Published online Dec 26, 2022. doi: 10.4252/wjsc.v14.i12.851
Peer-review started: September 11, 2022
First decision: October 20, 2022
Revised: October 29, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: December 26, 2022
Processing time: 100 Days and 23.1 Hours
Ischemic stroke is a condition in which an occluded blood vessel interrupts blood flow to the brain and causes irreversible neuronal cell death. Transplantation of regenerative stem cells has been proposed as a novel therapy to restore damaged neural circuitry after ischemic stroke attack. However, limitations such as low cell survival rates after transplantation remain significant challenges to stem cell-based therapy for ischemic stroke in the clinical setting. In order to enhance the therapeutic efficacy of transplanted stem cells, several biomaterials have been developed to provide a supportable cellular microenvironment or functional modification on the stem cells to optimize their reparative roles in injured tissues or organs.
To discuss state-of-the-art functional biomaterials that could enhance the therapeutic potential of stem cell-based treatment for ischemic stroke and provide detailed insights into the mechanisms underlying these biomaterial approaches.
The PubMed, Science Direct and Scopus literature databases were searched using the keywords of “biomaterial” and “ischemic stroke”. All topically-relevant articles were then screened to identify those with focused relevance to in vivo, in vitro and clinical studies related to “stem cells” OR “progenitor cells” OR “undifferentiated cells” published in English during the years of 2011 to 2022. The systematic search was conducted up to September 30, 2022.
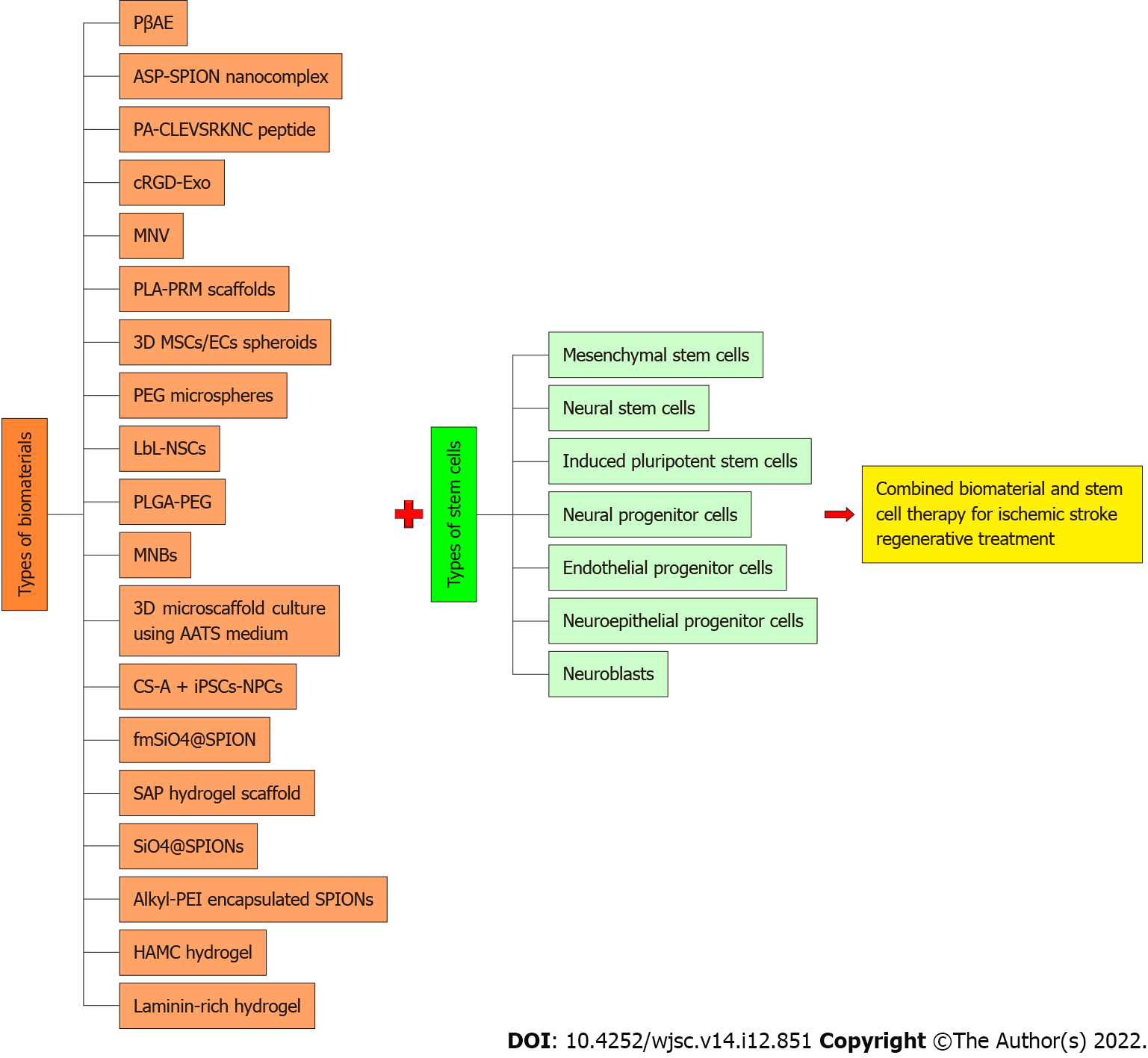
A total of 19 articles matched all the inclusion criteria. The data contained within this collection of papers comprehensively represented 19 types of biomaterials applied on seven different types of stem/progenitor cells, namely mesenchymal stem cells, neural stem cells, induced pluripotent stem cells, neural progenitor cells, endothelial progenitor cells, neuroepithelial progenitor cells, and neuroblasts. The potential major benefits gained from the application of biomaterials in stem cell-based therapy were noted as induction of structural and functional modifications, increased stem cell retention rate in the hostile ischemic microenvironment, and promoting the secretion of important cytokines for reparative mechanisms.
Biomaterials have a relatively high potential for enhancing stem cell therapy. Nonetheless, there is a scarcity of evidence from human clinical studies for the efficacy of this bioengineered cell therapy, highlighting that it is still too early to draw a definitive conclusion on efficacy and safety for patient usage. Future in-depth clinical investigations are necessary to realize translation of this therapy into a more conscientious and judicious evidence-based therapy for clinical application.
Core Tip: Ischemic stroke is becoming a significant health issue globally. An increasing number of studies have proposed the applications of regenerative stem cells for the treatment of this neurodegenerative disease. We critically reviewed the literature on biomaterial application to enhance the therapeutic potential of stem/progenitor cell therapy for ischemic stroke. Despite the limited evidence collected to translate this evidence into clinical practice, it is postulated that application of stem cells as regenerative treatment for stroke is practicable and beneficial for stroke patients, especially those in the chronic phase of stroke which could not be cured by any other established means.
- Citation: Mohd Satar A, Othman FA, Tan SC. Biomaterial application strategies to enhance stem cell-based therapy for ischemic stroke. World J Stem Cells 2022; 14(12): 851-867
- URL: https://www.wjgnet.com/1948-0210/full/v14/i12/851.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i12.851
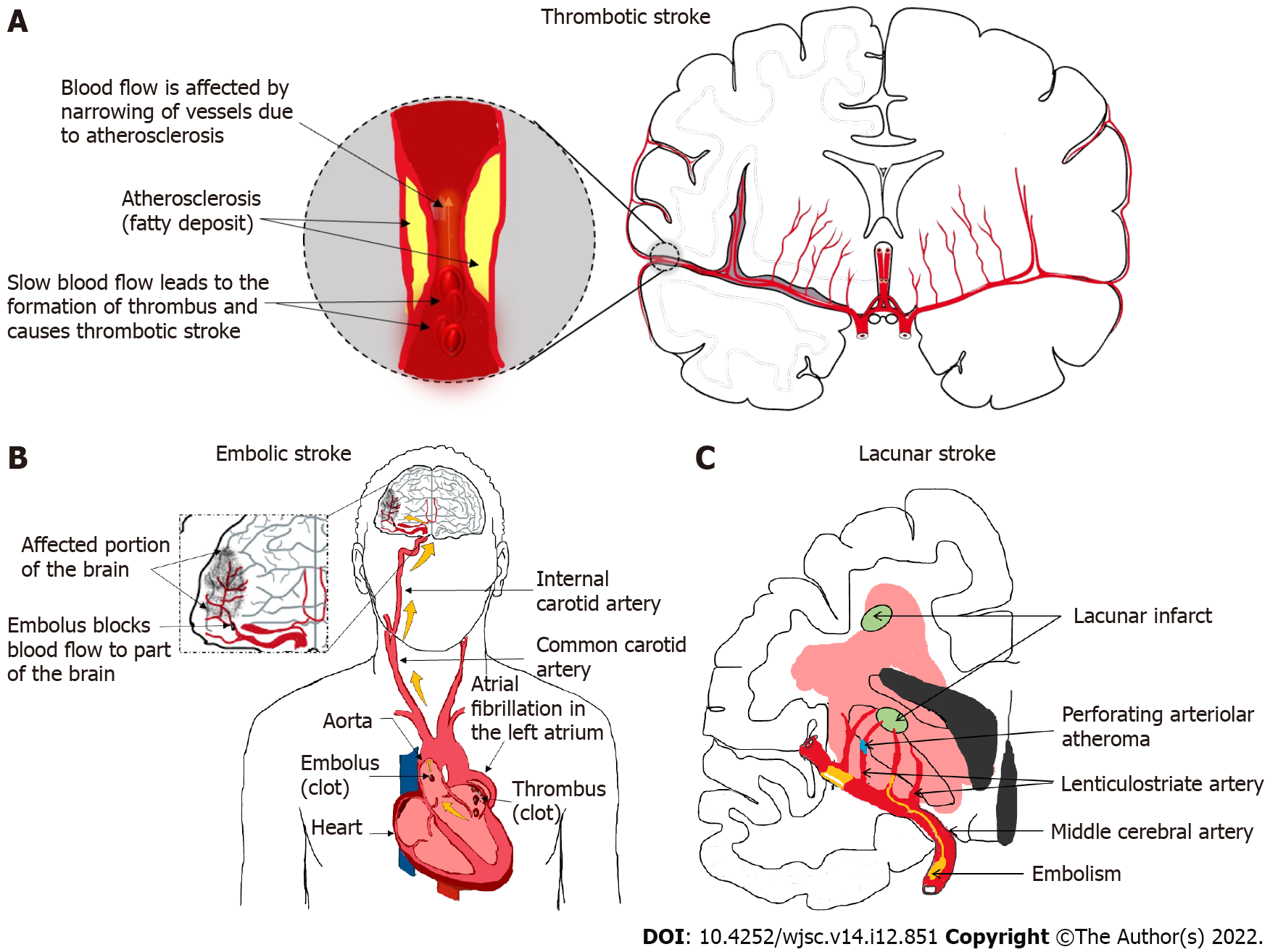
Ischemic stroke is a cerebrovascular disease that occurs when blood supply to part of a brain is interrupted, causing the affected brain portion to be deprived of oxygen and nutrients and subsequently leading to irreversible brain cell death. There are three major types of ischemic stroke, namely thrombotic stroke (Figure 1A), embolic stroke (Figure 1B), and lacunar stroke (Figure 1C). Thrombotic stroke occurs when a thrombus (blood clot) develops in the brain arteries and disrupts normal blood flow to the brain tissue that are supplied by those arteries. The thrombus normally develops due to atherosclerosis (deposit of a fatty substance called ‘plaque’ on the artery lining), which halts the flow of blood and causes the blood clumps to form blood clots[1]. On the other hand, embolic stroke is caused by an embolus (blood clot) being formed elsewhere in the body (e.g., heart or carotid arteries) and then traveling in the bloodstream until it reaches a blood vessel where its passage is blocked. Embolic stroke is usually associated with atrial fibrillation (i.e. abnormal heart rhythm, in which the atria does not beat effectively), itself eventually facilitating clot formation. It could also be caused by a clot dislodging from the atherosclerotic plaque formed in the aorta and carotid artery[2]. Last but not least, lacunar stroke happens when an occlusion occurs in a small artery in the brain that penetrates deep into the organ. Lacunar stroke is often associated with chronic hypertension, itself facilitating a small arteriole to become abnormal and susceptible to occlusion from micro-thrombi[3].
Human brain cells are permanent cells, being incapable of regeneration once matured. Brain cells also require a continuous supply of glucose and oxygen to support their heavy workload. These characteristics make brain cells vulnerable to any type of the ischemic stroke since any disruption of the blood supply to the brain, even within a few minutes, is sufficient to cause brain cell death and trigger permanent brain damage. Brain damage itself can lead to many devastating effects such as paralysis on one side of or the whole body, difficulty in speaking, inability to understand speech, and many other cognitive and neurological deficits. The adverse effect of stroke depends on which part of the brain is affected, whilst the severity of deficits depends on the extent of damage caused by the stroke.
Ischemic cell death could happen within minutes after a blood vessel occlusion. Due to acute onset and rapid deterioration, repairing ischemic damage in the brain has remained the most crucial, yet daunting, challenge of medical science. Immediate treatment is required in order to reduce complications from an ischemic stroke. Currently, clinical treatment for ischemic stroke disease mainly aims to restore blood flow to the ischemic penumbra in order to minimize the mortality and morbidity caused by brain damage and prevent recurrence or secondary complications. Nonetheless, such treatments cannot regenerate the brain tissues that have died. Therefore, the prognosis after current stroke treatments is very limited. According to the National Stroke Association, only 10% of stroke survivors exhibit full recovery, with an additional 25% showing mild disabilities and 50% showing moderate-to-severe disabilities which require special care[4]. This indicates that the current stroke treatments are not fully effective and are very limited according to their abilities to only minimize damage and reduce risk of stroke recurrence. Therefore, there is an urgent need for alternative treatments that will facilitate regeneration of damaged brain tissue for full recovery.
Recently, stem cells have emerged as a promising therapeutic agent for stroke patients due to their self-renewal and differentiation potentials[5]. Stem cells are present in many organs of the human body. These undifferentiated cells are capable of self-renewal and differentiate into specific functional cells depending on their lineages, in response to body conditions and requirements. Furthermore, under suitable stimulation in vitro, stem cells can also be induced to differentiate across cell type lineages. Using stem cells as regenerative treatment for stroke is, therefore, a practicable approach for ischemic stroke patients in the chronic phase of stroke.
Many stem cell types have been investigated to determine their feasibility, safety and efficacy in stem cell replacement therapy, for example, embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), bone marrow stem cells, umbilical cord blood stem cells, and neural stem cells (NSCs)[6,7]. On top of these, an emerging strategy was developed to enhance the potential of stem cells for ischemic stroke treatment by incorporating biomaterial application[8]. A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose. A carefully designed biomaterial could serve its purpose in the environment of the living body without causing toxicity to other bodily organs. Typically, a tissue engineering approach using a combination of biomaterial and stem cells involves growing stem cells on a three-dimensional scaffold material which could provide a supportable microenvironment for the stem cells to optimize their growth without affecting their therapeutic efficacy for regenerative treatments.
Due to the continuous research and progress in biomaterial sciences, there has been a rapid growth in applications of a vast variety of novel biomaterials in tissue engineering and regenerative medicine. However, there is no comprehensive report in the up-to-date literature that reviews the potential of all these biomaterials to enhance stem cell-based therapy. Therefore, we performed a systematic review of the literature databases to provide a comprehensive summarization of the recent findings regarding the efficacy of biomaterial strategies to enhance stem cell therapeutic potential for ischemic stroke treatment. The findings from this literature review will be beneficial due to its provision of collated updated information regarding the different types of biomaterials currently used by researchers, along with detailed descriptions of their key mechanisms, advantages and limitations, with an additional focus on stem cell-based ischemic stroke therapy.
A literature search was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify research documentation on application of biomaterials utilizing stem cell-based therapy for ischemic stroke diseases. Three carefully selected databases, namely PubMed, ScienceDirect, and Scopus, were used for the literature search using the keywords “biomaterial” and “ischemic stroke”. The resultant literature collection was further screened to identify all the articles relevant to in vivo, in vitro and clinical studies using “stem cell” OR “progenitor cells” OR “undifferentiated cells published in English during the years of 2011 to 2022. The systematic search was conducted up to September 30, 2022. When revising the manuscript, we applied the Reference Citation Analysis (https://www.referencecitationanalysis.com/) to supplement high quality research results.
Search results were limited to articles following the inclusion and exclusion criteria.
Inclusion criteria: (1) Full-text articles; (2) In vitro studies related to biomaterial application in stem cell-based therapy for ischemic stroke; (3) In vivo studies related to biomaterial application in stem cell-based therapy for ischemic stroke; and (4) Clinical studies related to biomaterial application in stem cell-based therapy for ischemic stroke.
Exclusion criteria: (1) Irrelevant titles and abstracts; (2) Duplicated studies; (3) Review articles/meta-analyses; (4) News/editorials/letters; (5) Case reports; and (6) Non-English language.
Three independent reviewers screened the databases and selected articles with potential relevance based on the inclusion and exclusion criteria stated above. For the first screening, the related articles were screened based on their titles and abstracts. Next, the remaining papers were checked for duplications. Finally, the selected full-text articles were checked by another reviewer according to the inclusion criteria for final validation.
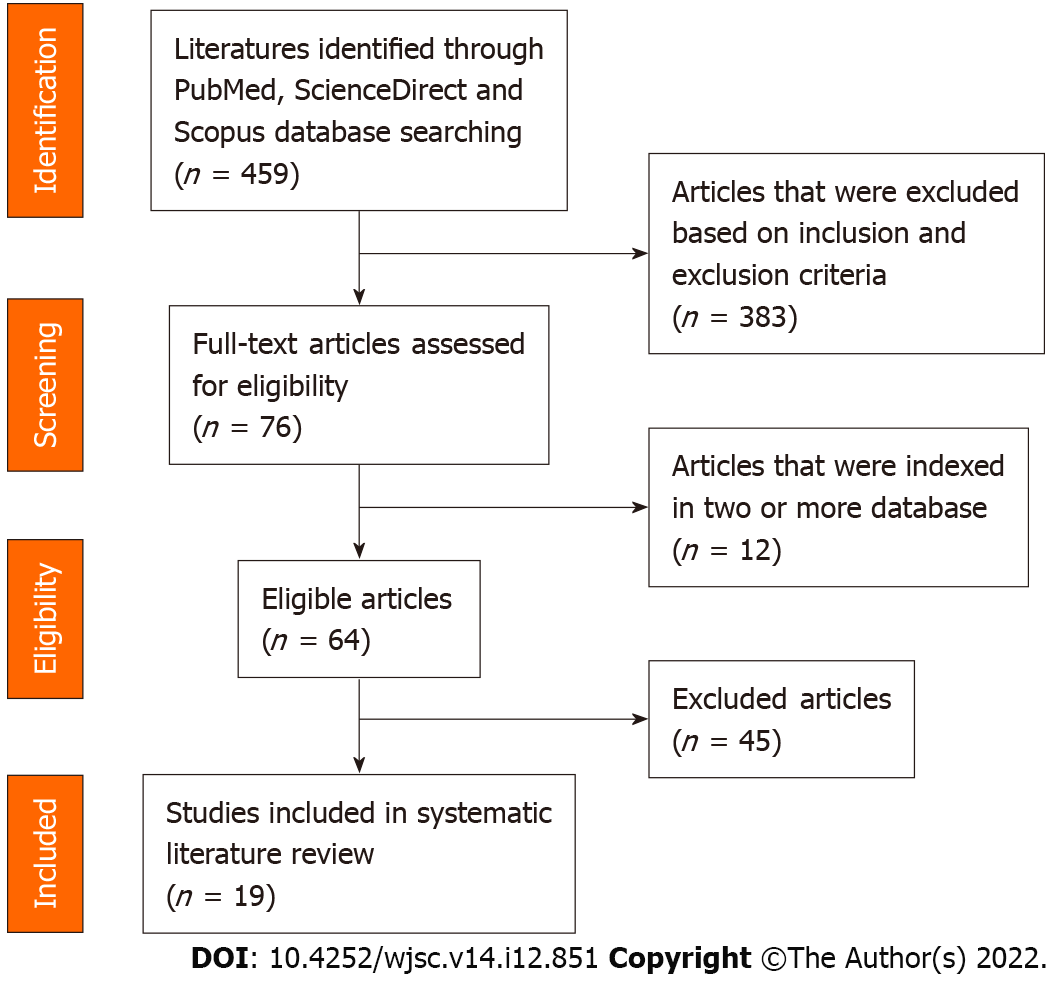
The primary search identified 459 articles, including 161 from PubMed, 257 from ScienceDirect and 41 from Scopus. Following the preliminary screening of the titles and abstracts, a total of 383 articles were excluded accordingly. Out of the 76 articles remaining, 12 were found to be present in two or more of the databases, and thus the duplicates were excluded, resulting in 64 articles eligible for further assessment. After a careful full-text analysis and validation of the content, a total of 19 articles that met all the screening criteria were selected for inclusion in the systematic review. A PRISMA diagram illustrating the flow of this systematic literature review search is shown in Figure 2.
Our systematic literature review analysis identified 19 articles that matched all the inclusion criteria. The selected articles were carefully read and analyzed. Based on the collected literature data, we identified a total of 19 different major types of materials applied on seven major types of stem cells, namely mesenchymal stem cells (MSCs), NSCs, iPSCs, neural progenitor cells (NPCs), endothelial progenitor cells (EPCs), neuroepithelial progenitor cells (NEPCs), and neuroblasts, respectively, to enhance their therapeutic potential for ischemic stroke treatment (Figure 3). The key findings reported in each article are presented in Table 1. Elaboration of the therapeutic benefits gained from each of these biomaterial engineered-stem cells for in vitro and in vivo stroke studies and detailed insights on the mechanisms underlying these cell-based therapies are provided in the Discussion section.
| Ref. | Type and source of stem cells | Type of biomaterials used | Biomaterial-based optimization strategy | Type of ischemic stroke model | Beneficial outcomes |
| Yang et al[12], 2015 | MSCs from human adipose tissue | PβAE | Genetically engineered hMSCs to overexpress GDNF using PβAE | Hypoxic-ischemic mice brains | Enhanced neurobehavioral functions of animals after transplantation in ischemic stroke model |
| Lin et al[22], 2017 | MSCs from SD rat bone marrow | ASP-SPION nanocomplex | Conjugation of cationic amylose and SPIONs to produce cationic nanoparticles | MCAO SD rats | A rapid and highly efficient approach for MSCs magnetic labelling, with no destructive effects on cells |
| Huang et al[24], 2017 | MSCs from bone marrow of 3 wk male SD rat | PA-CLEVSRKNC peptide | MSCs co-modified with PA-CLEVSRKNC peptide | MCAO SD rats | Effectively guide and homing a large amount of MSCs to the ischemic brain directly and enhanced miR-133b expression level |
| Tian et al[26], 2018 | MSC-derived exosomes from mouse | cRGD-Exo | [c(RGDyK)] peptide conjugated to MSCs exosome and loaded with curcumin | MCAO mice | Successfully targeted ischemic area, with inflammatory response and cellular apoptosis were suppressed effectively in the lesion region |
| Kim et al[34], 2020 | MSC-derived exosomes from rat | MNV | MNV derived from IONP-harboring MSCs | MCAO rats | Ischemic lesion targeting, and the therapeutic outcome improved |
| Zamproni et al[40], 2019 | MSCs from bone marrow from adult C57/BI6 mouse | PLA-PRM scaffolds | PRM microspheres produced by RJS and conjugated with the MSCs in the scaffold | MCAO mice | Retention time of MSCs in the injury site increased, possibly through upregulation of α6-integrin and CXCL12 |
| Hsu et al[43], 2021 | MSCs from mouse HUVEC and umbilical cord blood | 3D MSCs/ECs spheroids | Preassembled 3D spheroids with MSCs and vascular ECs | MCAO mice | Cell retention, structural and motor function recovery significantly improved in the ischemic stroke brain |
| Ghuman et al[49], 2021 | NSCs from SVZ of 2-mo-old mouse | PEG microspheres | PEG microsphere encapsulated the NSCs and ECs with suspension in ECM hydrogel | Photothrombotic and MCAO rats | Provision of ideal microenvironment, with highly efficient cell delivery to the damaged area |
| Ge et al[53], 2022 | NSCs from neocortices of day 14.5 mouse embryo | LbL-NSCs | LbL assembly of NSCs using gelatin and HA | Distal MCAO mice | LbL-NSCs engraftment enhanced NSC survival and neurogenesis; promotion of endogenous neuroblasts migration to the ischemic region |
| Shabani et al[56], 2022 | NSCs from 14-d-old murine embryo | PLGA-PEG | NSC encapsulation within PLGA-PEG and loaded with Reelin | Photothrombotic mice | PLGA-PEG with Reelin enhanced the NSCs proliferation, survival and differentiation |
| Li et al[59], 2022 | NSCs from hippocampus of fetal rat | MNBs | MNBs were fabricated through self-assembly of PSCE-modified (γ-Fe2O3SPIONPs) and internalized within NSCs in the ratio of 1:10 | Photothrombotic mice | MNBs activate Plezo1-Ca2+ -BMP2/Smad signaling pathway, inducing NSCs differentiation; efficiently tracking and imaging the MNBs-labelled NSCs using MRI and ultrasound |
| Zhang et al[67], 2021 | Mouse iPSC cell line | 3D microscaffold culture using AATS medium | Simplified and chemically defined AATS medium for robust production of iPSCs-derived ECs and SMCs | Permanent MCAO mice | ECs and SMCs successfully grown in simple, cost effective AATS medium, allowing GMP large scale production of these cells |
| McCrary et al[68], 2022 | Mouse iPSC cell line | CS-A + iPSCs-NPCs | iPSCs-derived NPCs were encapsulated in CS-A hydrogel | MCAO mice | Promote regenerative microglia/macrophage response via IL-10 accumulation; improvements of post-stroke neuropsychiatric deficits |
| Zhang et al[72], 2013 | NPCs from cerebellum of neonatal mouse | fmSiO4@SPION | SPIONs were modified with fmSiO4 to label and track the NPCs | MCAO mice | Labelled cells successfully migrating to the lesion with highly effective cell imaging and cell tracking |
| Somaa et al[73], 2017 | NPCs from human ESC | SAP hydrogel scaffold | SAP hydrogel scaffold was synthesized using laminin-derived epitope (IKVAV) | MCAO rats | Embedding in a self-assembling scaffold formed de novo "bio-bridges" between the lesions for prolonged survival, therapeutic efficacy and integration of neural grafts |
| Li et al[78], 2013 | EPCs from human umbilical cord blood | SiO4@ SPIONs | EPCs were labelled with SiO4@SPIONs and magnetic exterior to guide the cell migration to ischemic area | Transient MCAO mice | Dual benefit of cell homing and cell tracking were achieved; Atrophic volume of brain was reduced while the microvessel density and VEGF expression were increased |
| Wang et al[80], 2019 | EPCs from human umbilical cord blood | Alkyl-PEI encapsulated SPIONs | Alkyl-PEI/SPIONs were used to direct delivery of siRNA to EPCs | Photothrombotic mice | PHD2 silencing in EPCs improved the migration and survival of the cells through elevation of CXCR4 and HIF-1α expression |
| Payne et al[82], 2019 | NEPCs from human fibroblasts | HAMC hydrogel | HAMC as co-delivery agent to deliver cortically specified-NEPCs into a specific ischemic area | MCAO SD rats | HAMC led to cell survival along the migration process for more mature cells formation and increased host tissue repairs |
| Fujioka et al[86], 2017 | Neuroblasts | Laminin-rich hydrogel | Laminin-rich scaffold was developed with β1 integrins to facilitate neuronal migration toward an injured area | MCAO mice | Able to mimic the vasculature and increased neuroblasts chain formation and migration toward the infarct area of brain tissue |
MSCs are undifferentiated non-hematopoietic stromal cells present in almost all tissues such as bone marrow, adipose tissue, cartilage, trabecular bone, and arterial wall. MSCs from different tissues might exhibit different biological activities and cell markers depending on their tissue of origin. However, most MSCs share many common characteristics; for example, they have multilineage differentiation potential and thus are considered as a reservoir of reparative cells for numerous degenerative disorders including myocardial infarction, liver cirrhosis, limb ischemia and spinal cord injury[9]. The main features of this cell type is their ability to proliferate and differentiate into committed cell types and to mobilize to the site of injured tissues following specific signals in order to support the overall regeneration process[10]. Moreover, the protocols to isolate and expand MSCs in vitro are simple and do not pose ethical or tumorigenic concerns, unlike those for pluripotent ESCs[11]. Thus, the potential of MSCs have been explored over the last few decades extensively for tissue regeneration therapies in musculoskeletal, cardiovascular, liver, autoimmune, neurodegenerative and cancer-related diseases.
In biological cell-based therapeutic strategies for ischemic stroke, MSCs have been regarded as an ideal candidate due to their migratory capacity and expansive capabilities to regenerate neural cells after being grafted into the central nervous system (CNS). In recent years, the potential of MSCs for ischemic stroke treatment has been further enhanced by employing biomaterial tissue/cell engineering approaches. For example, in 2015, Yang et al[12] reported a method for genetically engineered human MSCs to overexpress glial cell-derived neurotrophic factor (GDNF) using a potent biodegradable cationic polymer known as poly (β-amino ester) (PβAE). PβAE is a synthetic polymer that has demonstrated extraordinary efficiency to overcome distinct cellular barriers for site-specific delivery[13]. PβAE is also widely used for gene transfer due to its demonstrated higher transfection efficiency and lower cytotoxicity in various cell and tissue types compared to several commercially available transfection reagents, including polyethylenimine and Lipofectamine 2000[14]. In that study[14], a GDNF plasmid was transfected into human MSCs (hMSCs) efficiently using PβAE nanoparticles and was found to successfully induce over-expression of GDNF protein in hMSCs. GDNF is a specific neurotrophic factor that is responsible for neuronal survival, differentiation and neurogenesis[15]. Transplantation of the GDNF-overexpressed hMSCs into a hypoxic-ischemic stroke animal model significantly improved the neurobehavioral functions of animals after the transplantation, indicating the potential of PβAE biomaterial in enhancing the MSC-based therapy for ischemic stroke.
Furthermore, tracking of transplanted MSCs in biological cell-based tissue therapy is essential in order to provide better understanding of the cellular proliferation dynamics, biodistribution, migrational dynamics, differentiation process and participation in the mechanisms of tissue repair[16]. Commonly used non-invasive imaging known as magnetic resonance imaging (MRI) is the right fit for a long-term imaging purpose, as this technology is clinically safe compared to ionizing radiation and capable of providing high three-dimensional (3D) resolution with deep tissue penetration[17]. In order to detect the cells of interest against the host tissue background using MRI, the cells need to be labelled with a substance to produce a strong contrast against the background tissue before transplantation. For in vivo MRI, this has been achieved mostly with superparamagnetic iron oxide nanoparticles (SPIONs) which could be incorporated into the cells to produce a strong signal loss in T2-weighted MRI by virtue of susceptibility differences to the adjacent environment[18]. However, most of the paramagnetic substances used for MRI contrast agents are composed of gadolinium (Gd3+) and manganese (Mn2+) which are cytotoxic, thus limiting their application in vivo[19]. Recent research by Lin and colleagues[20] developed a novel biocompatible nanocomplex known as spermine-modified amylose (ASP) to generate ASP-SPIONs. Amylose, present in starch along with amylopectin, is a linear polysaccharide, in which repeated glucose units are joined by α-1,4 glycosidic linkage. This naturally-occurring polysaccharide has been the subject of intense scientific investigation in the biomaterial field due to absence of toxic effects, biodegradability, biocompatibility, low-cost production, high tensile strength, and better flexibility. On the other hand, spermine, a natural polyamine required for cell physiology, has also been scientifically investigated in cationization of polymers for its potential action as a gene vector[21]. Lin et al[22] found that MSCs labelled with ASP-SPIONs have no detrimental toxicity effects on the cell and the labelled cells also retain the magnetic labels and remain viable up to 6 wk (when tested in vivo), suggesting the efficiency of this nanomaterial in stem cell tracking after transplantation.
MSCs have been demonstrated to exert therapeutic benefits for brain diseases. However, as previously reported, the percentage of homing cells is relatively low, and only a limited number of MSCs survive and engraft into the ischemic lesion because some cells die once exposed to unfavorable conditions[23]. Hence, improving the engraftment efficiency of MSCs into ischemic lesions is important. In a study done by Huang et al[24], a brain-targeting peptide known as palmitic acid (PA)-CLEVSRKNC was selected and coated onto the MSC surface via a lipid raft to induce the migration of MSCs to the ischemic lesion specifically. The CLEVSRKNC peptide could be generated from a phage peptide library based on T7 415-1b phage vector displaying CX7C[25]. This peptide has been shown to be able to guide cells to ischemic regions, making it an ideal stroke-homing peptide. Huang et al[24] reported that the PA-CLEVSRKNC peptide coating onto MSCs did not impose any cytotoxicity nor detrimental influence on cell differentiation. On the other hand, engraftment of PA-CLEVSRKNC-modified MSCs into the injured brain tissue was significantly enhanced. Moreover, with enhanced targeted cell homing, the number of cells trapped in other non-targeted organs (such as lung and liver) was reduced. This could decrease the risk of toxicity in other non-targeted organs.
The same strategy has also been applied by Tian et al[26], where a cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyK)] was conjugated to the MSC exosome surface, namely as cRGD-Exo to enhance the targeting capability of the exosomes. c(RGDyK) is a type of peptide sequence with an arginine-glycine-aspartic acid motif, where it possesses strong affinity for αvβ3 integrin. αvβ3 integrin is a transmembrane glycoprotein that plays an important role in angiogenesis, tumor metastasis and leucocyte migration, making it a suitable target for various inflammatory-related disease including ischemic stroke[27]. In addition, curcumin, a natural compound with antioxidant and anti-inflammatory properties[28] was also loaded onto the cRGD-Exo (cRGD-Exo-cur) to enhance the efficiency of this therapy. The data from this research revealed that the extents of suppression of inflammation and cellular apoptosis were higher than those of the therapy using exosomes or curcumin alone, demonstrating the promising potential of this strategy for ischemic stroke treatment.
Exosomes are extracellular vesicles secreted from various types of cells with diameter range of 40-150 nm[29]. It plays an important role for intercellular communication, facilitating crosstalk between cells located in a distant location via transfer of bioactive proteins, lipid and genetic material (i.e. RNA). This form of communication may affect many cellular processes such as immune response, antigen presentation and signal transduction[30,31]. Collective lines of evidence have highlighted the possibility of the MSC-derived exosomes as a potential substitute for biological MSC activity, as exosomes are not live cells and thus can overcome the poor survival limitation otherwise associated with MSC therapy[32]. While holding much promise, the use of exosomes for therapy has been hampered by their limited production yield, and thus require some modifications before they can be used efficiently as a therapeutic agent for stroke disease[33]. Based on a study reported by Kim et al[34] in the year 2020, the authors had managed to derive exosome-mimicking nanovesicles from MSCs, namely magnetic nanovesicles, via serial extrusion through 10 μm, 5 μm, 1 μm, and 400 nm pore-sized membrane filters. The nanovesicles produced were uniform in size (between 168.3 ± 48.3 nm and 194.2 ± 44.5 nm), spherical in shape and possessed lipid bilayers, mimicking the structure of exosomes. Furthermore, they also reported on having fabricated the localization of iron-oxide nanoparticles (IONPs) into the nanovesicles via the MSCs. An IONP is a chemical compound at nanoscale size that possesses unique properties including high specific surface area, super paramagnetism and biocompatibility. Therefore, the IONP is an ideal material for surface functionalization and modification[35]. Kim et al[34] also demonstrated that the transplantation of nanovesicles derived from IONP-incorporated MSCs displayed an attenuation in infarction volume and significantly improved motor function in ischemic stroke animal models.
Biomaterial scaffolds have been widely applied in regeneration therapy, as they can mimic the native microenvironment of a healthy brain region to support the regeneration of injured nervous system tissues. In a previous study, the incorporation of this biomaterial demonstrated promising results in CNS regeneration, specifically for tissue repair and functional recovery[36]. A 3D polymer scaffold made by either natural or synthetic material has also been shown to facilitate cell infiltration and proliferation, and aid in the regulation of cell behavior when combined with cell therapy[37]. Among the methods to fabricate the porous structure of 3D scaffolds are microfluidic fabrication, freeze drying, thermoforming, 3D printing, water emulsion, and electrospinning[38,39]. An alternative scaffold material using polylactic acid polymeric rough microfiber (PLA-PRM) scaffolding produced by rotary jet spinning (RJS) was designed by Zamproni et al[40] and reported in 2018. The novel RJS engineering strategy was designed without conventional electrospinning; the new method boasts a simple controlling rotation and may produce the functional rough and porous 3D structural support for MSCs to grow on it. In addition, the PLA-PRM 3D scaffold generated using the RJS technique was found to upregulate α6-integrin and C-X-C motif chemokine ligand 12 production of MSCs, which may underlie the mechanism for greater cell retention at the lesion site and may provide additional benefits to MSC transplantation procedures. Indeed, this was demonstrated by the survival and proliferation of MSCs being increased when the cells were transplanted into the ischemic brain region with the aid of a PLA-RPM scaffold, compared to direct injection of MSCs without a scaffold.
Increasing documented evidence has demonstrated the assembly of stem cells into 3D multicellular spheroids, which has many advantages over a traditional 2D monolayer cell culture. This is because the conventional 2D cell culture has losses of cell-cell contact, cell-matrix interaction as well as chemical and mechanical cues due to the lack of exposure to the extracellular matrix (ECM) environment, which may cause alteration in cell metabolism and protein expression[41]. A 3D multicellular spheroid overcomes these limitations by mimicking the in vivo cell-matrix interaction and physiological environment[42]. In addition, the bulky size of the spheroid configuration could also reduce the potential of cell leakage from the site of injection. In a study by Hsu and colleagues[43], the potential of this approach was investigated by preassembling the MSCs with vascular endothelial cells (ECs) into a 3D spherical configuration (to generate a MSC/EC spheroid). Using this approach, the transplanted 3D MSC/EC spheroid successfully displayed upregulation of neurotrophic factor, enhanced promotion of neovascularization, higher post-engraftment cell retention, and improved functional recovery in an ischemic stroke animal model.
Besides MSCs, NSCs are another potential stem cell type important for regenerative treatment. NSCs are multipotent cells that were first isolated from the CNS of embryonic and adult mice by Reynold, Weiss and colleagues[44], as reported in 1992. These cells have the capacity to self-renew and proliferate for extensive periods of time without limit, while maintaining a stable differentiation capacity. They are able to produce progeny cells that give rise to more specialized cells such as neurons, oligodendrocytes and astrocytes[45]. Endogenous adult NSCs reside in specific niches in the brain including the subventricular zone (SVZ), dentate gyrus of the hippocampus, and the olfactory bulb[45]. However, the turnover rate of endogenous NSCs for neurogenesis at the ischemic-injured area is extremely low, hampered by the harsh ischemic environment and its surrounding high concentration of pro-inflammatory cytokines[46]. Therefore, transplantation of exogenous NSCs has been proposed as an alternative strategy to obviate this issue[47].
Exogenous NSCs can be modified in vitro to enhance their therapeutic potential for ischemic stroke therapy prior to transplantation[48]. In a study reported by Ghuman et al[49], a combination therapy involving NSCs encapsulated in polyethylene glycol (PEG) microspheres was applied. The encapsulation of these cells into microspheres allowed for the avoidance of a cell mass that would otherwise hamper the stem cell-based therapeutic strategy, as the microsphere could maintain close cell-cell proximity[50]. Results of the study indicated that packaging of NSCs into a microsphere enhanced survival as well as migration after implantation into a stroke cavity. Furthermore, in order to achieve higher efficiency of NSC therapy and confer an effective cellular shield against host enzymatic degradation, the microspheres were packaged into ECM hydrogel. ECM is an essential non-cellular component of the tissue microenvironment, comprised of a complex and highly organized network of macromolecules and is a conglomerate of proteins[51] while hydrogel is a material commonly used in regenerative medicine because it could maintain its structural integrity by physically and chemically crosslinking the polymer chains[52]. As part of the strategy to enhance NSC-based therapy, ECM hydrogel is not only able to provide stability and structural strength to the cells but it can also mimic and provide an ideal microenvironment for cell adhesion, migration, proliferation, differentiation, and maturation. Moreover, the probable reason for use of hydrogel to construct the ECM in the study was its high water absorbing property and dimensional stability which could aid the NSC microsphere for better retention in the ischemic brain region.
Ge et al[53] also applied a feasible method to encapsulate NSCs as a layered structure composed of gelatin and hyaluronic acid (HA) biomaterials in order to enhance the therapeutic potential of the NSCs for ischemic stroke. HA is a widely used polyanion that can easily bind to gelatin via electrospinning. Moreover, HA can stabilize the components of ECM and trigger its reorganization and consequent remodeling of its original structure after CNS injury[54,55]. Simultaneously, HA facilitates NSCs’ direction into neurons, suppresses local excessive inflammation, and reduces cell necrosis and apoptosis at the injured site. The multiple neuroprotective effects demonstrated by HA indicate that layer-by-layer assembly of NSCs (LbL-NSCs) using gelatin and HA is a promising therapeutic strategy to increase the potential benefits of NSCs for ischemic stroke treatment. The researchers also showed that trans
On the other hand, Shabani et al[56] encapsulated NSCs with bioengineered Reelin-loaded polylactic coglycolic acidpolyethylene glycol (PLGA/PEG) micelles to retain the NSCs within the ischemic infarct cavity and accelerate the differentiation of these cells to generate de novo neuronal tissue after transplantation into a photothrombotic stroke model of mice[56]. Reelin, encoded by the RELN gene, is a large secreted ECM glycoprotein that regulates dendritic growth, dendritic spine development, synapse formation, and plasticity[57]. This protein is touted as a key player in the formation of the cerebral cortex and maintenance of adult synaptogenesis. Therefore, it was suggested that Reelin can reduce pathologies related to cerebral ischemia-reperfusion injury. In line with this claim, the researchers enriched the NSCs with Reelin and packaged the modified NSCs into PLGA, a United States Food and Drug Administration-approved biomaterial that can be injected directly into the injured sites. Due to the unique physicochemical features of PLGA, it is commonly used as a carrier to support stem cell delivery for the treatment of brain disorders[58]. In the study, the researchers further functionalized PLGA micelles using PEG polymer chains via the biochemical modification process known as PEGylation. The combination of PLGA copolymer with the non-cytotoxic and hydrophilic PEG substrate offers the desired microenvironment for the optimal growth of NSCs. The co-administration of NSCs with the Reelin-loaded PLGA/PEG micelles successfully promoted the commitment of NSCs toward a neural lineage and adult neurogenesis, resulting in reduction of the cavity size and alleviation of the functional behavior disorders after ischemic conditions.
Nanomaterial-assisted NSC therapy has led to significant progress in the treatment for ischemic stroke disease. A recent finding reported by Li et al[59] showed that a type of magnetic nanobubbles (MNBs) fabricated through the self-assembly of poly-glucose sorbitol carboxymethyl ether-modified (γ-Fe2O3)-SPIONPs can guide NSC differentiation fate by activation of the bone morphogenetic protein 2 (BMP2)/Smad biochemical signaling pathway. BMP signaling plays a pivotal role in brain development and NSC behavior, and it has been associated with a large number of related biological processes such as cell growth and cell differentiation[60]. Implantation of MNB-labeled NSCs into photothrombotic ischemic stroke mice also demonstrated that the MNB structure can direct NSC differentiation in vivo and in particular it can increase the efficiency of neuronal lineage differentiation.
iPSCs are a type of pluripotent stem cell derived from adult somatic cells that have been genetically reprogrammed to ESC-like state through the forced expression of pluripotency genes[61]. iPSCs are similar to ESCs in many aspects, including the expression of ESC markers, chromatin methylation patterns, embryoid body formation, and most importantly the pluripotency and the ability to differentiate into many different types of tissues in vitro. iPSCs were first generated from mouse fibroblasts by the Yamanaka lab at Kyoto University in 2006[62]. Shortly thereafter, in late 2007, human iPSCs were produced for the first time by Yamanaka’s and Thomson’s groups[63,64], both labs working independently and starting with human fibroblasts; these studies provided an invaluable reservoir of human pluripotent cells that could be genetically engineered and differentiated into target cells to treat various human genetic and degenerative diseases once transplanted. However, a major obstacle for using human iPSCs for cell therapy is the lack of simple, cost-saving, and scalable methods for cell production[65]. Moreover, the biological activity of the differentiated cells may be variable due to line-to-line variation (batch effect)[66].
In order to overcome this obstacle, Zhang et al[67] described a simplified 3D microscaffold culture using chemically defined medium composed of only four main ingredients, namely recombinant human albumin, L-ascorbic acid 2-phosphate, human apo-transferrin, and sodium selenite (AATS), for rapid, stable and highly efficient differentiation of human iPSCs into ECs and smooth muscle cells (SMCs). The researchers found that the ECs and SMCs generated in the AATS medium were similar to their counterpart primary cells. Moreover, the ECs and SMCs exhibit a strong revascularization ability upon transplantation to the permanent MCAO mouse model, which confirmed their therapeutic value in regenerative medicine. Such a simplified and robust design may facilitate a cost-effective and efficient system to create good manufacturing practices for the large-scale bioproduction of ECs and SMCs for human vascular diseases including the ischemic stroke.
On the other hand, McCarry et al[68] described the optimization of mouse iPSC- derived NPCs by encapsulating the cells in a chondroitin sulfate-A hydrogel (to generate CS-A + iPSC-NPCs) that can confer protection to the iPSC-NPCs and enhance their survival rate in ischemic brain. Treatment with CS-A + iPSC-NPCs were found to increase vascular density, improve angiogenesis, promote large vessel remodeling, augment cerebral blood flow recovery, and improve post-stroke sensorimotor deficits after transplantation into an ischemic mouse brain. CS-A was also found to interact with iPSC-NPCs synergically to increase the level of macrophage expression factors associated with tissue regeneration, such as monocyte chemo-attractant protein-1 which had been shown to recruit specific subsets of macrophages that participate in regeneration processes, including angiogenesis, arteriogenesis, and even neurogenesis[69,70].
NPCs are mostly present in the CNS of a developing embryo, but they are also found in the neonatal and mature adult brain. Initially, the NPCs were referred to as ‘’spongioblasts’’ and ‘’fetal glia’’ due to their non-neuronal nature and non-mature glial cell morphology[71]. In general, progenitor cells are multipotent precursor cells that have the capacity to differentiate into a subset of cell types. The difference between stem cells and progenitor cells is that the former are self-renewing and can replicate indefinitely to produce daughter cells, while the latter only divide a limited number of times. There are many types of progenitor cells throughout the human body with the capability to differentiate into cells that belong to the same tissue or organ. NPCs are descendants of NSCs that are destined to further differentiate into specialized brain cell types. Thus, the NPCs are postulated to play important roles in facilitating neuronal and functional recovery post-stroke.
Therapeutic strategies based on transplanted progenitor cells have strong promise for the treatment of diseases by implementing tissue regeneration and repair. However, one of the crucial points in this approach is the need to carry out long-term and noninvasive imaging of the transplanted cells in the host organ and to track their migration and distribution in vivo. Zhang et al[72] reported on a successful NPC labeling and tracking method using SPIONs that had been fabricated with template growth of silica on the surface to fluorescent mesoporous silica-coated superparamagnetic iron 1 oxide nanoparticles (fmSiO4@SPIONs) which were discrete and uniform in size, and had clear core-shell structure. The magnetic core size was about 10 nm and the fluorescent mesoporous silica coating layer was around 20 nm. The fmSiO4@SPIONs showed improved cell labeling efficiency, and hence increased MRI sensitivity to track the NPCs after transplantation. In the future, a useful innovation of this biomaterial will be resiliency for cell imaging, which will provide greater promise for cell tracking by MRI.
The long-term survival and integration of grafted NPCs remain challenges in a stroke-affected hostile environment, due to the ischemic condition and highly pro-inflammatory cues. A variety of natural and synthetic polymers such as collagen, chitosan, and poly (lactic acid) as well as decellularized scaffolds have been employed to support neural grafts in the ischemic brain. In a recent study reported by Somaa et al[73], a self-assembling peptide (SAP) scaffold was developed to provide physical and trophic support for long-term survival and functional maturation of NPCs generated from pluripotent stem cells. The SAP is a tissue (brain)-specific peptide and it was made using solid phase peptide synthesis. According to the results reported, the functionalized SAP scaffold was capable of restoring tissue structure within the lesion cavity, thereby reducing atrophy and cell loss within the damaged area after the stroke attack. Moreover, they also found that the SAP scaffold-supported cells were capable of prolonged restoration of motor function, providing evidence of sustained benefits of human stem cells and biomaterials in repair of the ischemic brain.
Recently, EPCs, the precursor of the mature endothelial blood vessel, have been studied extensively in the tissue regeneration and repair field. Isolation of EPCs was first reported by Asahara et al[74] in 1997, from peripheral blood circulation. These mononuclear cells are a subtype of progenitor cells originated from bone marrow. In general, EPCs could be identified by cell surface expression of hematopoietic marker proteins CD34 and CD133 and the endothelial marker vascular endothelial growth factor receptor 2. EPCs are able to promote and facilitate angiogenesis, vascular homeostasis, vascular repair, neovascularization and endothelial regeneration of ischemic tissues[75] because they express several angiogenic and vasculogenic factors such as platelet-derived growth factor BB, fibroblast growth factor, hepatocyte growth factor, vascular endothelial growth factor (VEGF) and angiopoietin-1[76,77]. Despite the potential of these cells to be applied in ischemic stroke treatment, however, similar to the other types of cell-based therapies, their low rates of migration and survival after engraftment are key limiting factors. Therefore, strategic modifications are required to enhance the migration and survival rates of this therapy.
Research efforts by Li et al[78] to enhance the migration of EPCs to an ischemic affected area in the brain involved labelling the EPCs with silica-coated SPIONs (SiO4@SPIONs-EPCs). Subsequently, an exterior magnetic field was applied to guide these cells directly to the targeted area. Silica was added to the unmodified SPION because this material has higher intracellular labelling efficiency and therefore was anticipated to have higher MRI sensitivity[17]. Moreover, silica has also been recognized in previous studies as a superior choice of coating material for SPIONs due to its biocompatibility, stability, and functionality[79]. SiO4@SPIONs-EPCs homing was found to be increased greatly in this study, with further promising findings of enhanced neurobehavioral activity, reduced infract volume, higher VEGF expression, and increased microvessel density. Besides its effect on homing to the infarcted region, silica also acts as a cell tracking agent, supporting its role as a tool to provide better understanding of cellular migration and survival after cell transplantation.
Another study carried out by Wang et al[80] involved the utilization of small interfering RNA (siRNA) to silence the hypoxia-inducible factor (HIF)-prolyl hydroxylase 2 (PHD2) gene in EPCs. The silencing was expected to stabilize the HIF-1 transcriptional factor, which is the key regulator of multiple genes that promote cell survival in a hypoxic environment such as glycolytic enzymes, erythropoietin, and VEGF[81]. Nonetheless, direct delivery of siRNA to EPCs may trigger rapid enzymatic digestion and poor cellular intake, representing a potential limitation of this strategy. Therefore, a delivery system using polyethylenimine (Alkyl-PEI)-encapsulated SPIONs was designed by this team to ensure effective delivery of the siRNA to the EPCs. Ultimately, this study demonstrated the effectiveness of PHD2 silencing in EPCs, with an elevation of CXCR4 and HIF-1α expression, which are important in homing, migration and survival of cells in an ischemic region. Furthermore, the observed reduction in infarct area and increases in functional activity and fractional anisotropy values suggested the potential of this strategy to enhance the effectiveness of EPCs for stroke treatment.
NEPCs are stem cells that differentiate into neurons and glia, essential components of the human CNS, following the process of neurogenesis. In a study reported by Payne et al[82], a biocompatible and bioresorbable hydrogel composed of hyaluronan and methylcellulose (HAMC) was used to deliver human cortically-specified NEPCs into stroke-injured rat brain. The authors then examined the survival and proliferation of the transplanted cells based on the expression of a specific NEPC marker, the human nuclear antigen (HuNu). They successfully found HuNu+ nuclei at the injected location 50 d after transplantation and that approximately 80% of animals that received transplants contained surviving cells, which was similar across all experimental groups. Their findings were similar to those of other studies in which HAMC hydrogel was found to improve cell survival and distribution in several CNS preclinical models of disease[83-85]. In conclusion, the HAMC hydrogel delivery vehicle used could co-deliver pro-survival or differentiation factors to promote the survival of NEPCs and/or direct their differentiation.
Neuroblasts, also known as immature neurons, are the precursors of neurons, which can be found in the neurogenic niche of the ventricular-SVZ in brain. Recent studies on implantation of neuroblasts have suggested their potential as an alternative therapy for ischemic stroke. Neuroblasts are capable of homing to an injured site and differentiating into a matured neuron; however, the molecular mechanism that regulates this migration process is unknown. Fujioka et al[86] suggested a role for β1-class integrins, transmembrane receptors for ECM proteins, in the neuroblast migration activity. They also developed an artificial laminin-containing scaffold to promote neuroblast chain formation and migration toward an injured area. To do this, they injected hydrogel with or without laminin (designated as laminin-hydrogel and control-hydrogel, respectively), which self-assembles from a soluble state into hydrated nanofibers, into the striatum of 10-d post-stroke brains. The migration of neuroblasts along the hydrogel toward the injured area was examined 8 d later. More migrating neuroblasts were observed on the laminin-hydrogel than on the control-hydrogel; additionally, chains were only formed on the laminin-hydrogel group, suggesting that the artificial laminin scaffold can efficiently mimic vasculature and facilitate neuronal migration toward an injured area. Thus, for the regeneration of brain tissue, artificial scaffolds containing laminin would be useful to promote neuroblast migration.
This review article provides new insights into the novel biomaterial applications that have been developed to enhance stem/progenitor cell-based therapy for ischemic stroke. According to the previously published data collected in this review, we can reasonably conclude that biomaterials could modify these cells to enhance their migration capacity to a targeted area of injury, increase their retention rate in the hostile ischemic microenvironment, and promote the secretion of importance cytokines for reparative mechanisms, such as neurogenic factors and angiogenic factors. In addition, biomaterials can be used also to track the cells in vivo, providing for a clearer understanding of the fate of the cells after transplantation. Some of these effects are direct and some are indirect, but each mechanism of action must be clearly understood in order to improve the efficacy of this therapy for stroke. There is currently a scarcity of published evidence on the efficacy of biomaterial-modified cell-based therapy in clinical studies. Therefore, more evidence-based clinical studies are required to verify the efficacy and safety of the combination of biomaterials and stem/progenitor cells as potential therapeutic agents for various human diseases such as ischemic stroke.
Low cell survival after transplantation has emerged as the biggest challenge of stem cell-based therapies for ischemic stroke in the clinical setting. Thus, biomaterials have been explored as a potential approach to provide a supportable cellular microenvironment or functional modification on the stem cells to optimize their reparative roles in injured tissues or organs.
Ischemic stroke remains a significant health issue globally. Stem/progenitor cells as regenerative treatment for stroke is practicable and beneficial for stroke patients, especially those in the chronic phase who could not be cured by any other means of currently available treatments.
This systematic review aimed to collect and present the current knowledge on state-of-art functional biomaterials that have been developed to enhance the therapeutic potential of stem cell-based treatments for ischemic stroke and to provide detailed insights of the mechanisms underlying these biomaterial-based approaches.
Publications indexed in the PubMed, Science Direct and Scopus literature databases were searched using the keywords “biomaterial” AND “ischemic stroke” AND “stem cells” OR “progenitor cells” OR “undifferentiated cells” to identify topically-relevant articles published in English during the years of 2011 to 2022. The systematic search was conducted up to September 30, 2022.
Ultimately, 19 types of biomaterials were identified that modify seven major stem/progenitor cell types to enhance their therapeutic potential for ischemic stroke.
Biomaterials can modify stem cells to enhance their migration capacity to a targeted area of injury, increase their retention rate, promote the secretion of important cytokines to support a reparative mechanism, and provide clearer understanding of the fate of transplanted cells via in vivo tracking. Biomaterials can enhance stem cell-based therapy for ischemic stroke.
It is crucial to study and define the mechanisms of state-of-art functional biomaterial-based approaches to maximize the therapeutic potential of stem cell-based treatments for ischemic stroke. Findings from future in-depth clinical investigations are expected to support the translation of this therapy into clinical application. Meta-analyses can be performed to generate a quantitative estimate of the effectiveness of the intervention.
The authors would like to thank the Universiti Sains Malaysia libraries, particularly Perpustakaan Hamdan Tahir and Perpustakaan Hamzah Sendut, for providing the resources to write this systematic review. Finally, the authors would like to express their appreciation to all the scientists whose previous work contributed to this review article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu J, China; Shao A, China; Ye Y, China S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Rojsanga W, Sawanyawisuth K, Chotmongkol V, Tiamkao S, Kongbonkiat K, Kasemsap N. Clinical risk factors predictive of thrombotic stroke with large cerebral infarction. Neurol Int. 2019;11:7941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Ntaios G, Papavasileiou V, Milionis H, Makaritsis K, Vemmou A, Koroboki E, Manios E, Spengos K, Michel P, Vemmos K. Embolic Strokes of Undetermined Source in the Athens Stroke Registry: An Outcome Analysis. Stroke. 2015;46:2087-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA Neurol. 2018;75:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 4. | Kwon S, Hartzema AG, Duncan PW, Min-Lai S. Disability measures in stroke: relationship among the Barthel Index, the Functional Independence Measure, and the Modified Rankin Scale. Stroke. 2004;35:918-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Baker EW, Kinder HA, West FD. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019;9: e01214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Li Z, Dong X, Tian M, Liu C, Wang K, Li L, Liu Z, Liu J. Stem cell-based therapies for ischemic stroke: a systematic review and meta-analysis of clinical trials. Stem Cell Res Ther. 2020;11:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Singh M, Pandey PK, Bhasin A, Padma MV, Mohanty S. Application of Stem Cells in Stroke: A Multifactorial Approach. Front Neurosci. 2020;14:473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Lin X, Li N, Tang H. Recent Advances in Nanomaterials for Diagnosis, Treatments, and Neurorestoration in Ischemic Stroke. Front Cell Neurosci. 2022;16:885190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 10. | Pountos I. Mesenchymal Stem Cells. In: Adult Stem Cells: Recent Advances. SM Group, 2017: 1-15. |
| 11. | Sandhaanam SD, Pathalam G, Dorairaj S, Savariar V. Mesenchymal stem cells (MSC): Identification, Proliferation and Differentiation-A review article. PeerJ PrePrints. 2013;1:1-13. [DOI] [Full Text] |
| 12. | Yang K, Park HJ, Han S, Lee J, Ko E, Kim J, Lee JS, Yu JH, Song KY, Cheong E, Cho SR, Chung S, Cho SW. Recapitulation of in vivo-like paracrine signals of human mesenchymal stem cells for functional neuronal differentiation of human neural stem cells in a 3D microfluidic system. Biomaterials. 2015;63:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Iqbal S, Zhao Z. Poly (β amino esters) copolymers: Novel potential vectors for delivery of genes and related therapeutics. Int J Pharm. 2022;611:121289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Park HJ, Lee J, Kim MJ, Kang TJ, Jeong Y, Um SH, Cho SW. Sonic hedgehog intradermal gene therapy using a biodegradable poly(β-amino esters) nanoparticle to enhance wound healing. Biomaterials. 2012;33:9148-9156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Zhang Z, Zhang N, Ding S. Glial cell line derived neurotrophic factor in brain repair after focal ischemic stroke. Neural Regen Res. 2022;17:1735-1736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Gera A, Steinberg GK, Guzman R. In vivo neural stem cell imaging: current modalities and future directions. Regen Med. 2010;5:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, Gu Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 19. | Yang X, Tian DC, He W, Lv W, Fan J, Li H, Jin WN, Meng X. Cellular and molecular imaging for stem cell tracking in neurological diseases. Stroke Vasc Neurol. 2021;6:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Clifton P, Keogh J. Starch. In: Benjamin Caballero, Paul M. Finglas FT. Encyclopedia of Food and Health. Academic Press, 2016: 146-151. |
| 21. | Pegg AE. The function of spermine. IUBMB Life. 2014;66:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 22. | Lin BL, Zhang JZ, Lu LJ, Mao JJ, Cao MH, Mao XH, Zhang F, Duan XH, Zheng CS, Zhang LM, Shen J. Superparamagnetic Iron Oxide Nanoparticles-Complexed Cationic Amylose for In Vivo Magnetic Resonance Imaging Tracking of Transplanted Stem Cells in Stroke. Nanomaterials (Basel). 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 24. | Huang B, Jiang XC, Zhang TY, Hu YL, Tabata Y, Chen Z, Pluchino S, Gao JQ. Peptide modified mesenchymal stem cells as targeting delivery system transfected with miR-133b for the treatment of cerebral ischemia. Int J Pharm. 2017;531:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Hong HY, Choi JS, Kim YJ, Lee HY, Kwak W, Yoo J, Lee JT, Kwon TH, Kim IS, Han HS, Lee BH. Detection of apoptosis in a rat model of focal cerebral ischemia using a homing peptide selected from in vivo phage display. J Control Release. 2008;131:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 810] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 27. | Vhora I, Bhatt SPPB, Misra A. Protein- and Peptide-Drug Conjugates: An Emerging Drug Delivery Technology. In: Donev R. Advances in Protein Chemistry and Structural Biology. Academic Press, 2015: 1-55. |
| 28. | Hewlings SJ, Kalman DS. Curcumin: A Review of Its Effects on Human Health. Foods. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 886] [Cited by in RCA: 1334] [Article Influence: 166.8] [Reference Citation Analysis (0)] |
| 29. | Heldring N, Mäger I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum Gene Ther. 2015;26:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 31. | Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics. 2015;15:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 32. | Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 33. | Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 1192] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 34. | Kim HY, Kim TJ, Kang L, Kim YJ, Kang MK, Kim J, Ryu JH, Hyeon T, Yoon BW, Ko SB, Kim BS. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243:119942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 35. | Ajinkya N, Yu X, Kaithal P, Luo H, Somani P, Ramakrishna S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Tan H, Hui X. Biomaterial Scaffolds in Regenerative Therapy of the Central Nervous System. Biomed Res Int. 2018;2018:7848901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Ellis-Behnke RG, Teather LA, Schneider GE, So KF. Using nanotechnology to design potential therapies for CNS regeneration. Curr Pharm Des. 2007;13:2519-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Tan Y, Richards DJ, Trusk TC, Visconti RP, Yost MJ, Kindy MS, Drake CJ, Argraves WS, Markwald RR, Mei Y. 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication. 2014;6:024111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Wu X, Liu Y, Li X, Wen P, Zhang Y, Long Y, Wang X, Guo Y, Xing F, Gao J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010;6:1167-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 40. | Zamproni LN, Grinet MAVM, Mundim MTVV, Reis MBC, Galindo LT, Marciano FR, Lobo AO, Porcionatto M. Rotary jet-spun porous microfibers as scaffolds for stem cells delivery to central nervous system injury. Nanomedicine. 2019;15:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Nath S, Devi GR. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol Ther. 2016;163:94-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 620] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 42. | Cui X, Hartanto Y, Zhang H. Advances in multicellular spheroids formation. J R Soc Interface. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 43. | Hsu TW, Lu YJ, Lin YJ, Huang YT, Hsieh LH, Wu BH, Lin YC, Chen LC, Wang HW, Chuang JC, Fang YQ, Huang CC. Transplantation of 3D MSC/HUVEC spheroids with neuroprotective and proangiogenic potentials ameliorates ischemic stroke brain injury. Biomaterials. 2021;272:120765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3870] [Cited by in RCA: 3842] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 45. | Lee VM, Louis SA, Reynolds BA. Neural Stem Cells. Stem Cell Technologies 2015; 1-6. |
| 46. | Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. 2014;2014:468748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 47. | Garzón-Muvdi T, Quiñones-Hinojosa A. Neural stem cell niches and homing: recruitment and integration into functional tissues. ILAR J. 2009;51:3-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Kornblum HI. Introduction to neural stem cells. Stroke. 38:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Ghuman H, Matta R, Tompkins A, Nitzsche F, Badylak SF, Gonzalez AL, Modo M. ECM hydrogel improves the delivery of PEG microsphere-encapsulated neural stem cells and endothelial cells into tissue cavities caused by stroke. Brain Res Bull. 2021;168:120-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Matta R, Gonzalez AL. Stroke repair via biomimicry of subventricular zone. Fornt Mater. 2018;5. [DOI] [Full Text] |
| 51. | Assunção M, Dehghan-Baniani D, Yiu CHK, Später T, Beyer S, Blocki A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front Bioeng Biotechnol. 2020;8:602009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 52. | Sackett SD, Tremmel DM, Ma F, Feeney AK, Maguire RM, Brown ME, Zhou Y, Li X, O'Brien C, Li L, Burlingham WJ, Odorico JS. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci Rep. 2018;8:10452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 53. | Ge H, Hu Q, Chen T, Yang Y, Zhang C, Zhong J, Yin Y, Jiang X, Zhou X, Wang S, Hu R, Li W, Feng H. Transplantation of layer-by-layer assembled neural stem cells tethered with vascular endothelial growth factor reservoir promotes neurogenesis and angiogenesis after ischemic stroke in mice. Appl Mater Today. 2022;28:101548. [DOI] [Full Text] |
| 54. | Shuborna NS, Chaiyasamut T, Sakdajeyont W, Vorakulpipat C, Rojvanakarn M, Wongsirichat N. Generation of novel hyaluronic acid biomaterials for study of pain in third molar intervention: a review. J Dent Anesth Pain Med. 2019;19:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Dovedytis M, Liu ZJ, Bartlett S. Hyaluronic acid and its biomedical applications: A review. Engineered Regeneration. 2020;1:102-113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 56. | Shabani Z, Rahbarghazi R, Karimipour M, Ghadiri T, Salehi R, Sadigh-Eteghad S, Farhoudi M. Transplantation of bioengineered Reelin-loaded PLGA/PEG micelles can accelerate neural tissue regeneration in photothrombotic stroke model of mouse. Bioeng Transl Med. 2022;7:e10264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Jossin Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 58. | Essa D, Kondiah PPD, Choonara YE, Pillay V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front Bioeng Biotechnol. 2020;8:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 59. | Li J, Zhang Y, Lou Z, Li M, Cui L, Yang Z, Zhang L, Gu N, Yang F. Magnetic Nanobubble Mechanical Stress Induces the Piezo1-Ca2+ -BMP2/Smad Pathway to Modulate Neural Stem Cell Fate and MRI/Ultrasound Dual Imaging Surveillance for Ischemic Stroke. Small. 2022;18:e2201123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Yan W, Chen ZY, Chen JQ, Chen HM. BMP2 promotes the differentiation of neural stem cells into dopaminergic neurons in vitro via miR-145-mediated upregulation of Nurr1 expression. Am J Transl Res. 2016;8:3689-3699. [PubMed] |
| 61. | Al Abbar A, Ngai SC, Nograles N, Alhaji SY, Abdullah S. Induced Pluripotent Stem Cells: Reprogramming Platforms and Applications in Cell Replacement Therapy. Biores Open Access. 2020;9:121-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 62. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18178] [Article Influence: 956.7] [Reference Citation Analysis (0)] |
| 63. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14305] [Article Influence: 841.5] [Reference Citation Analysis (0)] |
| 64. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7589] [Cited by in RCA: 7242] [Article Influence: 402.3] [Reference Citation Analysis (0)] |
| 65. | Youssef AA, Ross EG, Bolli R, Pepine CJ, Leeper NJ, Yang PC. The Promise and Challenge of Induced Pluripotent Stem Cells for Cardiovascular Applications. JACC Basic Transl Sci. 2016;1:510-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1023] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 67. | Zhang F, Zhu Y, Chen J, Kuang W, Huang R, Duan F, Li Y, Wang L, Qiu H, Chen X, Ming J, Liu P, Du Y, Chang SC, Chen L, Na J. Efficient endothelial and smooth muscle cell differentiation from human pluripotent stem cells through a simplified insulin-free culture system. Biomaterials. 2021;271:120713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | McCrary MR, Jiang MQ, Jesson K, Gu X, Logun MT, Wu A, Gonsalves N, Karumbaiah L, Yu SP, Wei L. Glycosaminoglycan scaffolding and neural progenitor cell transplantation promotes regenerative immunomodulation in the mouse ischemic brain. Exp Neurol. 2022;357:114177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3016] [Cited by in RCA: 2908] [Article Influence: 181.8] [Reference Citation Analysis (0)] |
| 70. | Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101:107598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 482] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 71. | Martínez-Cerdeño V, Noctor SC. Neural Progenitor Cell Terminology. Front Neuroanat. 2018;12:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 72. | Zhang L, Wang Y, Tang Y, Jiao Z, Xie C, Zhang H, Gu P, Wei X, Yang GY, Gu H, Zhang C. High MRI performance fluorescent mesoporous silica-coated magnetic nanoparticles for tracking neural progenitor cells in an ischemic mouse model. Nanoscale. 2013;5:4506-4516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Somaa FA, Wang TY, Niclis JC, Bruggeman KF, Kauhausen JA, Guo H, McDougall S, Williams RJ, Nisbet DR, Thompson LH, Parish CL. Peptide-Based Scaffolds Support Human Cortical Progenitor Graft Integration to Reduce Atrophy and Promote Functional Repair in a Model of Stroke. Cell Rep. 2017;20:1964-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 74. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6624] [Cited by in RCA: 6344] [Article Influence: 226.6] [Reference Citation Analysis (1)] |
| 75. | Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1101] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 76. | Park SJ, Moon SH, Lee HJ, Lim JJ, Kim JM, Seo J, Yoo JW, Kim OJ, Kang SW, Chung HM. A comparison of human cord blood- and embryonic stem cell-derived endothelial progenitor cells in the treatment of chronic wounds. Biomaterials. 2013;34:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Ribó M, Alvarez-Sabín J, Montaner J. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res. 2010;80:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Li Q, Tang G, Xue S, He X, Miao P, Li Y, Wang J, Xiong L, Wang Y, Zhang C, Yang GY. Silica-coated superparamagnetic iron oxide nanoparticles targeting of EPCs in ischemic brain injury. Biomaterials. 2013;34:4982-4992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Jana NR, Earhart C, Ying JY. Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem Mater. 2007;19:5074-5082. [DOI] [Full Text] |
| 80. | Wang C, Lin G, Luan Y, Ding J, Li PC, Zhao Z, Qian C, Liu G, Ju S, Teng GJ. HIF-prolyl hydroxylase 2 silencing using siRNA delivered by MRI-visible nanoparticles improves therapy efficacy of transplanted EPCs for ischemic stroke. Biomaterials. 2019;197:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Giusti S, Fiszer de Plazas S. Neuroprotection by hypoxic preconditioning involves upregulation of hypoxia-inducible factor-1 in a prenatal model of acute hypoxia. J Neurosci Res. 2012;90:468-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Payne SL, Tuladhar A, Obermeyer JM, Varga BV, Teal CJ, Morshead CM, Nagy A, Shoichet MS. Initial cell maturity changes following transplantation in a hyaluronan-based hydrogel and impacts therapeutic success in the stroke-injured rodent brain. Biomaterials. 2019;192:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 83. | Ballios BG, Cooke MJ, Donaldson L, Coles BL, Morshead CM, van der Kooy D, Shoichet MS. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports. 2015;4:1031-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 84. | Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010;31:2555-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 85. | Führmann T, Tam RY, Ballarin B, Coles B, Elliott Donaghue I, van der Kooy D, Nagy A, Tator CH, Morshead CM, Shoichet MS. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 86. | Fujioka T, Kaneko N, Ajioka I, Nakaguchi K, Omata T, Ohba H, Fässler R, García-Verdugo JM, Sekiguchi K, Matsukawa N, Sawamoto K. β1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine. 2017;16:195-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |