Published online Jul 26, 2022. doi: 10.4252/wjsc.v14.i7.435
Peer-review started: March 14, 2022
First decision: May 11, 2022
Revised: May 25, 2022
Accepted: June 20, 2022
Article in press: June 20, 2022
Published online: July 26, 2022
Processing time: 133 Days and 19.3 Hours
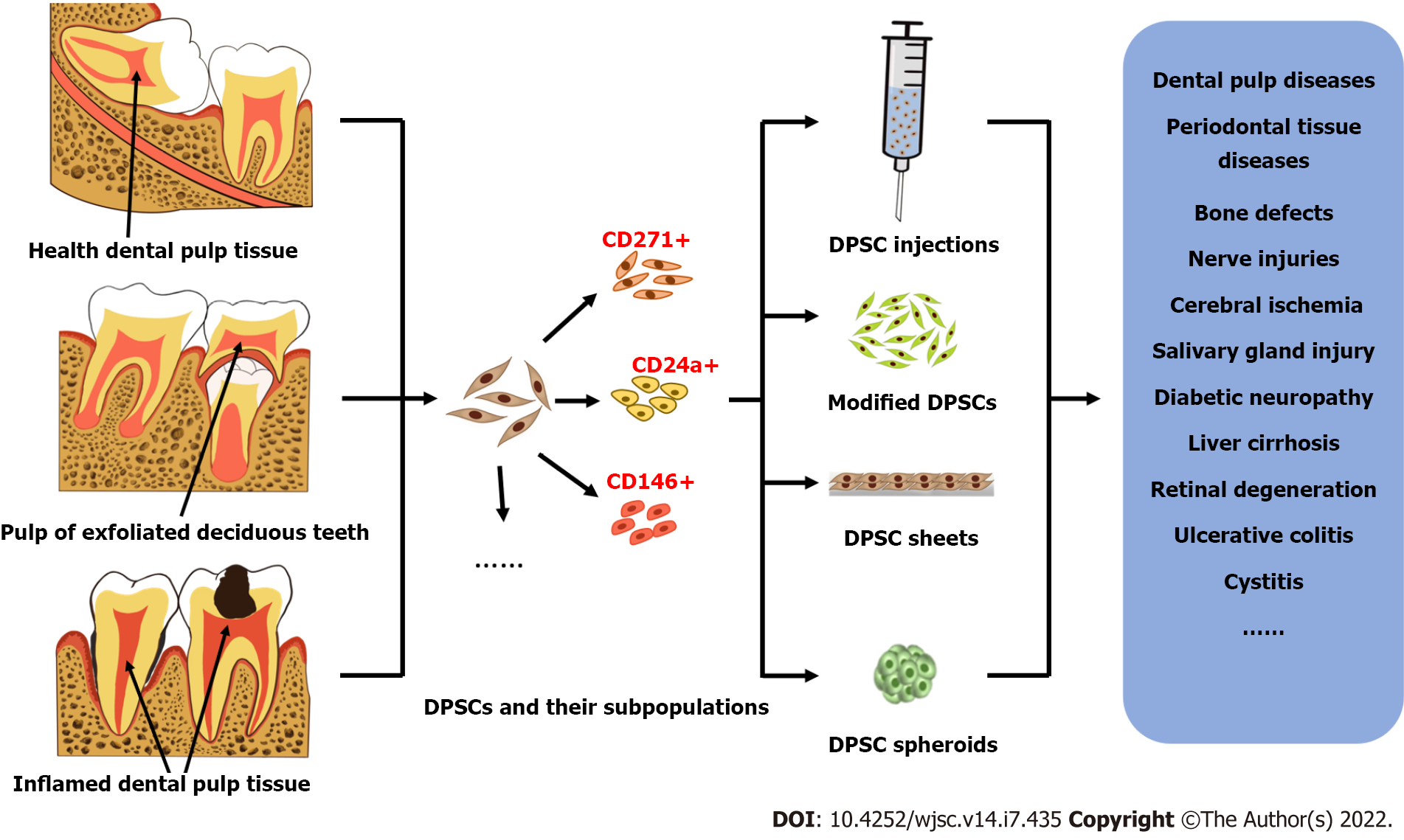
For more than 20 years, researchers have isolated and identified postnatal dental pulp stem cells (DPSCs) from different teeth, including natal teeth, exfoliated deciduous teeth, healthy teeth, and diseased teeth. Their mesenchymal stem cell (MSC)-like immunophenotypic characteristics, high proliferation rate, potential for multidirectional differentiation and biological features were demonstrated to be superior to those of bone marrow MSCs. In addition, several main application forms of DPSCs and their derivatives have been investigated, including stem cell injections, modified stem cells, stem cell sheets and stem cell spheroids. In vitro and in vivo administration of DPSCs and their derivatives exhibited beneficial effects in various disease models of different tissues and organs. Therefore, DPSCs and their derivatives are regarded as excellent candidates for stem cell-based tissue regeneration. In this review, we aim to provide an overview of the potential application of DPSCs and their derivatives in the field of regenerative medicine. We describe the similarities and differences of DPSCs isolated from donors of different ages and health conditions. The methodologies for therapeutic administration of DPSCs and their derivatives are introduced, including single injections and the transplantation of the cells with a support, as cell sheets, or as cell spheroids. We also summarize the underlying mechanisms of the regenerative potential of DPSCs.
Core Tip: In this review, we aim to outline the present understanding of the potential application of dental pulp stem cells (DPSCs) and their derivatives in the field of regenerative medicine. DPSCs have different properties and regenerative potentials according to the age and health condition of the donor. For therapeutic applications, DPSCs can be administered through different methodologies, including by single injections and the transplantation of the cells and their derivatives with a support, as cell sheets or as cell spheroids. The underlying mechanisms of the regenerative potential of DPSCs and their derivatives may occur through direct regulation and immunomodulatory and paracrine effects.
- Citation: Yuan SM, Yang XT, Zhang SY, Tian WD, Yang B. Therapeutic potential of dental pulp stem cells and their derivatives: Insights from basic research toward clinical applications. World J Stem Cells 2022; 14(7): 435-452
- URL: https://www.wjgnet.com/1948-0210/full/v14/i7/435.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i7.435
Dental pulp stem cells (DPSCs) have received major attention since they were first isolated in 2000 due to their easily accessible properties, lack of ethical problems, high proliferation ability and multidirectional differentiation potential[1-3]. Since then, numerous studies have emerged on the extraction and identification of DPSCs. Scholars have obtained DPSCs from a variety of dental sources and confirmed that they are superior to bone marrow mesenchymal stem cells (MSCs) in various characteristics, such as easier access, higher proliferation, and better neural differentiation[4-8].
In view of their excellent stem cell characteristics, DPSCs, as an important source of postnatal MSCs, have been widely studied in the field of regenerative medicine in the past two decades, including but not limited to cerebral ischemia[9], bone and dental loss defects[10,11], the nervous system[12], the digestive system[13] and the endocrine system[14], and many studies have achieved meaningful therapeutic effects. Meanwhile, in the application of regenerative medicine, with the continuous progress of cell culture and modification technologies, the application forms of MSCs continue to expand, such as the transformation from primitive cells to modified cells and the leap from single-cell preparations to multicell units[15-17]. These enhanced stem cell applications play an important role in improving the therapeutic effect of stem cells, which promotes the development of tissue engineering and regenerative medicine.
However, the mechanism by which DPSCs promote regeneration has not been fully revealed. At present, two main aspects of MSCs, immunomodulatory and paracrine effects, have been widely discussed[18-20]. MSCs have been suggested to possibly be involved in the process of immune regulation in the host by regulating the physiological functions of immune cells such as T cells, B cells, dendritic cells (DCs), and natural killer (NK) cells[21] or transmitting intercellular signals through paracrine pathways such as secretomes, exosomes and extracellular vesicles (EVs)[22-24], thereby inhibiting inflammation and promoting disease improvement. In addition, transplanted stem cells may directly promote the repair or regeneration of tissue injury by residing at the transplant site and differentiating into corresponding cells[25-29]. As a promising member of the MSC family, DPSCs may also play a role through the above mechanism in the process of promoting injury repair and reconstruction or disease improvement[18,30,31].
In this review, we introduce DPSCs from different sources and their characteristics and discuss several main applications of DPSCs in regenerative medicine in recent years, including cell injections (cell suspensions), modified cells, cell sheets and cell spheroids. We introduce the background, biological characteristics, representative examples and preliminary therapeutic effects of these various derivatives in regenerative medicine and briefly summarize the possible mechanisms of DPSCs in promoting regeneration.
Since their discovery, DPSCs have garnered extensive attention due to their easily accessible features and lack of ethical issues. In 2000, Gronthos et al[1] isolated clonogenic and highly proliferative cells from enzymatically disaggregated dental pulp tissue of normal human impacted third molars for the first time. The isolated cells were termed DPSCs[1]. Since then, many researchers have focused on DPSCs and successfully isolated these cells from dental pulp tissue of different ages and different states, as well as using cell sorting technologies to isolate subsets of cells with special phenotypes. These DPSCs have both the same characteristics and obvious differences.
To date, researchers have extracted DPSCs from teeth of different age groups, including children (deciduous teeth), adolescents, adults, aged (permanent teeth) and even infant donors (natal teeth)[1,32-34]. The most common are DPSCs from adult permanent teeth (generally less than 30 years old) and exfoliated deciduous teeth.
Adults (generally less than 30 years old): Adult human health dental pulp tissue derived dental pulp cells, as subsequently demonstrated by Gronthos et al[1], contained clonogenic cell populations within them that have the ability to form clones. Although colonies of dental pulp cells occurred at a similar frequency in comparison to bone marrow mesenchymal stem cells (BMSCs) from bone marrow aspirates flushed free of hematopoietic cells, DPSCs exhibited a higher proliferation rate than BMSCs in vitro, and they maintained their high rate of proliferation even after extensive subculturing[1]. In view of this phenomenon, the authors believe that DPSCs satisfy two of the criteria of a postnatal somatic stem cell: ex vivo expansion and clonogenicity[35]. Also, transplanted DPSCs can generate a dentin-pulp-like complex representative of the microenvironments from which they were derived in vivo, which underscores one of their stem cell natures again: Tissue reconstitution[1,35]. Later studies improved the immunophenotypic identification of DPSCs from adult healthy teeth and repeatedly confirmed their multidirectional differentiation ability, finding that DPSCs are positive for mesenchymal lineage markers (CD13, CD29, CD44, CD73, CD90 and CD105) and negative for monocytic (CD14) and hematopoietic lineage markers (CD34, and CD45). At the same time, DPSCs have the potential to differentiate into typical mesodermal cell lineages, such as osteogenic, chondrogenic, and adipogenic lineages[4-7]. In conclusion, DPSCs derived from adult healthy dental pulp have the phenotypic characteristics and multidirectional differentiation ability of MSCs and meet the criteria for postnatal somatic stem cells.
Children (deciduous teeth): In 2003, Miura et al[32], for the first time, isolated stem cells from exfoliated human deciduous teeth, named stem cells from human exfoliated deciduous teeth (SHED), which were also identified as a population of highly proliferative, clonogenic cells capable of differentiating into a variety of cell types, including odontoblasts, endothelia, neural cells and adipocytes[32,36,37]. They could also express MSC markers such as CD29, CD73, CD90, CD105, CD146, and STRO-1[32,38,39]. However, SHED showed higher expression of CD105 and CD146 than DPSCs, suggesting that SHED is a unique undifferentiated stem cell lineage and may have a higher capacity for differentiation[38]. Subsequent studies confirmed the differences between SHED and adult DPSCs, including that the SHED exhibited more colony forming units, shorter doubling time, higher proliferation rate, higher endothelial differentiation potential, stronger osteogenic and adipogenic differentiation ability in vitro and osteoinductive capacity in vivo[32,38,40,41] etc. Even under the adverse culture conditions of hypoxia, high glucose and low serum, the above characteristics of SHED were still better than the characteristics of DPSCs[42]. However, the neurogenic ability of SHED is lower than the neurogenic ability of DPSCs[43,44]. In short, from the current findings, SHED may be a better seed cell player than DPSCs in addition to neurogenic ability, which may be attributed to its younger physiological age.
Other ages: In addition to the above two common sources of DPSCs, another study reported that DPSCs obtained from natal teeth also have the immunophenotypic characteristics (expressed CD13, CD44, CD73, CD90, CD146, and CD166, but not CD3, CD8, CD10, CD11b, CD14, CD15, CD19, CD33, CD34, CD45, CD71, CD117, and HLA-DR) and multidirectional differentiation (adipogenic, osteogenic, chondrogenic, myogenic and neurogenic) potential of MSCs[45]. In addition, compared with the deciduous pulp, the expression of nestin and CD44 was stronger in the dental pulp of natal teeth. Positive immune expression of SOX2 (embryonic stem cell marker) was observed only in the dental pulp of natal teeth, which confirms the presence of a higher percentage of stem/progenitor cell population compared with the deciduous pulp[34]. In addition, Wu et al[33] specifically compared the growth and differentiation characteristics of DPSCs from patients of different ages, including children, adolescents, adults and aged donors. The results showed that although a large portion of cell surface markers was expressed in all DPSC lines, the expression of CD29 was downregulated in the DPSCs from aged teeth. At the same time, the doubling time of DPSCs from aged teeth was prolonged, and the number of apoptotic cells increased with propagation. Moreover, these DPSCs from aged teeth were completely or partially deprived of lineage differentiation capacity[33]. These results suggest that DPSCs from younger ages are more suitable as excellent candidates for regenerative medicine stem cell resource banks.
Although the sources of healthy dental pulp are very extensive, the sources of unhealthy dental pulp are more abundant with the trend of frequent occurrence of caries, dental pulp diseases and periodontitis. Therefore, some researchers have turned their attention to the field of dental pulp in different healthy states to expand the potential source range of DPSCs.
Yu et al[46] reported that stem cells from inflamed pulp of deciduous teeth (SCIDs) were positive for cell surface markers, including CD105, CD90, and CD146, and had high proliferation ability and osteogenic, adipogenic, and chondrogenic differentiation potentials. Except for SCIDs that secreted more tumor necrosis factor-α (TNF-α) protein, there was no significant difference in proliferation and differentiation potentials between SCIDs and SHED[46]. Pereira et al[47] also supported that the morphology, proliferation rate and differentiation potential of inflamed and normal DPSCs are similar[47].
However, Kim et al[48] and Alongi et al[49] reported different results: The abilities of colony forming and osteogenic differentiation in vitro and in vivo of stem cells from inflamed pulp tissue were decreased compared with normal DPSCs[48,49]. Another study also confirmed that DPSCs from carious teeth (DPSCs-CT) are not as efficient as normal DPSCs in differentiating into dopaminergic-like cells[50]. The colony forming capacity of stem cells isolated from pulp polyps (chronic hyperplastic pulpitis) and dental pulp with irreversible pulpitis was also lower than the colony forming capacity of healthy DPSCs, but both cell types (normal and pulpitis) have demonstrated the ability to form pulp/dentin complexes when transplanted into immunocompromised mice[51,52].
Interestingly, strong staining for CD146 was observed in inflamed pulps during the initial inflammatory response, and short-term TNF-α treatment has also been confirmed to enhance dental pulp cell function, including the ability to form cell colonies, migrate, and differentiate into odontogenic and adipogenic lineages[53]. Moreover, researchers have also identified and studied DPSCs isolated from teeth with different stages of aggressive periodontitis (representing different degrees of dental pulp inflammation) to explore the impact of periodontal infection during the progression of periodontitis on the pluripotency and regenerative potential of DPSCs within periodontally compromised dental pulp. The results showed that periodontal inflammation had a negative impact on the colony forming ability and proliferation of DPSCs, but the relationship between the effect on their pluripotency and the severity of the disease was uncertain. Although DPSCs from the inflamed dental pulp of teeth extracted due to aggressive periodontitis appear to have undergone some changes in terms of their stem cell properties, they still possess the capacity to differentiate into odontoblastic and osteoblastic phenotypes in vitro and form pulp- and dentin-like tissues in vivo[54]. These data indicate that the stem cell characteristics of unhealthy dental pulp-derived stem cells are unstable, which may be related to the degree of inflammation in the diseased dental pulp. Further characterization is needed to determine whether they can serve as a source of therapeutic cells for future regenerative therapies.
DPSCs showed heterogeneity in the earliest isolation and identification studies: In primary cultured DPSCs, many of the phenotypic markers were not uniformly expressed but were found in subsets of cells, which may represent different pulp cell lineages[1], indicating that there are different cell subpopulations in the primary DPSCs isolated and cultured directly, which may dominate the different cell fates of stem cells. The development and progress of cell sorting technologies make the separation of cell subsets possible.
Currently, DPSC subpopulations that have been isolated include but are not limited to side population cells with stem cell phenotypic characteristics[55], bromodeoxyuridine (BrdU)-labeled label-retaining cells for the localization of dental pulp stem/progenitor cells[56], ALDH1+ cell subpopulations reflecting DPSC niches[57], DPSCs mobilized by G-CSF (MDPCs) with high proliferation rates and stability[58], CD34−/CD34+ DPSCs with different neural differentiation potentials[59], thy-1-positive cells in the subodontoblastic layer with the ability to differentiate into hard tissue-forming cells[60], and CD271+ dental MSCs with high odontogenic potential[61]. These subsets reflect the origin or location of stem cells or have high potential in a particular direction of differentiation. For example, a group of unique multipotent stem cells were recently identified from mouse dental papilla called multipotent dental pulp regenerative stem cells, exhibiting enhanced osteogenic/odontogenic differentiation capabilities in vitro and in vivo and efficiently regenerating functional pulpo-dentinal complex-like tissues in vivo[62]. CD146+ human DPSCs have also been reported to be able to regenerate an increased area of dentin/pulp-like structures in vivo compared with their CD146- counterparts or mixtures of the two[63]. These results suggest that the use of DPSCs is flexible in tissue engineering, and taking advantage of a specific side group may be the pathway to achieve accurate and efficient tissue regeneration.
Regardless of the source of DPSCs, DPSCs are used in the later description of this article, and no special distinction is made when describing their applications in regenerative medicine.
Stem cell-based tissue engineering has been developed for many years, during which the application forms of stem cells have undergone major changes from primitive cells to genetically modified cells and from discrete stem cell suspensions to multicellular units, prompting regenerative medicine to take a big step forward. Here, we will discuss several main derivatives of DPSCs derived from long-term application, including cell injections, genetically modified cells, cell sheets and cell spheroids, and introduce their formation background, preparation techniques, biological characteristics and examples of their application in regenerative medicine.
The transplantation of DPSCs has been investigated as a potential therapy for a variety of injuries and diseases, including but not limited to stroke[64], spinal cord injury[65], cerebellar ataxia[66], retinal degeneration[15], diabetic neuropathy[67], parotid gland injury[68], cystitis[69], Sjögren syndrome[70], etc. In the treatment of these diseases using DPSCs, injection is one of the more commonly used forms. Stem cell injections are the easiest cell therapy products to prepare, and the commonly used vehicles at present for resuspension of stem cells is phosphate-buffered saline[69-73], Hank's Balanced Salt Solution[15], normal saline[14,67,74-76], and modified medium[66,68,77-79]. These mediators have no therapeutic effect and only serve as transport vectors for stem cells, so it is possible to observe the monotherapy effect of transplanted stem cells and rule out the influence of carriers. In addition, DPSC injections are usually administered by local injection[15,66,69,80], intramuscular injection[67,76,81] and intravenous injection[67,68,72,82]. Different methods of administration lead to different systemic distributions and different efficacies.
A study by Kim et al[83] indicated that intravenously injected human DPSCs via the tail vein in nude mice were distributed mostly to the lungs and rarely detected in other organs at all observed time points[83]. Another study confirmed that intravenously administered DPSCs did not show liver and kidney migration in Sprague-Dawley rats[72]. However, another study suggested that SHED transplantation via the tail vein in nonobese diabetic mice was observed mainly in the liver and spleen[70]. The mode of administration by intravenous injection can be seen to possibly cause drug distribution in multiple organs. However, locally administered DPSCs rarely migrated to other organs over time, e.g., DPSCs were transplanted into the pulp chamber[83]. This result indicated a differential distribution pattern of transplanted DPSCs between the intravenous and local injections. Both intravenously and locally injected DPSCs have been shown to improve symptoms of various lesions[15,72,76,80]. DPSCs have also been shown to migrate and integrate into the site of injuries[15,69,73,74] and differentiate into corresponding cells[68,72,75,79,84].
Shahani et al[71] traced the biodistribution of intramuscularly transplanted human DPSCs in immunocompetent healthy rats. The results showed that DPSCs started entering into the blood vessels adjacent to the muscle at hour 24 and gradually metastasized, but the signal intensity in the muscles at the injection site remained highest, serving as a repository for DPSCs in transplantation. Intramuscular injection also avoids the lung “first pass effect” compared with intravenous injection, prolonging the survival of transplanted stem cells in the body and thus providing a sustained delocalized benefit for systemic diseases[71]. Datta et al[67] compared the effects of intramuscular and intravenous injection of DPSCs on diabetic neuropathy, and a more rapid improvement in neuropathic symptoms was observed for DPSC intravenous transplantation. However, DPSC intramuscular injection, especially after repeated administration, maintained the improved inflammatory state[67]. The study of Hata et al[14] also confirmed that the therapeutic effects of DPSC transplantation with a single intramuscular injection lasted for prolonged periods[14], further demonstrating the continued efficacy of DPSC intramuscular administration. Another study demonstrated that the efficacies of DPSC intramuscular transplantation were limited to the administration site, but it was difficult to play a role on the opposite side[81], possibly related to the number of DPSCs that migrated.
In summary, intravenously administered DPSCs are distributed mainly in the lungs and can also be transferred to the injured area; however, locally injected DPSCs are less likely to migrate to other parts of the body, whereas intramuscularly injected DPSCs may enter into the blood vessels and metastasize but are distributed mainly in the local muscles and less in the lungs and persist longer in vivo. Intravenous administration works more quickly than intramuscular administration, but the effects of intramuscular transplantation last longer, while the effect of local injection is mostly limited to the site of administration. In conclusion, regardless of the injection mode, DPSC injections have shown certain therapeutic effects in the treatment of various diseases.
The combination of cell therapy and genetic engineering has resulted in genetically engineered cells, which are considered to have greater prospects of therapeutic potential and efficient treatment than nonengineered cell therapy approaches[85]. Although there are still many factors to consider before genetically modified stem cells can be directly used in regenerative medicine[86], gene therapy, as an increasingly mature discipline, has profoundly influenced the development of regenerative medicine. Genetically engineered cell therapy using primary cells that overexpress tissue-specific or therapeutic genes makes it possible to produce therapeutic proteins at sites of regeneration or to differentiate new cells into the desired cellular lineage and thus promote tissue regeneration[87]. Obtaining the carrier cells, genetically modifying and expanding the cells in vitro and then using the cells for disease therapeutic strategies in vivo is a common method of applying genetically engineered cell therapy[88]. Genetically engineered cell therapy often uses MSCs as gene delivery vectors due to their accessibility for genetic modification in vitro and their ability to be cultured and expanded in vitro[85,87-89]. DPSCs, a type of MSC, are characterized by self-renewal, multipotent differentiation potential and amplification in vitro, as well as easy access, low risk of immune rejection and fewer ethical issues; DPSCs are also considered ideal gene vehicles with wide application prospects[16].
Compared with unmodified DPSCs, genetically modified DPSCs have been shown to be more potent in treating various diseases. For instance, compared with DPSCs, DPSCs overexpressing hepatocyte growth factor have been shown to dramatically relieve the disease activity of dextran sulfate sodium-induced ulcerative colitis[16], promote improvements in postischemia/reperfusion brain injury[90], promote the grafted DPSC-induced hepatic functional recovery from liver cirrhosis in a rat model[91], significantly improve periodontal bone regeneration in swine[92] and have a stronger capacity to significantly reduce ovariectomy-induced bone loss in the trabecular bone of the distal femur metaphysis[93]. Similarly, Rizk et al[94] engineered sizable three-dimensional (3D) cartilage-like constructs using human DPSCs, and the results showed that constructs with transforming growth factor (TGF)-β3-DPSCs showed higher collagen type II and Sox9 mRNA expression than nontransduced DPSC constructs in vivo[94]. Gene therapy using Runx2-modified DPSCs has also been reported to be more effective in tibial distraction osteogenesis during bone deposition and new bone formation[95].
These studies indicated that genetically modified DPSCs can not only play the role of DPSCs themselves but also secrete specific therapeutic proteins to enhance their therapeutic effects, which is a major direction of innovative applications of DPSCs.
Recently, as a cell transplantation system that requires no scaffolds or carriers, cell sheet engineering has gradually become the research focus of regenerative medicine based on cell therapies and has been used for regenerative treatment of the esophagus, cornea, heart, etc[96-98]. In contrast to conventional tissue engineering approaches, cell sheet technology allows cell harvest as a continuous cell sheet with intact extracellular matrix (ECM) proteins and cell-cell junctions, which facilitates cell transplantation without any other artificial biomaterials[99]. Compared with the traditional tissue engineering of cell sus
According to previous reports, the initial study used temperature-responsive cell culture dishes to prepare the DPSC sheet and used it to successfully reconstruct the corneal epithelium[102]. The dish responds to temperature changes, allowing the formed DPSC sheets to automatically shed. Subsequent studies also confirmed that DPSC sheets can be prepared within 3-4 d using temperature-responsive cell culture dishes[103,104]. Although this method is not time-consuming, it requires the use of special materials and complicated production procedures, which limit its expanded use. Therefore, some researchers have developed a method of using vitamin C (VC) to induce DPSCs to form cell sheets[105], and it has gradually become the mainstream preparation method.
VC is an essential micronutrient for humans, a potent antioxidant and a cofactor for a family of biosynthetic and gene regulatory enzymes, and VC plays an important role in supporting the function of the immune system[106]. VC also plays a key role in the biosynthesis of collagen and other ECM constituents[107,108] and promotes the proliferation of stem cells without affecting their differentiation potential[109]. Therefore, some researchers have predicted that VC alone may induce cell sheet formation, streamline production procedures or avoid using special materials, and confirmed this prediction with their studies, developing a simple and inexpensive VC-mediated procedure to obtain MSC sheets[105]. The authors also explored the mechanism of VC-induced cell sheet formation, and the optimal dose of VC showed that VC is capable of inducing telomerase activity in MSCs, leading to upregulated expression of ECM and stem cell markers. Meanwhile, VC induces MSCs to form cell sheets in a dose-dependent manner, and 20 μg/mL VC is the optimal concentration for complete cell sheets with a high level of success. However, in the existing reports, the concentration of VC alone to induce DPSCs to form sheets ranged from 10 μg/mL to 100 μg/mL[110-114], incubated continuously for 10-15 d or until the edge of the cell sheet became slightly rolled up or spontaneously detached from the bottom of the dish, all of which resulted in the formation of operable sheets consisting of 2-3 layers of cells. It has even been reported that the harvested whole DPSC sheet contained five or six layers of cells[17]. Although the use of VC has the above characteristics, the time-consuming disadvantage is also very prominent.
In addition, some other studies have seeded cells on the surface of the amniotic membrane (AM)[115,116] or even directly cultured them in basic medium[117] to obtain DPSC sheets. For example, after DPSCs were seeded on the amniotic membrane and cultured for 2 wk, the cells became confluent and formed 1-3 layers of cell sheets that adhered to the basement membrane AM[115]. DPSC sheets have also been reported to be composed of multilayer cells forming after 4 wk of culture in basal medium[117]. In addition to the above cell sheet preparation techniques, more recently, scholars have also used techniques such as near infrared triggering, light induction, and the combination of rough surfaces with thermoresponsive polymers to accelerate or improve the formation of cell sheets[118-120]. These techniques need to be further applied to the preparation of DPSC sheets.
To date, use of DPSC sheets has been reported for the regenerative treatment of a variety of injuries, including but not limited to dental pulp diseases[112], periodontal tissue diseases[121], bone defects[122], nerve injuries[123,124], etc., and all of these applications have achieved significant results. In one representative clinical study, autologous DPSC sheets from deciduous teeth were transplanted into injured young permanent teeth due to trauma. This transplantation was able to regenerate whole dental pulp, increase the length of the root and reduce the width of the apical foramen at 12 mo after treatment[125]. Furthermore, the evaluation of DPSC sheets in a rat facial nerve crush injury model in vivo established that in comparison to untreated controls, nerves treated with dental pulp cell sheets had greater axon regeneration through the injury site and superior functional recovery as quantitatively assessed by compound muscle action potential measurements, possibly because the DPSC sheets can highly express neurotrophic factor (NTF) and continuously deliver the NTF to sites of peripheral nerve injury[123]. Moreover, bioengineered teeth using human DPSC aggregates combined with decellularized tooth matrix or avulsed teeth after traumatic dental injuries can regenerate 3D dental pulp and periodontium equipped with vasculature and innervation in both a preclinical pig model and a pilot clinical trial for treating tooth avulsion[126].
In summary, in cell-based regenerative medicine, the application of cells into the injured site using cell sheets shows a significant increase in the therapeutic effect compared to dissociated cell injections, which may be related to the form of cell sheets being able to provide a large number of seed cells and improve the survival rate of transplanted cells[127,128]. DPSC sheets have also been reported to be more effective in repairing periodontal bone defects and regenerating soft tissue than pulp stem cell injections[17]. Moreover, the applications of decellularized cell sheets suggest that in addition to the function of seed cells in the sheets, extracellular matrix may also play a certain role in regeneration[129]. For example, a decellularized matrix of DPSC sheets can promote the proliferation and osteogenic differentiation of inoculated human periodontal ligament stem cells[130].
Most studies in cell biology are performed on a two-dimensional (2D) culture basis, although these studies facilitate microscopic analysis and medium changes and sustain cell proliferation for most cell types. However, this is generally not considered the natural microenvironment of the cells[131]. A cell spheroid is a 3D aggregation of cells, which is considered to be closer to the microenvironment in vivo because its formation mode simulates the natural processes of cells undergoing biological self-assembly to form complex tissues with 3D architecture and intensive cell–cell contacts from the perspective of embryonic development[132]. Cell spheroids have been proven to be able to mimic the architectural and functional characteristics of native tissue. For example, liver spheroids constructed in vitro by liver cells and endothelial cells have an ultrastructure of liver tissue, such as bile canaliculus-like and Disse’s space-like structures, and show stable albumin secretion and ammonia removal activity[133]. 3D lung spheroids of outgrowth cells from healthy lung tissue explants can be expanded to a large quantity and can form alveoli-like structures and acquire mature lung epithelial phenotypes in vitro[134]. The introduction of endothelial cells can form capillary networks in spheroids from different kinds of cells, which is conducive to anastomosing with the host vasculature after transplantation and prolonging the survival time of cell spheroids[135-137]. In view of the above characteristics, cell spheroids are widely believed to be able to be used as excellent candidates for basic units of 3D tissue engineering constructs, thus providing new strategies for tissue defect repair and reconstruction.
At present, techniques to form cell spheroids include mainly pellet culture, spinner culture, hanging drop (HD), liquid overlay, rotating wall vessel, external force, microfluidics, micromolded nonadhesive hydrogels[138], microwell culture, medium regulation, bioreactors[139] and bioactive materials such as cellulose hydrogel film[140,141]. There are also methods to generate cell spheroids by using cell sheets as prophase tissues[142] or culturing in specific charged culture dishes based on polyion complex nanoparticles[143]. Some of these methods are still in the research stage and are not ready to be adopted for large-scale manufacturing. The manufacturing methods of DPSC spheroids have also emerged in an endless series, including but not limited to serum-free medium culture[144,145], culturing on Matrigel[146], special 3D Petri dish culture[135,147], low or ultralow attachment culture plates[62,148], culturing on gelatin methacrylamine/poly(ethylene glycol) diacrylate (GelMA/PEGDA) composite hydrogels[149], coculturing with microparticles with a leaf-stacked structure based on polycaprolactone[150], HD or molded parafilm-based methods[151]. Most of these methods belong to the classification of the aforementioned technologies.
Regardless of the method, the prepared DPSC spheroids basically have spherical or spheroid-like shapes, ranging in diameter from microns to millimeters. Since the typical viable rim of cells in spheroids is approximately 100–300 μm, cells die in the center of large spheroids due to the lack of oxygen and/or nutrients, accumulation of waste products and low pH[146]. Therefore, a larger spheroid diameter is not better. Histological examination of cell spheroids at the micron level revealed that spheroids were compact throughout with small single cells evenly distributed after 24 h of culture[147], while a diversity of nuclei in the spheroids after 1 wk of culture suggested that cells in the spheroid were multitype[145]. Real-time reverse transcriptase-polymerase chain reaction analysis also demonstrated that the stemness/pluripotency markers Oct4, Sox2, NANOG, TP63, and CD44 were expressed in 3D cultured DPSCs, and the expression level was significantly increased when compared to 2D cultured DPSCs[145,147]. In addition, compared to 2D cultured DPSCs, the osteogenic, adipogenic, odontogenic differentiation potential and migration ability of DPSC spheroids are also enhanced[145,147,152-154]. These results indicate that the stemness of 3D cultured DPSCs is maintained while the multilineage differentiation potential could be enhanced, which may be related to the improvement of signal transmissions between cells.
DPSC spheroids have been proved to be able to differentiate into specific phenotypic cells, or simulate the structural and functional characteristics of the corresponding tissues, and play a therapeutic role. For example, evidence that DPSC spheroids can differentiate into neuron-like cells with potential functions under neurogenic induction in vitro has been reported[155]. The expression of neuronal markers such as microtubule-associated protein 2 in DPSC spheroids was increased after culture in neurogenic maturation medium or with the addition of central nervous system mitogens such as EGF and bFGF[156,157]. These DPSC spheroids are able to differentiate into functional neuronal cells and stimulate neurogenesis in the adult mouse hippocampus through neurotrophic support in vitro[158]. Dissanayaka et al[135] also confirmed that DPSCs support the survival of the co-cultured endothelial cells, and they can self-assemble into microtissue spheroids within the microwells of an agarose mold. Combined with tooth-root slices, these prevascularized, scaffold-free, microtissue spheroids could successfully regenerate vascular dental pulp-like tissue in immunodeficient mice[135]. In addition to spherical cell spheroids, there are some irregular cell aggregates constructed by 3D cell culture technology, which are still multicellular units of DPSCs in nature and have corresponding regenerative therapeutic effects. For instance, some scholars obtained rod-like 3D DPSC constructs by shaping sheet-like aggregates of DPSCs with a thermoresponsive hydrogel, which could form blood vessel-rich pulp-like tissues in nude mice[159].
If the cell sheet is still a cell aggregate in a two-dimensional concept, cell spheroids are advancing the concept of 3D tissue engineering. In 3D cell spheroids, cells are in close contact with each other and surrounded by extracellular matrix, enabling the simulation of cell-to-cell interactions and cell-extracellular matrix interactions in vitro. These processes are very important for signal transmission between cells and guiding cell behaviors such as movement, proliferation and differentiation[138]. Current studies have confirmed that these characteristics can enhance the properties of DPSCs in 3D cell spheroids, more studies are needed to explore the therapeutic advantages of DPSC spheroids compared with other DPSC products.
The above derivatives based on DPSCs themselves can be used alone or in combination with bioscaffold materials for the treatment of diseases, which will not be discussed here. Figure 1 shows an overview of the main sources of DPSCs, their derivatives and examples of their applications in regenerative medicine.
For years, scholars have failed to fully understand the fate of implanted injured stem cells and their role in regeneration. The results of some studies show that the implanted stem cells can stay in situ and differentiate into corresponding cells, thus directly participating in the process of regeneration[25-29]. Researchers have preliminarily revealed this process through stem cell labeling techniques, such as green fluorescent protein (GFP)/BrdU labeling[26], superparamagnetic iron oxide[160], and fluorescence-based tracing[161]. For example, BrdU-labeled MSCs migrated into the entire periodontal tissue, including the periodontal ligament, alveolar bone, cementum and blood vessels, and differentiated into periodontal ligament fibroblasts and osteoblasts 6 wk after implantation in periodontal defects, confirming that MSC transplantation has the potential to regenerate periodontal tissue and that transplanted MSCs are at least partially directly involved in the formation of new tissue[29]. The findings of Hasegawa et al[25] also supported this conclusion[25]. Another example is the combination of Dil (a fluorescent dye) or GFP-prelabeled hESC-MSCs with the simulated tendon complex to form tissue-engineered tendons, which were then ectopically transplanted into the back of nude mice or orthotopically transplanted into the impaired rat Achilles tendon. Four weeks after transplantation, the transplanted MSCs partially survived and differentiated into the tenocyte lineage, and functional tendons were regenerated successfully[27].
Similarly, transplanted DPSCs have been proven to be partially involved directly in the process of tissue regeneration. For instance, 5 wk after DPSCs stably transduced with GFP, GFP-DPSCs were seeded into tooth slices/scaffolds and transplanted into the subcutaneous space in the dorsum of immunodeficient mice. The DPSCs were observed to differentiate into endothelial cells and form neovascularization anastomosed with host vessels by immunohistochemistry and immunofluorescence staining. This process may be related to the activation of the Wnt/β-catenin signaling pathway by vascular endothelial growth factor (VEGF)[162]. Luzuriaga et al[163] also confirmed that human CD31+/CD146+ and Nestin + DPSC-derived cells can survive 1 mo after grafting into the brains of nude mice, expressing CD31 and VEGF, forming full blood vessels of human origin and integrating into the host brain vasculature[163]. In short, these results suggest that transplanted stem cells can partially survive and directly participate in the process of tissue repair and regeneration.
In addition, stem cell transplantation has also shown promising results in the treatment of immune-related diseases[164,165], which suggests that stem cell transplantation may be involved in the process of immune regulation in the host. Previous studies have confirmed that MSCs have the characteristics of immunosuppression in vivo and in vitro and are capable of regulating immune cells, such as T cells, B cells, DCs, and NK cells[21]. MSCs can efficiently suppress the proliferation of T cells[166] and reduce the production and release of cytokines such as interferon (IFN)-γ and interleukin (IL)-17[167,168]. MSCs also efficiently inhibit the maturation of DCs and markedly impair a variety of functions of NK cells[169]. Furthermore, MSCs are able to inhibit the proliferation of B cells and their capacity to produce antibodies[20]. MSC-mediated immunosuppression may occur via the concerted action of chemokines and nitric oxide (NO). In the presence of IFN-γ, TNF-α, IL-1A, or IL-1β, MSCs can be stimulated to express several chemokines at high levels and inducible NO synthase. Chemokines drive T-cell migration into proximity with MSCs, where T-cell responsiveness is suppressed by NO, while blocking chemokine receptors could abolish immunosuppression[170]. However, there is a species variation in the mechanisms of MSC-mediated immunosuppression: Immunosuppression by cytokine-primed mouse MSCs is mediated by NO, whereas immunosuppression by cytokine-primed human MSCs is executed through indoleamine 2,3-dioxygenase (IDO). The similarity is that they both exert immunosuppression via the concerted action of chemokines and immune-inhibitory NO or IDO[19].
DPSCs have also been found to have immunomodulatory functions similar to those functions in MSCs. Therefore, DPSCs are considered promising candidates for cell therapy for a variety of immune- and inflammation-related diseases. Previous reports have demonstrated that DPSCs can suppress T cell proliferation and therefore might be suitable for preventing or treating T cell alloreactivity associated with hematopoietic or solid-organ allogeneic transplantation. The study also confirmed that DPSCs had a stronger inhibitory effect on the T cell response than BMSCs[171]. In addition, DPSCs can also inhibit acute allogeneic immune responses by their release of TGF-β as a result of allogeneic stimulation of T lymphocytes[172] and inhibit the proliferation of peripheral blood mononuclear cells (PBMCs) via the expression of soluble factors partly induced by the secretion of IFN-γ by activated PBMCs[173]. In another study, Toll-like receptors, key molecules that bridge the innate and adaptive immune responses, were shown to trigger the immunosuppression of DPSCs by upregulating the expression of TGF-β and IL-6[174]. In addition, DPSCs could induce activated T cell apoptosis in vitro and ameliorate inflammation-related tissue injuries in mice with colitis, which was associated with the expression of the Fas ligand (FasL). Knockdown of FasL expression reduced the immunoregulatory properties of DPSCs in the context of inducing T cell apoptosis[21]. These studies indicate that DPSCs exert their immunoregulatory functions mainly by inhibiting immune cells, especially T cells, including inhibiting proliferation, reducing the release of cellular inflammatory factors, and inducing apoptosis.
However, the proposal of secretomes, exosomes or EVs provides a new direction for us to understand how stem cells promote regeneration: Paracrine cues and derives a cell-free tissue engineering strategy. Many studies have confirmed that conditioned media/secretomes/exosomes/EVs from BMSCs, DPSCs or other types of stem cells can perform functions similar to the functions of stem cells themselves, promote the regeneration of damaged tissue or improve the severity of the disease[18,31,175,176]. Exosomes from different MSCs have been reported for the treatment of liver disease, kidney disease, cardiovascular disease, neurological disease, immune disease, and skin wounds[24,177]. Exosomes from MSCs may accomplish changes in the cellular microenvironment and the behavior of their neighboring cells by transferring factors that modulate different metabolic and signaling pathways, such as the maintenance of a dynamic and homeostatic environment and the ability to activate angiogenesis, proliferation, migration and differentiation of the main cell types involved in regeneration, thereby restoring tissue homeostasis and enabling cells within the tissue to recover, repair and regenerate[24,177]. In addition, some scholars believe that paracrine signaling is mainly responsible for the involvement of MSCs in the modulation of immune responses and the progression of diseases. Through the release of secretomes consisting of a diverse range of cytokines, chemokines, and EVs, MSCs convey regulatory messages to recipient immune cells in the microenvironment[178]. DPSC-derived exosomes have also been shown to alleviate cerebral ischemia–reperfusion injury by suppressing inflammatory responses, such as reducing the protein expression of IL-6, IL-1β, and TNF-α[18], suggesting that the immunomodulatory effect of stem cells may also be achieved through the paracrine pathway.
In conclusion, DPSCs may promote tissue repair and regeneration by directly differentiating into corresponding cells, exerting immunomodulatory effects, or by releasing paracrine substances such as exosomes to maintain microenvironment homeostasis and activate the functions of adjacent cells.
According to published data, DPSCs have become one of the important seed cells of regenerative medicine. Younger DPSCs, such as DPSCs from natal teeth or SHED, are more suitable as excellent candidates for regenerative medicine stem cell resource banks. However, due to the lack of abundant sources of natal teeth, SHED may become the most powerful source of DPSCs in the future. The establishment of a stem cell bank for SHED is an urgent problem to be solved. In addition, taking advantage of a specific side group of DPSCs may be the pathway to achieve accurate and efficient tissue regeneration. However, this does not mean that the use of the total population of DPSCs is meaningless. The possible reason could be that the tissue is composed of a variety of cells with different functions, and a certain subpopulation currently reflects only one or a few of its dominant functions. The use of a subpopulation may not be sufficient to regenerate well-organized native-like tissue. Therefore, the combined use of several side groups may be more sensible DPSCs can be used in the treatment of diseases in the form of single injections, cell sheets and cell spheroids, and their therapeutic effect can be enhanced by gene modifications. DPSC injections may be a useful method for the treatment of systemic diseases, but for the treatment of localized damaged tissue, which requires in situ tissue regeneration, how to keep the implanted DPSCs in situ is worth considering; therefore, cell sheets and cell spheroids with supports are more suitable in this case. Current data show that DPSCs may promote the improvement, repair and regeneration of diseased and injured tissues by means of immune regulation, paracrine signaling and direct differentiation into corresponding cells to occupy the injured site. However, the mechanism by which DPSCs promote regeneration is complex, and the above discussion does not address all of the mechanism. We should continue to pay attention to the new applications of DPSCs and improve the mechanism by which DPSCs promote diseased tissue recovery.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Capparè P, Italy; Moldovan C, Romania; Nath SG, India; Oliva J, United States S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3363] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 2. | Zhang SY, Ren JY, Yang B. Priming strategies for controlling stem cell fate: Applications and challenges in dental tissue regeneration. World J Stem Cells. 2021;13:1625-1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 3. | Potdar PD, Jethmalani YD. Human dental pulp stem cells: Applications in future regenerative medicine. World J Stem Cells. 2015;7:839-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (6)] |
| 4. | Yamada Y, Fujimoto A, Ito A, Yoshimi R, Ueda M. Cluster analysis and gene expression profiles: a cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials. 2006;27:3766-3781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Patil VR, Kharat AH, Kulkarni DG, Kheur SM, Bhonde RR. Long term explant culture for harvesting homogeneous population of human dental pulp stem cells. Cell Biol Int. 2018;42:1602-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Agha-Hosseini F, Jahani MA, Jahani M, Mirzaii-Dizgah I, Ali-Moghaddam K. In vitro isolation of stem cells derived from human dental pulp. Clin Transplant. 2010;24:E23-E28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Anitua E, Zalduendo M, Troya M. Autologous plasma rich in growth factors technology for isolation and ex vivo expansion of human dental pulp stem cells for clinical translation. Regen Med. 2019;14:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Kumar A, Kumar V, Rattan V, Jha V, Bhattacharyya S. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol Neurobiol. 2017;54:4672-4682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Sugiyama M, Iohara K, Wakita H, Hattori H, Ueda M, Matsushita K, Nakashima M. Dental pulp-derived CD31⁻/CD146⁻ side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2011;17:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Cristaldi M, Mauceri R, Tomasello L, Pizzo G, Pizzolanti G, Giordano C, Campisi G. Dental pulp stem cells for bone tissue engineering: a review of the current literature and a look to the future. Regen Med. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Capparè P, Tetè G, Sberna MT, Panina-Bordignon P. The Emerging Role of Stem Cells in Regenerative Dentistry. Curr Gene Ther. 2020;20:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Wang DR, Wang YH, Pan J, Tian WD. Neurotrophic effects of dental pulp stem cells in repair of peripheral nerve after crush injury. World J Stem Cells. 2020;12:1196-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, Kawase M, Go MJ, Adachi H, Yokota Y, Kirita T, Ohgushi H. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Hata M, Omi M, Kobayashi Y, Nakamura N, Miyabe M, Ito M, Ohno T, Imanishi Y, Himeno T, Kamiya H, Nakamura J, Miyachi H, Ozawa S, Miyazawa K, Mitani A, Nagao T, Goto S, Takebe J, Matsubara T, Naruse K. Sustainable Effects of Human Dental Pulp Stem Cell Transplantation on Diabetic Polyneuropathy in Streptozotocine-Induced Type 1 Diabetes Model Mice. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Alsaeedi HA, Koh AE, Lam C, Rashid MBA, Harun MHN, Saleh MFBM, Teh SW, Luu CD, Ng MH, Isa HM, Leow SN, Then KY, Bastion MC, Mok PL, Muthuvenkatachalam BS, Samrot AV, Swamy KB, Nandakumar J, Kumar SS. Dental pulp stem cells therapy overcome photoreceptor cell death and protects the retina in a rat model of sodium iodate-induced retinal degeneration. J Photochem Photobiol B. 2019;198:111561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Li N, Zhang Y, Nepal N, Li G, Yang N, Chen H, Lin Q, Ji X, Zhang S, Jin S. Dental pulp stem cells overexpressing hepatocyte growth factor facilitate the repair of DSS-induced ulcerative colitis. Stem Cell Res Ther. 2021;12:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Hu J, Cao Y, Xie Y, Wang H, Fan Z, Wang J, Zhang C, Wu CT, Wang S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 2016;7:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Li S, Luo L, He Y, Li R, Xiang Y, Xing Z, Li Y, Albashari AA, Liao X, Zhang K, Gao L, Ye Q. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 2021;54:e13093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 19. | Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Zhao S, Wehner R, Bornhäuser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Zhao Y, Wang L, Jin Y, Shi S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J Dent Res. 2012;91:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Scott SR, March KL, Wang IW, Singh K, Liu J, Turrentine M, Sen CK, Wang M. Bone marrow- or adipose-mesenchymal stromal cell secretome preserves myocardial transcriptome profile and ameliorates cardiac damage following ex vivo cold storage. J Mol Cell Cardiol. 2022;164:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 24. | Casado-Díaz A, Quesada-Gómez JM, Dorado G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front Bioeng Biotechnol. 2020;8:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 25. | Hasegawa N, Kawaguchi H, Hirachi A, Takeda K, Mizuno N, Nishimura M, Koike C, Tsuji K, Iba H, Kato Y, Kurihara H. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J Periodontol. 2006;77:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Demeter K, Herberth B, Duda E, Domonkos A, Jaffredo T, Herman JP, Madarász E. Fate of cloned embryonic neuroectodermal cells implanted into the adult, newborn and embryonic forebrain. Exp Neurol. 2004;188:254-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Chen JL, Yin Z, Shen WL, Chen X, Heng BC, Zou XH, Ouyang HW. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31:9438-9451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Jendelová P, Herynek V, Urdzíková L, Glogarová K, Kroupová J, Andersson B, Bryja V, Burian M, Hájek M, Syková E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Wei N, Gong P, Liao D, Yang X, Li X, Liu Y, Yuan Q, Tan Z. Auto-transplanted mesenchymal stromal cell fate in periodontal tissue of beagle dogs. Cytotherapy. 2010;12:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Hossein-Khannazer N, Hashemi SM, Namaki S, Ghanbarian H, Sattari M, Khojasteh A. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019;216:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Makino E, Nakamura N, Miyabe M, Ito M, Kanada S, Hata M, Saiki T, Sango K, Kamiya H, Nakamura J, Miyazawa K, Goto S, Matsubara T, Naruse K. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: Cell-free regenerative medicine for diabetic polyneuropathy. J Diabetes Investig. 2019;10:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 1983] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 33. | Wu W, Zhou J, Xu CT, Zhang J, Jin YJ, Sun GL. Derivation and growth characteristics of dental pulp stem cells from patients of different ages. Mol Med Rep. 2015;12:5127-5134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Shetty H, Kakade A, Shetty S, Neelakantan P, Nagar S, Desai RS, Beri K. Immunohistochemical characterization of stem cell and differentiation markers of the dental pulp of human natal teeth. Future Sci OA. 2018;4:FSO342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Robey PG. Stem cells near the century mark. J Clin Invest. 2000;105:1489-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, Santos CF, Nör JE. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 37. | Gonmanee T, Thonabulsombat C, Vongsavan K, Sritanaudomchai H. Differentiation of stem cells from human deciduous and permanent teeth into spiral ganglion neuron-like cells. Arch Oral Biol. 2018;88:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen LY, Liu SY, Chen L, Ding Y, Xuan K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. 2012;57:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Zhu SY, Yuan CY, Lin YF, Liu H, Yang YQ, Wong HM, Zhang CF, Wang PL, Gu M. Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs) and Dental Pulp Stem Cells (DPSCs) Display a Similar Profile with Pericytes. Stem Cells Int. 2021;2021:8859902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Xu JG, Gong T, Wang YY, Zou T, Heng BC, Yang YQ, Zhang CF. Inhibition of TGF-β Signaling in SHED Enhances Endothelial Differentiation. J Dent Res. 2018;97:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Hagar MN, Yazid F, Luchman NA, Ariffin SHZ, Wahab RMA. Comparative evaluation of osteogenic differentiation potential of stem cells derived from dental pulp and exfoliated deciduous teeth cultured over granular hydroxyapatite based scaffold. BMC Oral Health. 2021;21:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Kanafi MM, Ramesh A, Gupta PK, Bhonde RR. Influence of hypoxia, high glucose, and low serum on the growth kinetics of mesenchymal stem cells from deciduous and permanent teeth. Cells Tissues Organs. 2013;198:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Govindasamy V, Abdullah AN, Ronald VS, Musa S, Ab Aziz ZA, Zain RB, Totey S, Bhonde RR, Abu Kasim NH. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J Endod. 2010;36:1504-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Hollands P, Aboyeji D, Orcharton M. Dental pulp stem cells in regenerative medicine. Br Dent J. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Karaöz E, Doğan BN, Aksoy A, Gacar G, Akyüz S, Ayhan S, Genç ZS, Yürüker S, Duruksu G, Demircan PC, Sariboyaci AE. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010;133:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Yu S, Diao S, Wang J, Ding G, Yang D, Fan Z. Comparative analysis of proliferation and differentiation potentials of stem cells from inflamed pulp of deciduous teeth and stem cells from exfoliated deciduous teeth. Biomed Res Int. 2014;2014:930907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Pereira LO, Rubini MR, Silva JR, Oliveira DM, Silva IC, Poças-Fonseca MJ, Azevedo RB. Comparison of stem cell properties of cells isolated from normal and inflamed dental pulps. Int Endod J. 2012;45:1080-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Kim J, Park JC, Kim SH, Im GI, Kim BS, Lee JB, Choi EY, Song JS, Cho KS, Kim CS. Treatment of FGF-2 on stem cells from inflamed dental pulp tissue from human deciduous teeth. Oral Dis. 2014;20:191-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, Tuan RS, Huang GT. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010;5:617-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 50. | Gnanasegaran N, Govindasamy V, Abu Kasim NH. Differentiation of stem cells derived from carious teeth into dopaminergic-like cells. Int Endod J. 2016;49:937-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Attar A, Eslaminejad MB, Tavangar MS, Karamzadeh R, Dehghani-Nazhvani A, Ghahramani Y, Malekmohammadi F, Hosseini SM. Dental pulp polyps contain stem cells comparable to the normal dental pulps. J Clin Exp Dent. 2014;6:e53-e59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Wang Z, Pan J, Wright JT, Bencharit S, Zhang S, Everett ET, Teixeira FB, Preisser JS. Putative stem cells in human dental pulp with irreversible pulpitis: an exploratory study. J Endod. 2010;36:820-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Ueda M, Fujisawa T, Ono M, Hara ES, Pham HT, Nakajima R, Sonoyama W, Kuboki T. A short-term treatment with tumor necrosis factor-alpha enhances stem cell phenotype of human dental pulp cells. Stem Cell Res Ther. 2014;5:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Sun HH, Chen B, Zhu QL, Kong H, Li QH, Gao LN, Xiao M, Chen FM, Yu Q. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials. 2014;35:9459-9472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Wang J, Wei X, Ling J, Huang Y, Gong Q, Huo Y. Identification and characterization of side population cells from adult human dental pulp after ischemic culture. J Endod. 2012;38:1489-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Ishikawa Y, Ida-Yonemochi H, Nakakura-Ohshima K, Ohshima H. The relationship between cell proliferation and differentiation and mapping of putative dental pulp stem/progenitor cells during mouse molar development by chasing BrdU-labeling. Cell Tissue Res. 2012;348:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Machado CV, Passos ST, Campos TM, Bernardi L, Vilas-Bôas DS, Nör JE, Telles PD, Nascimento IL. The dental pulp stem cell niche based on aldehyde dehydrogenase 1 expression. Int Endod J. 2016;49:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Murakami M, Horibe H, Iohara K, Hayashi Y, Osako Y, Takei Y, Nakata K, Motoyama N, Kurita K, Nakashima M. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials. 2013;34:9036-9047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Pisciotta A, Carnevale G, Meloni S, Riccio M, De Biasi S, Gibellini L, Ferrari A, Bruzzesi G, De Pol A. Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev Biol. 2015;15:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 60. | Hosoya A, Hiraga T, Ninomiya T, Yukita A, Yoshiba K, Yoshiba N, Takahashi M, Ito S, Nakamura H. Thy-1-positive cells in the subodontoblastic layer possess high potential to differentiate into hard tissue-forming cells. Histochem Cell Biol. 2012;137:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Alvarez R, Lee HL, Hong C, Wang CY. Single CD271 marker isolates mesenchymal stem cells from human dental pulp. Int J Oral Sci. 2015;7:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Chen H, Fu H, Wu X, Duan Y, Zhang S, Hu H, Liao Y, Wang T, Yang Y, Chen G, Li Z, Tian W. Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a+ stem cells. Sci Adv. 2020;6:eaay1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 63. | Matsui M, Kobayashi T, Tsutsui TW. CD146 positive human dental pulp stem cells promote regeneration of dentin/pulp-like structures. Hum Cell. 2018;31:127-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Nagpal A, Kremer KL, Hamilton-Bruce MA, Kaidonis X, Milton AG, Levi C, Shi S, Carey L, Hillier S, Rose M, Zacest A, Takhar P, Koblar SA. TOOTH (The Open study Of dental pulp stem cell Therapy in Humans): Study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int J Stroke. 2016;11:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Bonaventura G, Incontro S, Iemmolo R, La Cognata V, Barbagallo I, Costanzo E, Barcellona ML, Pellitteri R, Cavallaro S. Dental mesenchymal stem cells and neuro-regeneration: a focus on spinal cord injury. Cell Tissue Res. 2020;379:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Aliaghaei A, Boroujeni ME, Ahmadi H, Bayat AH, Tavirani MR, Abdollahifar MA, Pooyafar MH, Mansouri V. Dental pulp stem cell transplantation ameliorates motor function and prevents cerebellar atrophy in rat model of cerebellar ataxia. Cell Tissue Res. 2019;376:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Datta I, Bhadri N, Shahani P, Majumdar D, Sowmithra S, Razdan R, Bhonde R. Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy. 2017;19:1208-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Al-Serwi RH, El-Kersh AOFO, El-Akabawy G. Human dental pulp stem cells attenuate streptozotocin-induced parotid gland injury in rats. Stem Cell Res Ther. 2021;12:577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Hirose Y, Yamamoto T, Nakashima M, Funahashi Y, Matsukawa Y, Yamaguchi M, Kawabata S, Gotoh M. Injection of Dental Pulp Stem Cells Promotes Healing of Damaged Bladder Tissue in a Rat Model of Chemically Induced Cystitis. Cell Transplant. 2016;25:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Du ZH, Ding C, Zhang Q, Zhang Y, Ge XY, Li SL, Yu GY. Stem cells from exfoliated deciduous teeth alleviate hyposalivation caused by Sjögren syndrome. Oral Dis. 2019;25:1530-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 71. | Shahani P, Kaushal A, Waghmare G, Datta I. Biodistribution of Intramuscularly-Transplanted Human Dental Pulp Stem Cells in Immunocompetent Healthy Rats through NIR Imaging. Cells Tissues Organs. 2020;209:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Song M, Lee JH, Bae J, Bu Y, Kim EC. Human Dental Pulp Stem Cells Are More Effective Than Human Bone Marrow-Derived Mesenchymal Stem Cells in Cerebral Ischemic Injury. Cell Transplant. 2017;26:1001-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 73. | Zordani A, Pisciotta A, Bertoni L, Bertani G, Vallarola A, Giuliani D, Puliatti S, Mecugni D, Bianchi G, de Pol A, Carnevale G. Regenerative potential of human dental pulp stem cells in the treatment of stress urinary incontinence: In vitro and in vivo study. Cell Prolif. 2019;52:e12675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Sanches EF, Valentim L, de Almeida Sassi F, Bernardi L, Arteni N, Weis SN, Odorcyk FK, Pranke P, Netto CA. Intracardiac Injection of Dental Pulp Stem Cells After Neonatal Hypoxia-Ischemia Prevents Cognitive Deficits in Rats. Neurochem Res. 2018;43:2268-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Zhang X, Zhou Y, Li H, Wang R, Yang D, Li B, Fu J. Intravenous administration of DPSCs and BDNF improves neurological performance in rats with focal cerebral ischemia. Int J Mol Med. 2018;41:3185-3194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Omi M, Hata M, Nakamura N, Miyabe M, Ozawa S, Nukada H, Tsukamoto M, Sango K, Himeno T, Kamiya H, Nakamura J, Takebe J, Matsubara T, Naruse K. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res Ther. 2017;8:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Merckx G, Lo Monaco M, Lambrichts I, Himmelreich U, Bronckaers A, Wolfs E. Safety and Homing of Human Dental Pulp Stromal Cells in Head and Neck Cancer. Stem Cell Rev Rep. 2021;17:1619-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Kremer KL, Smith AE, Sandeman L, Inglis JM, Ridding MC, Koblar SA. Transcranial Magnetic Stimulation of Human Adult Stem Cells in the Mammalian Brain. Front Neural Circuits. 2016;10:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | El-Kersh AOFO, El-Akabawy G, Al-Serwi RH. Transplantation of human dental pulp stem cells in streptozotocin-induced diabetic rats. Anat Sci Int. 2020;95:523-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Lam C, Alsaeedi HA, Koh AE, Harun MHN, Hwei ANM, Mok PL, Luu CD, Yong TK, Subbiah SK, Bastion MC. Human Dental Pulp Stem Cells (DPSCs) Therapy in Rescuing Photoreceptors and Establishing a Sodium Iodate-Induced Retinal Degeneration Rat Model. Tissue Eng Regen Med. 2021;18:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Kanada S, Makino E, Nakamura N, Miyabe M, Ito M, Hata M, Yamauchi T, Sawada N, Kondo S, Saiki T, Minato T, Miyazawa K, Goto S, Matsubara T, Naruse K. Direct Comparison of Therapeutic Effects on Diabetic Polyneuropathy between Transplantation of Dental Pulp Stem Cells and Administration of Dental Pulp Stem Cell-Secreted Factors. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Nitahara-Kasahara Y, Kuraoka M, Guillermo PH, Hayashita-Kinoh H, Maruoka Y, Nakamura-Takahasi A, Kimura K, Takeda S, Okada T. Dental pulp stem cells can improve muscle dysfunction in animal models of Duchenne muscular dystrophy. Stem Cell Res Ther. 2021;12:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Kim S, Lee S, Jung HS, Kim SY, Shin SJ, Kang MK, Kim E. Evaluation of the Biodistribution of Human Dental Pulp Stem Cells Transplanted into Mice. J Endod. 2018;44:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Fang CZ, Yang YJ, Wang QH, Yao Y, Zhang XY, He XH. Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2013;8:e66748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Sheyn D, Mizrahi O, Benjamin S, Gazit Z, Pelled G, Gazit D. Genetically modified cells in regenerative medicine and tissue engineering. Adv Drug Deliv Rev. 2010;62:683-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Porteus M. Translating the lessons from gene therapy to the development of regenerative medicine. Mol Ther. 2011;19:439-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM, Gazit D. Review: gene- and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 2007;13:1135-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Bao Q, Zhao Y, Niess H, Conrad C, Schwarz B, Jauch KW, Huss R, Nelson PJ, Bruns CJ. Mesenchymal stem cell-based tumor-targeted gene therapy in gastrointestinal cancer. Stem Cells Dev. 2012;21:2355-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21:1513-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 90. | Sowa K, Nito C, Nakajima M, Suda S, Nishiyama Y, Sakamoto Y, Nitahara-Kasahara Y, Nakamura-Takahashi A, Ueda M, Kimura K, Okada T. Impact of Dental Pulp Stem Cells Overexpressing Hepatocyte Growth Factor after Cerebral Ischemia/Reperfusion in Rats. Mol Ther Methods Clin Dev. 2018;10:281-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 91. | Cao XF, Jin SZ, Sun L, Zhan YB, Lin F, Li Y, Zhou YL, Wang XM, Gao L, Zhang B. Therapeutic effects of hepatocyte growth factor-overexpressing dental pulp stem cells on liver cirrhosis in a rat model. Sci Rep. 2017;7:15812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Cao Y, Liu Z, Xie Y, Hu J, Wang H, Fan Z, Zhang C, Wang J, Wu CT, Wang S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res Ther. 2015;6:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 93. | Kong F, Shi X, Xiao F, Yang Y, Zhang X, Wang LS, Wu CT, Wang H. Transplantation of Hepatocyte Growth Factor-Modified Dental Pulp Stem Cells Prevents Bone Loss in the Early Phase of Ovariectomy-Induced Osteoporosis. Hum Gene Ther. 2018;29:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 94. | Rizk A, Rabie AB. Human dental pulp stem cells expressing transforming growth factor β3 transgene for cartilage-like tissue engineering. Cytotherapy. 2013;15:712-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Feng G, Zhang J, Feng X, Wu S, Huang D, Hu J, Zhu S, Song D. Runx2 modified dental pulp stem cells (DPSCs) enhance new bone formation during rapid distraction osteogenesis (DO). Differentiation. 2016;92:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 96. | Kanai N, Yamato M, Okano T. Cell sheets engineering for esophageal regenerative medicine. Ann Transl Med. 2014;2:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 97. | Sakaguchi K, Shimizu T, Okano T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J Control Release. 2015;205:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 98. | Hayashi R, Yamato M, Takayanagi H, Oie Y, Kubota A, Hori Y, Okano T, Nishida K. Validation system of tissue-engineered epithelial cell sheets for corneal regenerative medicine. Tissue Eng Part C Methods. 2010;16:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Baek J, Cho Y, Park HJ, Choi G, Lee JS, Lee M, Yu SJ, Cho SW, Lee E, Im SG. A Surface-Tailoring Method for Rapid Non-Thermosensitive Cell-Sheet Engineering via Functional Polymer Coatings. Adv Mater. 2020;32:e1907225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Guo R, Morimatsu M, Feng T, Lan F, Chang D, Wan F, Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 101. | Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, Okano T. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415-6422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 437] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 102. | Gomes JA, Geraldes Monteiro B, Melo GB, Smith RL, Cavenaghi Pereira da Silva M, Lizier NF, Kerkis A, Cerruti H, Kerkis I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 103. | Lee JM, Kim HY, Park JS, Lee DJ, Zhang S, Green DW, Okano T, Hong JH, Jung HS. Developing palatal bone using human mesenchymal stem cell and stem cells from exfoliated deciduous teeth cell sheets. J Tissue Eng Regen Med. 2019;13:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Watanabe M, Ohyama A, Ishikawa H, Tanaka A. Three-dimensional bone formation including vascular networks derived from dental pulp stem cells in vitro. Hum Cell. 2019;32:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Wei F, Qu C, Song T, Ding G, Fan Z, Liu D, Liu Y, Zhang C, Shi S, Wang S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227:3216-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 106. | Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 662] [Cited by in RCA: 983] [Article Influence: 122.9] [Reference Citation Analysis (0)] |
| 107. | Franceschi RT. The role of ascorbic acid in mesenchymal differentiation. Nutr Rev. 1992;50:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Villacorta L, Azzi A, Zingg JM. Regulatory role of vitamins E and C on extracellular matrix components of the vascular system. Mol Aspects Med. 2007;28:507-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Mekala NK, Baadhe RR, Rao Parcha S, Prameela Devi Y. Enhanced proliferation and osteogenic differentiation of human umbilical cord blood stem cells by L-ascorbic acid, in vitro. Curr Stem Cell Res Ther. 2013;8:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Yang X, Ma Y, Guo W, Yang B, Tian W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics. 2019;9:2694-2711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 111. | Pedroni ACF, Diniz IMA, Abe GL, Moreira MS, Sipert CR, Marques MM. Photobiomodulation therapy and vitamin C on longevity of cell sheets of human dental pulp stem cells. J Cell Physiol. 2018;233:7026-7035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Chen YJ, Zhao YH, Zhao YJ, Liu NX, Lv X, Li Q, Chen FM, Zhang M. Potential dental pulp revascularization and odonto-/osteogenic capacity of a novel transplant combined with dental pulp stem cells and platelet-rich fibrin. Cell Tissue Res. 2015;361:439-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Meng H, Hu L, Zhou Y, Ge Z, Wang H, Wu CT, Jin J. A Sandwich Structure of Human Dental Pulp Stem Cell Sheet, Treated Dentin Matrix, and Matrigel for Tooth Root Regeneration. Stem Cells Dev. 2020;29:521-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Garrido PR, Pedroni ACF, Cury DP, Moreira MS, Rosin F, Sarra G, Marques MM. Effects of photobiomodulation therapy on the extracellular matrix of human dental pulp cell sheets. J Photochem Photobiol B. 2019;194:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Honjo K, Yamamoto T, Adachi T, Amemiya T, Mazda O, Kanamura N, Kita M. Evaluation of a dental pulp-derived cell sheet cultured on amniotic membrane substrate. Biomed Mater Eng. 2015;25:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Takizawa S, Yamamoto T, Honjo KI, Sato Y, Nakamura K, Yamamoto K, Adachi T, Uenishi T, Oseko F, Amemiya T, Yamamoto Y, Kumagai W, Kita M, Kanamura N. Transplantation of dental pulp-derived cell sheets cultured on human amniotic membrane induced to differentiate into bone. Oral Dis. 2019;25:1352-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Tatsuhiro F, Seiko T, Yusuke T, Reiko TT, Kazuhito S. Dental Pulp Stem Cell-Derived, Scaffold-Free Constructs for Bone Regeneration. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 118. | Xu W, Chen S, Yao M, Jiang X, Lu Q. A Near-Infrared-Triggered Dynamic Wrinkling Biointerface for Noninvasive Harvesting of Practical Cell Sheets. ACS Appl Mater Interfaces. 2021;13:32790-32798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | Stanton MM, Lambert CR. A thermoresponsive, micro-roughened cell culture surface. Acta Biomater. 2015;15:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 120. | Jiang Z, Xi Y, Lai K, Wang Y, Wang H, Yang G. Laminin-521 Promotes Rat Bone Marrow Mesenchymal Stem Cell Sheet Formation on Light-Induced Cell Sheet Technology. Biomed Res Int. 2017;2017:9474573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 121. | Magalhães FD, Sarra G, Carvalho GL, Pedroni ACF, Marques MM, Chambrone L, Gimenez T, Moreira MS. Dental tissue-derived stem cell sheet biotechnology for periodontal tissue regeneration: A systematic review. Arch Oral Biol. 2021;129:105182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 122. | Pedroni ACF, Sarra G, de Oliveira NK, Moreira MS, Deboni MCZ, Marques MM. Cell sheets of human dental pulp stem cells for future application in bone replacement. Clin Oral Investig. 2019;23:2713-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Ahmed MN, Shi D, Dailey MT, Rothermund K, Drewry MD, Calabrese TC, Cui XT, Syed-Picard FN. Dental Pulp Cell Sheets Enhance Facial Nerve Regeneration via Local Neurotrophic Factor Delivery. Tissue Eng Part A. 2021;27:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 124. | Drewry MD, Dailey MT, Rothermund K, Backman C, Dahl KN, Syed-Picard FN. Promoting and Orienting Axon Extension Using Scaffold-Free Dental Pulp Stem Cell Sheets. ACS Biomater Sci Eng. 2022;8:814-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 125. | Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 126. | Guo H, Li B, Wu M, Zhao W, He X, Sui B, Dong Z, Wang L, Shi S, Huang X, Liu X, Li Z, Guo X, Xuan K, Jin Y. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials. 2021;279:121223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 127. | Sekine H, Shimizu T, Dobashi I, Matsuura K, Hagiwara N, Takahashi M, Kobayashi E, Yamato M, Okano T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A. 2011;17:2973-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 128. | Takeuchi R, Kuruma Y, Sekine H, Dobashi I, Yamato M, Umezu M, Shimizu T, Okano T. In vivo vascularization of cell sheets provided better long-term tissue survival than injection of cell suspension. J Tissue Eng Regen Med. 2016;10:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 129. | Gilpin A, Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int. 2017;2017:9831534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 483] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 130. | Huang JP, Wu YM, Liu JM, Zhang L, Li BX, Chen LL, Ding PH, Tan JY. Decellularized matrix could affect the proliferation and differentiation of periodontal ligament stem cells in vitro. J Periodontal Res. 2021;56:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, de Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 724] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 132. | Laschke MW, Menger MD. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017;35:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 133. | Lee D, Yoon H, Lee J, Lee K, Lee S, Kim S, Choi J, Kim Y, Park J. Enhanced liver-specific functions of endothelial cell-covered hepatocyte hetero-spheroids. Biochem Eng J. 2004;20:181-187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 134. | Henry E, Cores J, Hensley MT, Anthony S, Vandergriff A, de Andrade JB, Allen T, Caranasos TG, Lobo LJ, Cheng K. Adult Lung Spheroid Cells Contain Progenitor Cells and Mediate Regeneration in Rodents With Bleomycin-Induced Pulmonary Fibrosis. Stem Cells Transl Med. 2015;4:1265-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C. Scaffold-free Prevascularized Microtissue Spheroids for Pulp Regeneration. J Dent Res. 2014;93:1296-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 136. | Kim EM, Lee YB, Kim SJ, Park J, Lee J, Kim SW, Park H, Shin H. Fabrication of core-shell spheroids as building blocks for engineering 3D complex vascularized tissue. Acta Biomater. 2019;100:158-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 137. | Inamori M, Mizumoto H, Kajiwara T. An approach for formation of vascularized liver tissue by endothelial cell-covered hepatocyte spheroid integration. Tissue Eng Part A. 2009;15:2029-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012;12:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 375] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 139. | Liu D, Chen S, Win Naing M. A review of manufacturing capabilities of cell spheroid generation technologies and future development. Biotechnol Bioeng. 2021;118:542-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 140. | Tseng TC, Wong CW, Hsieh FY, Hsu SH. Biomaterial Substrate-Mediated Multicellular Spheroid Formation and Their Applications in Tissue Engineering. Biotechnol J. 2017;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 141. | Kim HJ, Castañeda R, Kang TH, Kimura S, Wada M, Kim U. Cellulose hydrogel film for spheroid formation of human adipose-derived stemcells. Cellulose. 2018;25:2589-2598. [DOI] [Full Text] |
| 142. | Byun H, Bin Lee Y, Kim EM, Shin H. Fabrication of size-controllable human mesenchymal stromal cell spheroids from micro-scaled cell sheets. Biofabrication. 2019;11:035025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 143. | Iwai R, Nemoto Y, Nakayama Y. Preparation and characterization of directed, one-day-self-assembled millimeter-size spheroids of adipose-derived mesenchymal stem cells. J Biomed Mater Res A. 2016;104:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 144. | Zheng Y, Jiang LI, Yan M, Gosau M, Smeets R, Kluwe L, Friedrich RE. Optimizing Conditions for Spheroid Formation of Dental Pulp Cells in Cell Culture. In Vivo. 2021;35:1965-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 145. | Xiao L, Tsutsui T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: stem cells in the core. J Cell Biochem. 2013;114:2624-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 146. | Xiao L, Kumazawa Y, Okamura H. Cell death, cavitation and spontaneous multi-differentiation of dental pulp stem cells-derived spheroids in vitro: a journey to survival and organogenesis. Biol Cell. 2014;106:405-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 147. | Son YB, Bharti D, Kim SB, Jo CH, Bok EY, Lee SL, Kang YH, Rho GJ. Comparison of Pluripotency, Differentiation, and Mitochondrial Metabolism Capacity in Three-Dimensional Spheroid Formation of Dental Pulp-Derived Mesenchymal Stem Cells. Biomed Res Int. 2021;2021:5540877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 148. | Kawashima N, Noda S, Yamamoto M, Okiji T. Properties of Dental Pulp-derived Mesenchymal Stem Cells and the Effects of Culture Conditions. J Endod. 2017;43:S31-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 149. | Han X, Tang S, Wang L, Xu X, Yan R, Yan S, Guo Z, Hu K, Yu T, Li M, Li Y, Zhang F, Gu N. Multicellular Spheroids Formation on Hydrogel Enhances Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells Under Magnetic Nanoparticles Induction. Int J Nanomedicine. 2021;16:5101-5115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 150. | Min TJ, Kim MJ, Kang KJ, Jeoung YJ, Oh SH, Jang YJ. 3D Spheroid Formation Using BMP-Loaded Microparticles Enhances Odontoblastic Differentiation of Human Dental Pulp Stem Cells. Stem Cells Int. 2021;2021:9326298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 151. | Kim H, Han Y, Suhito IR, Choi Y, Kwon M, Son H, Kim HR, Kim TH. Raman Spectroscopy-Based 3D Analysis of Odontogenic Differentiation of Human Dental Pulp Stem Cell Spheroids. Anal Chem. 2021;93:9995-10004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 152. | Yamamoto M, Kawashima N, Takashino N, Koizumi Y, Takimoto K, Suzuki N, Saito M, Suda H. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch Oral Biol. 2014;59:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 153. | Zhang S, Buttler-Buecher P, Denecke B, Arana-Chavez VE, Apel C. A comprehensive analysis of human dental pulp cell spheroids in a three-dimensional pellet culture system. Arch Oral Biol. 2018;91:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 154. | Hsieh HY, Young TH, Yao CC, Chen YJ. Aggregation of human dental pulp cells into 3D spheroids enhances their migration ability after reseeding. J Cell Physiol. 2018;234:976-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 155. | Li D, Zou XY, El-Ayachi I, Romero LO, Yu Z, Iglesias-Linares A, Cordero-Morales JF, Huang GT. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell Rev Rep. 2019;15:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 156. | Gervois P, Struys T, Hilkens P, Bronckaers A, Ratajczak J, Politis C, Brône B, Lambrichts I, Martens W. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev. 2015;24:296-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 157. | Rafiee F, Pourteymourfard-Tabrizi Z, Mahmoudian-Sani MR, Mehri-Ghahfarrokhi A, Soltani A, Hashemzadeh-Chaleshtori M, Jami MS. Differentiation of dental pulp stem cells into neuron-like cells. Int J Neurosci. 2020;130:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 158. | Xiao L, Ide R, Saiki C, Kumazawa Y, Okamura H. Human Dental Pulp Cells Differentiate toward Neuronal Cells and Promote Neuroregeneration in Adult Organotypic Hippocampal Slices In Vitro. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 159. | Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J Dent Res. 2018;97:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 160. | Farrell E, Wielopolski P, Pavljasevic P, Kops N, Weinans H, Bernsen MR, van Osch GJ. Cell labelling with superparamagnetic iron oxide has no effect on chondrocyte behaviour. Osteoarthritis Cartilage. 2009;17:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 161. | Bergenheim F, Seidelin JB, Pedersen MT, Mead BE, Jensen KB, Karp JM, Nielsen OH. Fluorescence-based tracing of transplanted intestinal epithelial cells using confocal laser endomicroscopy. Stem Cell Res Ther. 2019;10:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 162. | Zhang Z, Nör F, Oh M, Cucco C, Shi S, Nör JE. Wnt/β-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. Stem Cells. 2016;34:1576-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 163. | Luzuriaga J, Pastor-Alonso O, Encinas JM, Unda F, Ibarretxe G, Pineda JR. Human Dental Pulp Stem Cells Grown in Neurogenic Media Differentiate Into Endothelial Cells and Promote Neovasculogenesis in the Mouse Brain. Front Physiol. 2019;10:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 164. | Sarsenova M, Issabekova A, Abisheva S, Rutskaya-Moroshan K, Ogay V, Saparov A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 165. | Matheakakis A, Batsali A, Papadaki HA, Pontikoglou CG. Therapeutic Implications of Mesenchymal Stromal Cells and Their Extracellular Vesicles in Autoimmune Diseases: From Biology to Clinical Applications. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 166. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2363] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 167. | Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K, Basik M, Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994-6002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 168. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3276] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 169. | Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 170. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1570] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 171. | Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 172. | Kwack KH, Lee JM, Park SH, Lee HW. Human Dental Pulp Stem Cells Suppress Alloantigen-induced Immunity by Stimulating T Cells to Release Transforming Growth Factor Beta. J Endod. 2017;43:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 173. | Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 174. | Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G, Colic M. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 2011;20:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 175. | Fujio M, Xing Z, Sharabi N, Xue Y, Yamamoto A, Hibi H, Ueda M, Fristad I, Mustafa K. Conditioned media from hypoxic-cultured human dental pulp cells promotes bone healing during distraction osteogenesis. J Tissue Eng Regen Med. 2017;11:2116-2126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 176. | Mancuso P, Raman S, Glynn A, Barry F, Murphy JM. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front Bioeng Biotechnol. 2019;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 177. | Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 178. | Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |