Published online Jul 26, 2022. doi: 10.4252/wjsc.v14.i7.453
Peer-review started: March 17, 2022
First decision: April 18, 2022
Revised: May 2, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: July 26, 2022
Processing time: 130 Days and 15.7 Hours
The use of mesenchymal stem-cells (MSC) in cell therapy has received considerable attention because of their properties. These properties include high expansion and differentiation in vitro, low immunogenicity, and modulation of biological processes, such as inflammation, angiogenesis and hematopoiesis. Curiously, the regenerative effect of MSC is partly due to their paracrine activity. This has prompted numerous studies, to investigate the therapeutic potential of their secretome in general, and specifically their extracellular vesicles (EV). The latter contain proteins, lipids, nucleic acids, and other metabolites, which can cause physiological changes when released into recipient cells. Interestingly, contents of EV can be modulated by preconditioning MSC under different culture conditions. Among them, exposure to hypoxia stands out; these cells respond by activating hypoxia-inducible factor (HIF) at low O2 concentrations. HIF has direct and indirect pleiotropic effects, modulating expression of hundreds of genes involved in processes such as inflammation, migration, proliferation, differentiation, angiogenesis, metabolism, and cell apoptosis. Expression of these genes is reflected in the contents of secreted EV. Interestingly, numerous studies show that MSC-derived EV conditioned under hypoxia have a higher regenerative capacity than those obtained under normoxia. In this review, we show the implications of hypoxia responses in relation to tissue regeneration. In addition, hypoxia preconditioning of MSC is being evaluated as a very attractive strategy for isolation of EV, with a high potential for clinical use in regenerative medicine that can be applied to different pathologies.
Core Tip: Mesenchymal stem-cells (MSC)-derived EV have a high therapeutic interest. The composition of extracellular vesicles (EV) depends on the state of source cells, generating physiological changes in recipient cells. MSC culture preconditioning affects the cargos of EV. Thus, hypoxia exposition leads to hypoxia-inducible factor induction and regulation of hundreds of genes involved in processes such as inflammation, migration, proliferation, differentiation, angiogenesis, metabolism, and apoptosis. This affects the contents of secreted EV. Accordingly, numerous studies have shown that EV from MSC under hypoxia have a higher regenerative capacity than those obtained under normoxia. Therefore, the former have a high clinical potential in different pathologies.
- Citation: Pulido-Escribano V, Torrecillas-Baena B, Camacho-Cardenosa M, Dorado G, Gálvez-Moreno MÁ, Casado-Díaz A. Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J Stem Cells 2022; 14(7): 453-472
- URL: https://www.wjgnet.com/1948-0210/full/v14/i7/453.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i7.453
Mesenchymal stem-cells or mesenchymal stromal-cells (MSC) derived from adult tissues are characterized by their low immunogenicity, high proliferation capacity, differentiation capabilities, and modulation of physiological processes such as inflammation, hematopoiesis, and angiogenesis[1-3]. MSC can be isolated from different tissues for their culture and expansion in vitro. Therefore, they are currently considered an important therapeutic tool in the field of regenerative medicine[4,5]. However, one of the main limitations of their use is the need to obtain and expand MSC in vitro, which cannot always be obtained from the same patient to be treated. Unfortunately, MSC manipulations may cause cell-functionality loss and genetic instability when performed outside their natural niches[6]. Moreover, one risk of the application of cell therapy in regenerative medicine is that MSC may remain undifferentiated and produce tumors[7].
Recently, numerous studies have shown that the regenerative capacity of MSC mainly depends on their paracrine functions. Therefore, an alternative or complement to cell therapy in regenerative medicine is the use of media enriched in cytokines and other factors secreted by in vitro cultures of progenitor cells[8]. The MSC secretome is composed by soluble factors and extracellular vesicles (EV)[9]. The main functions of EV are cell communications and interactions. Their contents depend on their cellular origin and the physiological conditions in which they are produced[10]. Therefore, preconditioning MSC under conditions that increase their regenerative power, like hypoxia, may induce the production of EV with enhanced regenerative potential. In the presence of damage, tissues normally undergo ischemic processes. These reduce the supply of O2 and nutrients to damaged areas. This causes cellular responses that induce the release of factors, promoting vessel formation and tissue regeneration[11]. Indeed, MSC preconditioning in hypoxia can induce this response, leading to the production of EV rich in angiogenic factors and inducers of tissue regeneration. In this context, the main aim of this review is to describe the effects that preconditioning in hypoxia may have on MSC, mainly in the contents of their EV, and how this strategy has a great potential for regenerative medicine.
MSC are multipotent cells first discovered by Friedenstein et al[12] in 1970. They have fibroblast-like morphology, behaving as colony-forming units-fibroblasts. These cells originate in the mesoderm, having the ability to differentiate into different cell types including osteoblasts, adipocytes, and chondrocytes[13]. The minimum characteristics that a cell must have to be considered MSC, according to the International Society for Cellular Therapy, are: (1) Adhere to plastic under standard culture conditions; (2) Exhibit several clusters of differentiation (CD): CD-73, CD-90, and CD-105, lacking CD-11b, CD-14, CD-19, CD-34, CD-45, CD-79a, and human leukocyte antigen–D-related isotype; and (3) Differentiation potency into osteoblasts, adipocytes, or chondrocytes in vitro[14]. Isolated MSC may have different origins, such as adipose tissue, placenta, umbilical-cord blood or Wharton’s jelly, synovium, periodontal ligament, menstrual blood, and bone marrow, the latter being one of the essential sources of these cells for research and clinical applications[15-17].
MSC are involved in tissue regeneration, being necessary for maintaining vital functions and delaying aging. The application of MSC in regenerative therapies is gaining great interest due to their advantages. Thus, these cells can be isolated and cultured in vitro, have the capacity to undergo multilineage differentiation, and also possess anti-inflammatory and immunosuppressive properties[5]. Indeed, such cells have great potential to treat various pathologies, including those of the nervous system, bone, skin, myocardium, and liver, among others[4,18-20]. In this regard, multiple clinical trials related to these pathologies have demonstrated the potential of MSC in human clinical practice[21-23]. Nevertheless, despite the potential and good results obtained in cell therapy, the risks involved when using cells in regenerative medicine should be considered, as indicated above. Therefore, in the last few years, cell-free therapies have gained attention, becoming the preferred options in many instances.
It is well known that MSC-based cell therapies have beneficial therapeutic effects in different pathologies. Nevertheless, some studies suggest that these benefits may not be due to the cells themselves, but mainly linked to their paracrine effects. For instance, at the site of injury[13,24,25], the EV secreted by these cells are the key players[26]. Thus, the use of MSC-derived EV has been found to be beneficial to improve cartilage repair and regeneration, cardiac repair after myocardial infarction, wound healing, and lung repair, among other clinical applications[27-30].
According to their size, biogenesis, release pathway, function, and content, EV have been classified into microvesicles, exosomes, and apoptotic bodies. Microvesicles range between 100 to 1000 nm in diameter and are formed through outward outgrowth. Exosomes are vesicles generating after fusion of multivesicular bodies with plasma membranes, ranging between 40 to 100 nm[10,31]. They should not be confused with RNA-degrading complexes with the same name found in both archaea and eukaryotes. On the other hand, apoptotic bodies are released during early apoptosis and are larger than 1000 nm[32,33]. However, there is a lack of consensus about classification and biochemical markers characterizing the different EV types. Therefore, the International Society for EV stated the following in the “Minimal Information for Studies of Extracellular Vesicles 2018” (MISEV2018), in relation to the EV nomenclature: “EV is the preferred generic term for the subject of our investigations, and subtypes should be defined by physical and biochemical characteristics and/or conditions/sources. When other terms are used, careful definition is required”[34].
EV may contain proteins, nucleic acids (including coding and non-coding RNA), lipids, and other metabolites. Normally, the content is rich in cytoskeletal proteins (such as TSG10 or CD63 tetraspanins), integrins, and major histocompatibility complex molecules[35]. Depending on cell types and microenvironments in which they are secreted, contents of EV may change. Thus, EV reflect physiological states of cells generating them. For this reason, MSC growth under different conditions, such as hypoxia, presence of trophic and physical factors, or chemical and pharmacological agents, may stimulate secretion of EV enriched in certain cytokines, growth factors, or non-coding RNA like microRNA (miRNA)[36].
In relation to the functionality of EV, at first it was thought that they were a mechanism for cells to get rid of unwanted material. It was later demonstrated that EV play a fundamental role in cellular homeostasis, being key elements in cell-to-cell communications[33,37]. Thus, these vesicles regulate different physiological processes such as cell proliferation, differentiation, and migration[38].
EV can be isolated from various sources, including blood, urine, breast milk, amniotic fluid, and synovial fluid, among others, as well as supernatants from cell cultures such as endothelial, epithelial, cancer, MSC, etc[39]. There are different purification approaches such as differential and density-gradient ultracentrifugation, ultrafiltration, size-exclusion chromatography, precipitation, immunoaffinity, and microfluidic-based methods[33]. Likewise, isolated EV can be characterized by different techniques like electron microscopy, flow cytometry, nanoparticle tracking analysis, dynamic light scattering, tunable-resistive pulse sensing, and atomic-force microscopy, among others[38,40].
Using EV in regenerative medicine has some advantages in comparison with whole-cell therapies[41] including: (1) Can be easily stored, being immediately available for clinical applications; (2) Production of large quantities of cells is not required; (3) Can be evaluated for safety, dosage, and activity in a manner similar to conventional pharmaceutical agents; (4) Are stable, exhibiting a long half-life; indeed, the lipid bilayers of their membranes protect their contents from degradation in vivo; (5) Can be more easily applied for clinical purposes than proliferative cells; for example, they can be intravenously injected, circulate through the smallest capillaries, and cross the blood-brain barrier; (6) Risks of immune rejection, cellular dedifferentiation, or tumor formation are lower than in whole-cell therapies; and (7) EV can be manipulated for more precise effects as therapeutic agents[10,41]. Therefore, the use of EV in therapy has become a great tool for regenerative medicine in recent years.
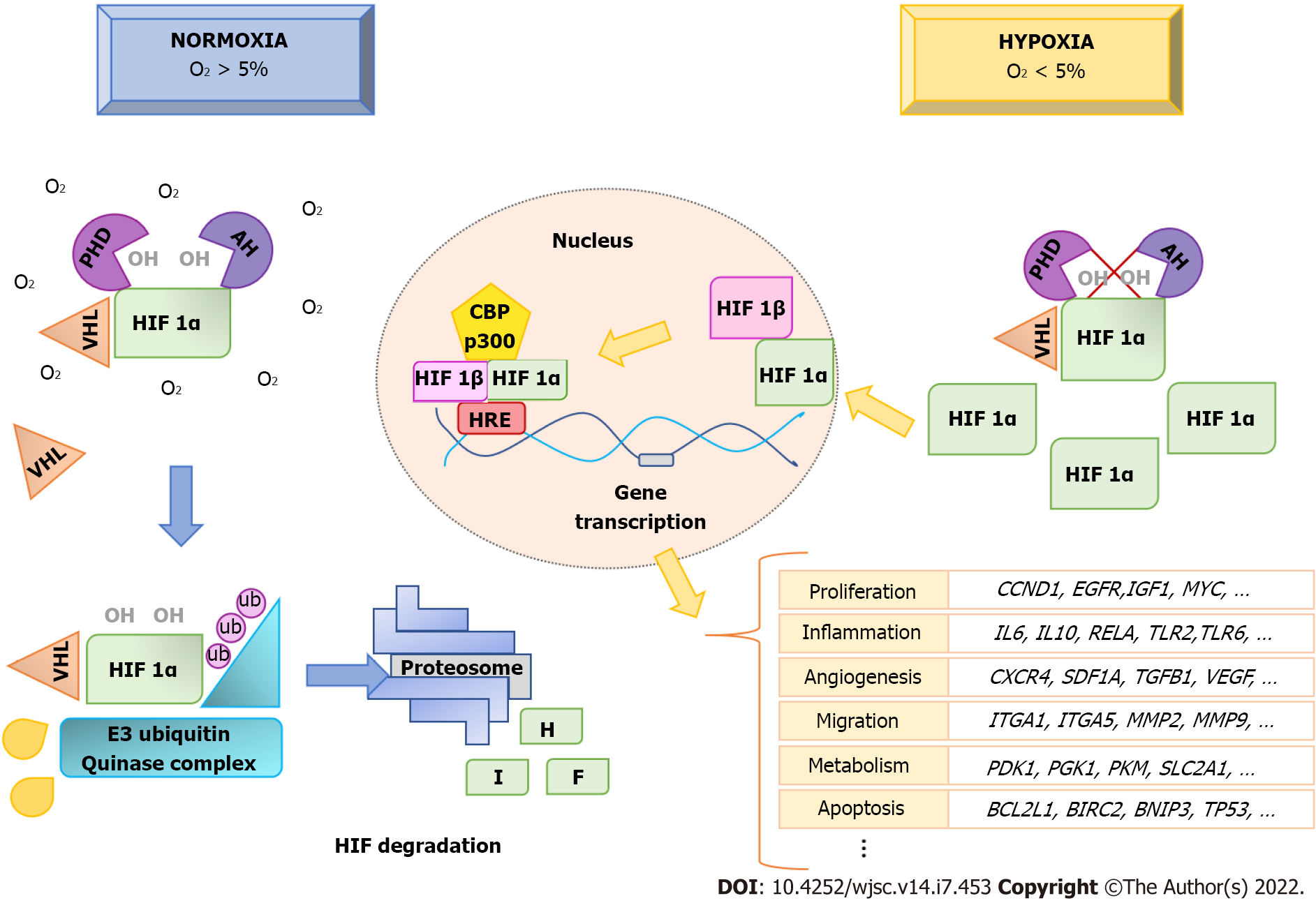
When oxygen concentrations decrease to less than 5% in tissues, cells have to adapt their metabolism and functions to such hypoxic conditions. Moderate (< 5% to > 2% O2), severe (≤ 2% to ≥ 0.1% O2), and anoxia (< 0.1% O2) are hypoxia levels equal or below 5% oxygen concentration. Depending on O2 concentration and hypoxia time, cells show different responses, as observed in human embryonic-derived MSC[42]. That occurs mainly through activation of hypoxia-inducible factor (HIF). This is a transcription factor consisting in a heterodimer of two basic helix-loop-helix proteins: Alpha (HIFA) and beta (HIFB)[43,44]. While expression of the gene encoding alpha subunits is induced by hypoxia, the gene encoding HIFB, also known as aryl hydrocarbon-receptor nuclear translocator, is constitutively expressed[45]. There are three alpha subunits (HIF1A, HIF2A, and HIF3A),with the first two well-known. HIF1A and HIF2A have 48% amino acid sequence identity and similar protein structures. Although they share functions, they can regulate the expression of different genes[46].
HIF2A, which is also known as endothelial PAS (Period, Aryl-hydrocarbon-receptor, Single minded) domain protein-1, was originally associated with endothelial development and regulation. Its encoding gene exhibits a more restricted expression relative to the one of HIF1A[47]. Furthermore, whereas HIF1A requires very low O2 concentrations for stabilization, H2FA can be activated at less severe levels of hypoxia (approximately 5%). Therefore, HIF1A would act in the initial response, whereas HIF2A would regulate the response to long periods of hypoxia[48,49]. On the other hand, HIF3A has three isoforms (HIF3A, neonatal and embryonic PAS, and inhibitory PAS protein). They inhibit the transcriptional activity of HIF1A and HIF2A by preventing their heterodimerization with HIF1B[50,51].
Under normoxia, HIF1A proteins in the cytoplasm are continuously degraded, through the proteasome pathway[52]. However, when the O2 concentration decreases, HIF1A proteins are not degraded, but rather they are accumulated and translocated into the nucleus (Figure 1). Regulation of HIF1A levels depends on the presence of an oxygen-dependent degradation domain in the protein. This domain is constituted by Fe2+ and two prolyl residues (Pro402 and Pro564). Such residues undergo hydroxylation through prolyl hydroxylases (PHD1, PHD2, and PHD3) in the presence of oxygen and α-ketoglutarate, allowing HIF1A to be recognized by the von Hippel-Lindau tumor suppressor protein, a component of the E3 ubiquitin-ligase complex. That way, it is degraded by the ubiquitin-proteosome pathway[53,54] (Figure 1).
In addition to PHD, another enzyme, called factor inhibiting HIF1 (FIH), can inhibit the transcriptional activity of HIF1A. In this case, FIH hydrolyzes residues within the C-terminal transactivation domain of HIF1A, preventing their binding to coactivators to initiate transcription in the nucleus[55]. Under hypoxic conditions, prolyl hydroxylation is inhibited, and, thus, the degradation of HIF1A is also inhibited. They accumulate and translocate into the nucleus, where they form heterodimers with HIF1B. That way, they can induce gene transcription through binding to pentanucleotide sequences (A/GCGTG) called hypoxic-response elements in the promoters of target genes. For transcription of target genes to occur, coactivators are recruited, mainly p300/CBP[56] (Figure 1).
It has been described that more than 1000 genes can be directly or indirectly regulated by HIF. These genes are involved in adaptation of cells to hypoxic conditions. They affect different physiological processes including metabolism, angiogenesis, inflammatory response, cell differentiation, migration, and apoptosis[57]. In order to present an overview of the various functions of genes regulated by HIF1α and HIF2α, we have analyzed the information contained in Qiagen Ingenuity Pathway Analysis (Qiagen IPA) web-based software application (https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa)[58]. This platform allows querying information gathered from databases and findings described in the literature for a given gene. Information from 493 references related to genes regulated by HIF1A and 215 for HIF2A were integrated in the description of functions of human HIF at the time of writing. From the information of these references, the application shows 191 genes regulated by HIF1α and 111 by HIF2α. Among them, 72 are common to both.
Functional analyses of HIF1A- and HIF2A-regulated genes with IPA show categories and functional annotations in which they are involved. Tables 1 and 2 show these data together with P values and the number of genes identified for each of the categories obtained from Qiagen IPA. Regarding the functional annotations, a maximum of the five most significant annotations in each category are shown. The list of genes for HIF1A and HIF2A obtained from such application, as well as genes corresponding to each of the categories presented in Tables 1 and 2, are shown in elsewhere (Supplementary Tables 1-
| Categories | P value | Top five functional annotations | Number of genes |
| Carbohydrate Metabolism | 3.50E-20 | Glycolysis of cells | 22 |
| Cardiovascular System Development and Function | 3.31E-15 to 4.96E-54 | Angiogenesis, Vasculogenesis, Growth of blood vessel | 84 |
| Cell Cycle | 7.42E-16 to 1.82E-38 | Binding of DNA, Cell cycle progression, Interphase, Binding of protein binding site, Arrest in interphase | 99 |
| Cell Death and Survival | 3.79E15 to 7.3E-74 | Apoptosis, Necrosis, Cell death of tumor cell lines, Apoptosis of tumor cell lines, Cell viability | 150 |
| Cell Morphology | 1.66E-16 to 3.21E-24 | Morphology of tumor cell lines, Tubulation of cells, Transmembrane potential of mitochondria, Cell spreading, Orientation of cells | 77 |
| Cell Signaling | 2.83E-19 to 1.1E-26 | Cytokine and chemokine mediated signaling pathway, Quantity of Ca2+ | 48 |
| Cell-To-Cell Signaling and Interaction | 3.31E15 to 1.99E-36 | Binding of tumor cell lines, Binding of blood cells, Adhesion of blood cells, Binding of leukocytes, Adhesion of immune cells | 105 |
| Cellular Assembly and Organization | 5.56E-16 to 1.42E-17 | Organization of cytoskeleton, Microtubule dynamics, Fibrogenesis | 52 |
| Cellular Development | 3.47-15 to 2.85E-73 | Cell proliferation of tumor cell lines, Cell proliferation of carcinoma cell lines, Cell proliferation of breast cancer cell lines, Proliferation of muscle cells, Assembly of cells | 151 |
| Cellular Function and Maintenance | 7.07E-32 | Cellular homeostasis | 67 |
| Cellular Growth and Proliferation | 9.07E-27 to 2.12E-30 | Proliferation of vascular cells, Colony formation, Proliferation of connective tissue cells, Proliferation of lymphatic system cells, Proliferation of epithelial cells | 101 |
| Cellular Movement | 3.31E-15 to 3.35E-67 | Cell movement, Migration of cells, Invasion of cells, Cell movement of tumor cell lines, Migration of tumor cell lines | 132 |
| Connective Tissue Development and Function | 1.36E-16 to 1.73E-30 | Growth of connective tissue, Quantity of connective tissue | 46 |
| DNA Replication, Recombination, and Repair | 1.67E-18 to 8.06E-31 | Synthesis of DNA, Metabolism of DNA, Degradation of DNA | 47 |
| Drug Metabolism, Lipid Metabolism, Small Molecule Biochemistry | 2.36E-16 | Synthesis of prostaglandin E2 | 16 |
| Free Radical Scavenging | 6.09E-16 to 5.5E-33 | Synthesis of reactive oxygen species, Production of reactive oxygen species, Generation of reactive oxygen species, Quantity of reactive oxygen species | 43 |
| Gene Expression | 1.373-18 to 4.01E-31 | Expression of RNA, Transcription, Transcription of RNA, Transactivation, Transactivation of RNA | 88 |
| Inflammatory Response | 5.48E-20 to 2.72E-26 | Inflammation of absolute anatomical region, Inflammation of organ, Inflammatory response, Inflammation of body cavity, Immune response of cells | 77 |
| Lipid Metabolism | 6.24E-16 to 9.37E-24 | Synthesis of eicosanoid, Metabolism of eicosanoid, Fatty acid metabolism, Synthesis of fatty acid, Synthesis of prostaglandin | 48 |
| Organismal Survival | 9.55E-37 to 2.76E-39 | Organismal death, Survival of organism | 81 |
| Post-Translational Modification | 1.86E-18 | Phosphorylation of protein | 34 |
| Protein Synthesis | 2.10E-15 | Metabolism of protein | 44 |
| Tissue Development | 1.65E-19 to 4.13E-46 | Growth of epithelial tissue, Development of epithelial tissue, Growth of nervous tissue | 62 |
| Tissue Morphology | 3.02E-17 to 3.06E-26 | Quantity of cells, Quantity of tumor cell lines | 42 |
| Categories | P value | Top five functional annotations | Number of genes |
| Carbohydrate Metabolism | 2.45E-12 to 2.81E-17 | Quantity of carbohydrate, Uptake of D-glucose, Synthesis of polysaccharide, Glycolysis, Uptake of monosaccharide | 44 |
| Cardiovascular System Development and Function | 1.00E-11 to 8.41E-31 | Angiogenesis, Development of vasculature, Vasculogenesis, Endothelial cell development, Proliferation of endothelial cells | 68 |
| Cell Cycle | 6.82E-12 to 4.39E-19 | Mitogenesis, Binding of DNA, Interphase, Arrest in interphase, Cell cycle progression | 53 |
| Cell Death and Survival | 1.08E-11 to 5.12E-36 | Cell death of tumor cell lines, Apoptosis of tumor cell lines, Cell viability, Cell survival, Apoptosis | 88 |
| Cell Morphology | 1.11E-11 to 4.76E-19 | Tubulation of cells, Morphology of tumor cell lines, Autophagy of cells, Formation of cellular protrusions, Autophagy | 67 |
| Cell Signaling | 6.16E-12 to 1.73E-12 | Quantity of Ca2+, Synthesis of nitric oxide | 29 |
| Cell-To-Cell Signaling and Interaction | 7.17E-12 to 1.36E-26 | Activation of cells, Interaction of tumor cell lines, Binding of tumor cell lines, Activation of blood cells, Binding of professional phagocytic cells | 62 |
| Cell-mediated Immune Response | 4.19E-14 to 7.70E-15 | T cell development, T cell homeostasis | 26 |
| Cellular Assembly and Organization | 1.74E-12 to 3.51E-20 | Microtubule dynamics, Organization of cytoskeleton, Organization of cytoplasm, Development of cytoplasm | 52 |
| Cellular Development | 1.10E-11 to 2.98E-39 | Cell proliferation of tumor cell lines, Colony formation of cells, Proliferation of smooth muscle cells, Cell proliferation of carcinoma cell lines, Cell proliferation of breast cancer cell lines | 89 |
| Cellular Function and Maintenance | 4.81E-13 to 6.37E-27 | Cellular homeostasis, Lymphocyte homeostasis, Function of blood cells | 63 |
| Cellular Growth and Proliferation | 8.67E-13 to 3.51E-27 | Colony formation, Proliferation of connective tissue cells, Proliferation of vascular cells, Proliferation of lymphatic system cells, Proliferation of epithelial cells | 72 |
| Cellular Movement | 9.45E-12 to 2.99E-30 | Invasion of cells, Cellular infiltration, Cell movement of myeloid cells, Migration of cells, Cell movement of tumor cell lines | 72 |
| Connective Tissue Development and Function | 1.56E-16 to 1.63E-25 | Growth of connective tissue, Quantity of connective tissue cells, Quantity of connective tissue, Inflammation of joint, Rheumatic Disease | 50 |
| DNA Replication, Recombination, and Repair | 4.08E-22 | Synthesis of DNA | 29 |
| Digestive System Development and Function | 1.42E-13 | Morphology of digestive system | 28 |
| Embryonic Development | 1.52E-12 to 1.2E-22 | Development of body trunk, Development of abdomen, Growth of embryo, Formation of lymphoid tissue, Formation of lung | 60 |
| Free Radical Scavenging | 3.11E-13 to 1.77E-17 | Metabolism of reactive oxygen species, Synthesis of reactive oxygen species, Production of reactive oxygen species | 29 |
| Hair and Skin Development and Function | 1.33E-13 | Growth of skin | 17 |
| Hematological System Development and Function | 1.17E-11 to 8.08E-21 | Quantity of blood cells, Quantity of leukocytes, Quantity of lymphocytes, Quantity of myeloid cells, Quantity of T lymphocytes | 52 |
| Inflammatory Response | 9.18E-22 to 4E-24 | Inflammation of absolute anatomical region, Inflammatory response, Inflammation of body cavity | 59 |
| Lipid Metabolism | 8.00E-12 to 3.77E-16 | Concentration of lipid, Synthesis of lipid | 41 |
| Lymphoid Tissue Structure and Development | 3.73E-18 | Quantity of lymphatic system cells | 35 |
| Molecular Transport | 7.49E-15 | Transport of molecule | 44 |
| Nervous System Development and Function | 4.54E-12 | Sensory system development | 22 |
| Organ Development, Renal and Urological System Development | 8.50E-12 to 3.25E-12 | Growth of kidney, Growth of renal glomerulus | 11 |
| Organ Morphology | 1.78E-13 | Morphology of gland | 22 |
| Organismal Development | 1.92E-12 to 7.70E-25 | Morphology of body cavity, Formation of vessel, Morphology of head, Development of genitourinary system, Growth of organism | 73 |
| Organismal Survival | 7.68E-26 to 1.24E-28 | Organismal death, Survival of organism | 75 |
| Post-Translational Modification | 4.05E-12 to 1.23E-15 | Phosphorylation of protein, Activation of protein | 31 |
| Skeletal and Muscular System Development and Function | 1.08E-11 to 1.22E-26 | Morphology of muscle, Function of muscle, Growth of smooth muscle | 39 |
| Tissue Development | 1.73E-13 to 3.23E-30 | Development of epithelial tissue, Growth of epithelial tissue, Growth of nervous tissue, Accumulation of cells, Formation of epithelial tissue | 58 |
| Tissue Morphology | 3.33E-14 to 2.65E-24 | Quantity of cells, Quantity of progenitor cells | 60 |
The importance of HIF1A in tissue regeneration has been further demonstrated using a Murphy Roths large mouse model. These animals are characterized by high basal expression of HIF1A gene which has been associated with the ability of these animals to regenerate significant ear lesions without the appearance of fibrotic areas[59]. Indeed, HIF1A induction upregulates genes such as vascular-endothelial growth factor (VEGF), stromal cell-derived factor-1 alpha protein (SDF-1A), transforming growth factor beta 1 (TGFB1), platelet-derived growth factor (PDGF), and matrix metallopeptidase 9 (MMP9), among others. All of them have important functions in healing processes. Therefore, upregulating HIF1A can accelerate wound healing. This has been observed in hyperbaric oxygen therapy (HBOT) treatments of diabetic skin ulcers. Interestingly, HBOT treatments increased HIF1A levels[60], probably due to high reactive oxygen species (ROS) concentrations produced by increased O2 in the tissue, which may inhibit PHD and FIH, thus stabilizing HIF1A. In fact, HIF1A activity is decreased in diabetics, being associated with wound healing difficulty in these patients[61].
However, if hypoxia is maintained, wounds may become chronic, and fibrotic processes may appear. This is because, among the genes regulated by the HIF pathway, there are some that encode pro-fibrotic enzymes, producing an excess of extracellular matrix. Some of these genes are related to collagen biosynthesis like collagen type–IV, V, IX and XVIII–Alpha–1 and 2–chains (COL4A1, COL4A2, COL5A1, COL9A1 and COL18A1, accordingly), and the ones encoding enzymes that produce modifications in collagen, such as procollagen PHD and lysyl hydroxylases[62].
Inflammation is the first phase activated by injuries, and hypoxia is related to inflammatory responses. Several protein-encoding genes of nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) cell complex, such as reticuloendotheliosis (REL)-Associated (RELA) proto-oncogene (transcription factor p65, also known as nuclear factor NF-kappa-B p65 subunit p65, involved in NF-κB heterodimer formation, nuclear translocation, and activation) are induced by HIF1A[63]. NF-κB is a family of transcription factors whose activation regulates different physiological processes. They include inflammatory response, as well as cell differentiation, proliferation, and survival[64]. Among the genes that NF-κB regulates is HIF1A, thus producing a reciprocal regulation[65]. The HIF1A proteins also induce expression of genes encoding proteins belonging to the Toll-Like Receptor (TLR) family. Thus, it enhances the activation of NF-κB[66]. This is because TLR have the capacity to recognize pathogen-associated molecules, inducing immune responses through activation of transcription factors, such as NF-κB[67].
Upon injury, the resulting hypoxia promotes macrophage recruitment, through regulation of Sphingosine 1-Phosphate (S1P) levels. It acts as a signal for recruitment, activation, differentiation, and polarization of macrophages[68]. This may be mediated by induction of expression of genes such as sphingosine kinase 1. this gene is involved in the last step of S1P synthesis. It has been described that HIF1A and HIF2A act on M1 and M2 macrophages through different pathways. While HIF1A induces the gene encoding inducible nitric-oxidase synthase, HIF2A acts through arginase-1, maintaining nitric oxide homeostasis during inflammation. In the case of HIF1A, its overexpression induces glycolysis metabolism, resulting in macrophage polarization to M1 (proinflammatory)[69]. However, although HIF2A has also been associated with the M1 phenotype, other studies have shown that it may promote anti-inflammatory and pro-resolving/regenerative M2 macrophages[70]. HIF1A may also produce immunosuppression through induction of Programmed Death-ligand 1 (PD-L1) encoding gene (CD274). Binding of PD-L1 to its programmed cell death protein 1 receptor on activated T cells inhibits immunity by counteracting T cell-activating signals[71].
Adaptation to hypoxia also requires metabolic changes. Cells must reduce mitochondrial oxygen consumption. In this sense, glycolysis is activated as the only way to produce adenosine triphosphate (ATP) under such hypoxic conditions. Not surprisingly, HIF1A upregulates genes related to glucose metabolism. Among them is solute carrier family 2 member A1, encoding glucose transporter-1, necessary for glucose uptake by cells[72]. Also, the genes encoding phosphoglycerate kinase 1 and pyruvate kinase M1/2 are transcriptionally upregulated by HIF1A[73,74]. Additionally, in the adaptation to hypoxia, the tricarboxylic acid (TCA) (or Krebs cycle) must be suppressed to prevent accumulation of ROS in mitochondria. For this purpose, HIF1A induces the gene encoding pyruvate dehydrogenase (PDH) kinase 1. This inactivates PDH, which is responsible for converting pyruvate to acetyl-CoA in the TCA[75].
In response to hypoxia caused by tissue damage, cells produce angiogenic factors to induce generation of vessels, restore oxygen levels, and increase nutrient delivery. HIF1A and HIF2A induce expression of genes encoding these factors. Among them, VEGF, SDF-1A, C-X-C chemokine-Receptor type 4 (CXCR4), angiopoietin-2 (ANG-2), PDGF, and TGFB[76] stand out. These factors favor endothelial-cell proliferation, differentiation, and migration for vessel formation. That also involves the mobilization and recruitment of endothelial progenitor cells (EPC) from bone marrow[61]. Mobilization of EPC is mediated by production of SDF-1 in hypoxic tissues. It acts as a chemoattractant of EPC expressing the CXCR4 receptor[77]. On the other hand, regulation of EPC migration to ischemic tissues through CXCR4/SDF1 axis is specific to HIF2A[78].
Tissue regeneration also induces cell proliferation and migration processes. HIF activation can affect cell-cycle progression due to regulation of genes such as cyclin D1 and cellular myelocytomatosis (c-MYC or MYC)[79,80]. Interestingly, while HIF1A downregulates c-MYC expression and results in cell-cycle arrest[79], HIF2A upregulates c-MYC expression, promoting cell-cycle progression and proliferation[81]. Regarding cell migration, HIF regulates genes encoding integrin–alpha and beta–1, 3 and 5 (ITGA1, ITGA5, ITGAV, ITGB3, and ITGB5, accordingly) and MMP2, MMP7, and MMP9, which are important in such processes[82]. The induction of cell migration by hypoxia is essential under physiological conditions for tissue regeneration after injury. This favors recruitment and homing of inflammatory and precursor cells, eliminating pathogens and cellular debris, further regenerating damaged tissues[68].
Hypoxia causes important changes in cellular microenvironments that might condition cell viability. Therefore, another set of important genes regulated by hypoxia are related to cell survival and death. Thus, HIF1A regulates genes activators of apoptosis such as the ones encoding tumor protein p53 (TP53; antioncogene) and B-Cell Lymphoma 2 (BCL2)/adenovirus E1B 19 kDa protein-interacting protein 3[83], as well as anti-apoptotic genes, such as baculoviral inhibitor of apoptosis protein (IAP) repeat containing 2 and BCL2[83,84]. The balance of expression of these genes, and thus cell survival, will depend on the adaptation of cells to hypoxic conditions. Thus, cell survival may predominate under mild hypoxia, but apoptosis is preferentially activated under severe hypoxia[85].
MSC reside in areas of 3%-9% of oxygen tension, allowing this hypoxic niche it’s capacities for self-renewal, proliferation, migration, and ultimately, their differentiation[86,87]. Based on this, MSC in culture have been grown at low levels of oxygen to condition or acclimate them before their therapeutic use[88]. These cells exposed to hypoxic conditions activate protein kinase B (also known as Akt, name derived from Ak mouse strain with thymoma transforming tumors) or AKT signaling pathway mediated by HIF-1 activation to improve their survival and proliferation[89]. However, different modes, severity, and duration of hypoxic exposure could provoke different responses on MSC. Indeed, cells can become stressed and even undergo apoptosis under extreme (< 1.5%) oxygen levels[87]. Furthermore, if hypoxic exposures are maintained, internal energy reserves of glucose are rapidly consumed. That is due to glycolysis characteristic of MSC causing poor survival after implantation[90].
Ischemic conditions could be solved by providing glucose supplementation to hypoxic MSC. That allows them to retain their proliferative capacity and differentiation potency[91]. Therefore, survival of MSC could be improved by preconditioning them at 1%-4% O2 for 24 to 48 h prior to implantation[88]. Hypoxia could also reduce cell viability and proliferation of MSC. Nevertheless, reoxygenation processes might promote recovery of cells, enhancing expression of pro-survival genes, as well as various trophic factors[92], further promoting multipotency of MSC[93,94]. Therefore, maintenance of MSC cultures in hypoxia may influence processes such as proliferation[87,94,95], migration[87], differentiation[93,95], metabolism[87], and apoptosis[88,96], which may affect their regenerative capacity. Interestingly, cyclic hypoxic exposure, defined as periodic exposure to hypoxia interrupted by normoxic exposure or lower levels of hypoxia[97], could have positive effects on proliferation and migration abilities of MSC[98].
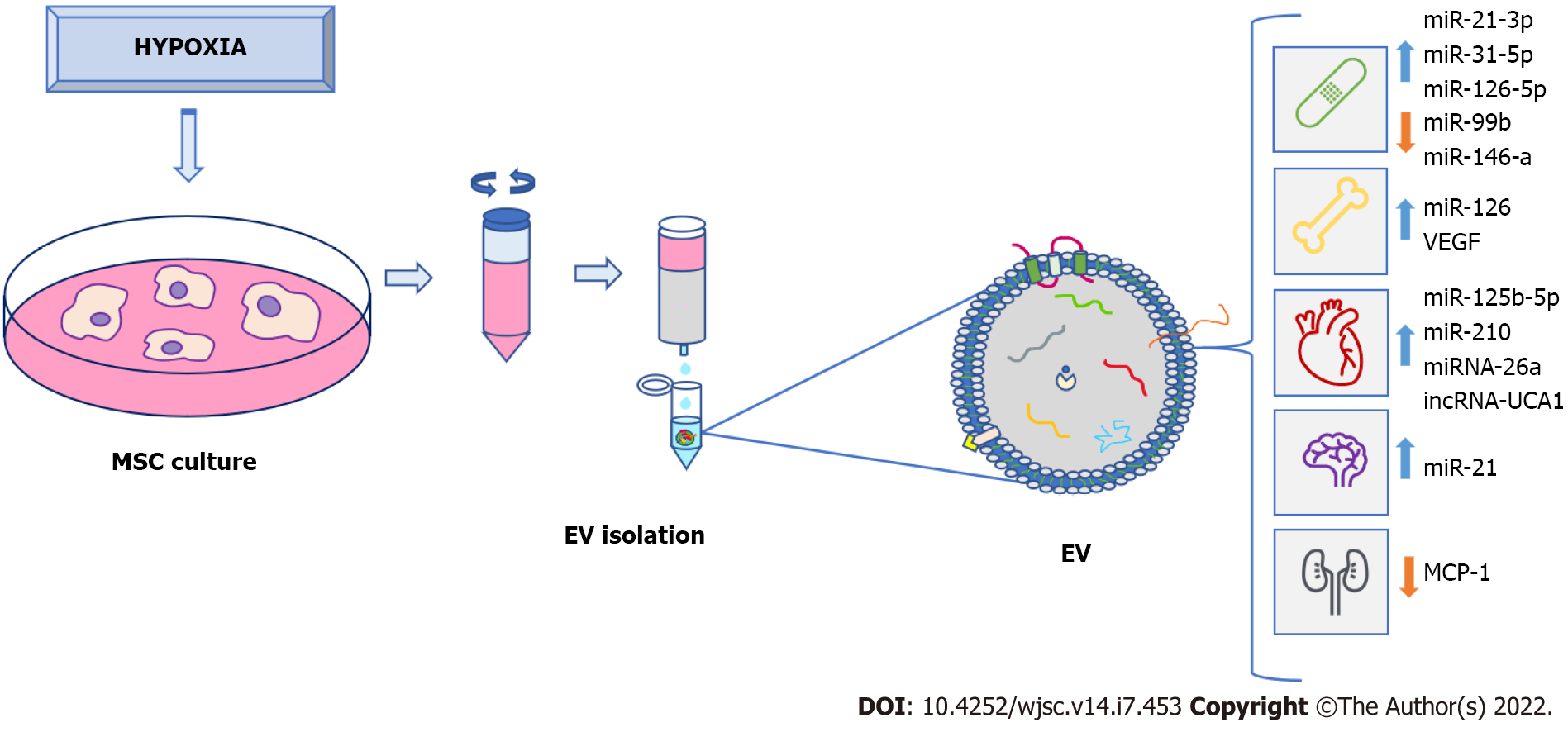
Microenvironments in which MSC are cultivated are extremely important for their proliferation, differentiation, and therapeutic potential. Factors, such as time in culture, oxygen levels, medium composition, or cell-material interactions, should be considered[99]. As indicated in previous sections, many factors induced by hypoxia are involved in processes related to tissue regeneration, such as inflammation, angiogenesis, cell proliferation, and migration[100]. Thus, priming MSC in hypoxia favors generation of EV enriched in hypoxia-induced factors. Their functions include alterations of microenvironments for tissue adaptations to low O2 concentrations[101-103]. Production and isolation of these EV for use in regenerative medicine is of great interest from a clinical point of view. Therefore, numerous studies have evaluated their potential therapeutic applications. In this scenario, time exposure and degree of hypoxia may represent relevant factors influencing contents and therapeutic properties of EV (Table 3).
| Source of MSC | Culture | Hypoxic preconditions, O2%, percentage | Time of exposition | Major findings | Ref. |
| Human umbilical-cord | α-Mem deprived of FBS | 1 | 72 h | Proangiogenic effects with an increase in UPAR, angiogenin, VEGF, IGF, Tie-2/TEK and IL-6 expression | [104] |
| α-Mem deprived of FBS | 1 | 72 h | Promoted angiogenesis in vitro and in vivo | [103] | |
| DMEM/high glucose media with 10% Exo depleted FBS and 1% penicillin/streptomycin | 1 | 48 h | Enhanced of miRNA-126 exerting a pro-angiogenic effect in endothelial cells thereby activating Spred 1/Ras/Erk pathway | [117] | |
| α-Mem 10% EV free FBS | 1 | Not defined | EV encapsulated in a hyaluronic acid adhesive hydrogel have angiogenic properties and nerve regeneration effects after traumatic spinal cord injury | [107] | |
| Olfactory mucosa | DMEM supplemented with 10% EV-depleted FBS | 3 | 48 h | Promoted angiogenesis via miR-612 transfer | [112] |
| Adipose tissue | α-Mem 10% EV free serum | 5 | 48 h | Promoted vessel formation in vitro. Enhanced angiogenesis, neovascularization and graft survival in vivo. Activation of VEGF/VEGF-R | [105,106] |
| EV depleted standard medium | 5 | 72 h | Promoted angiogenesis | [32] | |
| RPMI medium | 1 | 72 h | Promoted angiogenesis, inhibition of apoptosis, immunomodulation, intracellular ATP recovery and reduction of ROS | [122] | |
| Microvascular endothelial cell growth medium 2 media deprived of FBS with supplement of 1× serum | 1 | 24 h | Improved diabetic wound healing. Enhanced fibroblasts proliferation and migration activating PI3K/Akt pathway | [132] | |
| DMEM/F12 with 10% EV-free FBS | 0–20 (5 cycles) | Hypoxia 60 min–reoxygenation 30 min | miRNA-224-5p in EV decreases TXNIP expression in cardiomyocytes and protects them from hypoxia mediated injury | [128] | |
| Bone marrow | DMEM with low glucose containing inactivated 15% FBS | — | 12 h | Increased of miRNA-21. Synaptic dysfunction restoration, inactivation of STAT3 and NF-kB, reduced plaque deposition and amyloid-β. Regulation of inflammatory responses in APP/PS1 mouse model | [114] |
| DMEM with 10% FBS and 1% penicillin-streptomycin | 5 | 6 d | High HMGB1 expression. Activation of JNK pathway and induction of HIF-1α/VEGF expression promoting angiogenesis | [111] | |
| Exosome-depleted fetal bovine serum | 1 | 48 h | Increased exosomal levels of miRNA-216a-5p. Inhibition of TLR4/NF-κB and activation of PI3KAKT signaling pathway shifting microglial M1/M2 polarization | [115] | |
| α-Mem 10% exosomes-depleted FBS | 2 | 48 h | Promoted angiogenesis | [118] | |
| Mesenchymal Stem Cells Medium (Sciencell) 5% exosomes-depleted FBS | 1 | 48 h | Alleviate intervertebral disc degeneration by delivering miR-17-5p | [119] | |
| DMEM/F12 10% exosomes-depleted FBS | 3 | 48 h | promote cartilage regeneration via the miR-205–5p/PTEN/AKT pathway | [120] | |
| DMEM/F12 10% exosomes-depleted FBS | 5 | 48 h | EV improved chondrocyte proliferation and migration and suppressed chondrocyte apoptosis. miRNA-18-3P/JAK/STAT or miRNA-181c-5p/MAPK signaling pathway may be involved | [121] | |
| DMEM low glucose 10% platelet lysate | 1 | 48 h | EV increase angiogenesis, reduced neuronal degeneration, brain atrophy and improved neurological recovery | [116] | |
| Murine bone | α-Mem 10% Exo-removed FBS | 0.5 | 24 h | Significant enrichment of miRNA-210. Promoted survival and recovery of cardiac functions. Also, reduced apoptosis and fibrosis and increased the mobilization of cardiac progenitor cells | [124] |
| DMEM/F12 supplemented with 10% fetal bovine serum | 1 | 72 h | Overexpression of miR-210 regulated PI3K/AKT and p53 signaling by targeting AIFM3 reducing apoptosis and tissue death after a myocardial infarction | [125] | |
| α-Mem 10% Exo-removed FBS | 1 | 72 h | Overexpression of miR-125b-5p. Ability to modify the direction of exosomes to ischemic tissue | [126] |
Hypoxia is an important inducer of angiogenesis, which plays a key role in tissue regeneration. Therefore, diverse studies have analyzed whether MSC-derived EV exposed to low levels of O2 are enriched in angiogenic factors, and likewise, whether this has an impact on their ability to induce vessel formation. One of these studies showed that MSC cultivated for 72 h under hypoxic conditions (1% O2) produced exosomes with proangiogenic effects, through overexpression of genes encoding urokinase receptor [also known as urokinase plasminogen-activator surface receptor (uPAR)], angiogenin (ANG), VEGF, insulin-like growth Factor (IGF), angiopoietin receptor tyrosine kinase with immunoglobulin-like and epidermal growth factor (EGF)-like domains 2 (Tie-2) [also known as tyrosine endothelial kinase (TEK)], and interleukin 6 (IL-6)[104]. Additionally, umbilical cord MSC-derived EV have the ability to enhance endothelial-cell angiogenesis in vitro and in a rat hindlimb ischemia model, being able to restore blood flow[103]. Preconditioning adipose-derived MSC in moderate hypoxia (5% O2) also produced EV with capacity to increase formation of tubular structures in human umbilical-vein endothelial cells (HUVEC) with respect to EV obtained in normoxia. On the other hand, effects of EV were greater than those produced by media obtained after isolation of microvesicles. This indicates that EV, rather than soluble factors in the media, are responsible for angiogenic induction[32].
In vivo studies have also shown the potential of EV derived from MSC grown under hypoxia on angiogenesis. For example, in a mouse model of fat grafting, co-transplantation of exosomes in subcutaneous fat grafting enhanced angiogenesis, neovascularization, and graft survival[105]. A significant rise in protein synthesis of EGF, fibroblast growth-factors, VEGF/VEGF receptors (VEGF-R), Ang-1, and angiopoietin receptor tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (Tie-1) were shown in grafted animals, 30 d after transplantation[106]. Inclusion in hydrogels that allow local release of EV with high angiogenic capacity has also been used for treatment of spinal-cord injuries[107]. One of the proteins that has been found to be over synthesized in MSC-derived EV under hypoxia, compared to those obtained in normoxia, is jagged-1 (JAG1). This is one of the notch ligands. The notch pathway modulates processes such as angiogenesis, embryonic development, and hematopoietic stem-cell (HSC) biology[108,109]. HSC from umbilical-cord blood were treated in vitro with EV from MSC preconditioned in 1% O2 for 48 h. As expected, their expansion capacity, self-renewal, and clonogenic potential was increased through JAG1/notch pathway regulation[110].
Treatment of endothelial cells with hypoxia-conditioned MSC-derived EV modulates angiogenesis-related signaling pathways. For instance, it has recently been described that EV obtained from MSC maintained in 5% O2 for 6 d induced angiogenesis in HUVEC. That was accomplished through increased synthesis of high-mobility group box 1. It activates c-Jun N-terminal Kinases pathway (name derived from viral homolog v-jun, discovered in avian sarcoma virus 17 and named ju-nana, the Japanese word for 17), and consequently upregulated HIF1A/VEGF expression[111]. The angiogenic effects of MSC-derived EV exposed to hypoxia are mediated, in part, by their cargos, specifically by certain miRNA. One of these miRNAs is miR-612, which inhibits translation of TP53 mRNA, favoring the activity of HIF-1A-VEGF signaling, and consequently angiogenesis[112].
According to the properties of MSC-derived EV under hypoxia, their applications may be useful in multiple disease treatments (Figure 2). Among them is Alzheimer’s disease, which is characterized by neuronal and synaptic loss caused by deposition of beta-amyloid peptides[113] due to erroneous protein folding. Experiments have been carried out with an Alzheimer’s transgenic mouse model overexpressing mutated forms of human amyloid-precursor protein (APP) and presenilin 1 (PS1). Interestingly, they improved learning and memory functions after treatment with exosomes from MSC preconditioned for 12 h under hypoxia. These improvements could be due to reduced β-amyloid accumulation through increased levels of miR-21 in the brain, synthesis of synaptic proteins, and a decrease of inflammatory factors[114].
Repair of traumatic injuries of spinal cords have also been studied using exosomes released during 48 h under hypoxia (1% O2). An enrichment of miR-216a-5p in exosomes was observed involving TLR4/NF-κB/phosphoinositide 3-kinase (PI3K)/AKT signaling cascades. These miR-216a-5p-enriched exosomes promoted functional behavioral recovery using both in vitro and in vivo models carried out by shifting microglial polarization from classically-activated macrophage (M1) to alternatively-activated macrophage (M2) phenotype, effectively switching from pro-inflammatory to non-inflammatory states[115]. Also, application of EV derived from bone-marrow MSC preconditioned in hypoxia (1% O2) reduced neuronal degeneration, brain atrophy, and improved neurological recovery. These effects were due to the EV effects on angiogenesis in a mouse model of cerebral ischemia[116].
In relation to the skeletal system, EV may be used in bone-fracture healing. Thus, exosomes generated by MSC obtained from human umbilical cord were exposed to 1% O2 during 48 h. They promoted bone fracture healing in an animal model. These exosomes are enriched in miR-126 by the action of HIF1A exerting proangiogenic effects by means of sprouty-related, N-terminal enabled/vasodilator-stimulated phosphoprotein homology-1 domain-containing protein 1/Ras (name derived from Rat sarcoma-virus protein)/mitogen-activated protein kinase (originally called extracellular signal-regulated kinase or Erk) pathway activation[117].
Also, treatment with EV, released from MSC preconditioned in 2% of oxygen, prevented bone loss, increasing blood-vessel formation in a rat model of steroid-induced osteonecrosis of femoral head[118]. Additionally, other studies have showed that MSC-derived EV, grown in hypoxia, protect from intervertebral-disc degeneration through their content in mir-17-5p, which modulates proliferation of nucleus-pulposus cell (NPC) matrix, via the TLR4/PI3K/AKT pathway[119]. Furthermore, preconditioning in hypoxia also increased the capacity of MSC-derived EV in cartilage regeneration by positively acting on chondrocytes. Thus, in vivo assays have shown that an injectable silk-fibroin hydrogel, containing articular chondrocytes and MSC-derived EV in hypoxia promoted cartilage regeneration[120]. Several miRNA were involved in this process, including miR-18a-3p, miR-181c-5p, miR-205-5p, miR-337-5p, and miR-376a-5p[120,121].
Treatment with EV derived from MSC has also been proposed for kidney injury. Thus, EV from adipose tissue-derived MSC cultured 72 h under hypoxia (1% O2) or normoxia conditions were compared in treatment of kidney injury induced by ischemia in a rat model. Both conditions reduced tissue damage, but renal regeneration was higher under hypoxia conditions, triggering antiapoptotic, angiogenetic, immunomodulatory, and anti-oxidative stress responses. This could be due to differences in proteomic profiles of EV types[122].
On the other hand, EV derived from MSC cultured in hypoxia have been applied, using models of myocardial infarction, in several studies. Generally, protective effects of cardiac tissues from ischemic injury were observed. They were due, at least in part, to the ability of these EV to promote blood-vessel formation[123]. Additionally, exosomes from conditioned bone-marrow MSC cultured in hypoxia (24 h, 0.5% O2) or normoxia were used. They were injected intramyocardially into infarcted hearts of C57 Black 6 inbred mice strain. Treatment with hypoxia-derived exosomes produced interesting results: (1) Decrease in fibrotic tissue and apoptotic cardiomyocytes; and (2) Increase in cardiac-progenitor cells. These exosomes, compared to normoxia ones, had a significant increase in expression of miR-210, which had positive effects on endothelial cells and cardiomyocytes[124].
Such miRNA were also abundant in EV secreted by rat bone-marrow MSC, cultured in 1% O2 for 72 h. Their antiapoptotic effects in cardiomyocytes have also been demonstrated in a rat model of myocardium infarction[125]. Other EV derived from MSC cultured under hypoxia were also enriched in miRNA, showing antiapoptotic activity in cardiomyocytes. They include miR-125b-5p, which works through repression of p53 and BCL2 Antagonist/Killer 1[126]. It has also been shown that EV obtained from MSC cultured in hypoxia were enriched in miR-26a in relation to EV obtained in normoxia. Such miRNA is involved in upregulating glycogen-synthase kinase 3 beta (GSK3B) expression, which enhanced the beta-catenin pathway, reducing ischemia-reperfusion injury in a rat model[127].
Other miRNA enriched in EV, derived from adipose and bone-marrow MSC preconditioned in hypoxia were miR-224-5p and miR-24. The former decreased synthesis of thioredoxin-interacting protein, which facilitates degradation of HIF1A. EV enriched in miR-224-5p favored adaptation of cardiomyocytes to hypoxia, therefore protecting them against myocardial infarction[128]. On the other hand, miR-24 decreased in infarcted myocardium of rats. Thus, application of EV containing this miRNA protected cardiomyocytes from apoptosis, reducing infarct size, and improving cardiac function[129].
In addition to miRNA, other RNA types have also been identified, showing cardioprotective effects in EV generated by MSC, under hypoxia conditions. This is the case of long non-coding RNA of urothelial carcinoma-associated 1, which is related to the anti-apoptotic miR-873-5p/X-linked inhibitor of apoptosis protein/phosphorylated AMP-activated protein kinase pathway[130].
Exosomes derived from MSC grown under hypoxia may be also useful for treatments of chronic skin-ulcers. They are associated with pathologies such as diabetes. Their healing is difficult and isa serious problem for patients and public health systems[131]. Recently, a study has evaluated the potential application of EV obtained from adipose-tissue stem cells maintained at 1% O2 for 24 h. In vitro assays showed that they promoted fibroblast proliferation and migration. That was accomplished by activating PI3K/AKT pathway in a more effective way than when EV obtained under normoxia were used. Differential expression analyses of miRNA contents between both types of EV showed upregulated miR-21-3p, miR-31-5p, and miR-126-5p and downregulated miR-99b and miR-146a. They may be involved in signaling pathways related to fibroblast proliferation and migration, modulating immune responses. Indeed, treatment with hypoxia-derived EV improved healing in a diabetic nude mice model of wound healing, which was carried out via downregulation of IL-6, upregulation of VEGF and modulation of extracellular matrix[132]. Additionally, EV derived from umbilical cord MSC exposed to 1% O2 for 3 to 6 h were used in a full-thickness skin-injury mouse model, improving wound healing with respect to EV obtained in normoxia. In this case, it was demonstrated that EV in hypoxia had anti-apoptotic effects on endothelial cells due to miR-125b, which suppressed expression of TP53-inducible nuclear-protein 1[133].
In recent years, the therapeutic potential of using MSC-derived EV has become apparent. This is because the regenerative effects of MSC are partly due to their paracrine activity. Besides, the contents of EV can be modulated through preconditioning of MSC under different culture conditions. Among they, exposure to hypoxia stands out. HIF activation affects hundreds of genes involved in processes such as inflammation, migration, proliferation, differentiation, metabolism, and cell apoptosis. That is related to the contents of secreted EV, and thus their therapeutic potential, which is better than the one of EV obtained under normoxic conditions. Therefore, hypoxia preconditioning of MSC is a very attractive strategy for isolation of therapeutic EV. They have a high potential for use in regenerative medicine and can be applied to different pathologies. However, studies published to date show a great variability. That includes sources of MSC, culture media, O2 concentrations, and exposure times to hypoxia, as well as methods of EV isolation. Such factors may influence the degree of induction of HIF1A and HIF2A, and therefore MSC responses and EV cargos. Thus, it would be necessary to perform studies to optimize and standardize conditions for obtaining EV in the future according to their therapeutic applications. Also, in vivo studies carried out so far have been performed mainly in animal models. Only two active MSC-derived EV clinical trials in recruitment phase in which hypoxia is being evaluated are shown in ClinicalTrials (https://clinicaltrials.gov): “Treatment of Severe COVID-19 Patients Using Secretome of Hypoxia-Mesenchymal Stem Cells in Indonesia” (ID: NCT04753476) and “Regeneration of Posterior Cruciate Ligament Injury Using Hypoxic Conditioned Allogenic Adipose Mesenchymal Stem Cell and Condition Medium” (ID: NCT04889963). Therefore, in order to ascertain the greater potential effectiveness of EV obtained from MSC preconditioned in hypoxia, it would be necessary to carry out a greater number of properly designed clinical trials.
Using EV in regenerative medicine is very promising, as shown above. Yet, possible adverse effects associated with the use of the ones derived from MSC in human clinical practice must be taken into account. One of them is that the contents of EV may enhance tumor-cell activity[134]. In any case, that should be significantly lower –if it exists– than using whole stem-cells. Therefore, these risks should be properly evaluated in animal models and potential clinical trials. In this regard, there are several challenges for the use of MSC-derived EV in regenerative medicine that must be properly addressed beforehand. These include: (1) Identification of the most suitable MSC sources for each pathology; (2) Optimization and consensus of culture methods and conditions to obtain EV with greater regenerative capacity; (3) Scaling up of production for clinical use; (4) Control of variability and stability of produced EV; (5) Increase in clinical trials to make them statistically significant; and (6) A better understanding of pharmacokinetics and biodistribution of applied EV[135].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: “Sociedad Española de Investigación Ósea y del Metabolismo Mineral”, No. 547.
Specialty type: Cell and tissue engineering
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LJ, China; Miceli V, Italy S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 2. | Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2810] [Cited by in RCA: 2581] [Article Influence: 172.1] [Reference Citation Analysis (0)] |
| 3. | Xu X, Zhu F, Zhang M, Zeng D, Luo D, Liu G, Cui W, Wang S, Guo W, Xing W, Liang H, Li L, Fu X, Jiang J, Huang H. Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs. 2013;197:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 5. | Dzobo K. Recent Trends in Multipotent Human Mesenchymal Stem/Stromal Cells: Learning from History and Advancing Clinical Applications. OMICS. 2021;25:342-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 7. | Bernardo ME, Cometa AM, Pagliara D, Vinti L, Rossi F, Cristantielli R, Palumbo G, Locatelli F. Ex vivo expansion of mesenchymal stromal cells. Best Pract Res Clin Haematol. 2011;24:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Tamama K, Kerpedjieva SS. Acceleration of Wound Healing by Multiple Growth Factors and Cytokines Secreted from Multipotential Stromal Cells/Mesenchymal Stem Cells. Adv Wound Care (New Rochelle). 2012;1:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | González-González A, García-Sánchez D, Dotta M, Rodríguez-Rey JC, Pérez-Campo FM. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J Stem Cells. 2020;12:1529-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (6)] |
| 10. | Casado-Díaz A, Quesada-Gómez JM, Dorado G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front Bioeng Biotechnol. 2020;8:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 11. | Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells. 2014;37:637-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 949] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 13. | Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 881] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 14. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 15. | Huang S, Leung V, Peng S, Li L, Lu FJ, Wang T, Lu W, Cheung KM, Zhou G. Developmental definition of MSCs: new insights into pending questions. Cell Reprogram. 2011;13:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Chen L, Qu J, Cheng T, Chen X, Xiang C. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. 2019;10:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 17. | Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour AA, Yousefi M, Talebi M, Shamsasenjan K. Regenerative potential of Wharton's jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J Cell Physiol. 2020;235:9230-9240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Liu P, Deng Z, Han S, Liu T, Wen N, Lu W, Geng X, Huang S, Jin Y. Tissue-engineered skin containing mesenchymal stem cells improves burn wounds. Artif Organs. 2008;32:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Maranda EL, Rodriguez-Menocal L, Badiavas EV. Role of Mesenchymal Stem Cells in Dermal Repair in Burns and Diabetic Wounds. Curr Stem Cell Res Ther. 2017;12:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 2015;48:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Harris VK, Vyshkina T, Sadiq SA. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy. 2016;18:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Petrou P, Kassis I, Levin N, Paul F, Backner Y, Benoliel T, Oertel FC, Scheel M, Hallimi M, Yaghmour N, Hur TB, Ginzberg A, Levy Y, Abramsky O, Karussis D. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143:3574-3588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 23. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, He W, Geng H, Jin L, Liu Z, Wang FS. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 24. | Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1645] [Cited by in RCA: 1545] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 25. | Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 27. | Tan SSH, Tjio CKE, Wong JRY, Wong KL, Chew JRJ, Hui JHP, Toh WS. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng Part B Rev. 2021;27:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Khan K, Caron C, Mahmoud I, Derish I, Schwertani A, Cecere R. Extracellular Vesicles as a Cell-free Therapy for Cardiac Repair: a Systematic Review and Meta-analysis of Randomized Controlled Preclinical Trials in Animal Myocardial Infarction Models. Stem Cell Rev Rep. 2022;18:1143-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Prasai A, Jay JW, Jupiter D, Wolf SE, El Ayadi A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J Invest Dermatol. 2022;142:662-678.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Khalaj K, Figueira RL, Antounians L, Lauriti G, Zani A. Systematic review of extracellular vesicle-based treatments for lung injury: are EVs a potential therapy for COVID-19? J Extracell Vesicles. 2020;9:1795365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6572] [Article Influence: 1314.4] [Reference Citation Analysis (0)] |
| 32. | Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, Egger D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro. Front Bioeng Biotechnol. 2019;7:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 33. | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 2141] [Article Influence: 356.8] [Reference Citation Analysis (35)] |
| 34. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7702] [Article Influence: 1100.3] [Reference Citation Analysis (1)] |
| 35. | Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab. 2017;28:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 36. | Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed Res Int. 2019;2019:9742765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 37. | Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126:1139-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 38. | Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 786] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 39. | Rani S, Ritter T. The Exosome - A Naturally Secreted Nanoparticle and its Application to Wound Healing. Adv Mater. 2016;28:5542-5552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 40. | Chuo ST, Chien JC, Lai CP. Imaging extracellular vesicles: current and emerging methods. J Biomed Sci. 2018;25:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 41. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 881] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 42. | Lee SM, Jun DW, Kang HT, Oh JH, Saeed WK, Ahn SB. Optimal Hypoxic Preconditioning of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells (hES-MSCs) and Their Characteristics. Int J Stem Cells. 2021;14:221-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4725] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 44. | Yellowley CE, Genetos DC. Hypoxia Signaling in the Skeleton: Implications for Bone Health. Curr Osteoporos Rep. 2019;17:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 721] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 46. | Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 261] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 47. | Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 1005] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 48. | Lin Q, Cong X, Yun Z. Differential hypoxic regulation of hypoxia-inducible factors 1alpha and 2alpha. Mol Cancer Res. 2011;9:757-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | Kumar H, Choi DK. Hypoxia Inducible Factor Pathway and Physiological Adaptation: A Cell Survival Pathway? Mediators Inflamm. 2015;2015:584758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 50. | Yamashita T, Ohneda O, Nagano M, Iemitsu M, Makino Y, Tanaka H, Miyauchi T, Goto K, Ohneda K, Fujii-Kuriyama Y, Poellinger L, Yamamoto M. Abnormal heart development and lung remodeling in mice lacking the hypoxia-inducible factor-related basic helix-loop-helix PAS protein NEPAS. Mol Cell Biol. 2008;28:1285-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Hara S, Hamada J, Kobayashi C, Kondo Y, Imura N. Expression and characterization of hypoxia-inducible factor (HIF)-3alpha in human kidney: suppression of HIF-mediated gene expression by HIF-3alpha. Biochem Biophys Res Commun. 2001;287:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 439] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 53. | Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4121] [Cited by in RCA: 4311] [Article Influence: 179.6] [Reference Citation Analysis (0)] |
| 54. | Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3582] [Cited by in RCA: 3712] [Article Influence: 154.7] [Reference Citation Analysis (0)] |
| 55. | Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1173] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 56. | Kaluz S, Kaluzová M, Stanbridge EJ. Regulation of gene expression by hypoxia: integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin Chim Acta. 2008;395:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | DeFrates KG, Franco D, Heber-Katz E, Messersmith PB. Unlocking mammalian regeneration through hypoxia inducible factor one alpha signaling. Biomaterials. 2021;269:120646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2902] [Cited by in RCA: 4218] [Article Influence: 351.5] [Reference Citation Analysis (0)] |
| 59. | Naviaux RK, Le TP, Bedelbaeva K, Leferovich J, Gourevitch D, Sachadyn P, Zhang XM, Clark L, Heber-Katz E. Retained features of embryonic metabolism in the adult MRL mouse. Mol Genet Metab. 2009;96:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Sunkari VG, Lind F, Botusan IR, Kashif A, Liu ZJ, Ylä-Herttuala S, Brismar K, Velazquez O, Catrina SB. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015;23:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 61. | Catrina SB, Zheng X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab Res Rev. 2016;32 Suppl 1:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 62. | Strowitzki MJ, Ritter AS, Kimmer G, Schneider M. Hypoxia-adaptive pathways: A pharmacological target in fibrotic disease? Pharmacol Res. 2019;147:104364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 63. | Luo R, Zhang W, Zhao C, Zhang Y, Wu H, Jin J, Grenz A, Eltzschig HK, Tao L, Kellems RE, Xia Y. Elevated Endothelial Hypoxia-Inducible Factor-1α Contributes to Glomerular Injury and Promotes Hypertensive Chronic Kidney Disease. Hypertension. 2015;66:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8:227-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 756] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 65. | Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1563] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 66. | Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS One. 2007;2:e1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 2013;5:a011247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 68. | Hutami IR, Izawa T, Khurel-Ochir T, Sakamaki T, Iwasa A, Tanaka E. Macrophage Motility in Wound Healing Is Regulated by HIF-1α via S1P Signaling. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 69. | Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1α-Induced Glycolysis Metabolism Is Essential to the Activation of Inflammatory Macrophages. Mediators Inflamm. 2017;2017:9029327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 70. | Takeda N, O'Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 502] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 71. | Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1369] [Cited by in RCA: 1600] [Article Influence: 228.6] [Reference Citation Analysis (0)] |
| 72. | Xu O, Li X, Qu Y, Liu S, An J, Wang M, Sun Q, Zhang W, Lu X, Pi L, Zhang M, Shen Y. Regulation of glucose transporter protein-1 and vascular endothelial growth factor by hypoxia inducible factor 1α under hypoxic conditions in Hep-2 human cells. Mol Med Rep. 2012;6:1418-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757-23763. [PubMed] |
| 74. | Xu C, Gu L, Kuerbanjiang M, Wen S, Xu Q, Xue H. Thrombospondin 2/Toll-Like Receptor 4 Axis Contributes to HIF-1α-Derived Glycolysis in Colorectal Cancer. Front Oncol. 2020;10:557730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2899] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 76. | Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int. 2015;2015:549412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 77. | Hoenig MR, Bianchi C, Sellke FW. Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis. Curr Drug Targets. 2008;9:422-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Tu TC, Nagano M, Yamashita T, Hamada H, Ohneda K, Kimura K, Ohneda O. A Chemokine Receptor, CXCR4, Which Is Regulated by Hypoxia-Inducible Factor 2α, Is Crucial for Functional Endothelial Progenitor Cells Migration to Ischemic Tissue and Wound Repair. Stem Cells Dev. 2016;25:266-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 80. | Baba M, Hirai S, Yamada-Okabe H, Hamada K, Tabuchi H, Kobayashi K, Kondo K, Yoshida M, Yamashita A, Kishida T, Nakaigawa N, Nagashima Y, Kubota Y, Yao M, Ohno S. Loss of von Hippel-Lindau protein causes cell density dependent deregulation of CyclinD1 expression through hypoxia-inducible factor. Oncogene. 2003;22:2728-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 625] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 82. | Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 462] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 83. | Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 598] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 84. | Kilic M, Kasperczyk H, Fulda S, Debatin KM. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene. 2007;26:2027-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 1293] [Article Influence: 215.5] [Reference Citation Analysis (0)] |
| 86. | Basciano L, Nemos C, Foliguet B, de Isla N, de Carvalho M, Tran N, Dalloul A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011;12:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 87. | Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P, Molaeipour Z, Saleh M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv Pharm Bull. 2015;5:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 88. | Sylakowski K, Bradshaw A, Wells A. Mesenchymal Stem Cell/Multipotent Stromal Cell Augmentation of Wound Healing: Lessons from the Physiology of Matrix and Hypoxia Support. Am J Pathol. 2020;190:1370-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 89. | Lee JH, Yoon YM, Lee SH. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 90. | Moya A, Paquet J, Deschepper M, Larochette N, Oudina K, Denoeud C, Bensidhoum M, Logeart-Avramoglou D, Petite H. Human Mesenchymal Stem Cell Failure to Adapt to Glucose Shortage and Rapidly Use Intracellular Energy Reserves Through Glycolysis Explains Poor Cell Survival After Implantation. Stem Cells. 2018;36:363-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 91. | Hawkins KE, Sharp TV, McKay TR. The role of hypoxia in stem cell potency and differentiation. Regen Med. 2013;8:771-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 92. | Kim YS, Noh MY, Cho KA, Kim H, Kwon MS, Kim KS, Kim J, Koh SH, Kim SH. Hypoxia/Reoxygenation-Preconditioned Human Bone Marrow-Derived Mesenchymal Stromal Cells Rescue Ischemic Rat Cortical Neurons by Enhancing Trophic Factor Release. Mol Neurobiol. 2015;52:792-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Boyette LB, Creasey OA, Guzik L, Lozito T, Tuan RS. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med. 2014;3:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 94. | Kheirandish M, Gavgani SP, Samiee S. The effect of hypoxia preconditioning on the neural and stemness genes expression profiling in human umbilical cord blood mesenchymal stem cells. Transfus Apher Sci. 2017;56:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Samal JRK, Rangasami VK, Samanta S, Varghese OP, Oommen OP. Discrepancies on the Role of Oxygen Gradient and Culture Condition on Mesenchymal Stem Cell Fate. Adv Healthc Mater. 2021;10:e2002058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 96. | Antebi B, Rodriguez LA 2nd, Walker KP 3rd, Asher AM, Kamucheka RM, Alvarado L, Mohammadipoor A, Cancio LC. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 97. | Powell FL, Garcia N. Physiological effects of intermittent hypoxia. High Alt Med Biol. 2000;1:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 99. | Patel DB, Gray KM, Santharam Y, Lamichhane TN, Stroka KM, Jay SM. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med. 2017;2:170-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 100. | Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 538] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 101. | Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8:e68451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 102. | Yu J, Liu XL, Cheng QG, Lu SS, Xu XQ, Zu QQ, Liu S. G-CSF and hypoxic conditioning improve the proliferation, neural differentiation and migration of canine bone marrow mesenchymal stem cells. Exp Ther Med. 2016;12:1822-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Zhang HC, Liu XB, Huang S, Bi XY, Wang HX, Xie LX, Wang YQ, Cao XF, Lv J, Xiao FJ, Yang Y, Guo ZK. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 104. | Chen J, Liu Z, Hong MM, Zhang H, Chen C, Xiao M, Wang J, Yao F, Ba M, Liu J, Guo ZK, Zhong J. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS One. 2014;9:e115316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 105. | Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 106. | Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 107. | Mu J, Li L, Wu J, Huang T, Zhang Y, Cao J, Ma T, Chen J, Zhang C, Zhang X, Lu T, Kong X, Sun J, Gao J. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater Sci. 2022;10:1803-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 108. | Lomelí H, Castillo-Castellanos F. Notch signaling and the emergence of hematopoietic stem cells. Dev Dyn. 2020;249:1302-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 110. | Niazi V, Ghafouri-Fard S, Verdi J, Jeibouei S, Karami F, Pourhadi M, Ahani M, Atarodi K, Soleimani M, Zali H, Zomorrod MS. Hypoxia preconditioned mesenchymal stem cell-derived exosomes induce ex vivo expansion of umbilical cord blood hematopoietic stem cells CD133+ by stimulation of Notch signaling pathway. Biotechnol Prog. 2022;38:e3222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Gao W, He R, Ren J, Zhang W, Wang K, Zhu L, Liang T. Exosomal HMGB1 derived from hypoxia-conditioned bone marrow mesenchymal stem cells increases angiogenesis via the JNK/HIF-1α pathway. FEBS Open Bio. 2021;11:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 112. | Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y, Duan D, Hu Z, Chen P, Lu M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnology. 2021;19:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 113. | Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL. Alzheimer's disease prevention: from risk factors to early intervention. Alzheimers Res Ther. 2017;9:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 428] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 114. | Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 115. | Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 116. | Gregorius J, Wang C, Stambouli O, Hussner T, Qi Y, Tertel T, Börger V, Mohamud Yusuf A, Hagemann N, Yin D, Dittrich R, Mouloud Y, Mairinger FD, Magraoui FE, Popa-Wagner A, Kleinschnitz C, Doeppner TR, Gunzer M, Meyer HE, Giebel B, Hermann DM. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res Cardiol. 2021;116:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 117. | Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 118. | Yuan N, Ge Z, Ji W, Li J. Exosomes Secreted from Hypoxia-Preconditioned Mesenchymal Stem Cells Prevent Steroid-Induced Osteonecrosis of the Femoral Head by Promoting Angiogenesis in Rats. Biomed Res Int. 2021;2021:6655225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | Zhou ZM, Bao JP, Peng X, Gao JW, Vlf C, Zhang C, Sun R, Kun-Wang, Wu XT. Small extracellular vesicles from hypoxic mesenchymal stem cells alleviate intervertebral disc degeneration by delivering miR-17-5p. Acta Biomater. 2022;140:641-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 120. | Shen K, Duan A, Cheng J, Yuan T, Zhou J, Song H, Chen Z, Wan B, Liu J, Zhang X, Zhang Y, Xie R, Liu F, Fan W, Zuo Q. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205-5p/PTEN/AKT pathway. Acta Biomater. 2022;143:173-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 121. | Zhang B, Tian X, Qu Z, Hao J, Zhang W. Hypoxia-Preconditioned Extracellular Vesicles from Mesenchymal Stem Cells Improve Cartilage Repair in Osteoarthritis. Membranes (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 122. | Collino F, Lopes JA, Corrêa S, Abdelhay E, Takiya CM, Wendt CHC, de Miranda KR, Vieyra A, Lindoso RS. Adipose-Derived Mesenchymal Stromal Cells Under Hypoxia: Changes in Extracellular Vesicles Secretion and Improvement of Renal Recovery after Ischemic Injury. Cell Physiol Biochem. 2019;52:1463-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 123. | Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 540] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 124. | Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J, Lin Y, Xiang P, Tang Y, Hu X, Chen J, Zhu W, Webster KA, Wang J, Yu H. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol. 2018;46:1659-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 125. | Cheng H, Chang S, Xu R, Chen L, Song X, Wu J, Qian J, Zou Y, Ma J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 126. | Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, Wang KK, Shen H, Zhang GG, Bai YP. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163-6177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 127. | Park H, Park H, Mun D, Kang J, Kim H, Kim M, Cui S, Lee SH, Joung B. Extracellular Vesicles Derived from Hypoxic Human Mesenchymal Stem Cells Attenuate GSK3β Expression via miRNA-26a in an Ischemia-Reperfusion Injury Model. Yonsei Med J. 2018;59:736-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 128. | Mao CY, Zhang TT, Li DJ, Zhou E, Fan YQ, He Q, Wang CQ, Zhang JF. Extracellular vesicles from hypoxia-preconditioned mesenchymal stem cells alleviates myocardial injury by targeting thioredoxin-interacting protein-mediated hypoxia-inducible factor-1α pathway. World J Stem Cells. 2022;14:183-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 129. | Zhang CS, Shao K, Liu CW, Li CJ, Yu BT. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci. 2019;23:6691-6699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 130. | Sun L, Zhu W, Zhao P, Wang Q, Fan B, Zhu Y, Lu Y, Chen Q, Zhang J, Zhang F. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis. 2020;11:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 131. | Raghav A, Khan ZA, Labala RK, Ahmad J, Noor S, Mishra BK. Financial burden of diabetic foot ulcers to world: a progressive topic to discuss always. Ther Adv Endocrinol Metab. 2018;9:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 132. | Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, Xiao Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology. 2021;19:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 133. | Zhang XF, Wang T, Wang ZX, Huang KP, Zhang YW, Wang GL, Zhang HJ, Chen ZH, Wang CY, Zhang JX, Wang H. Hypoxic ucMSC-secreted exosomal miR-125b promotes endothelial cell survival and migration during wound healing by targeting TP53INP1. Mol Ther Nucleic Acids. 2021;26:347-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 134. | Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, Li G, Tang J, Xiang J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 427] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 135. | Fuloria S, Subramaniyan V, Dahiya R, Dahiya S, Sudhakar K, Kumari U, Sathasivam K, Meenakshi DU, Wu YS, Sekar M, Malviya R, Singh A, Fuloria NK. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |