Published online Jul 26, 2022. doi: 10.4252/wjsc.v14.i7.513
Peer-review started: March 17, 2022
First decision: April 25, 2022
Revised: May 18, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: July 26, 2022
Processing time: 130 Days and 14.8 Hours
Mesenchymal stem cells (MSCs) have gained wide-ranging reputation in the medical research community due to their promising regenerative abilities. MSCs can be isolated from various resources mostly bone marrow, Adipose tissues and Umbilical cord. Huge advances have been achieved in comprehending the possible mechanisms underlying the therapeutic functions of MSCs. Despite the proven role of MSCs in repairing and healing of many disease modalities, many hurdles hinder the transferring of these cells in the clinical settings. Among the most reported problems encountering MSCs therapy in vivo are loss of tracking signal post-transplantation, insufficient migration, homing and engraftment post-infusion, and undesirable differentiation at the site of injury. Magnetic nano
Core Tip: Mesenchymal stem cells (MSCs) have been thoroughly investigated in many disease models and they showed great therapeutic potential. Despite the confirmed therapeutic abilities of MSCs, many challenges still exist which hinder the transfer of these cells to the treatment guidelines. The incorporation of magnetic nanoparticles (MNPs) with MSCs has been reported to increase the therapeutic outcomes of MSCs by solving major challenges that impede their long–term regenerative effects. MNPs are able to improve the ability to track and deliver MSCs and to increase their migration, homing, survival and differentiation in vitro and in vivo. This may help increase the success rate of MSCs transplantation and thus increase the chance to include these cells in the treatment guidelines used in different clinical settings.
- Citation: Abu-El-Rub E, Khasawneh RR, Almahasneh F. Prodigious therapeutic effects of combining mesenchymal stem cells with magnetic nanoparticles. World J Stem Cells 2022; 14(7): 513-526
- URL: https://www.wjgnet.com/1948-0210/full/v14/i7/513.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i7.513
Mesenchymal stem cells (MSCs) are the mostly investigated stem cells due to their enchanting, wide-range therapeutic and regenerative potential[1]. Since their discovery by Friedenstein in 1970, MSCs have been thoroughly analyzed and characterized to discover the mechanistic explanations for their therapeutic abilities[2]. MSCs are easily reached stem cells and can be isolated from many sources including bone marrow (BM), adipose tissues and umbilical cord (UC)[3]. These cells are extensively studied compared to other types of stem cells because they are ethically benign and have low teratogenic tendency[3]. In addition, MSCs have an acceptable safety profile and less likely to cause serious side effects[3]. MSCs beneficial effects have been linked primarily to the ability of MSCs to secrete a cocktail of therapeutically active paracrine factors[4]. These paracrine factors secreted by MSCs can attenuate many pathological processes including apoptosis, necrosis, fibrosis, and inflammation and initiate repairing mechanisms in the damaged organs[4]. MSCs immunomodulatory functions also contribute strongly to their curative potential[5]. Moreover, MSCs can exert actual regeneration of the injured tissues by adopting the intrinsic machinery and differentiating to many functional cell types such as osteocytes, chondrocytes, adipocytes, and cardiomyocytes-like cells[6]. Endogenous or exogenous MSCs must migrate and home in the damaged tissues in order to gain their therapeutic benefits[3]. After homing in the damaged tissues, MSCs should endure the harsh microenvironment that may present[7]. Despite the numerous studies that highlighted the therapeutic efficiency of MSCs, many serious obstacles encumber the shift of MSCs from bench to bedside and delay their presence in the treatment guidelines[8]. The most reported post-transplantation challenges that researchers bump into when they use MSCs in clinical studies: (1) The disparities in the differentiation potential between in vitro and in vivo[9]; (2) The shift in their immunological characteristics and cytokines secretion profile under different stress microenvironments that may exist at the site of injury mainly Hypoxia and inflammation[5]; (3) The poor homing and migratory abilities of administered MSCs which may vary based on the route of injection and microenvironment status[10]; and (4) The loss of signal emitted from labelled cells due to the leakage of contrast agent after being injected, leads to difficulties in tracking and monitoring of these cells[11].
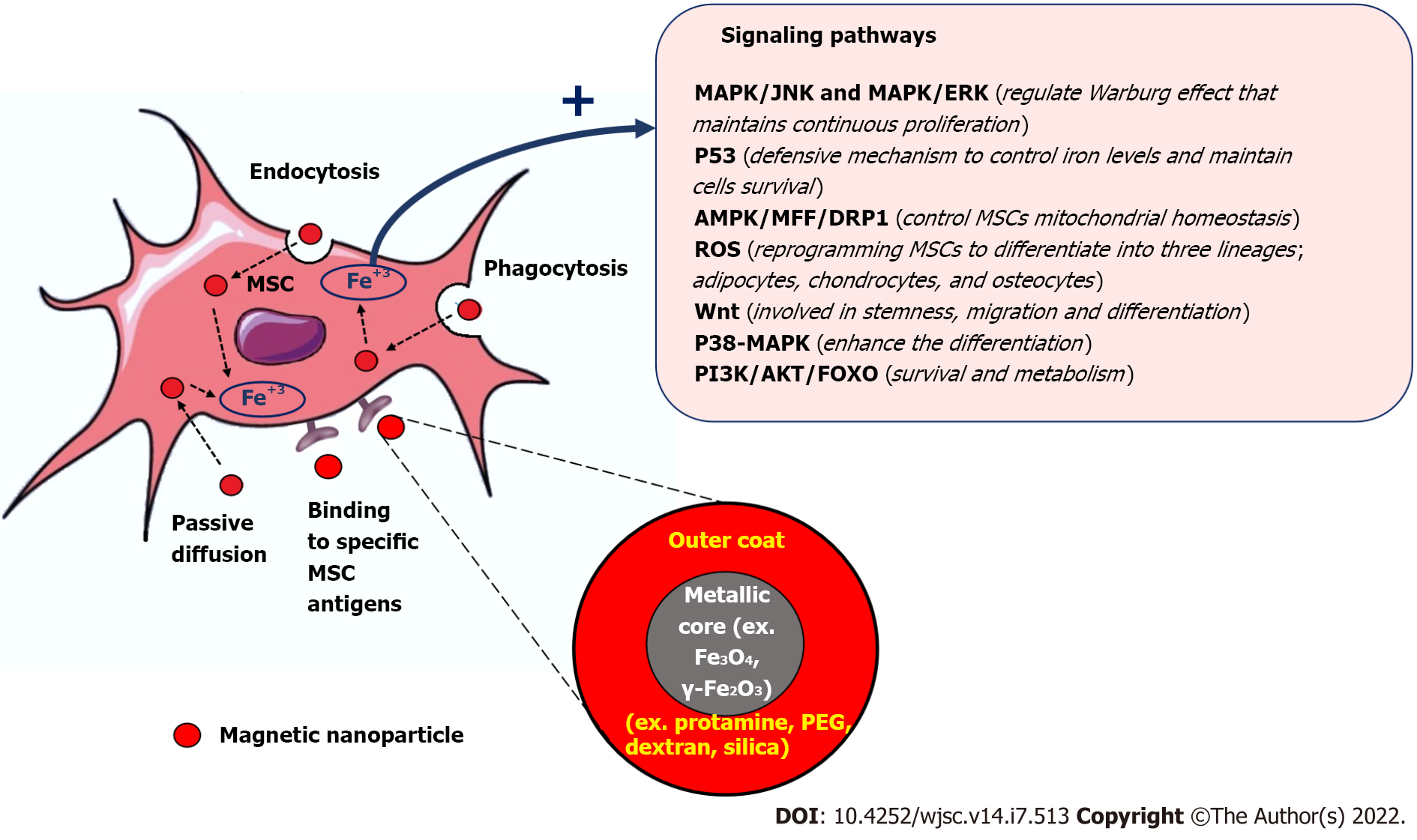
Magnetic nanoparticles (MNPs) have gained great attention among the medical researchers due to their unique biochemical and physical characteristics, their intrinsic biocompatibility and being biodegradable through normal cellular pathways which make them suitable for wide range of biomedical applications[12]. The intrinsic magnetic field elicited by the MNPs, which can be modulated externally by an applied magnetic field , is the basis for using these MNPs as contrast agents for biomedical imaging[13], biomarkers and biosensors[14], and targeted drug[15], cell and gene delivery[16]. Combining MNPs with stem cells was found to enhance their therapeutic performance and solve many challenges that hamper their regenerative potential and delay their clinical applications[17]. There are many types of MNPs that have been fabricated, but the most non-toxic and non- immunogenic MNPs that have been used with MSCs are iron oxide nanoparticles (IONs) such as magnetite (Fe3O4) or its oxidized form maghemite (γ-Fe2O3)[18-20]. These iron oxides based MNPs can be synthesized with different particles’ diameters such as Superparamagnetic iron oxide (SPIO) nanoparticles (50–200 nm diameter)[21] and ultra-small SPIO (USPIO) nanoparticles (around 35 nm diameter)[22] and different types of stabilizing non-toxic coating substrates such as dextran, polyethylene glycol, and Silica[23]. In general, the uptake of MNPs by MSCs is mediated mostly through endocytosis. MNPs usually are engulfed by MSCs to form endosomes, which then transformed into Mature multivesicular endosomes (MVEs). The MVEs then combined with lysosomes and get digested and decomposed into Fe3+. The free iron released into the cytoplasm of MSCs modified many cellular pathways to induce and promote their survival, migration, homing, anti-apoptosis and anti-inflammatory, and differentiation. These magnetized MSCs can be further modulated and guided to enhance their therapeutic outcomes by external magnetic fields. The internalization of MNPs inside MSCs can be also achieved by passive diffusion if their particle size is small and by using MNPs that bind specific cell surface immune marker found on MSCs. The prodigious power of using MNPs with MSCs to potentiate their tracking, migration and homing, differentiation and regenerative abilities will be the focus of this review.
The use of MSCs in the clinical settings requires more accurate tracking methods of MSCs after transplantation to determine their destinations, survival and final differentiated fates[24]. To visualize transplanted MSCs using imaging modalities importantly the computed tomography, positron emission tomography, and magnetic resonance imaging (MRI), these cells must be labelled with contrast agents[25-28]. The problem with the traditional contrast agents is the high leakage rate which causes the loss of emitted signal after short time course[29]. The contrast features of MNPs and their high safety profile encouraged many researchers to use them for labeling MSCs prior to injection[18]. MSCs labelled with MNPs have less leaking tendency and do not affect their stemness[30], rate of proliferation and the differentiation potential beside providing higher contrast-to-noise ratio for effective imaging[31,32].
IONs are the most commonly used MNPs for labelling and tracking MSCs due to their non-toxic and non-immunogenic features, high spatial resolution and penetration depth, and the non-ionizing radiation characteristics[33]. Superparamagnetic iron oxide nanoparticles (SPIONs), ultra-small SPIO-poly (acrylic acid) (USPIO-PAA)[34], glucosamine-modified USPIO-PAA (USPIO-PAA-GlcN)[35], and microgel iron oxide (MGIO)[36] are the most studied MNPs for MSCs labelling and tracking by multiple imaging methods. Using SPIONs for stem cell labeling and tracking is a relatively new application. Recently, ferumoxytol (Feraheme®, AMAG Pharmaceuticals), an ultrasmall SPION used clinically as an MRI contrast agent[37]. Ferumoxytol colloidal particle size is less than 50 nm and can be phagocytized efficiently by the MSCs–which have an inherited phagocytosis property- and can then be imaged and tracked by MRI[37]. FeraTrackTM, a dextran coated SPIONs, have a positive surface charge making it cell penetrable through a vesicular endocytosis route[38]. FeraTrackTM has gained utility as a biocompatible MRI contrast agent for cell tracking purposes due to their high safety profile[38]. Mesentier-Louro et al[39] used FeraTrack to track BM-MSCs at site of injury in a rodent model of optical nerve injury[39]. They reported that the after injecting FeraTrackTM labeled MSCs intravitreously, they migrated to the site of optical nerve injury and remained there for up to 18 wk which is suitable to monitor their integration with the host tissues at the site of injury using MRI. The incorporation of cationic compounds such as poly-l-lysine and protamine onto the surface coating of SPIONs can enhance labeling of MSCs by promoting interactions with the negatively charged cell surface[39]. Guldris et al[35] studied the contrast characteristics of SPIOs and USPIOs coated with PAA, and USPIO-PAA-GlcN as labeling agents for MSCs in vitro[35]. A portion of these MNPs was cultured with MSCs for 24 h at a concentration of 100 μg mL–1. In the second group, the conditions were maintained, but polylysine (PLL) was used to promote particle uptake. The study found that in the absence of PLL, SPIO-PAA showed a very low and non-homogeneous labeling efficiency. USPIO-PAA and USPIO-PAA-GlcN showed little to no internalization by MSCs, while combining USPIO-PAA-GlcN with polylysine enhances their biocompatibility with MSCs and increases the detection sensitivity by MRI in both in vitro and in vivo experiments[35]. Studies also reported that using an external pulsed magnetic field opened channels in the cell membrane and increased the uptake of SPIONs by MSCs which intensified the contrast signal[40,41]. Interestingly, Ngen and Artemov[42] developed a dual-contrast agent by combining SPIONs and gadolinium chelate to monitor and track MSCs[42]. This dual contrast agent generates powerful positive contrast and increases the signal gain[42]. Furthermore, this dual-contrast agent was also able to distinguish between dead and live cells at the site of injury. This helps in estimating the percentage of MSCs survival rate, as Gadolinium dependent positive contrast is expunged in the live cells, whereas enhanced contrast level found in dead cells[42].
MGIO particles were studied to track human fetal MSCs through using 1.5T MRI[43]. MGIO particles were found to achieve high detection sensitivity with low cellular toxicity through a simple incubation protocol, which makes them useful for cellular tracking using standard MRI scanners[43]. These results were similar to that reported by Mailänder et al[44] using adult BM-MSCs[44]. The tracking efficacy achieved by MGIO was higher than that achieved with USPIO particles and the larger polystyrene particles[43]. Extracellular vesicles (EVs) are secreted lipid bilayered vesicles containing enzymes, nucleic acids and lipoproteins that are involved in intercellular communication. MSCs can activate various repairing machineries by secreting EVs[45]. SPIONs were also used to facilitate the labelling and imaging of EVs derived from MSCs. Dabrowska et al[46] labeled these EVs derived from MSCs using the fluorescent lipophilic stain PKH26 and SPION nanoparticles conjugated with rhodamine (Molday ION Rhodamine B™) which was found to be highly biocompatible with EVs to be imaged using MRI[46]. The prospective use of MNPs in MSCs tracking is highly encouraging. MRI and MNPs are complementary and provide integrated information, like tracking and monitoring MSCs transplanting and engulfing overtime, and this will provide more information to guide further therapy.
Most of MSCs curative applications require injecting these cells directly to the injured tissues or delivering them intravenously, which requires their migration and homing in the damaged tissues[47,48]. MSCs homing is one of the major challenges in clinical settings because only a small percentage of delivered MSCs reaches the desired injury site and integrates with host tissues, while the majority of the administrated cells are trapped in the draining organs and get washed.
Recently, MNPs have been used to improve the homing percentage of transplanted MSCs at the site of injury[49]. Among all nanoparticles, SPIONs are the most extensively used nanomaterials to increase MSCs homing tendency without affecting their viability, proliferation and differentiation[31,32]. MSCs labeled with SPIONs exhibit enhanced homing due to magnetic attraction[50]. Several research groups have investigated the homing and tracking of MSCs after being labelled with SPIONs. Meng et al[51] used SPIONs and green fluorescent protein (GFP) reporter gene to create a double labelling of Wharton’s Jelly human umbilical cord-derived MSCs (WJ-MSCs)[51]. These cells were injected to a nude mouse with cutaneous tissue injury. In this work, they used 25 μg/mL of SPION, and they divided the nude mice into three groups: The first group treated with WJ-MSCs only, the second group treated with GFP/SPIONs-positive WJ-MSCs, and the third group treated with SPIONs/GFP-positive WJ-MSCs and exposed to an external magnetic field (0.5T)[51]. In all three groups, MSCs were injected subcu
A recent study by Braniste et al[52] in which they created a semiconductor nanoparticle by combining nanometer scale GaN thin layers with a sacrificial zinc ferrite core (ZnFe2O4)[52]. Braniste et al[52] incubated MSCs with (10 mg/mL) semiconductor nanoparticles and applied a remote magnetic field to control the direction of their movement. They found that these semiconductor nanoparticles were effectual to redistribute and rearrange MSCs according to the remote magnetic field intensity, thus enhanced the long term tracking and monitoring of the injected cells in vivo[52].
Silva et al[53] fabricated gold and maghemite nanoparticles that were functionalized with 2,3-dimercaptosuccinic acid (DMSA) (Au-DMSA and γ-Fe2O3-DMSA)[53]. These nanoparticles were incubated with human MSCs and these labelled MSCs were inoculated through intranasal route and tracked using standard computed microtomography. Despite the high biocompatibility of these nanoparticles with MSCs, γ-Fe2O3-DMSA and Au-DMSA based contrast was not strong enough for tracking MSCs in vivo by standard computed microtomography[53]. An innovative iron-doped hydroxyapatite nanoparticles (FeHA NPs) were prepared by Panseri et al[54] and were found to be superior to SPIONs in improving the survival of MSCs due to rapid degradation and lower resulting intracellular iron content[54]. The unique magnetic properties of FeHA NPs make them a suitable carrier for delivering MSCs to the injury site and other therapeutically active products such as drugs, growth factors, and miRNA[54].
Moayeri et al[55] used a poly-L-lysine hydrobromide coated SPIONs to label adipose-derived stem cells (ADSC-SPION/PLL)[55]. These labeled ADSCs were injected in the medial forebrain bundle in a rat model of Parkinson’s disease (PD), and simultaneously an external magnetic field were placed on the top of rat skull for 2 wk[55]. The results of this study showed a significant improvement in the migration and homing of these labeled ADSCs in the damaged sites of substantia nigra[55]. These abovementioned studies provided strong evidence about the importance of these non-toxic and biocompatible MNPs in potentiating the homing percentage of transplanted MSCs which may improve the successful rate of MSCs transplantation in different disease models.
Migration and subsequent engraftment following the infusion of MSCs are essential to enkindle the regenerative power of MSCs. The desultory, undirected movement of MSCs and poor accumulation at the injured site can hinder their therapeutic abilities. It has been found that MNPs can improve the migratory features of MSCs and directed them to the target site[56]. Dextran-coated iron oxide nanoparticles have been reported by Chung et al[57] to boost MSCs migration and the subsequent trans-differentiation into dopaminergic like neurons in a mouse model of PD[57].
Li et al[33] also examined the in vitro migration of rat BM-MSCs to an injury site in the presence or absence of polydopamine (PDA)-capped Fe3O4 (Fe3O4@PDA) superparticles[33]. The results showed a significant difference in the number of migrated cells between control MSCs and MSCs labeled with these superparticles[33]. Iron oxide nanoparticles were also found to increase the number of MSCs in the S-phase, their proliferation index, migration ability and secretion of vascular endothelial growth factor[47]. This suggests that labeling with iron oxide nanoparticles increased MSCs migration, while the cell cycle progression was unaffected. It was also demonstrated that labeling MSCs with Fe3O4@PDA NPs increase their migration towards laser burn injury sites in a living rat model, as well as their expression of CXCR4[47]. The latter could explain the increased migration ability of labeled MSCs. Indeed, previous studies had showed that the migration process is heavily dependent on the interaction between SDF-1α and CXCR4, and the internalization of magnetic iron oxide nanoparticles elevates CXCR4 levels in MSCs[58,59]. Furthermore, SPIONs have been found to activate the hepatocyte growth factor/tyrosine-protein kinase Met pathway in MSCs to regulate their migratory and engraftment properties[60].
The superparamagnetic properties of MNPs are not only suitable for improving the homing and migration properties of MSCs, studies found that MNPs can potentiate the MSCs survival and differentiation[61,62]. Several studies demonstrated a substantial enhancement of MSCs differentiation when these cells are combined with magnetic iron oxide nanoparticles, magnetic field and a specialized differentiation medium. MNPs improve the engraftment of MSCs at the injury site which is an essential step to adopt the cellular and molecular machinery required to initiate the differentiation to committed cell type[63-66]. MNPs can be also used to enhance the quality of MSCs cryopreservation and survival after thawing these cells[67]. Naseroleslami et al[68] transplanted a SPIONs-labelled human-derived MSCs (hAMSCs) in a rat model of isoproterenol-induced myocardial injury[68]. They reported that SPIONs-labeled hAMSCs produce a remarkable activation of cardiac repair machinery in the presence of magnetic field through suppressing nuclear factor-kappaB/mitogen-activated protein kinases dependent inflammation[68]. Zhang et al[69] reconstructed a Fe3O4 MNPs by adding graphene oxide (GO) to generate Fe3O4@GO magnetic nanocomposites (MNCs) that were loaded with bone morphogenetic protein-2 (BMP2)[69]. This Fe3O4@GO MNCs were able to mitigate the cell damage caused by oxidative stress and through delivering BMP2, they also improved the osteogenic differentiation abilities of MSCs[69]. Wang et al[70] created a magnetic lanthanum-doped HA/CS scaffolds (MLaHA/CS)[70]. They found after placing the MLaHA/CS scaffolds into rats with calvarial defects, it significantly enhances the recruitment of endogenous MSCs and facilitated regeneration of new bone matrix[70]. The dose of internalized MNPs found to have a great influence on the preferential differentiation of MSCs. When less than 10 pgFe/cell was used, the differentiation of MSCs into chondrocytes, adipocytes or osteocytes using citrate-coated maghemite nanoparticles was similar to that of control unlabeled cells[71]. On the other hand, when higher dose of 30 to 60 pgFe/cell was used, the chondrogenesis was significantly turned off while the adipogenesis and osteogenesis were turned on. Intriguingly, the source of MSCs may also govern their response to certain MNPs[72]. Labusca et al[73] showed some discrepancies in the response of ADSCs and WJ-MSCs to uncoated MNPs, with average size of 20 nm[73]. When external magnetic field was applied, the chondrogenic differentiation was more pronounced in the ADSCs cell culture but not in WJ-MSCs cell culture[73]. The possible explanation for these findings was the presence of an active senescent protective mechanism in WJ-MSCs. Fan et al[74] studied the differences in intracellular iron content, labeling efficiency, cell viability, and Adipogenic and osteogenic differentiation potentials between AD-MSCs and BM-MSCs after labelling them with SPIOs. They found that SPIO-labeled AD-MSCs and SPIO-labelled BM-MSCs were similar in their labeling efficiency, intracellular iron level, survival, proliferation, differentiation potentials, and MRI imaging[74]. Since the presence of an external magnetic field can dictate the differentiation fate of MSCs, the same group of investigators, Labusca et al[75], also studied the effect of duration, intensity and frequency of magnetic field on the differentiation abilities of ADSCs labeled with MNPs[75]. These scientists revealed that using an intermittent low intensity magnetic field (0.5 MT) for short time (2 d) triggered their differentiation to adipocytes, while applying intermittent high intensity magnetic field (21.6 MT) for short time (2 d) or continuous low intensity magnetic field (0.5 MT) for longer time (7 d) activated the osteogenic machinery[75]. Wang et al[76] injected SPION-labeled ADSCs in a rat model of stress urinary incontinence. These magnetically labeled MSCs found to have a high survival rate post-transplantation and efficiently enhanced the repairing process of the non-functional sphincter[76]. In the similar context, Xu et al[77] showed that UC-MSCs labelled with SPIONs can tolerate the inflammatory microenvironment in mouse model of sepsis by enhancing their immunomodulatory abilities and the expression of heme oxygenase-1 and tumor necrosis factor receptor-associated factor (TRAF1)[77]. These findings highlighted the advantageous outcomes of incorporating MNPs with MSCs therapy which may ultimately potentiate the success rate of MSCs transplantation and increase the chance to shift these cells toward bedside. Future studies should be designed to extensively investigate the long-term efficacy and safety of these MNPs labeled MSCs, and in parallel clinical trials must be conducted to reveal the translational possibilities of these MNPs–labeled MSCs. Table 1 summarizes the different studies that used MNPs to improve the transplantation characteristics of MSCs. Combining Nanotechnology with MSCs opens new avenues to enhance their therapeutic outcomes and long-term regenerative abilities. The incorporation of MNPs with MSCs has been extensively investigated and it revealed great chances to increase their survival, promote their homing and retention at the site of injury, improve their tolerance to stress microenvironments and enhance their integration with host tissues and trigger their differentiation. The use of MNPs with MSCs still in need for further investigation to answer many concerns surrounding their combination. Some of these concerns are related to assessing the safety profile of MNPs on the long-run, determining the optimal non-toxic dose that can be added to MSCs based on the type of pathology and the ultimate target to be achieved, finding the best coating substrate to be used with MNPs without affecting their therapeutic functions, exploring the possibility of combining more than one MNPs for synergistic effects, finding the exact molecular mechanisms that are exerted by MNPs to alter the cellular pathways in MSCs, and studying the impact of the internal microenvironment which varies based on and the type of disease in influencing the uptake of MNPs by MSCs and their ultimate response. Future studies should also focus on addressing the role of MNPs in solving other MSCs therapy challenges including cellular heterogeneity which highly depends on the source of MSCs and the culturing procedures being used, the undesirable pre-transplantation differentiation, and the switch in their immunological characteristics under stress microenvironments. A Schematic summary depicted the role of MNPs in improving the transplantation and biological characteristics of MSCs can be found in Figure 1.
| No. | Ref. | Magnetic nanoparticle | Source of MSCs | Application | Outcomes |
| 1 | Maggio et al[78], 2016 | Iron MNP with poly(epsilon-lysine) dendrons exposing carboxybetaine residue (CB-MNP) | hBM-MSCs | Viability and differentiation | Survival, Adipogenic and osteogenic differentiation were significantly improved |
| 2 | Hu et al[79], 2021 | 3D printing Magnetic nanoparticles scaffold made from Ferumoxytol (γ-Fe2O3@PSC) and polylysine | AD-MSCs | Bone tissue engineering and Osteogenesis | Upregulated the MAPK signaling and PI3K-Akt signaling and increased the levels of RUNX2, ALP and SMAD 1/5/8 which promoted the Osteogenic differentiation |
| 3 | Huang et al[80], 2017 | Magnetic nanoparticle composite scaffold formulated using the magnetic nanoparticles Fe2O3, Nano-hydroxyapatite and l-polylactic acid | BM-MSCs | Osteogenic differentiation of MSCs | The expression of type I collagen gene increased in MSCs with noticeable enhancement in their Osteogenic differentiation without toxic effects |
| 4 | Andrzejewska et al[30], 2019 | Molday ION Rhodamine B™ | hBM-MSCs | Tracking of transplanted MSCs | Basic hBM-MSC characteristics and functions might be affected by labeling. Molday ION Rhodamine B™ labeling had a better profile than other vital stains |
| 5 | Kono et al[81], 2021 | Magnetic anionic liposome/atelocollagen complexes | mBM-MSCs | Sarcopenia mouse model | Magnetized MSCs have higher retention rate in the skeletal muscles after their local injection with significant enhancement in their immunomodulation abilities marked by upregulating IL-6 and IL-10 and downregulating TNF-α and IL-1β in the inflamed skeletal muscle which may be useful for effective Sarcopenia treatment |
| 6 | Guldris et al[35], 2017 | (1) SPIO-PAA; (2) USPIO-PAA; and (3) USPIO-PAA-GlcN | Rat MSCs | Cell tracking by MRI | SPIO-PAA combined with polylysine showed non-homogeneous cell internalization. USPIO-PAA showed no uptake. USPIO-PAA-GlcN featured high cellular uptake, bio-compatibility, and sensitive in vitro and in vivo |
| 7 | Lee et al[36], 2010 | MGIO | Primary endothelial progenitor cells | In vivo tracking of stem cells after transplantation | MGIO is an efficient label for the studying of relaxation induced by magnetic particles and cellular tracking by MRI |
| 8 | Thu et al[37], 2012 | Self-assembling ferumoxytol- HPF nanocomplexes | (1) Hematopoietic stem cells; (2) Bone marrow stromal cells; and (3) Neural stem cells | Cell tracking by MRI | HPF labeling facilitates the monitoring of infused or implanted cells by MRI |
| 9 | Unterweger et al[82], 2017 | Dextran-coated SPIONDex | Human endothelial and monocytic cells | MRI imaging | SPIONDex are extremely safe and represents a promising candidate for further clinical development |
| 10 | Han et al[83], 2021 | 3D-printed poly(lactic-co-glycolic acid) scaffolds coated with IONPs | rBM-MSCs | Rat Calvarial bone defect model to investigate Osteogenic differentiation | Increased the adhered cell number, and promoted cell spreading by upregulating the expression of integrin α1 and β1 and their downstream signaling molecules FAK and ERK1/2. ALP levels and Osteogenesis also significantly increased |
| 11 | Lee et al[43], 2009 | MGIOs | Human fetal mesenchymal stem cells | MSC tracking by MRI | The use of M600 particles may be useful for cellular tracking using MRI |
| 12 | Mailänder et al[44], 2008 | Carboxylated superparamagnetic iron oxide particles | MSC | Monitor trafficking of transplanted MSCs cells by MRI without transfection agents | Feasibility and efficiency of labeling MSC with SPIONs was determined |
| 13 | Dabrowska et al[46], 2018 | Superparamagnetic iron oxide nanoparticles conjugated with rhodamine (Molday ION Rhodamine B™) | Human bone marrow MSCs EVs | Imaging of EVs | Molday ION is biocompatible with EVs. Labeling did not interfere with the capability of EVs to re-enter hBM-MSCs. IONs have magnetic properties useful for imaging by MRI |
| 14 | Li et al[59], 2019 | Fe3O4@PDA | Rat bone marrow-derived MSCs | Migration and homing of MSCs | Iron oxide nanoparticles increased the expression of CXCR4 in MSCs and improved their homing and ant-inflammatory abilities |
| 15 | Yun et al[48], 2018 | SPIONs with rhodamine B | Mouse bone marrow-derived MSCs | Enhanced homing effect in a model of olfactory injury | SPIONs-labeled MSCs produced better homing effects of MSCs in vivo |
| 16 | Meng et al[51], 2017 | SPIONs (Molday ION Rhodamine B™) | WJ-MSCs | Gene carrying into cutaneous injury sites | Exposure to an external magnetic field increases transportation of SPIONs-labeled WJ-MSCs in vivo |
| 17 | Braniste et al[52], 2020 | ZnFe2O nanoparticles based on iron covered with a chemically stable crystalline GaN film | Rat bone marrow MSCs | Long term monitoring of tracked MSCs | These nanoparticles are compatible with MSCs. Increasing concentrations of nanoparticles inhibit proliferation of MSCs. GaN growth on zinc ferrite nanoparticles increases the chemical stability of the material |
| 18 | Silva et al[53], 2016 | Gold and maghemite nanoparticles functionalized with DMSA: (1) Au-DMSA; and (2) γ-Fe2O3-DMSA | Dental pulp derived MSCs | Tracking of MSCs in vivo | γ-Fe2O3-DMSA and Au-DMSA can be used as tracers for MSCs. Au-DMSA is not suitable for visualization and tracking. γ-Fe2O3-DMSA is a promising agent for MSC magnetic targeting |
| 19 | Moayeri et al[55], 2020 | PLL hydrobromide coated SPIONs | Rat ADSC | Delivery and homing of transplanted MSCs in the target tissue | Transfection of ADSC by SPION/PLL is an appropriate protocol for cell therapy |
| 20 | Chung et al[57], 2018 | Dex-IO NPs | hMSCs | Accelerate and optimize MSC therapeutics for Parkinson disease | NPs enhance the migration of hMSCs toward damaged DA-like cells, induce hMSCs to differentiate to DA-like neurons and promote the protection/regeneration effects of hMSCs |
| 21 | Li et al[84], 2020 | Fe3O4@PDA NPs | Mouse bone marrow MSCs | Optimization of MSC-based therapeutic strategies for burn wound healing | NPs effectively incorporated into the MSCs without negative effects on cell properties and enhanced their migration ability |
| 22 | Dai et al[61], 2019 | MIONs | mESCs | Induction of neural differentiation of stem cells | MIONs promoted the differentiation of the embryonic stem cells into nerve cells |
| 23 | Hachani et al[85], 2017 | 3,4-dihydroxyhydrocinnamic acid (DHCA) functionalized IONPs | hBM-MSCs | Imaging and contrast | It was significantly phagocytized by MSCs and produced significant contrast enhancement for proper tracking |
| 24 | Daquinag et al[66], 2013 | Iron oxide (Fe2O3) and gold (Au) nanoparticles cross-linked with PLL | WAT ASC | WAT transplantation applications and WAT-based cell therapy | This NP-based 3D methodology potentially enhance WAT transplantation efficacy |
| 25 | Wang et al[67], 2016 | Superparamagnetic Fe3O4 nanoparticles | hUCM-MSCs | Long-term banking of living cells | Magnetic induction heating in a magnetic field with Fe3O4 nanoparticles facilitates rewarming and cryopreservation outcome of hUCM-MSCs |
| 26 | Naseroleslami et al[68], 2021 | SPIONs | hUCM-MSCs | Protection against myocardial injury | SPION-labeled MSCs in the presence of magnetic field reduces inflammation following myocardial injury |
| 27 | Zhang et al[69], 2020 | Fe3O4@GO MNCs | Rat bone marrow mesenchymalstem cells | Bone tissue regeneration | Fe3O4@GO MNCs reduced cell damage caused by ROS, improved the activity of MSCs and promote osteogenic differentiation |
| 28 | Hamid et al[86], 2022 | Combining Static Magnetic field with Samarium Cobalt (SmCO5) | hUC-MSCs | Proliferative properties o MSCs | Enhancement of MSCs proliferation without changing their stemless and immunophenotype |
| 29 | Van de Walle et al[72], 2019 | Citrate coated iron oxide (maghemite) nanoparticles | hBM-MSCs | The long-term intracellular fate of MNP in MSCs and differentiation status | Intracellular de novo synthesis of magnetic nanoparticles was demonstrated due to the overexpression of H-subunit of ferritin. This process could prevent long-term cytotoxicity and enhance MSCs differentiation |
| 30 | Labusca et al[73], 2021 | Fe3O4 MNP | (1) Human primary adipose derived MSCs; and (2) hWJMSCs | Cartilage engineering | Exposure to magnetic field increases ADSC-MNP chondrogenesis in ADSC, but not in WJMSC |
| 31 | Labusca et al[75], 2020 | Fe3O4 magnetite MNP | Primary human ADSCs | Treatment of osteoporosis | Parameters of magnetic field and the exposure way interfere with ADSCs differentiation in terms of adipogenic and osteogenic conversion. |
| 32 | Ishmukhametov et al[87], 2022 | Citrate-stabilized MNPs that are Functionalized with calf thymus DNA solution (50 μg/mL) and immobilized on glass surface | Human ADSCs | Differentiation of MSCs | Enhanced the Chondrogenesis and Osteogenesis in hTERT-transduced MSCs and the use of glass surface increased the chondrogenesis rate and reduced the need to high level of growth factors in the differentiation medium |
| 33 | Hao et al[88], 2021 | Magnetic Scaffold made from Chitosan, Laponite and Fe3O4 | hUC-MSCs | Proliferation and Osteogenesis | Enhanced the proliferation of hUC-MSCs and increased Osteogenesis markers; ALP, OCN and type I collagen |
| 34 | Zhang et al[89], 2022 | 3D magnetic scaffolds fabricated by incorporating MNPs into electrospun gelatin nanofibers coated with either citric acid or polyvinylpyrrolidone | BM-MSCs | Osteogenesis and Chondrogenesis | Chondrogenesis-related genes COL2A1 and ACAN were selectively enhanced by magnetic scaffolds with citric acid-coated MNPs (CAG). Osteogenesis-related genes (RUNX2 and SPARC were selectively upregulated by magnetic scaffolds with polyvinylpyrrolidone-coated MNPs |
| 35 | Ohki et al[90], 2020 | SPIO and USPIO | hUC-MSCs | Labelling, Proliferation and differentiation | Remarkable increase in the signal intensity, proliferation and three-lineage differentiation (Osteogenesis, Adipogenesis, and Chondrogenesis) |
| 36 | Theruvath et al[91], 2021 | Ferumoxytol and Ascorbic acid | BM-MSCs | Knee cartilage regeneration in minipigs | Hyaline-like cartilage regeneration in the knee joints of minipigs and improved Chondrogenesis were observed with significant upregulation in the amount of collagen type II |
| 37 | Xu et al[77], 2021 | SPIOs | hUC-MSCs | Survival and Immunomodulation in Mouse Sepsis model | Enhanced the survival and immunomodulatory abilities of MSCs by increasing the levels of HO-1 and TRAF1 and promoted the polarization of macrophages to the M2 type. This was found to improve the liver- related injury in Sepsis |
| 38 | Liu et al[92], 2021 | Fe3O4@PDA | hUC-MSCs | Homing and differentiation in rat model of Sciatic Nerve Chronic Compression Injury | Fe3O4@PDA-labeled MSCs showed better homing to the spinal cord under magnetic field guidance and decreases decreased spinal nerve demyelination and c-Fos expression |
The regenerative abilities of MSCs have been thoroughly investigated and discussed. Despite the great improvement in understanding the curative mechanisms of MSCs, many challenges are still there which slow down the transferring of these cells in the treatment guidelines. Loss of tracking signal, poor migration and homing to the injury site, and undesirable differentiation are the most reported hurdles that thwart the therapeutic outcomes of MSCs in clinical trials. The new strategy of combining MSCs with MNPs has been proven to boost the success rate of MSCs transplantation. MNPs have been employed as an effective contrast agent for long term tracking and monitoring of injected MSCs. MNPs also increase the migration and homing tendency of MSCs and enhance the committed differentiation of these cells. Future studies should be designed to investigate the long term safety profile of these MNPs and determine the suitable formulation and doses based on the specificity of each disease model and the source of MSCs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Jordan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen H, China; Xia P, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1248] [Cited by in RCA: 1126] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 3. | Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 912] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 4. | Burlacu A, Grigorescu G, Rosca AM, Preda MB, Simionescu M. Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev. 2013;22:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 6. | Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8:54-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Mollentze J, Durandt C, Pepper MS. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021;2021:9919361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 428] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 11. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1209] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 12. | Ali A, Shah T, Ullah R, Zhou P, Guo M, Ovais M, Tan Z, Rui Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front Chem. 2021;9:629054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 13. | Singh D, McMillan JM, Liu XM, Vishwasrao HM, Kabanov AV, Sokolsky-Papkov M, Gendelman HE. Formulation design facilitates magnetic nanoparticle delivery to diseased cells and tissues. Nanomedicine (Lond). 2014;9:469-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Haun JB, Yoon TJ, Lee H, Weissleder R. Magnetic nanoparticle biosensors. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Yang HW, Hua MY, Liu HL, Huang CY, Wei KC. Potential of magnetic nanoparticles for targeted drug delivery. Nanotechnol Sci Appl. 2012;5:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Majidi S, Zeinali Sehrig F, Samiei M, Milani M, Abbasi E, Dadashzadeh K, Akbarzadeh A. Magnetic nanoparticles: Applications in gene delivery and gene therapy. Artif Cells Nanomed Biotechnol. 2016;44:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Baldari S, Di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Dasari A, Xue J, Deb S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Akbarzadeh A, Samiei M, Davaran S. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett. 2012;7:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 20. | Ganapathe LS, Mohamed MA, Mohamad Yunus R, Berhanuddin DD. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry. 2020;6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 21. | Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 1007] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 22. | Suzuki M, Bachelet-Violette L, Rouzet F, Beilvert A, Autret G, Maire M, Menager C, Louedec L, Choqueux C, Saboural P, Haddad O, Chauvierre C, Chaubet F, Michel JB, Serfaty JM, Letourneur D. Ultrasmall superparamagnetic iron oxide nanoparticles coated with fucoidan for molecular MRI of intraluminal thrombus. Nanomedicine (Lond). 2015;10:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Zhu N, Ji H, Yu P, Niu J, Farooq MU, Akram MW, Udego IO, Li H, Niu X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials (Basel). 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 24. | Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells. 2008;1:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Andreas K, Georgieva R, Ladwig M, Mueller S, Notter M, Sittinger M, Ringe J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials. 2012;33:4515-4525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Cheng SH, Yu D, Tsai HM, Morshed RA, Kanojia D, Lo LW, Leoni L, Govind Y, Zhang L, Aboody KS, Lesniak MS, Chen CT, Balyasnikova IV. Dynamic In Vivo SPECT Imaging of Neural Stem Cells Functionalized with Radiolabeled Nanoparticles for Tracking of Glioblastoma. J Nucl Med. 2016;57:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Guo B, Feng Z, Hu D, Xu S, Middha E, Pan Y, Liu C, Zheng H, Qian J, Sheng Z, Liu B. Precise Deciphering of Brain Vasculatures and Microscopic Tumors with Dual NIR-II Fluorescence and Photoacoustic Imaging. Adv Mater. 2019;31:e1902504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Patrick PS, Kolluri KK, Zaw Thin M, Edwards A, Sage EK, Sanderson T, Weil BD, Dickson JC, Lythgoe MF, Lowdell M, Janes SM, Kalber TL. Lung delivery of MSCs expressing anti-cancer protein TRAIL visualised with 89Zr-oxine PET-CT. Stem Cell Res Ther. 2020;11:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int J Nanomedicine. 2015;10:1727-1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 30. | Andrzejewska A, Jablonska A, Seta M, Dabrowska S, Walczak P, Janowski M, Lukomska B. Labeling of human mesenchymal stem cells with different classes of vital stains: robustness and toxicity. Stem Cell Res Ther. 2019;10:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2902] [Cited by in RCA: 2755] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 32. | Alvarim LT, Nucci LP, Mamani JB, Marti LC, Aguiar MF, Silva HR, Silva GS, Nucci-da-Silva MP, DelBel EA, Gamarra LF. Therapeutics with SPION-labeled stem cells for the main diseases related to brain aging: a systematic review. Int J Nanomedicine. 2014;9:3749-3770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, Gu Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 34. | Frascione D, Diwoky C, Almer G, Opriessnig P, Vonach C, Gradauer K, Leitinger G, Mangge H, Stollberger R, Prassl R. Ultrasmall superparamagnetic iron oxide (USPIO)-based liposomes as magnetic resonance imaging probes. Int J Nanomedicine. 2012;7:2349-2359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Guldris N, Argibay B, Gallo J, Iglesias-Rey R, Carbó-Argibay E, Kolen'ko YV, Campos F, Sobrino T, Salonen LM, Bañobre-López M, Castillo J, Rivas J. Magnetite Nanoparticles for Stem Cell Labeling with High Efficiency and Long-Term in Vivo Tracking. Bioconjug Chem. 2017;28:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Lee ES, Shuter B, Chan J, Chong MS, Ding J, Teoh SH, Beuf O, Briguet A, Tam KC, Choolani M, Wang SC. The use of microgel iron oxide nanoparticles in studies of magnetic resonance relaxation and endothelial progenitor cell labelling. Biomaterials. 2010;31:3296-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Thu MS, Bryant LH, Coppola T, Jordan EK, Budde MD, Lewis BK, Chaudhry A, Ren J, Varma NR, Arbab AS, Frank JA. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat Med. 2012;18:463-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Unterweger H, Dézsi L, Matuszak J, Janko C, Poettler M, Jordan J, Bäuerle T, Szebeni J, Fey T, Boccaccini AR, Alexiou C, Cicha I. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: evaluation of size-dependent imaging properties, storage stability and safety. Int J Nanomedicine. 2018;13:1899-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 39. | Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueirêdo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One. 2014;9:e110722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Min KA, Shin MC, Yu F, Yang M, David AE, Yang VC, Rosania GR. Pulsed magnetic field improves the transport of iron oxide nanoparticles through cell barriers. ACS Nano. 2013;7:2161-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Prijic S, Scancar J, Romih R, Cemazar M, Bregar VB, Znidarsic A, Sersa G. Increased cellular uptake of biocompatible superparamagnetic iron oxide nanoparticles into malignant cells by an external magnetic field. J Membr Biol. 2010;236:167-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Ngen EJ, Artemov D. Advances in Monitoring Cell-Based Therapies with Magnetic Resonance Imaging: Future Perspectives. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Lee ES, Chan J, Shuter B, Tan LG, Chong MS, Ramachandra DL, Dawe GS, Ding J, Teoh SH, Beuf O, Briguet A, Tam KC, Choolani M, Wang SC. Microgel iron oxide nanoparticles for tracking human fetal mesenchymal stem cells through magnetic resonance imaging. Stem Cells. 2009;27:1921-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Mailänder V, Lorenz MR, Holzapfel V, Musyanovych A, Fuchs K, Wiesneth M, Walther P, Landfester K, Schrezenmeier H. Carboxylated superparamagnetic iron oxide particles label cells intracellularly without transfection agents. Mol Imaging Biol. 2008;10:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3959] [Cited by in RCA: 4132] [Article Influence: 413.2] [Reference Citation Analysis (0)] |
| 46. | Dabrowska S, Del Fattore A, Karnas E, Frontczak-Baniewicz M, Kozlowska H, Muraca M, Janowski M, Lukomska B. Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int J Nanomedicine. 2018;13:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Li X, Wei Z, Lv H, Wu L, Cui Y, Yao H, Li J, Zhang H, Yang B, Jiang J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int J Nanomedicine. 2019;14:573-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 48. | Yun WS, Choi JS, Ju HM, Kim MH, Choi SJ, Oh ES, Seo YJ, Key J. Enhanced Homing Technique of Mesenchymal Stem Cells Using Iron Oxide Nanoparticles by Magnetic Attraction in Olfactory-Injured Mouse Models. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Ahn YJ, Kong TH, Choi JS, Yun WS, Key J, Seo YJ. Strategies to enhance efficacy of SPION-labeled stem cell homing by magnetic attraction: a systemic review with meta-analysis. Int J Nanomedicine. 2019;14:4849-4866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Ahn YJ, Yun WS, Choi JS, Kim WC, Lee SH, Park DJ, Park JE, Key J, Seo YJ. Biodistribution of poly clustered superparamagnetic iron oxide nanoparticle labeled mesenchymal stem cells in aminoglycoside induced ototoxic mouse model. Biomed Eng Lett. 2021;11:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Meng Y, Shi C, Hu B, Gong J, Zhong X, Lin X, Zhang X, Liu J, Liu C, Xu H. External magnetic field promotes homing of magnetized stem cells following subcutaneous injection. BMC Cell Biol. 2017;18:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Braniste T, Cobzac V, Ababii P, Plesco I, Raevschi S, Didencu A, Maniuc M, Nacu V, Ababii I, Tiginyanu I. Mesenchymal stem cells proliferation and remote manipulation upon exposure to magnetic semiconductor nanoparticles. Biotechnol Rep (Amst). 2020;25:e00435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Silva LH, da Silva JR, Ferreira GA, Silva RC, Lima EC, Azevedo RB, Oliveira DM. Labeling mesenchymal cells with DMSA-coated gold and iron oxide nanoparticles: assessment of biocompatibility and potential applications. J Nanobiotechnology. 2016;14:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Panseri S, Montesi M, Iafisco M, Adamiano A, Ghetti M, Cenacchi G, Tampieri A. Magnetic Labelling of Mesenchymal Stem Cells with Iron-Doped Hydroxyapatite Nanoparticles as Tool for Cell Therapy. J Biomed Nanotechnol. 2016;12:909-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Moayeri A, Darvishi M, Amraei M. Homing of Super Paramagnetic Iron Oxide Nanoparticles (SPIONs) Labeled Adipose-Derived Stem Cells by Magnetic Attraction in a Rat Model of Parkinson's Disease. Int J Nanomedicine. 2020;15:1297-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Labusca L, Herea DD, Mashayekhi K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World J Stem Cells. 2018;10:43-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 57. | Chung TH, Hsu SC, Wu SH, Hsiao JK, Lin CP, Yao M, Huang DM. Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of Parkinson's disease. Nanoscale. 2018;10:2998-3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 58. | Dong F, Harvey J, Finan A, Weber K, Agarwal U, Penn MS. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation. 2012;126:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 59. | Li X, Wei Z, Li B, Li J, Lv H, Wu L, Zhang H, Yang B, Zhu M, Jiang J. In vivo migration of Fe3O4@polydopamine nanoparticle-labeled mesenchymal stem cells to burn injury sites and their therapeutic effects in a rat model. Biomater Sci. 2019;7:2861-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 61. | Dai R, Hang Y, Liu Q, Zhang S, Wang L, Pan Y, Chen H. Improved neural differentiation of stem cells mediated by magnetic nanoparticle-based biophysical stimulation. J Mater Chem B. 2019;7:4161-4168. |
| 62. | Du V, Luciani N, Richard S, Mary G, Gay C, Mazuel F, Reffay M, Menasché P, Agbulut O, Wilhelm C. A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Nat Commun. 2017;8:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 63. | Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-18001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 64. | Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622-9629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 422] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 65. | Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys Appl Phys. 2003;36:R167-R181. [DOI] [Full Text] |
| 66. | Daquinag AC, Souza GR, Kolonin MG. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng Part C Methods. 2013;19:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 67. | Wang J, Zhao G, Zhang Z, Xu X, He X. Magnetic induction heating of superparamagnetic nanoparticles during rewarming augments the recovery of hUCM-MSCs cryopreserved by vitrification. Acta Biomater. 2016;33:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Naseroleslami M, Aboutaleb N, Mokhtari B. Amniotic membrane mesenchymal stem cells labeled by iron oxide nanoparticles exert cardioprotective effects against isoproterenol (ISO)-induced myocardial damage by targeting inflammatory MAPK/NF-κB pathway. Drug Deliv Transl Res. 2021;11:242-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Zhang H, Li S, Liu Y, Yu Y, Lin S, Wang Q, Miao L, Wei H, Sun W. Fe3O4@GO magnetic nanocomposites protect mesenchymal stem cells and promote osteogenic differentiation of rat bone marrow mesenchymal stem cells. Biomater Sci. 2020;8:5984-5993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Wang Q, Tang Y, Ke Q, Yin W, Zhang C, Guo Y, Guan J. Magnetic lanthanum-doped hydroxyapatite/chitosan scaffolds with endogenous stem cell-recruiting and immunomodulatory properties for bone regeneration. J Mater Chem B. 2020;8:5280-5292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 71. | Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Arniego P, Ho KL, Chopp M. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003;53:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Van de Walle A, Plan Sangnier A, Abou-Hassan A, Curcio A, Hémadi M, Menguy N, Lalatonne Y, Luciani N, Wilhelm C. Biosynthesis of magnetic nanoparticles from nano-degradation products revealed in human stem cells. Proc Natl Acad Sci U S A. 2019;116:4044-4053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 73. | Labusca L, Herea DD, Emanuela Minuti A, Stavila C, Danceanu C, Plamadeala P, Chiriac H, Lupu N. Magnetic Nanoparticles and Magnetic Field Exposure Enhances Chondrogenesis of Human Adipose Derived Mesenchymal Stem Cells But Not of Wharton Jelly Mesenchymal Stem Cells. Front Bioeng Biotechnol. 2021;9:737132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Fan J, Tan Y, Jie L, Wu X, Yu R, Zhang M. Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Res Ther. 2013;4:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Labusca L, Herea DD, Danceanu CM, Minuti AE, Stavila C, Grigoras M, Gherca D, Stoian G, Ababei G, Chiriac H, Lupu N. The effect of magnetic field exposure on differentiation of magnetite nanoparticle-loaded adipose-derived stem cells. Mater Sci Eng C Mater Biol Appl. 2020;109:110652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Wang Y, Zhou S, Yang R, Rahman M, Sequeira RC, Cao N, Zhang Y, Zhao W, Fu Q. Magnetic targeting of super-paramagnetic iron oxide nanoparticle labeled myogenic-induced adipose-derived stem cells in a rat model of stress urinary incontinence. Nanomedicine. 2020;30:102281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Xu Y, Liu X, Li Y, Dou H, Liang H, Hou Y. SPION-MSCs enhance therapeutic efficacy in sepsis by regulating MSC-expressed TRAF1-dependent macrophage polarization. Stem Cell Res Ther. 2021;12:531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Maggio ND, Martella E, Meikle S, Columbaro M, Lucarelli E, Santin M, Banfi A. Rapid and efficient magnetization of mesenchymal stem cells by dendrimer-functionalized magnetic nanoparticles. Nanomedicine (Lond). 2016;11:1519-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Hu K, Yu T, Tang S, Xu X, Guo Z, Qian J, Cheng Y, Zhao Y, Yan S, Zhang H, Wan M, Du C, Feng Y, Liu Q, Gu Z, Chen B, Zhang F, Gu N. Dual anisotropicity comprising 3D printed structures and magnetic nanoparticle assemblies: towards the promotion of mesenchymal stem cell osteogenic differentiation. NPG Asia Mater. 2021;13:19. [DOI] [Full Text] |
| 80. | Huang J, Wang D, Chen J, Liu W, Duan L, You W, Zhu W, Xiong J. Osteogenic differentiation of bone marrow mesenchymal stem cells by magnetic nanoparticle composite scaffolds under a pulsed electromagnetic field. Saudi Pharm J. 2017;25:575-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Kono Y, Takegaki J, Ohba T, Matsuda K, Negoro R, Fujita S, Fujita T. Magnetization of mesenchymal stem cells using magnetic liposomes enhances their retention and immunomodulatory efficacy in mouse inflamed skeletal muscle. Int J Pharm. 2021;596:120298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Unterweger H, Janko C, Schwarz M, Dézsi L, Urbanics R, Matuszak J, Őrfi E, Fülöp T, Bäuerle T, Szebeni J, Journé C, Boccaccini AR, Alexiou C, Lyer S, Cicha I. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: a biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int J Nanomedicine. 2017;12:5223-5238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Han L, Guo Y, Jia L, Zhang Q, Sun L, Yang Z, Dai Y, Lou Z, Xia Y. 3D magnetic nanocomposite scaffolds enhanced the osteogenic capacities of rat bone mesenchymal stem cells in vitro and in a rat calvarial bone defect model by promoting cell adhesion. J Biomed Mater Res A. 2021;109:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Li X, Wang Y, Shi L, Li B, Li J, Wei Z, Lv H, Wu L, Zhang H, Yang B, Xu X, Jiang J. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnology. 2020;18:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 85. | Hachani R, Birchall MA, Lowdell MW, Kasparis G, Tung LD, Manshian BB, Soenen SJ, Gsell W, Himmelreich U, Gharagouzloo CA, Sridhar S, Thanh NTK. Assessing cell-nanoparticle interactions by high content imaging of biocompatible iron oxide nanoparticles as potential contrast agents for magnetic resonance imaging. Sci Rep. 2017;7:7850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Hamid HA, Ramasamy R, Mustafa MK, Hosseinpour Sarmadi V, Miskon A. Magnetic exposure using Samarium Cobalt (SmCO5) increased proliferation and stemness of human Umbilical Cord Mesenchymal Stem Cells (hUC-MSCs). Sci Rep. 2022;12:8904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Ishmukhametov I, Batasheva S, Rozhina E, Akhatova F, Mingaleeva R, Rozhin A, Fakhrullin R. DNA/Magnetic Nanoparticles Composite to Attenuate Glass Surface Nanotopography for Enhanced Mesenchymal Stem Cell Differentiation. Polymers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Hao M, Xiong M, Liu Y, Tan W, Cai H. Magnetic-driven dynamic culture promotes osteogenesis of mesenchymal stem cell. Bio Biopro. 2021;8:15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Zhang J, Zhang M, Lin R, Du Y, Wang L, Yao Q, Zannettino A, Zhang H. Chondrogenic preconditioning of mesenchymal stem/stromal cells within a magnetic scaffold for osteochondral repair. Biofabrication. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Ohki A, Saito S, Fukuchi K. Magnetic resonance imaging of umbilical cord stem cells labeled with superparamagnetic iron oxide nanoparticles: effects of labelling and transplantation parameters. Sci Rep. 2020;10:13684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Theruvath AJ, Mahmoud EE, Wu W, Nejadnik H, Kiru L, Liang T, Felt S, Daldrup-Link HE. Ascorbic Acid and Iron Supplement Treatment Improves Stem Cell-Mediated Cartilage Regeneration in a Minipig Model. Am J Sports Med. 2021;49:1861-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Liu M, Yu W, Zhang F, Liu T, Li K, Lin M, Wang Y, Zhao G, Jiang J. Fe3O4@Polydopamine-Labeled MSCs Targeting the Spinal Cord to Treat Neuropathic Pain Under the Guidance of a Magnetic Field. Int J Nanomedicine. 2021;16:3275-3292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |