Published online Sep 26, 2021. doi: 10.4252/wjsc.v13.i9.1231
Peer-review started: March 1, 2021
First decision: July 18, 2021
Revised: July 26, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: September 26, 2021
Processing time: 200 Days and 15.9 Hours
Cardiovascular diseases represent the world’s leading cause of death. In this heterogeneous group of diseases, ischemic cardiomyopathies are the most devastating and prevalent, estimated to cause 17.9 million deaths per year. Despite all biomedical efforts, there are no effective treatments that can replace the myocytes lost during an ischemic event or progression of the disease to heart failure. In this context, cell therapy is an emerging therapeutic alternative to treat cardiovascular diseases by cell administration, aimed at cardiac regeneration and repair. In this review, we will cover more than 30 years of cell therapy in cardio
Core Tip: The challenge to regenerate an adult heart has stimulated the field of stem cell therapies to search for a therapeutic alternative to promote robust cardiac repair. In this review, we will discuss several types of cell therapy, which have been used in cardiology, such as adult somatic cells and endogenous progenitor cells, presenting future perspectives with the use of cardiomyocytes derived from pluripotent stem cells and their extracellular vesicles.
- Citation: Kasai-Brunswick TH, Carvalho AB, Campos de Carvalho AC. Stem cell therapies in cardiac diseases: Current status and future possibilities. World J Stem Cells 2021; 13(9): 1231-1247
- URL: https://www.wjgnet.com/1948-0210/full/v13/i9/1231.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i9.1231
Cardiovascular diseases represent the world’s leading cause of death, and in this heterogeneous group of diseases, ischemic cardiomyopathies are the most prevalent, accounting for 17.9 million deaths per year[1]. Coronary artery occlusion or reduction of blood flow results in irreversible death of cardiac cells with consequent functional cardiac impairment. Despite improvements in the clinical-surgical management of these patients, the main effect of ischemic heart disease — the death of cardiomyocytes — is not reversed. Acute interventions can restore blood flow, avoiding the death of more cardiac cells. However, this neither contributes to the recovery of the function of the damaged tissue nor stops the progression of ischemic disease[2]. Due to the limited cardiac regenerative capacity, the lost cardiomyocytes are replaced by fibrotic scarring, leading to cardiac remodeling and heart failure. The therapeutic option for heart failure patients is an organ transplant, but the demand is far greater than the avail
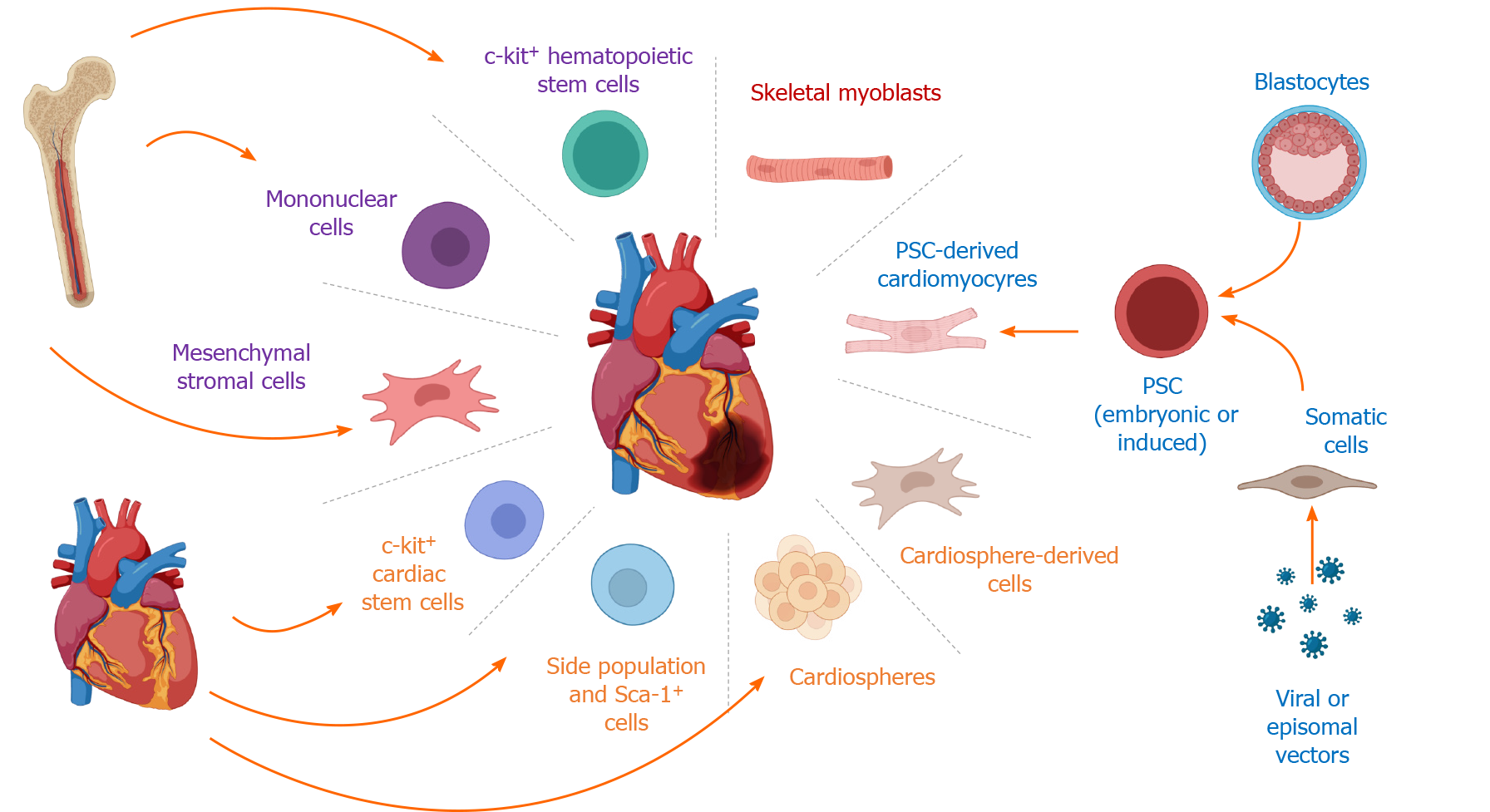
In the 1990s, the idea to replace the cardiomyocytes lost due to ischemic diseases with an external cell source paved the way for cell therapies for regenerative medicine in cardiology[4]. In this review, we will discuss the various cell therapies that were applied to treat cardiac diseases, the questionable existence of an endogenous cardiac stem cell (CSC) and their putative cardiac regenerative potential, and, finally, what we envisage as the future in the field which is using cardiomyocytes derived from pluripotent stem cells or their derivatives, such as exosomes and microvesicles (Figure 1).
Skeletal myoblasts (SM) were the first cell type used to treat ischemic cardiac diseases. This was a natural choice as SM were contractile cells with autologous availability, simple isolation, high in vitro proliferation, and resistance to ischemia[5-7].
Preclinical studies showed that SM could survive and engraft after cell transplan
Phase I and II clinical trials failed to demonstrate the functional benefits observed in experimental studies, and the presence of arrhythmias was also observed in some patients[14]. These unfavorable outcomes stimulated the search for other cell types for cardiac cell therapy.
Orlic et al[15], showed for the first time, that mouse c-kit+ progenitor bone marrow cells could transdifferentiate into cardiomyocytes and improve cardiac function in ischemic hearts. The group injected c-kit+ bone marrow-derived cells from a male GFP (green fluorescent protein) mouse into a wild-type infarcted female, claiming that the marrow-derived GFP cells expressed sarcomeric, endothelial, and smooth muscle proteins[15]. The same group achieved similar results by administering stem cell factor and granulocyte-colony stimulating factor into infarcted mice, showing bone marrow-derived cell homing into the infarcted area, differentiation into cardiomyocytes, and increased cardiac function[16].
The capability of mononuclear bone marrow cells to transdifferentiate into cardiomyocytes was disputed by independent groups, which showed that, after cell transplantation, these cells could only differentiate into mature blood cells[17,18]. In addition, some authors attributed the improvement in cardiac function after mononuclear bone marrow cell transplantation into ischemic hearts to a fusion process between bone marrow cells and cardiomyocytes[19,20]. However, this fusion mech
Equally important to understanding the mechanism involved in cardiac repair was the search for the subset of bone marrow cells that could be more effective. The potential for cardiac repair of mesenchymal stromal cells (MSC), a subpopulation of mononuclear bone marrow cells, was starting to be investigated. Initially, MSC transdifferentiation into cardiomyocytes was also proposed, but these data were also disputed, and the benefits of MSC treatments were also attributed to paracrine effects[22-26].
The preclinical results with bone marrow-derived cells drove several clinical trials to pursue the same positive results in the clinical setting. The clinical trials conducted to treat heart diseases using bone marrow cells were safe and feasible, but the results were controversial regarding the functional benefits. A large number of diverse clinical studies of different designs made the comparison of results difficult. They differed on time to intervention and severity of the treated disease, subset of cells used, number of cells administered, injection pathway, endpoints, functional analyses methods, number of subjects, randomization, blinding, single or multicenter trials, among others. Due to this heterogeneity, even metanalyses were not able to draw definitive conclusions[27,28]. The only common aspect of these trials was that none could reproduce the highly significant preclinical improvements seen on cardiac function, nor could they demonstrate new cardiomyocyte generation. Therefore, scientists continued with their search for the ideal cell type to treat ischemic heart disease.
The regenerative capacity of the heart has been the subject of intense and controversial investigations for 150 years. However, the paradigm that the heart is a post-mitotic organ and therefore incapable of regenerating itself was challenged in 2009 when Bergmann et al[29] proved that the heart could regenerate itself during an individual’s lifespan. Many nuclear tests were performed during the Cold War, leading to an increase in atmospheric Carbon 14 (C14) concentration. The C14 was absorbed by plants and entered our food chain, marking the DNA of dividing cells. After the interruption of the nuclear tests in 1963, the atmospheric concentration of this isotope decreased dramatically. Bergmann’s group compared the concentration of C14 present in cardiomyocyte DNA to the atmospheric C14 in the year that the individual was born, thus identifying the “age” of the cardiomyocytes. Using this elegant strategy, they showed that there was indeed cardiac cell renovation after birth and, by mathematical modeling, demonstrated that 50% of cardiomyocytes were renewed during the entire lifespan and that this renovation rate was age-dependent (1% per year at age 25, decreasing to 0.45% at age 75)[29].
This paradigm shift led researchers to pose a new question: what cell type was responsible for this cardiac regeneration capability? Hsieh et al[30] addressed this question using a double transgenic MerCreMer-ZEG mouse with the myosin heavy chain 6 promoter driving Cre-recombinase expression and constitutively expressing β-galactosidase (β-gal) flanked by loxP sequences followed by GFP. In this model, after administration of 4-hydroxy tamoxifen, most cardiomyocytes became GFP+ and non-cardiomyocytes remained β-gal+. Since stem cells were not labeled with GFP, if the percentage of β-gal+ cardiomyocytes increased, the group would assume that the new cardiomyocytes originated from resident stem cells. The proportion of GFP+/β-gal+ cardiomyocytes was evaluated during aging and after ischemic heart injury. One year after the tamoxifen pulse, the GFP+/β-gal+ ratio was not altered, suggesting that nonmyocytes (possibly stem cells) have no contribution to heart regeneration with aging. On the other hand, after myocardial infarction, the group observed a decrease in GFP+ and an increase of β-gal+ cardiomyocytes, suggesting that regeneration was due to resident stem cells[30]. This article supported, at that time, the idea that cardiac repair was promoted by endogenous CSC.
Beltrami et al[31] suggested that cardiac regeneration was driven by CSC located in special niches in the heart. These CSC had self-renewal properties, expressed the c-kit protein, had a clonal origin, and could differentiate in vitro into cardiomyocytes, endothelial and smooth muscle cells. When injected into an infarcted border zone, the c-kit+ CSC differentiated in vivo into cardiomyocytes[31]. Human c-kit+ CSC, when injected into immunodeficient mice and immunosuppressed rats, formed a chimeric heart improving the cardiac function of infarcted animals (increased ejection fraction and attenuated left ventricular dilation)[32]. The same group reported promising results of cell therapy using c-kit+ CSC derived from rats, dogs, and humans[32-35].
They also reported an expressive cardiac renovation of the human adult heart dependent on c-kit+ CSC. Contradicting the low regenerative rates demonstrated by Bergmann[29], Kajstura et al[36] reported that cardiomyocyte renovation rate was greater in women and increased with aging (10%-20% per year at age 20 and 40% per year at age 100), suggesting that all cardiomyocytes were replaced 15 times in women and 11 times in men during one’s lifespan.
The regenerative potential of c-kit+ CSC was evaluated in a phase I clinical trial coordinated by Bolli et al[37]. The c-kit+ CSC were obtained from the appendage of the right atria of patients with chronic ischemic heart disease submitted to coronary artery bypass surgery. The cells were isolated and cultured three weeks before injection. An increase in ejection fraction (30.3% to 38.5%) and ventricular mass (24% to 30%) of the c-kit-treated group was reported after four months of follow-up[37]. In addition, after 12 mo, the group reported a reduction of the infarcted area in the c-kit+ treated group compared to placebo[38].
Independent groups raised a cautionary note about the regenerative potential of c-kit+ CSC. They failed to demonstrate the putative cardiac regeneration of c-kit+ CSC in animal models and questioned the very existence of these endogenous cell populations in the adult heart[39,40]. Pouly et al[41] identified very few cells expressing c-kit on human atrial biopsies and these cells co-expressed CD45 and tryptase, indicating that they were not stem cells but rather mast cells. In transgenic mice expressing eGFP under the control of the c-kit promoter, c-kit-eGFP expression was observed during different stages of development in embryonic hearts, increasing in number until reaching maximum expression in the postnatal period (2 d of life). After this stage, the expression of c-kit-eGFP cells declined, and they were rarely found in adult hearts. In addition, Tallini et al[39] reported no evidence that adult c-kit+ cells differentiated into cardiomyocytes, suggesting that the c-kit+ expression shown by other groups after injury was only due to c-kit re-expression in preexisting cardiomyocytes. Jesty et al[42] evaluated the contribution of c-kit+ CSC to cardiac regeneration by injecting c-kit+-GFP CSC into infarcted neonatal and adult mice. They observed differentiation of c-kit+-GFP CSC with cardiomyogenic fate only in neonatal animals. In adults, these cells did not contribute to tissue repair[42]. Zaruba et al[40] also showed that only c-kit+ CSC derived from neonatal animals could differentiate into cardiomyocytes, promote cardiac repair and engraft in healthy hearts. They suggested that the c-kit+ CSC potential to differentiate and repair has age limitations and was not present in c-kit+ CSC derived from adults[40].
The controversy surrounding the existence of c-kit+ CSC motivated groups to conduct lineage-tracing studies, considering that the main findings which sustained the cardiomyogenic ability of c-kit+ CSC cells were based on immunofluorescence assays. These assays used an indirect strategy (primary and secondary antibodies) and therefore could produce false-positive results.
Ellison et al[43] used a lentiviral system that expressed cre-recombinase under the control of a c-kit promoter to evaluate myocardial repair in yellow fluorescent protein (YFP) reporter mice. After isoproterenol injury, the group showed the presence of new YFP+ myocytes (3.4% to 7.7%). Since only cells infected by the virus and that expressed c-kit became YFP+, the presence of YFP+ cardiomyocytes led the group to state that adult c-kit+ CSC were necessary and sufficient for functional cardiac regeneration and repair. They postulated that the key mechanism of cardiac regeneration after isoproterenol-induced heart injury was by c-kit+ CSC differentiation into cardiomyocytes[43]. However, this study was criticized due to methodology issues, including the fact that the partial c-kit promoter used could not properly recapitulate c-kit regulatory elements[44,45].
Other groups used a Cre knockin c-kit transgene approach to identify the contribution of cardiac c-kit+ cells for differentiation into cardiomyocytes during mouse development, aging, and after cardiac injury. van Berlo et al[46] showed that endogenous c-kit+ CSC contributed minimally to generate cardiomyocytes (approximately 0.003% or less if cellular fusion was considered) and concluded that this rare phenomenon could not significantly impact cardiac function. Sultana et al[47] used multiple reporter genes in mice to show that there was no c-kit co-localized with Nkx2.5 — a gene expressed by cardiac progenitor cells — nor cardiac troponin T — a gene expressed by cardiomyocytes. The group showed that c-kit+ cells in murine hearts are not cardiac progenitors but rather endothelial cell progenitors[47]. Liu et al[48] studied the cells immediately after Cre-recombinase induction using an instant c-kit lineage tracing model. Using this system, they described that 50% to 70% of labeled cardiomyocytes expressed c-kit 24 h to 48 h after myocardial injury. In this short time, it was unlikely that a progenitor could differentiate into a cardiomyocyte. Moreover, the group showed that c-kit was expressed by cardiomyocytes in adult hearts and concluded that new cardiomyocytes generated after injury were derived from preexisting c-kit+ cardiomyocytes and not from CSC[48].
The technical limitations of the chosen tools and models to study c-kit biology, fate, and function are still subject to intense debate[49,50]. To add to the controversy, Vicinanza et al[51] argued that the previously reported Cre knockin c-kit models had major limitations, such as the inability to identify cells that express c-kit in low levels and the fact that these animal constructions caused haploinsufficiency of the c-kit gene, impairing normal biological regulation and causing a severe defect in CSC-dependent myogenesis[46-48,51].
To bypass these technical issues, He et al[52] developed a system that used two new kit-Cre drivers. This system allowed labeling of all c-kit+ cells (even those with low expression) and did not affect the endogenous c-kit gene expression. In agreement with previous studies, the group showed that neither in homeostasis nor after an injury did c-kit+ CSC contribute to the generation of new cardiomyocytes[46-48,52].
Thus, despite the many studies describing c-kit+ cardiac cells published in the last 20 years, the role of c-kit+ CSC in cardiac regeneration and even their existence in adult hearts is highly questionable. Therefore, cardiac regeneration by other endogenous CSC candidates would have to be explored.
Cardiospheres and cardiosphere-derived cells (CDC) are a heterogeneous cell population obtained from explant culture of heart biopsies. Cardiospheres are originated from small phase bright cells, which detach spontaneously from the explants. These structures mimic the tridimensional tissue architecture and preserve the concept that resident stem cells are located in niches inside the organs. Messina et al[53] described them as clonal, with c-kit in the core and MSC at the periphery. Cardiospheres were obtained from mice and humans and, when injected in the peri-infarcted area in mice, induced cardiac regeneration, cell coupling, and improvement of cardiac function[53].
The expansion of cardiospheres as an adherent cell culture showed that a large number of CDC could be obtained from a small piece of heart biopsy, an important goal to translate CDC therapy to the clinical setting. The group reported that CDC differentiated into cardiomyocytes and presented spontaneous beating in vitro after ten days of co-culture with rat neonatal cardiomyocytes. When injected at the peri-infarcted heart zone, CDC improved the ejection fraction of infarcted animals by directly differentiating into cardiomyocytes and by paracrine effects[54-57]. CDC obtained from children, or newborn cardiac biopsies showed a superior regenerative cardiac capacity to treat infarcted animals compared to those obtained from adults[58,59].
Clinical trials using autologous CDC were conducted to treat patients who suffered recent myocardial infarctions (30 d), aiming to reverse ventricular dysfunction. The intracoronary administration of autologous CDC was safe and showed a discrete decrease of the fibrotic scar when analyzed by magnetic resonance imaging, accompanied by an increase in left ventricular mass with no effect on ejection fraction or end-diastolic or end-systolic volumes at four months and one year after treatment[60,61].
Negative results after administration of cardiospheres or CDC were also observed when utilizing this cell therapy to repair the heart. Li et al[62] failed to observe mouse cardiac improvement after administration of CDC derived from adult hearts. Takehara et al[63] treated four groups of infarcted pigs: (1) Treated with human CDC (hCDC); (2) Treated with a low release β-fibroblast growth factor (FGF) gel; (3) Treated with both; and (4) Treated with placebo. Group 2 showed cardiac regeneration and improved cardiac function. Group 3 had the same effects reported for group 2 but with higher magnitudes. The group that received isolated hCDC did not exhibit cardiac improvement[63]. In agreement with this, other groups showed that autologous CDC did not improve cardiac function in small or large animal models of myocardial infarction, with CDC, at most, attenuating cardiac remodeling[64-67]. Lineage-tracing of the cardiac explant-derived cells (EDC) was performed using the ventricular myosin light chain MLC2v-Cre/ZEG model. Transgenic EDC were analyzed in vitro by morphology and immunofluorescence for cardiac proteins and in vivo by engraftment and cardiac differentiation. EDC were engrafted into murine hearts but failed to generate cardiomyocytes, suggesting that the strategy to identify cardiac progenitor cells exclusively by morphology was inadequate[68].
Cardiospheres and CDC, similar to c-kit+ cells, generated controversial preclinical results and failed to demonstrate robust cardiomyocyte differentiation or improved cardiac function in clinical trials. Thus, the search for the identity of cardiac progeni
The capacity to extrude Hoechst33342 and the expression of cell surface ATP binding cassette sub-family G member 2 (ABCG2) are characteristics shared among stem cells present in various tissues, called side population (SP) cells. These cells were also considered putative cardiac progenitor cells with cardiac, endothelial, and smooth muscle differentiation capabilities[69]. However, lineage-tracing using an ABCG2 CreER model demonstrated that this differentiation capacity was present only in embryonic phases and was lost in adulthood, refuting that these cells were CSC candidates responsible for homeostasis and injury response[70,71].
The presence of stem cell antigen-1 (Sca-1) in heart cells was first described by Oh et al[72]. This surface marker, also called lymphocyte activation protein-6A (Ly-6A), consists of a glycosylphosphatidylinositol-anchored cell surface protein (GP-AP) of the Ly6 gene family that is a popular marker used to enrich samples with murine adult hematopoietic stem cells[73]. Sca-1+ adult mouse heart cells were negative for CD45, CD34, c-kit, GATA-2, Lmo2, and Flk-1, presenting a distinct phenotype from hematopoietic stem cells, progenitor endothelial, and muscle satellite cells. In addition, Sca-1+ cultured cells were clonal, expressed contractile proteins, and presented spontaneous beating[72,74,75].
Transplantation of Sca-1+ cardiac cells showed improved heart function in infarcted mice, promoted by direct differentiation into cardiomyocytes and the release of cytokines such as a soluble VCAM-1, which stimulated angiogenesis, migration, and survival in vivo[76].
The Sca-1+ population was described as a heterogeneous cell population. The subpopulation that expressed Sca-1 in high levels did not differentiate into multiple cell types, while the Sca-1low showed direct differentiation into endothelial cells and cardiomyocytes in vitro and in vivo, decreased infarct size, and preserved ventricular function in infarcted mice[77]. Another subpopulation, the Sca-1+CD31-, also showed cardiomyogenic potential in co-culture through a process mediated by cellular coupling with adult cardiomyocytes. This cell population could home to an ischemic heart area using the SDF-1α/CXCR4 pathway and attenuated post-infarct structural ventricular remodeling by direct endothelial and cardiomyocyte differentiation[78-80]. Similar properties were observed for Sca-1+CD45- Isl1+ cells obtained from cardiospheres derived from middle-aged mice[81]. The Bmi1+ cells, another Sca-1 subpopulation, demonstrated remarkable cardiac regeneration after cell therapy in infarcted mice. Approximately 14% of new mouse cardiomyocytes were observed after myocardial infarction[29,82], contradicting the low rates of cardiac regeneration previously demonstrated in humans.
Noseda et al[83] further refined the study of Sca-1+ murine heart subpopulations using a single-cell expression profile to identify a definitive phenotype for the cardiac stem/progenitor cells. They evaluated the expression of Sca-1, CD31, PDGFRα, and the ability to extrude Hoechst33342 and identified that only SP+ Sca-1+ CD31- PDGFRα+ cells were clonogenic cardiac progenitors.
Even though Sca-1 is not expressed in humans, Goumans et al[84] isolated cardiac progenitor cells from human fetal and adult cardiac biopsies using an antibody that recognized a mouse Sca-1 epitope as a target. These progenitor cells also differentiated in vitro into spontaneous beating cardiomyocytes and endothelial cells. Furthermore, when Sca-1+ cells derived from fetal tissue were injected in immunodeficient infarcted mice, they also improved cardiac function by direct differentiation into cardio
Bailey et al[85] studied a Sca-1 knockout mouse to understand the role of Sca-1 in heart development and cardiac regeneration. These animals showed defects in ventricular contractility and repair, suggesting that the genetic deletion of Sca-1 compromised resident progenitor cells responsible for cardiac repair[85]. A triple transgenic mouse based on the Tet-off Cre system showed that Sca-1+ cells played an important role in the generation of cardiomyocytes during homeostasis and after heart injury[86]. These data were not confirmed by independent groups using lineage-tracing and fate-mapping studies by multiple sophisticated tools used to genetically trace Sca-1+ cells. They proved that these cells did not contribute to cardiac homeo
Considering all these studies, it is unlikely that the beneficial results promoted by therapy using Sca-1+ cells could be attributed to direct cardiomyocyte differentiation. It is more likely they are linked to angiogenesis stimulated by a paracrine effect. These data reinforce the current leading theory that the generation of new cardiomyocytes during adult life is derived from the proliferation of preexisting cardiomyocytes than from progenitor cells, as already demonstrated in neonatal mice and zebrafish[92-94]. For more details, we suggest the review written by He et al[95].
All putative CSC listed in this review have been discarded as true cardiac stem/ progenitor cells by detailed lineage tracing experiments using sophisticated transgenic models. Furthermore, controversial results surrounded their proposed benefits in preclinical studies, and none resisted the test when applied in the clinical setting. The central idea to replace cardiomyocytes lost due to ischemic or chronic injury persists, but the efforts in the field are now redirected towards obtaining these cardiomyocytes in vitro, from pluripotent stem cells, and then transplant them into the injured heart.
Pluripotent stem cells are self-renewing cells that can differentiate into the three embryonic germ layers upon specific stimuli. Until 2006, the sole available source of pluripotent stem cells were embryonic stem cells (ESC). ESC was obtained from the blastocyst's internal mass and could be cultured in vitro as an immortalized lineage[96]. In 2006, Takahashi and Yamanaka[97] made a revolutionary discovery showing how to generate pluripotent stem cells from a somatic cell. They reprogramed fibroblasts, first from mice and, in the next year, from humans, by overexpressing Oct-3/4, Sox-2, Klf-4, and c-Myc transcription factors and obtained induced pluripotent stem cells (iPSC), which share the same unique ESC properties[97,98]. Furthermore, the differentiation protocols of pluripotent stem cells into adult cells were improved based on lessons learned from developmental biology[99]. These advances allowed pluripotent stem cells to be efficiently differentiated into cardiomyocytes in vitro by modulating the Wnt pathway, representing an almost inexhaustible source of animal-specific, including human, cardiomyocytes to be used for cell therapy[100].
Transplantation of cardiomyocytes derived from human ESC (hESC-CM) engrafted into infarcted hearts, partially remuscularized myocardial infarctions, improved cardiac performance, and attenuated the remodeling process in infarcted rats and guinea pigs[101,102]. Yu et al[103] proposed that the anti-inflammatory effect promoted by the administration of hESC-CM on immunodeficient female mice submitted to permanent ischemia was a therapeutic mechanism by which these cells improved cardiac function. Human ESC-CM were also evaluated in a non-human primate model submitted to ischemia and reperfusion injury to evaluate the safety, feasibility, and efficacy in a large animal model. As shown in small animals, the administration of 1 billion hESC-CM via intramyocardial injection improved cardiac function through remuscularization in the non-human primate model[104]. Arrhythmias are a significant concern in cardiac cell therapies. They can result either from the fetal-like phenotype of cardiomyocytes derived from pluripotent stem cells or from a dysfunctional electromechanical coupling between the graft and host cells — as seen with SM[14,105-107]. In this context, the maturation of grafted cells after three months of follow-up, the presence of electromechanical junctions leading to synchronic regular calcium transients between transplanted and host cells, and the absence of fatal ventricular arrhythmias were important observations by Chong et al[104].
The administration of human cardiomyocytes derived from iPSC (hiPSC-CM) also showed promising results in murine, porcine, and non-human primate ischemic cardiomyopathy models[108-110]. Kawamura et al[108] showed that hiPSC-CM therapy improved cardiac function and attenuated ventricular remodeling of immunosuppressed minipigs submitted to permanent occlusion of the left anterior descending coronary artery after eight weeks of administration of 25 million purified hiPSC-CM. This work suggested that a paracrine mechanism was responsible for the observed results instead of the direct muscularization observed by Chong et al[104]. This conclusion was based on blood flow increase at the infarcted myocardium border due to angiogenesis, probably induced by basic FGF and vascular endothelial growth factor secreted by hiPSC-CM. Also, the majority of the iPSC-CM survived for a short time in infarcted hearts (2 wk), even though some were identified eight weeks after treatment. Therefore, the authors hypothesized that low engraftment was due to insufficient immunosuppressive therapy[108]. The low engraftment and paracrine activity of hiPSC-CM (release of proangiogenic and antiapoptotic cytokines) in the acute myocardial infarction model was corroborated by Ong et al[109], who also demonstrated improved cardiac function by magnetic resonance imaging.
A major concern in regenerative medicine is how to avoid immunologic rejection and how to induce immunologic tolerance to cells used in cell therapy, considering that autologous transplantation has to meet manufacture temporal challenges in a clinical setting. The use of major histocompatibility complex (MHC)-matched cells between host and graft was addressed by Shiba et al[110]. They treated five immuno
During myocardial infarction, aside from cardiomyocytes, other important cell types are also lost, such as endothelial, smooth muscle cells, and fibroblasts. Cell therapy using these cell types was successfully performed in a porcine model of acute myocardial infarction. Endothelial cells (hEC-ESC) and smooth muscle cells derived from human ESC (hSMC-ESC) were transplanted in a fibrin-gel patch into five infarcted pigs submitted to ischemia and reperfusion. Resonance magnetic imaging showed left ventricular functional improvement after a 4-wk follow-up period. The success of vascular cell therapy was attributed to direct neovascularization[111]. The combined use of cardiomyocytes, endothelial and smooth muscle cells derived from hiPSC to treat porcine myocardial infarction was also performed by Ye et al[112]. They showed that this cell combination resulted in cardiomyocyte engraftment and coupling to the host tissue, increased angiogenesis, improved left ventricular function and myocardial metabolism while reducing apoptosis and infarct size with no ventricular arrhythmias observed after four weeks of follow-up.
The end product of all these preclinical experiments with cardiomyocytes derived from pluripotent stem cells was that, for the first time, cardiomyocytes could be produced in vitro on a large scale to meet the requirements for cell therapy, presented robust engraftment of newly-generated units, and promoted angiogenesis, an important additional effect for cardiac regeneration, resulting in improved cardiac function. Despite these significant advances, there are still obstacles to overcome before moving to the clinic, such as managing the risk of arrhythmias, improving cell viability and consequently engraftment, eliminating immune rejection, and identifying the combination of secreted factors that could be responsible for the paracrine effect. Another major problem, not yet completely solved, is the maturation of the PSC-derived cardiomyocytes. Cardiomyocytes derived from pluripotent stem cells exhibit an immature phenotype that can be shifted to a more mature state by different approaches such as: in vivo grafting, three-dimensional constructs using scaffolds and electrostimulation, and manipulation of metabolic pathways. A detailed analysis of such maneuvers is beyond the scope of this review and can be found in Karbassi et al[113].
Cardiac cell therapy has changed significantly in the past 30 years. We have lived through the rise and fall of the endogenous CSC paradigm, whose existence is still subject to debate. Cardiomyocytes derived from pluripotent stem cells emerged as a promising therapeutic alternative, and this cell technology should continue to be investigated to meet the required conditions for clinical application. IPSC technology in human diseases has already been demonstrated to be safe, feasible and showed exciting first results in a patient with macular degeneration[114].
As the paracrine effect has also been suggested as a possible cardiac function improvement mechanism produced by cell therapy, the soluble factors secreted by cells have been investigated. Microvesicles and exosomes — collectively known as extracellular vesicles — were described as small carriers of bioactive products (such as RNA, DNA, proteins, lipids, and cytokines). They are released by cells in different contexts, exerting modulatory effects in diverse biological processes[115]. In ischemic cardiac diseases, extracellular vesicles derived from iPSC-cardiac progenitors and MSC have shown cardioprotective effects in infarcted mice by modulating the inflammatory response and promoting tissue regeneration via microRNAs[116-118].
Another emerging approach in cardiac therapy is the in situ direct reprogramming of fibroblasts into cardiomyocytes. The injection of transcription factors Gata4, Mef2c, and Tbx5 directly into mouse myocardium generated new induced cardiomyocytes (iCM), which coupled with the host’s heart cells and decreased the infarct area. When the transcriptomes of iCM were compared to the adult heart cells, they were found to be more similar to those heart cells than to cardiomyocytes generated by the same method in vitro[119-120]. The direct reprogramming of human cells, which involves another molecular cocktail and epigenetic modulation, is still under investigation[121-124].
Strategies to stimulate the proliferation of endogenous cardiomyocytes are another possibility to regenerate infarcted hearts. MicroRNAs, such as the miR-15 family, regulate the cell cycle of cardiomyocytes. Treatment of infarcted mice with inhibitors of this family (anti-miR-15) has resulted in mitotic cardiomyocytes and improved cardiac function[125-127]. The potential of anti-miR-15, commercially named MGN-1374, is being evaluated in a clinical trial[128]. The small molecule MSI-1436 has also shown interesting results, accelerating heart regeneration in zebrafish and mouse infarction models by the same mechanism — stimulation of preexisting cardiomyocyte proliferation[129].
Thirty years of research have taken us a long way in the understanding of cardiac regeneration mechanisms. Unfortunately, this long journey has not yet resulted in the salutary effects of cell-based therapies in the clinical setting. Nonetheless, the long and winding road up to here has shown, as mentioned above, that many options still remain to be explored. Undoubtedly, learning from previous mistakes, we will reach efficacious cell-based therapies to repair and regenerate the injured heart.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haque N, Jiang W S-Editor: Gao CC L-Editor: Webster JR P-Editor: Ma YJ
| 1. | World of Health Organization. Cardiovascular diseases. [cited 21 February 2021]. In: World of Health Organization [Internet]. Available from: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1. |
| 2. | Butler D. UN targets top killers. Nature. 2011;477:260-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, Wertheim JA, Rothblatt M, Boyden ES, Eidbo E, Lee WPA, Pomahac B, Brandacher G, Weinstock DM, Elliott G, Nelson D, Acker JP, Uygun K, Schmalz B, Weegman BP, Tocchio A, Fahy GM, Storey KB, Rubinsky B, Bischof J, Elliott JAW, Woodruff TK, Morris GJ, Demirci U, Brockbank KGM, Woods EJ, Ben RN, Baust JG, Gao D, Fuller B, Rabin Y, Kravitz DC, Taylor MJ, Toner M. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol. 2017;35:530-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 352] [Article Influence: 44.0] [Reference Citation Analysis (1)] |
| 4. | Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 378] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Marelli D, Desrosiers C, el-Alfy M, Kao RL, Chiu RC. Cell transplantation for myocardial repair: an experimental approach. Cell Transplant. 1992;1:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 130] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Koh GY, Klug MG, Soonpaa MH, Field LJ. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993;92:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 174] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995;60:12-18. [PubMed] |
| 8. | Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 680] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 9. | Scorsin M, Hagège A, Vilquin JT, Fiszman M, Marotte F, Samuel JL, Rappaport L, Schwartz K, Menasché P. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J Thorac Cardiovasc Surg. 2000;119:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Rajnoch C, Chachques JC, Berrebi A, Bruneval P, Benoit MO, Carpentier A. Cellular therapy reverses myocardial dysfunction. J Thorac Cardiovasc Surg. 2001;121:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100:7808-7811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Rubart M, Soonpaa MH, Nakajima H, Field LJ. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest. 2004;114:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagège AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 615] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 15. | Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3891] [Cited by in RCA: 3547] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 16. | Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344-10349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1568] [Cited by in RCA: 1449] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 17. | Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1200] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 18. | Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1658] [Cited by in RCA: 1490] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 19. | Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1160] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 20. | Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1499] [Cited by in RCA: 1375] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 21. | Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 719] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 22. | Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 588] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 23. | Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1548] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 24. | Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 751] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 25. | Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 541] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 27. | Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, Howard JP, Cole GD, Francis DP; DAMASCENE writing group. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 28. | Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moyé LA, Sürder D, Corti R, Huikuri H, Miettinen J, Wöhrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G; ACCRUE Investigators. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 29. | Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2522] [Cited by in RCA: 2232] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 30. | Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 568] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 31. | Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2692] [Cited by in RCA: 2438] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 32. | Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068-14073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 704] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 33. | Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Dawn B, Zuba-Surma EK, Abdel-Latif A, Tiwari S, Bolli R. Cardiac stem cell therapy for myocardial regeneration. A clinical perspective. Minerva Cardioangiol. 2005;53:549-564. [PubMed] |
| 35. | Linke A, Müller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Böhm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966-8971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 406] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1059] [Cited by in RCA: 975] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 38. | Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 39. | Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 40. | Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992-2000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasché P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci U S A. 2012;109:13380-13385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 43. | Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfò M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 44. | Cairns LA, Moroni E, Levantini E, Giorgetti A, Klinger FG, Ronzoni S, Tatangelo L, Tiveron C, De Felici M, Dolci S, Magli MC, Giglioni B, Ottolenghi S. Kit regulatory elements required for expression in developing hematopoietic and germ cell lineages. Blood. 2003;102:3954-3962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Molkentin JD, Houser SR. Are resident c-Kit+ cardiac stem cells really all that are needed to mend a broken heart? Circ Res. 2013;113:1037-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 619] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 47. | Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 48. | Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y, Qiao Z, Wang QD, Lui KO, Zhou B. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016;26:119-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 49. | Gude NA, Firouzi F, Broughton KM, Ilves K, Nguyen KP, Payne CR, Sacchi V, Monsanto MM, Casillas AR, Khalafalla FG, Wang BJ, Ebeid DE, Alvarez R, Dembitsky WP, Bailey BA, van Berlo J, Sussman MA. Cardiac c-Kit Biology Revealed by Inducible Transgenesis. Circ Res. 2018;123:57-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Zhou B, Wu SM. Reassessment of c-Kit in Cardiac Cells: A Complex Interplay Between Expression, Fate, and Function. Circ Res. 2018;123:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Vicinanza C, Aquila I, Cianflone E, Scalise M, Marino F, Mancuso T, Fumagalli F, Giovannone ED, Cristiano F, Iaccino E, Marotta P, Torella A, Latini R, Agosti V, Veltri P, Urbanek K, Isidori AM, Saur D, Indolfi C, Nadal-Ginard B, Torella D. Kitcre knock-in mice fail to fate-map cardiac stem cells. Nature. 2018;555:E1-E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 52. | He L, Han M, Zhang Z, Li Y, Huang X, Liu X, Pu W, Zhao H, Wang QD, Nie Y, Zhou B. Reassessment of c-Kit+ Cells for Cardiomyocyte Contribution in Adult Heart. Circulation. 2019;140:164-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1024] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 54. | Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 844] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 55. | Davis DR, Ruckdeschel Smith R, Marbán E. Human cardiospheres are a source of stem cells with cardiomyogenic potential. Stem Cells. 2010;28:903-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration vs paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 513] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 57. | Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marbán L, Marbán E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 357] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 58. | Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, Backer CL, Dudley SC Jr, Wold LE, Kaushal S. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 59. | Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, Kaushal S. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation. 2012;126:S46-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1058] [Article Influence: 81.4] [Reference Citation Analysis (1)] |
| 61. | Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 62. | Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 63. | Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, Takahashi T, Takamatsu T, Fukushima M, Komeda M, Yamagishi M, Yaku H, Tabata Y, Matsubara H, Oh H. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Zhao ZA, Han X, Lei W, Li J, Yang Z, Wu J, Yao M, Lu XA, He L, Chen Y, Zhou B, Hu S. Lack of Cardiac Improvement After Cardiosphere-Derived Cell Transplantation in Aging Mouse Hearts. Circ Res. 2018;123:e21-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Kasai-Brunswick TH, Costa AR, Barbosa RA, Farjun B, Mesquita FC, Silva Dos Santos D, Ramos IP, Suhett G, Brasil GV, Cunha ST, Brito JO, Passipieri JD, Carvalho AB, Campos de Carvalho AC. Cardiosphere-derived cells do not improve cardiac function in rats with cardiac failure. Stem Cell Res Ther. 2017;8:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marbán E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075-1083, 7 p following 1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 67. | Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, Makkar R, Marbán E. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 68. | Shenje LT, Field LJ, Pritchard CA, Guerin CJ, Rubart M, Soonpaa MH, Ang KL, Galiñanes M. Lineage tracing of cardiac explant derived cells. PLoS One. 2008;3:e1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 70. | Maher TJ, Ren Y, Li Q, Braunlin E, Garry MG, Sorrentino BP, Martin CM. ATP-binding cassette transporter Abcg2 Lineage contributes to the cardiac vasculature after oxidative stress. Am J Physiol Heart Circ Physiol. 2014;306:H1610-H1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Doyle MJ, Maher TJ, Li Q, Garry MG, Sorrentino BP, Martin CM. Abcg2-Labeled Cells Contribute to Different Cell Populations in the Embryonic and Adult Heart. Stem Cells Dev. 2016;25:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313-12318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1358] [Cited by in RCA: 1229] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 73. | Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 74. | Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384-11391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 453] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 75. | Tateishi K, Ashihara E, Takehara N, Nomura T, Honsho S, Nakagami T, Morikawa S, Takahashi T, Ueyama T, Matsubara H, Oh H. Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J Cell Sci. 2007;120:1791-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Takamiya M, Haider KH, Ashraf M. Identification and characterization of a novel multipotent sub-population of Sca-1⁺ cardiac progenitor cells for myocardial regeneration. PLoS One. 2011;6:e25265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Pfister O, Jain M, Liao R. Cell therapy in heart failure. Heart Fail Clin. 2005;1:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 80. | Liang SX, Tan TY, Gaudry L, Chong B. Differentiation and migration of Sca1+/CD31- cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol. 2010;138:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Ye J, Boyle A, Shih H, Sievers RE, Zhang Y, Prasad M, Su H, Zhou Y, Grossman W, Bernstein HS, Yeghiazarians Y. Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One. 2012;7:e30329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | Valiente-Alandi I, Albo-Castellanos C, Herrero D, Sanchez I, Bernad A. Bmi1 (+) cardiac progenitor cells contribute to myocardial repair following acute injury. Stem Cell Res Ther. 2016;7:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Noseda M, Harada M, McSweeney S, Leja T, Belian E, Stuckey DJ, Abreu Paiva MS, Habib J, Macaulay I, de Smith AJ, al-Beidh F, Sampson R, Lumbers RT, Rao P, Harding SE, Blakemore AI, Jacobsen SE, Barahona M, Schneider MD. PDGFRα demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat Commun. 2015;6:6930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 84. | Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, Verhaar MC, van der Heyden MA, de Kleijn D, Mummery CL, van Veen TA, Sluijter JP, Doevendans PA. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 85. | Bailey B, Fransioli J, Gude NA, Alvarez R Jr, Zhang X, Gustafsson ÅB, Sussman MA. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ Res. 2012;111:750-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K, Braun T. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports. 2013;1:397-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 87. | Tang J, Li Y, Huang X, He L, Zhang L, Wang H, Yu W, Pu W, Tian X, Nie Y, Hu S, Wang QD, Lui KO, Zhou B. Fate Mapping of Sca1+ Cardiac Progenitor Cells in the Adult Mouse Heart. Circulation. 2018;138:2967-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Vagnozzi RJ, Sargent MA, Lin SJ, Palpant NJ, Murry CE, Molkentin JD. Genetic Lineage Tracing of Sca-1+ Cells Reveals Endothelial but Not Myogenic Contribution to the Murine Heart. Circulation. 2018;138:2931-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 89. | Zhang L, Sultana N, Yan J, Yang F, Chen F, Chepurko E, Yang FC, Du Q, Zangi L, Xu M, Bu L, Cai CL. Cardiac Sca-1+ Cells Are Not Intrinsic Stem Cells for Myocardial Development, Renewal, and Repair. Circulation. 2018;138:2919-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 90. | Neidig LE, Weinberger F, Palpant NJ, Mignone J, Martinson AM, Sorensen DW, Bender I, Nemoto N, Reinecke H, Pabon L, Molkentin JD, Murry CE, van Berlo JH. Evidence for Minimal Cardiogenic Potential of Stem Cell Antigen 1-Positive Cells in the Adult Mouse Heart. Circulation. 2018;138:2960-2962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 91. | Soonpaa MH, Lafontant PJ, Reuter S, Scherschel JA, Srour EF, Zaruba MM, Rubart-von der Lohe M, Field LJ. Absence of Cardiomyocyte Differentiation Following Transplantation of Adult Cardiac-Resident Sca-1+ Cells Into Infarcted Mouse Hearts. Circulation. 2018;138:2963-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1141] [Cited by in RCA: 1035] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 93. | Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 1053] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 94. | Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2137] [Cited by in RCA: 1950] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 95. | He L, Nguyen NB, Ardehali R, Zhou B. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress. Circulation. 2020;142:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 96. | Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5956] [Cited by in RCA: 5428] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 97. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18135] [Article Influence: 954.5] [Reference Citation Analysis (0)] |
| 98. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14287] [Article Influence: 840.4] [Reference Citation Analysis (0)] |
| 99. | Parikh A, Wu J, Blanton RM, Tzanakakis ES. Signaling Pathways and Gene Regulatory Networks in Cardiomyocyte Differentiation. Tissue Eng Part B Rev. 2015;21:377-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848-E1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1269] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 101. | Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1672] [Cited by in RCA: 1597] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 102. | Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 558] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 103. | Yu Y, Qin N, Lu XA, Li J, Han X, Ni X, Ye L, Shen Z, Chen W, Zhao ZA, Lei W, Hu S. Human embryonic stem cell-derived cardiomyocyte therapy in mouse permanent ischemia and ischemia-reperfusion models. Stem Cell Res Ther. 2019;10:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 104. | Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1103] [Cited by in RCA: 1041] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 105. | Guo Y, Pu WT. Cardiomyocyte Maturation: New Phase in Development. Circ Res. 2020;126:1086-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 106. | Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 107. | Menasché P, Hagège AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 714] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 108. | Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 109. | Ong SG, Huber BC, Lee WH, Kodo K, Ebert AD, Ma Y, Nguyen PK, Diecke S, Chen WY, Wu JC. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation. 2015;132:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 110. | Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 585] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 111. | Xiong Q, Hill KL, Li Q, Suntharalingam P, Mansoor A, Wang X, Jameel MN, Zhang P, Swingen C, Kaufman DS, Zhang J. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells. 2011;29:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 112. | Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 360] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 113. | Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, Murry CE. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 2020;17:341-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 114. | Souied E, Pulido J, Staurenghi G. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med. 2017;377:792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 115. | Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 677] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 116. | Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 117. | Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 545] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 118. | Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, Ni C, Li Q, Xiang C, Zhang L, Wu R, Zhu W, Yu H, Hu X, Wang J. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl Med. 2017;6:209-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 227] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 119. | Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, Wada R, Katsumata Y, Kaneda R, Nakade K, Kurihara C, Obata Y, Miyake K, Fukuda K, Ieda M. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 120. | Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1000] [Cited by in RCA: 1029] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 121. | Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, Ma T, Xu T, Shi G, Srivastava D, Ding S. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352:1216-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 122. | Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1:235-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 123. | Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci U S A. 2013;110:12667-12672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 124. | Muraoka N, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Isomi M, Nakashima H, Akiyama M, Wada R, Inagawa K, Nishiyama T, Kaneda R, Fukuda T, Takeda S, Tohyama S, Hashimoto H, Kawamura Y, Goshima N, Aeba R, Yamagishi H, Fukuda K, Ieda M. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014;33:1565-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 125. | Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 587] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 126. | Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW 2nd, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 358] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 127. | Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 128. | Chakraborty C, Sharma AR, Sharma G, Lee SS. Therapeutic advances of miRNAs: A preclinical and clinical update. J Adv Res. 2021;28:127-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 279] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 129. | Smith AM, Maguire-Nguyen KK, Rando TA, Zasloff MA, Strange KB, Yin VP. The protein tyrosine phosphatase 1B inhibitor MSI-1436 stimulates regeneration of heart and multiple other tissues. NPJ Regen Med. 2017;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |