrochemical measurement of endothelial permeability in a 3D hydrogel-based microfluidic vascular model. Biosens Bioelectron. 2020;147:111757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Hassani F, Ebrahimi B, Moini A, Ghiaseddin A, Bazrafkan M, Hassanzadeh GH, Valojerdi MR. Chitosan hydrogel supports integrity of ovarian follicles during in vitro culture: a preliminary of a novel biomaterial for three dimensional culture of ovarian follicles. Cell J. 2020;21:479-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Singh A, Tayalia P. Three-dimensional cryogel matrix for spheroid formation and anti-cancer drug screening. J Biomed Mater Res A. 2020;108:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | He D, Wang RX, Mao JP, Xiao B, Chen DF, Tian W. Three-dimensional spheroid culture promotes the stemness maintenance of cranial stem cells by activating PI3K/AKT and suppressing NF-κB pathways. Biochem Biophys Res Commun. 2017;488:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Marupanthorn K, Tantrawatpan C, Kheolamai P, Tantikanlayaporn D, Manochantr S. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int J Mol Med. 2017;39:654-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Ye K, Liu D, Kuang H, Cai J, Chen W, Sun B, Xia L, Fang B, Morsi Y, Mo X. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J Colloid Interface Sci. 2019;534:625-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Chaicharoenaudomrung N, Kunhorm P, Noisa P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J Stem Cells. 2019;11:1065-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 255] [Article Influence: 42.5] [Reference Citation Analysis (7)] |
| 8. | Enderami SE, Ahmadi SF, Mansour RN, Abediankenari S, Ranjbaran H, Mossahebi-Mohammadi M, Salarinia R, Mahboudi H. Electrospun silk nanofibers improve differentiation potential of human induced pluripotent stem cells to insulin producing cells. Mater Sci Eng C Mater Biol Appl. 2020;108:110398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Imamura A, Kajiya H, Fujisaki S, Maeshiba M, Yanagi T, Kojima H, Ohno J. Three-dimensional spheroids of mesenchymal stem/stromal cells promote osteogenesis by activating stemness and Wnt/β-catenin. Biochem Biophys Res Commun. 2020;523:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Ryu NE, Lee SH, Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 11. | Gao W, Wu D, Wang Y, Wang Z, Zou C, Dai Y, Ng CF, Teoh JY, Chan FL. Development of a novel and economical agar-based non-adherent three-dimensional culture method for enrichment of cancer stem-like cells. Stem Cell Res Ther. 2018;9:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Radtke AL, Herbst-Kralovetz MM. Culturing and applications of rotating wall vessel bioreactor derived 3D epithelial cell models. J Vis Exp. 2012;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Nakod PS, Kim Y, Rao SS. Three-dimensional biomimetic hyaluronic acid hydrogels to investigate glioblastoma stem cell behaviors. Biotechnol Bioeng. 2020;117:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Darouie S, Ansari Majd S, Rahimi F, Hashemi E, Kabirsalmani M, Dolatshahi-Pirouz A, Arpanaei A. The fate of mesenchymal stem cells is greatly influenced by the surface chemistry of silica nanoparticles in 3D hydrogel-based culture systems. Mater Sci Eng C Mater Biol Appl. 2020;106:110259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Scalzone A, Ferreira AM, Tonda-Turo C, Ciardelli G, Dalgarno K, Gentile P. The interplay between chondrocyte spheroids and mesenchymal stem cells boosts cartilage regeneration within a 3D natural-based hydrogel. Sci Rep. 2019;9:14630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Zuo X, Zhang H, Zhou T, Duan Y, Shou H, Yu S, Gao C. Spheroids of endothelial cells and vascular smooth muscle cells promote cell migration in hyaluronic acid and fibrinogen composite hydrogels. Research (Wash D C). 2020;2020:8970480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Petrova VA, Chernyakov DD, Poshina DN, Gofman IV, Romanov DP, Mishanin AI, Golovkin AS, Skorik YA. Electrospun bilayer chitosan/hyaluronan material and its compatibility with mesenchymal stem cells. Materials (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Huang GS, Hsieh PS, Tseng CS, Hsu SH. The substrate-dependent regeneration capacity of mesenchymal stem cell spheroids derived on various biomaterial surfaces. Biomater Sci. 2014;2:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Han HW, Asano S, Hsu SH. Cellular spheroids of mesenchymal stem cells and their perspectives in future healthcare. Appl Sci. 2019;9:627. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Mo M, Zhou Y, Li S, Wu Y. Three-dimensional culture reduces cell size by increasing vesicle excretion. Stem Cells. 2018;36:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Los DA, Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta. 2004;1666:142-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 575] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 22. | Mondal D, Dutta R, Banerjee P, Mukherjee D, Maiti TK, Sarkar N. Modulation of membrane fluidity performed on model phospholipid membrane and live cell membrane: revealing through spatiotemporal approaches of flim, faim, and trfs. Anal Chem. 2019;91:4337-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Gupta A, Marzinek JK, Jefferies D, Bond PJ, Harryson P, Wohland T. The disordered plant dehydrin Lti30 protects the membrane during water-related stress by cross-linking lipids. J Biol Chem. 2019;294:6468-6482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Dawaliby R, Trubbia C, Delporte C, Noyon C, Ruysschaert JM, Van Antwerpen P, Govaerts C. Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells. J Biol Chem. 2016;291:3658-3667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 25. | Edmond V, Dufour F, Poiroux G, Shoji K, Malleter M, Fouqué A, Tauzin S, Rimokh R, Sergent O, Penna A, Dupuy A, Levade T, Theret N, Micheau O, Ségui B, Legembre P. Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene. 2015;34:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Mao H, Joddar B, Umeki N, Sako Y, Wada K, Nishioka C, Takahashi E, Wang Y, Ito Y. The significance of membrane fluidity of feeder cell-derived substrates for maintenance of iPS cell stemness. Sci Rep. 2015;5:11386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Matsuzaki T, Matsumoto S, Kasai T, Yoshizawa E, Okamoto S, Yoshikawa HY, Taniguchi H, Takebe T. Defining lineage-specific membrane fluidity signatures that regulate adhesion kinetics. Stem Cell Reports. 2018;11:852-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Chatgilialoglu A, Rossi M, Alviano F, Poggi P, Zannini C, Marchionni C, Ricci F, Tazzari PL, Taglioli V, Calder PC, Bonsi L. Restored in vivo-like membrane lipidomics positively influence in vitro features of cultured mesenchymal stromal/stem cells derived from human placenta. Stem Cell Res Ther. 2017;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Fu CY, Chuang WT, Hsu SH. A biodegradable chitosan-polyurethane cryogel with switchable shape memory. ACS Appl Mater Interfaces. 2021;13:9702-9713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Vorwald CE, Joshee S, Leach JK. Spatial localization of endothelial cells in heterotypic spheroids influences Notch signaling. J Mol Med (Berl). 2020;98:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Martín-Saavedra F, Crespo L, Escudero-Duch C, Saldaña L, Gómez-Barrena E, Vilaboa N. Substrate microarchitecture shapes the paracrine crosstalk of stem cells with endothelial cells and osteoblasts. Sci Rep. 2017;7:15182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, Lamperska K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14:910-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 577] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 33. | Tseng TC, Wong CW, Hsieh FY, Hsu SH. Biomaterial substrate-mediated multicellular spheroid formation and their applications in tissue engineering. Biotechnol J. 2017;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Sunshine H, Iruela-Arispe ML. Membrane lipids and cell signaling. Curr Opin Lipidol. 2017;28:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 35. | Lockshon D, Olsen CP, Brett CL, Chertov A, Merz AJ, Lorenz DA, Van Gilst MR, Kennedy BK. Rho signaling participates in membrane fluidity homeostasis. PLoS One. 2012;7:e45049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Sohn J, Lin H, Fritch MR, Tuan RS. Influence of cholesterol/caveolin-1/caveolae homeostasis on membrane properties and substrate adhesion characteristics of adult human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Noutsi BK. Correlation between membrane fluidity cellular development and stem cell differentiation. Diss. 2016. |
| 38. | Petrenko Y, Syková E, Kubinová Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 39. | Murakami T, Otsuki S, Okamoto Y, Nakagawa K, Wakama H, Okuno N, Neo M. Hyaluronic acid promotes proliferation and migration of human meniscus cells via a CD44-dependent mechanism. Connect Tissue Res. 2019;60:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 731] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 41. | Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3378] [Cited by in RCA: 3537] [Article Influence: 321.5] [Reference Citation Analysis (0)] |
| 42. | Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 43. | Potter DR, Miyazawa BY, Gibb SL, Deng X, Togaratti PP, Croze RH, Srivastava AK, Trivedi A, Matthay M, Holcomb JB, Schreiber MA, Pati S. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg. 2018;84:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 44. | Emam SE, Ando H, Abu Lila AS, Shimizu T, Ukawa M, Okuhira K, Ishima Y, Mahdy MA, Ghazy FS, Ishida T. A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biol Pharm Bull. 2018;41:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | Zartner L, Garni M, Craciun I, Einfalt T, Palivan CG. How can giant plasma membrane vesicles serve as a cellular model for controlled transfer of nanoparticles? Biomacromolecules. 2021;22:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A. 2007;104:3165-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 634] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 47. | Säälik P, Niinep A, Pae J, Hansen M, Lubenets D, Langel Ü, Pooga M. Penetration without cells: membrane translocation of cell-penetrating peptides in the model giant plasma membrane vesicles. J Control Release. 2011;153:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Gaur D, Yogalakshmi Y, Kulanthaivel S, Agarwal T, Mukherjee D, Prince A, Tiwari A, Maiti TK, Pal K, Giri S. Osteoblast‐derived giant plasma membrane vesicles induce osteogenic differentiation of human mesenchymal stem cells. Adv Biosyst. 2018;2:1800093. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 346] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 50. | Holliday LS, Faria LP, Rody WJ Jr. Actin and actin-associated proteins in extracellular vesicles shed by osteoclasts. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Moujaber O, Stochaj U. The Cytoskeleton as Regulator of Cell Signaling Pathways. Trends Biochem Sci. 2020;45:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Wong CW, Han HW, Tien YW, Hsu SH. Biomaterial substrate-derived compact cellular spheroids mimicking the behavior of pancreatic cancer and microenvironment. Biomaterials. 2019;213:119202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Han HW, Hou YT, Hsu SH. Angiogenic potential of co-spheroids of neural stem cells and endothelial cells in injectable gelatin-based hydrogel. Mater Sci Eng C Mater Biol Appl. 2019;99:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |