Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.214
Peer-review started: November 5, 2021
First decision: December 4, 2021
Revised: December 12, 2021
Accepted: February 16, 2022
Article in press: February 16, 2022
Published online: February 26, 2022
Processing time: 111 Days and 21 Hours
Recently, we read with interest the article entitled “Unveiling the Morphogenetic Code: A New Path at the Intersection of Physical Energies and Chemical Signaling”. In this paper, the investigation into the systematic and comprehensive bio-effects of physical energies prompted us to reflect on our research. We believe that ultrasound, which possesses a special physical energy, also has a certain positive regulatory effect on macrophages, and we have already obtained some preliminary research results that support our hypothesis.
Core Tip: Because physical energies can contribute to the recovery of tissue damage in multiple aspects, it is widely used in clinical practice. The unique insights of the article “Unveiling the Morphogenetic Code: A New Path at the Intersection of Physical Energies and Chemical Signaling” inspired the direction of our experiments concerning the impact of physical energies on stem cells. In the future, we will conduct experiments and analytical techniques to reveal the mechanism of the regulatory effects behind ultrasound.
- Citation: Qin HC, Luo ZW, Zhu YL. Physical energy-based ultrasound shifts M1 macrophage differentiation towards M2 state. World J Stem Cells 2022; 14(2): 214-218
- URL: https://www.wjgnet.com/1948-0210/full/v14/i2/214.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i2.214
Recently, the article named “Unveiling the Morphogenetic Code: A New Path at the Intersection of Physical Energies and Chemical Signaling” contributed by the Editor-in-Chief Carlo Ventura[1] motivated us to reconsider the biological roles of physical energies. In that article, the authors provided a detailed summary of the developmental history of physical energy, especially bioelectricity, as well as its applications and prospects in stem cell research. We strongly support the proposition about the high efficacy of physical energy in tissue repair. An article recently released in “Science Translational Medicine” described how massage, which is also a physical stimulus, regulates muscle repair[2]. The systematic and accurate explanation of physical energies by the editor echoes our dedicated research, which is the effect of therapeutic ultrasound on macrophages.
As early as the 1920s, Wood and Loomis began to investigate ultrasound as a therapeutic intervention[3]. In recent years, one particular type of ultrasound, low-intensity pulsed ultrasound (LIPUS), has gained much attention. This is due to its non-thermal effects, such as acoustic cavitation and “cellular massage”, which produce a range of biological effects[4]. Clinically, LIPUS can be used as a non-invasive adjunctive therapy for a variety of diseases, such as fractures, muscle injury, osteoarthritis, as well as nerve injury[4]. Besides, LIPUS has received considerable attention in the discipline of stem cell research. Salgarella et al[5] proved the bio-effect of ultrasound therapy on the proliferation and differentiation of C2C12 myoblasts in vitro, while the research of Wang et al[6] indicated that LIPUS promotes the production of mesenchymal stem cells (MSC) derived mainly from bone marrow differentiation. Both of these studies verified the positive bio-effects of this therapeutic technique. Tan et al[7] listed recent studies regarding the action of LIPUS on various neural stem cells and concluded that LIPUS can stimulate stem cells in vitro, promote stem cell proliferation, differentiation, and migration, and maintain stem cell activity. The findings of Wu et al[8] suggested that LIPUS regulates the Notch signalling pathway in the central nervous system, causing neural stem cell proliferation and differentiation. Additionally, LIPUS can also accelerate tissue repair[9] and promote the dissipation of inflammation[10], angiopoiesis[11], etc.
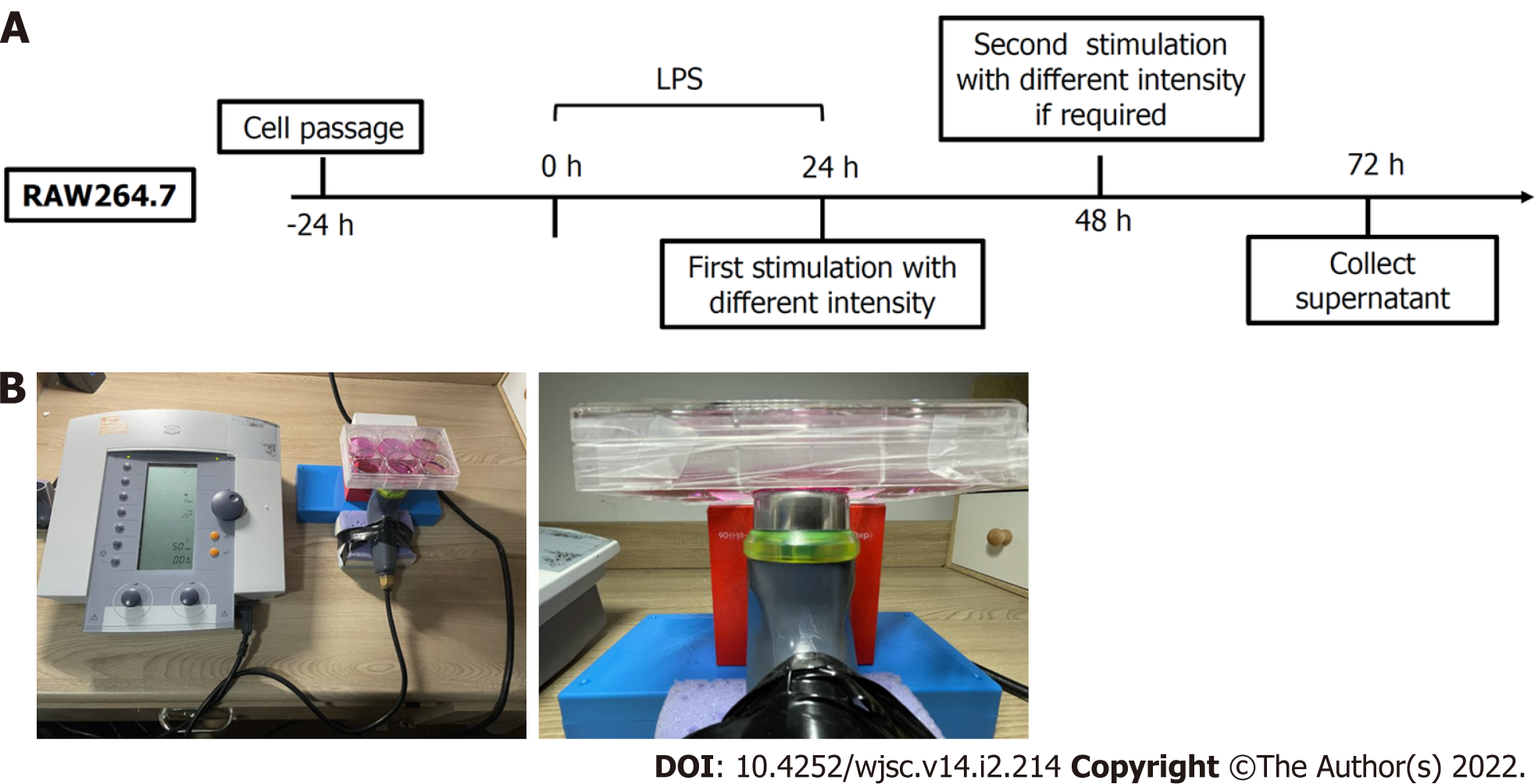
Our research focused on discovering how sound waves, a kind of physical energy, exert potential effects on macrophage polarisation, which is of great significance during the inflammation stage. To ensure accurate muscle regeneration, a balance between M1 and M2 activity (pro- and anti-inflammatory, respectively) shifting over time is required[12]. Previous studies have found that ultrasound may regulate macrophages in the spinal fusion model of male Sprague Dawley (SD) rats[13]. However, very few studies have explored the direct effect of ultrasound on macrophages in vitro. We induced macrophages into M1 macrophages using lipopolysaccharide to mimic the inflammatory microenvironment in vivo. Then, we collected and analysed the secretion composition and gene expression following ultrasound therapy. Details of the experiments are presented in Figure 1.
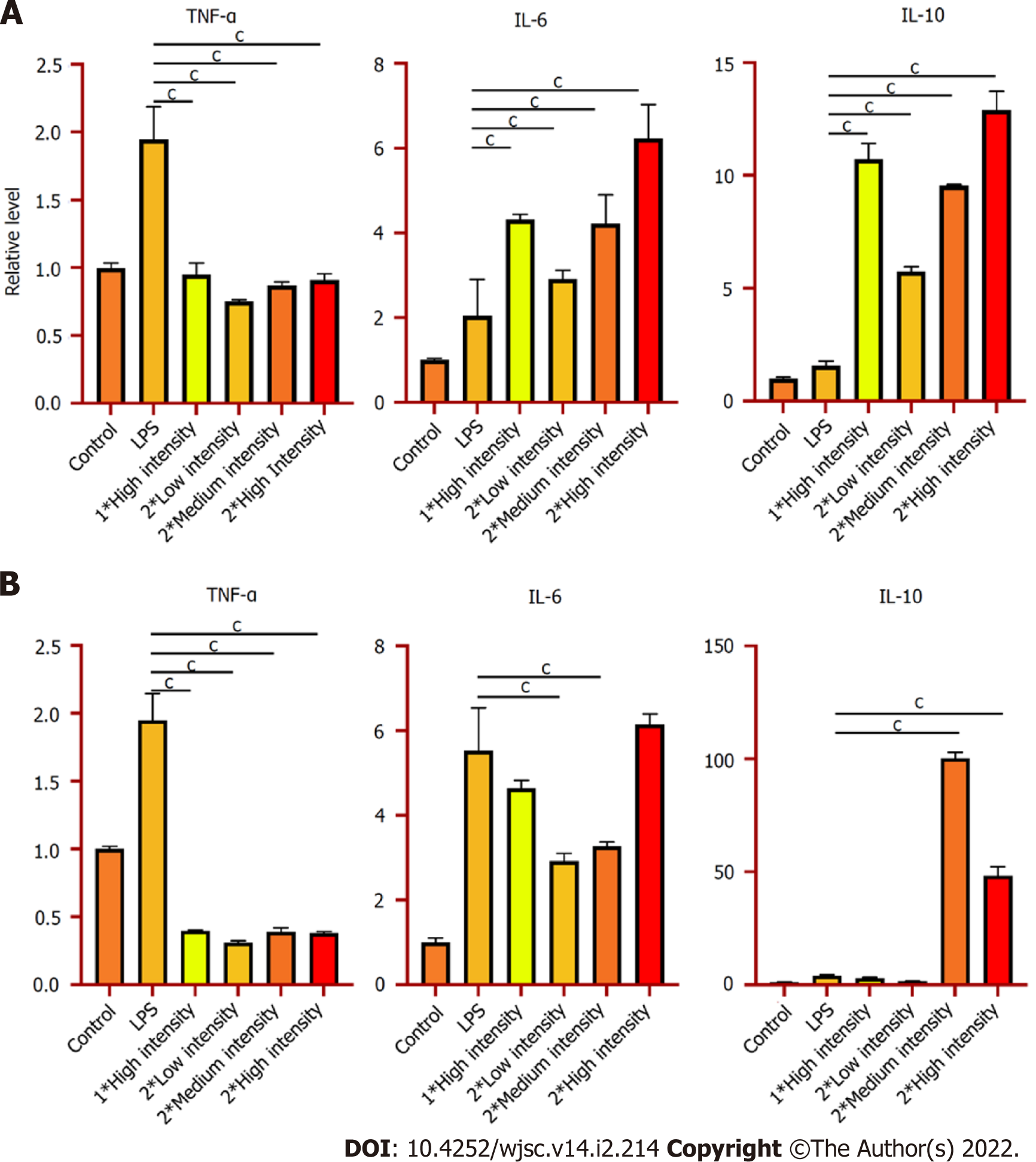
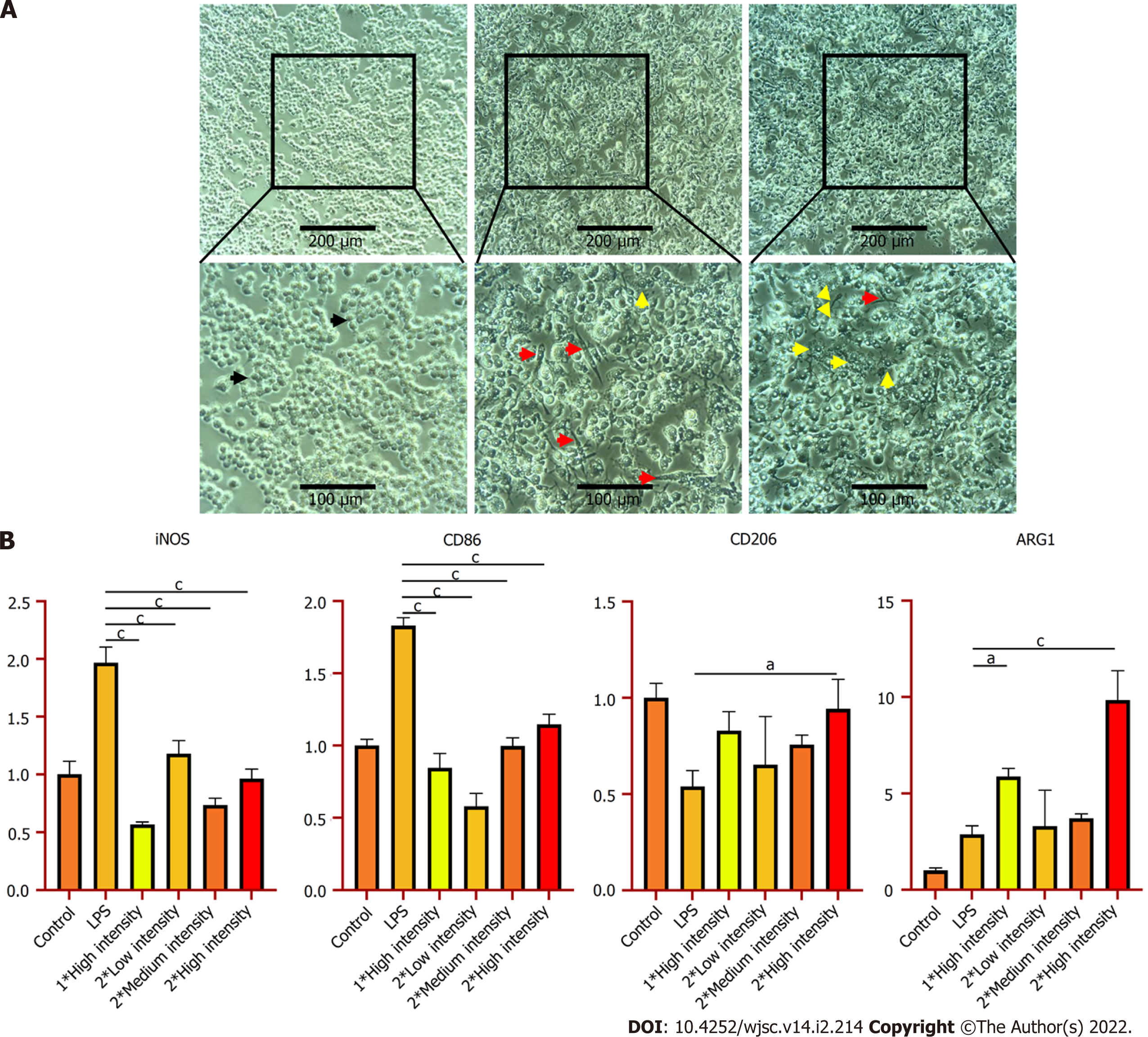
So far, we have already obtained some preliminary results. As Figure 2 indicates, after LIPUS treatment, the secretion of anti-inflammatory cytokine interleukin (IL)-10 significantly increased, while quantities of pro-inflammatory cytokines tumor necrosis factor-α and IL-6 fell substantially at genetic and secreted proteins levels. Besides, we determined that the phenotypes of the macrophages are polarised into M2 after ultrasound stimulation (Figure 3). According to the above experimental data, we can conclude that ultrasound facilitates the transition of macrophages from the M1 to M2 phenotype in vitro, which is consistent with da Silva Junior's discovery[14]. For subsequent research, we will further investigate the underlying mechanism of macrophage polarisation caused by LIPUS, and the potentially affected molecular pathways. Moreover, we will conduct both in vivo (skeletal muscle contusion model)[15] and in vitro experiments to verify the mechanism and ascertain how LIPUS exerts a series of downstream bio-effects.
Various studies have established that physical energies can modulate inflammation and further promote tissue repair. As a conventional form of physical energy, ultrasound has extensive application prospects due to its non-thermal mechanical effect. Accordingly, it warrants further investigation to elucidate its influence on cell signalling. We predict that subsequent research can be extended from the aspect of monotypic cell regulation to the integral tissue, by employing single-cell transcriptomic analysis and spatial transcriptomic analysis[16]. Thus, we will gain an increasingly comprehensive insight into the role of ultrasound in tissue repair.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ankrah AO, Prasetyo EP S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Tassinari R, Cavallini C, Olivi E, Taglioli V, Zannini C, Ventura C. Unveiling the morphogenetic code: A new path at the intersection of physical energies and chemical signaling. World J Stem Cells. 2021;13:1382-1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Seo BR, Payne CJ, McNamara SL, Freedman BR, Kwee BJ, Nam S, de Lázaro I, Darnell M, Alvarez JT, Dellacherie MO, Vandenburgh HH, Walsh CJ, Mooney DJ. Skeletal muscle regeneration with robotic actuation-mediated clearance of neutrophils. Sci Transl Med. 2021;13:eabe8868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, Chen J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans Biomed Eng. 2019;66:2704-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Lin G, Reed-Maldonado AB, Lin M, Xin Z, Lue TF. Effects and Mechanisms of Low-Intensity Pulsed Ultrasound for Chronic Prostatitis and Chronic Pelvic Pain Syndrome. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Salgarella AR, Cafarelli A, Ricotti L, Capineri L, Dario P, Menciassi A. Optimal Ultrasound Exposure Conditions for Maximizing C2C12 Muscle Cell Proliferation and Differentiation. Ultrasound Med Biol. 2017;43:1452-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Wang X, Lin Q, Zhang T, Wang X, Cheng K, Gao M, Xia P, Li X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res Ther. 2019;10:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Tan Y, Guo Y, Reed-Maldonado AB, Li Z, Lin G, Xia SJ, Lue TF. Low-intensity pulsed ultrasound stimulates proliferation of stem/progenitor cells: what we need to know to translate basic science research into clinical applications. Asian J Androl. 2021;23:602-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Wu Y, Gao Q, Zhu S, Wu Q, Zhu R, Zhong H, Xing C, Qu H, Wang D, Li B, Ning G, Feng S. Low-intensity pulsed ultrasound regulates proliferation and differentiation of neural stem cells through notch signaling pathway. Biochem Biophys Res Commun. 2020;526:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Yang B, Li M, Lei H, Xu Y, Li H, Gao Z, Guan R, Xin Z. Low Intensity Pulsed Ultrasound Influences the Myogenic Differentiation of Muscle Satellite Cells in a Stress Urinary Incontinence Rat Model. Urology. 2019;123:297.e1-297.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Zheng C, Wu SM, Lian H, Lin YZ, Zhuang R, Thapa S, Chen QZ, Chen YF, Lin JF. Low-intensity pulsed ultrasound attenuates cardiac inflammation of CVB3-induced viral myocarditis via regulation of caveolin-1 and MAPK pathways. J Cell Mol Med. 2019;23:1963-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Li J, Guo W, Yu F, Liu L, Wang X, Li L, Fang B, Xia L. Low-intensity pulsed ultrasound promotes angiogenesis via the AKT pathway and DNA methylation in human umbilical vein endothelial cells. Ultrasonics. 2022;118:106561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | De Santa F, Vitiello L, Torcinaro A, Ferraro E. The Role of Metabolic Remodeling in Macrophage Polarization and Its Effect on Skeletal Muscle Regeneration. Antioxid Redox Signal. 2019;30:1553-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Zhang ZC, Yang YL, Li B, Hu XC, Xu S, Wang F, Li M, Zhou XY, Wei XZ. Low-intensity pulsed ultrasound promotes spinal fusion by regulating macrophage polarization. Biomed Pharmacother. 2019;120:109499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | da Silva Junior EM, Mesquita-Ferrari RA, França CM, Andreo L, Bussadori SK, Fernandes KPS. Modulating effect of low intensity pulsed ultrasound on the phenotype of inflammatory cells. Biomed Pharmacother. 2017;96:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Luo Z, Lin J, Sun Y, Wang C, Chen J. Bone Marrow Stromal Cell-Derived Exosomes Promote Muscle Healing Following Contusion Through Macrophage Polarization. Stem Cells Dev. 2021;30:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 16. | van den Brink SC, Alemany A, van Batenburg V, Moris N, Blotenburg M, Vivié J, Baillie-Johnson P, Nichols J, Sonnen KF, Martinez Arias A, van Oudenaarden A. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature. 2020;582:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |