Published online Sep 26, 2021. doi: 10.4252/wjsc.v13.i9.1197
Peer-review started: February 27, 2021
First decision: April 20, 2021
Revised: April 21, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: September 26, 2021
Processing time: 202 Days and 10.7 Hours
Despite a vast amount of different methods, protocols and cryoprotective agents (CPA), stem cells are often frozen using standard protocols that have been optimized for use with cell lines, rather than with stem cells. Relatively few comparative studies have been performed to assess the effects of cryopreservation methods on these stem cells. Dimethyl sulfoxide (DMSO) has been a key agent for the development of cryobiology and has been used universally for cryopreservation. However, the use of DMSO has been associated with in vitro and in vivo toxicity and has been shown to affect many cellular processes due to changes in DNA methylation and dysregulation of gene expression. Despite studies showing that DMSO may affect cell characteristics, DMSO remains the CPA of choice, both in a research setting and in the clinics. However, numerous alternatives to DMSO have been shown to hold promise for use as a CPA and include albumin, trehalose, sucrose, ethylene glycol, polyethylene glycol and many more. Here, we will discuss the use, advantages and disadvantages of these CPAs for cryopreservation of different types of stem cells, including hematopoietic stem cells, mesenchymal stromal/stem cells and induced pluripotent stem cells.
Core Tip: The manuscript is an overview of current cryopreservation protocols used for cold storage of hematopoietic stem cells, mesenchymal stem cells and induced pluripotent stem cells. Although dimethyl sulfoxide (DMSO) is commonly used in cryopreservation of cell lines, primary cells and stem cells, the use of DMSO has been associated with certain toxicity, both directly on the cells, as well as upon infusion with the stem cell product. As a result of this many groups have undertaken efforts to find suitable replacements for DMSO that are equally potent but less toxic. In this review, we summarize the current status quo of stem cell freezing protocols and we describe the most commonly used cryoprotective agents and their effects on stem cells and stem cell function.
- Citation: Erol OD, Pervin B, Seker ME, Aerts-Kaya F. Effects of storage media, supplements and cryopreservation methods on quality of stem cells. World J Stem Cells 2021; 13(9): 1197-1214
- URL: https://www.wjgnet.com/1948-0210/full/v13/i9/1197.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i9.1197
Although optimization of stem cell culture, expansion and differentiation methods has been the main focus of stem cell research, an equally important and largely ignored topic in stem cell research is long term storage and cryopreservation. No matter the quality of the stem cell cultures, without optimization and careful control of cryopreservation, reproducibility and clinical (side) effects may be difficult to interpret. Furthermore, effects may be unexpected and suboptimal if cells are not stored, frozen and thawed under the most favorable conditions. Cryopreservation of cells, tissues and embryos has been common practice since the 1950s and took flight with the development of in vitro fertilization practices and hematopoietic stem cell (HSC) transplantation.
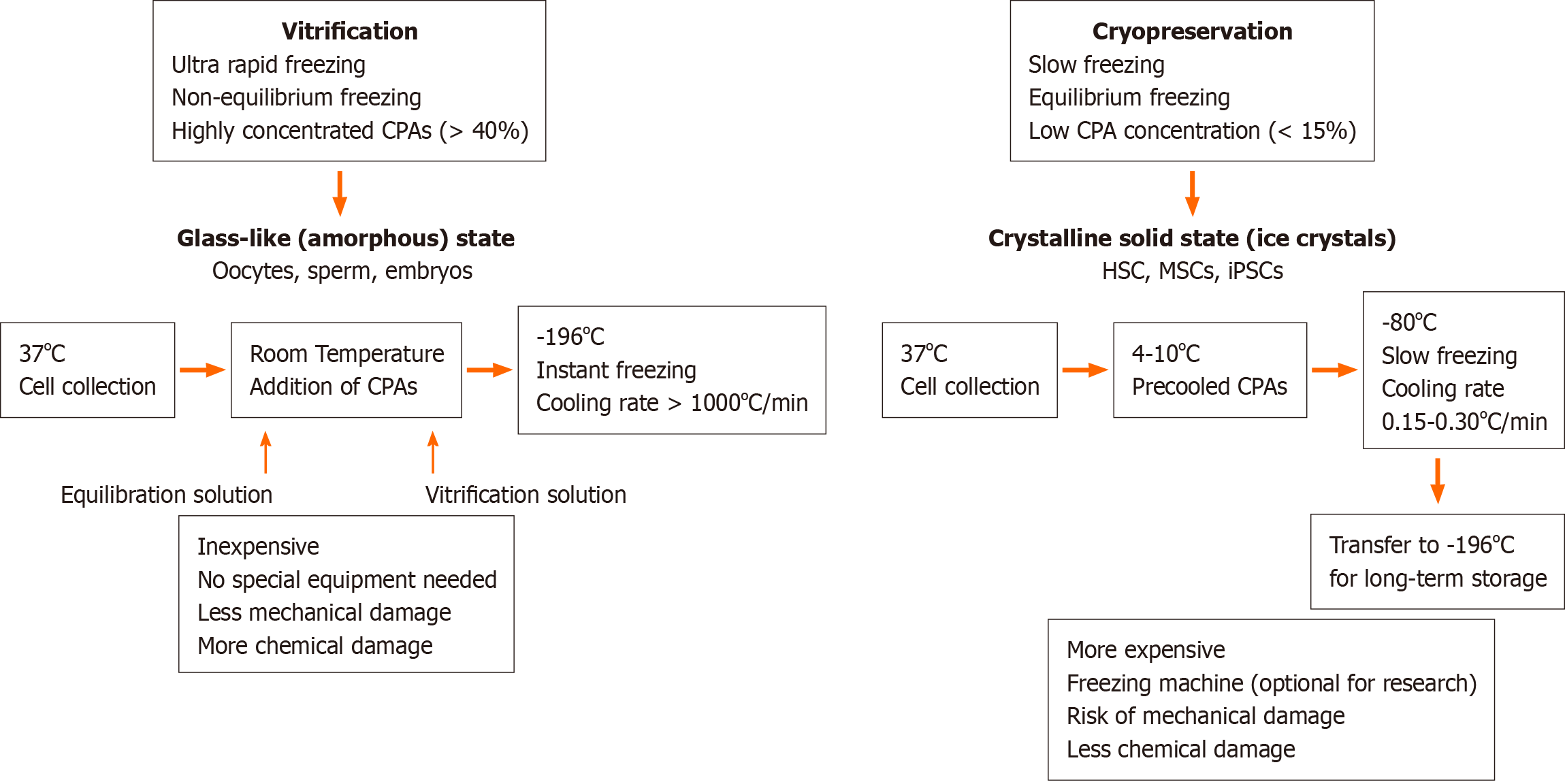
Storage under low temperature conditions reduces the rates of intracellular enzymatic and chemical reactions that may be harmful and allows the cells to be stored long-term without damage. The basic principle underlying successful cell cryopreservation is prevention of the formation of intra- and extracellular ice crystals during freezing, since this is the primary cause of cell damage[1]. Cryopreservation methods can be classified into slow freezing and fast freezing (vitrification) procedures. Both methods are based on the freezing or solidification of the cells or tissues and may cause cell injury in the process. However, the mechanisms that cause cell damage are quite distinct. Whereas rapid cooling results in the formation of intracellular ice crystals causing physical stress to the cells and mechanical breakdown, slow cooling causes osmotic changes in the cells and mechanical stress due to the formation of extracellular ice[2]. During vitrification a liquid is transformed into a glass-like non-crystalline solid state due to overcooling without freezing. Its most important feature is the prevention of ice formation[3,4]. During vitrification, cells kept in cryoprotectant solutions are briefly exposed to nitrogen vapor and subsequently immersed in liquid nitrogen[5] and usually a permeable cryoprotectant [dimethyl sulfoxide (DMSO) or glycerol] and an impermeable cryoprotectant [hydroxyethyl starch (HES), polyvinyl alcohol, trehalose] are used together[6,7]. During slow freezing, extracellular ice crystals may cause an increase in cellular osmolality and dehydration, and therefore the cooling rate during freezing should be sufficiently slow to allow a suitable amount of water to leave the cell[8,9]. The optimal cooling rate depends on cell size, sample size, water permeability and the presence of nucleating agents, which initiate and catalyze the freezing process. In addition, the cryoprotectant used, the temperature and surface/volume ratio should also be taken into consideration to determine the optimal cooling rate[10]. A cooling rate of 1-3 ℃/min during the initial freezing phase (+4 ℃ to -40 ℃) is optimal for most mammalian cells when frozen in the presence of cryoprotective agents, such as glycerol or DMSO[11]. Automated freezing devices, such as KRYO 10 series III (Planer Products, Sunbury-on-Thames, United Kingdom)[12], CryoMed 1010 (Forma Scientific, Marjetta, OH, United States)[13] and Cryomed (New Baltimore, MD, United States)[14] provide a temperature decrease at a controlled rate. Differences between vitrification and cryopreservation are depicted schematically in Figure 1.
Despite a vast amount of different methods, protocols and cryoprotectants, stem cells are often frozen using protocols optimized for cell lines and relatively few comparative studies have been performed to assess the effects of cryopreservation methods and supplements on stem cell quality and viability. A list of commercially available cryopreservation media is provided as Supplement 1. Here, we summarize the use, advantages and disadvantages of cryopreservation methods used for different types of stem cells, including HSCs, mesenchymal stem cells (MSC) and induced pluripotent stem cells (iPSC).
In order to serve as an effective cellular cryoprotective agent (CPA), the compound should have certain properties, including (1) High water solubility, even at low temperatures; (2) Free penetration of cell membranes; and (3) Low toxicity. Although many compounds may have these properties, including the most commonly used agents DMSO and glycerol, the choice of the compound may differ depending on the type of cell. CPAs are often used in combination with a carrier solution, which may provide different concentrations of (nutritional) salts, a variety of buffers, osmogens and/or apoptosis inhibitors. The contents of this carrier solution further help the cells maintain an isotonic concentration (300 milliosmoles) to prevent swelling or shrinking during the freezing process[15].
DMSO has been a key agent for the development of cryobiology. For cryopreservation of HSCs, use of DMSO, in combination with a temperature-controlled freezing technique followed by a rapid thawing procedure of 1-2 °C/min, is considered the clinical standard[16]. The use of DMSO as a CPA to prevent freezing-related cell damage was first proposed by Lovelock and Bishop[17], who used it during slow cooling of bull sperm. Due to its low hydrophilicity and molecular weight, DMSO freely penetrates cell membranes. It can disrupt ice crystal nucleation by forming hydrogen bonds with intracellular water molecules and prevents dehydration by reducing the amount of water absorbed into ice crystals[18]. However, prolonged exposure to DMSO negatively affects cellular function and growth by interfering with metabolism, enzymatic activity, cell cycle and apoptosis[19]. DMSO is also thought to modulate intracellular calcium concentrations[19,20] and may induce or inhibit cell apoptosis and differentiation, depending on the cell type, the stage of cell growth and differentiation, the concentration of DMSO (typically 5%-10%), duration of exposure and temperature[21,22]. Whereas high concentrations of DMSO may cause instant hemolysis, white cell stacking and fibrinogen precipitation, intravenous administration of DMSO has been associated with local irritation and necrosis[23]. Infusion of cell products that contain DMSO is associated with a wide range of gastrointestinal side effects (nausea, vomiting, abdominal pain, diarrhea)[24-26]; cardiovascular effects (hypertension, bradycardia, tachycardia)[25-27]; respiratory (dyspnea) and dermatological effects (urticaria, itching, and redness)[28,29]. Furthermore, even very low concentrations of DMSO can affect cellular processes by causing differential expression of thousands of genes, changing DNA methylation profiles and tissue-specific deregulation of miRNAs[30,31], and may affect stem cell fate by inducing unwanted differentiation[32].
Glycerol is a simple polyol compound. Its cryoprotective effects have been known since the early 1950s, when glycerol was first tested on fowl spermatozoa, rabbit red blood cells and water amoeba[33,34]. Glycerol is a colligative CPA that prevents dehydration damage by increasing the total solute concentration, including sodium ions, thus preventing ice formation and reducing the amount of water absorbed by ice crystals[7,35]. Although glycerol at low concentrations (< 20%) is not sufficient to prevent crystallization completely, it does protect different cells from cell death. High concentrations (70%) of glycerol were used without significant toxicity and were shown to provide substantial protection[36].
Hydroxyethyl starch was synthesized by Ziese W in 1934. The hydroxyethyl starch molecule is a high molecular weight synthetic polymer and can be purified from corn or potatoes[37]. Since high molecular weight CPAs are generally unable to enter cells, HES accumulates in the extracellular space. Here, it regulates water flow during cooling and heating and provides cryoprotection by absorbing the water molecules and keeping them thermally inert. Although HES remains extracellulary, it can minimize intracellular ice crystal formation and provides membrane stabilization[38]. By increasing the extracellular viscosity it further prevents osmotic stress and damage, reducing the rate at which water is withdrawn from the cells during cooling[39,40].
Trehalose is a non-toxic disaccharide and helps maintaining the structural integrity of cells during freezing and thawing[41,42]. Trehalose has high water retaining properties and is found in a large number of organisms, such as nematodes and yeasts that can survive freezing and drying[43] and can be isolated from yeasts, plants and fungi[42,44]. However, trehalose does not display any significant cryoprotective potential by itself and should therefore be used in combination with other CPAs[45].
The albumin protein consists of three homologous domains, each with specific structural and functional properties[46]. Human serum albumin (HSA) is present in serum at high quantities and serves as a buffer or depot for hormones, growth factors, fatty acids and metals. Due to its stabilizing function, albumin is an important component of common preservation and cell culture media. During freezing, albumin is used for its ability to coat surfaces, buffer function and binding capacity[47], but, similar to trehalose, albumin is only used as a supplementary cryoprotective agent during freezing of cells and tissues[48].
Dextran is a branched polysaccharide with α-1.6 glycosidic links between glucose molecules[49]. Dextran can interact with lipoproteins, enzymes and cells, and has the ability to stabilize proteins[50]. Dextran is non-toxic, only weakly antigenic and usually used at a concentration of 10%[51,52]. Dextran has been used as a cryoprotect during freezing of HSCs and sperm[53,54]. Similar to albumin and trehalose, dextran is only used in combination with other CPAs, such as DMSO or glycerol.
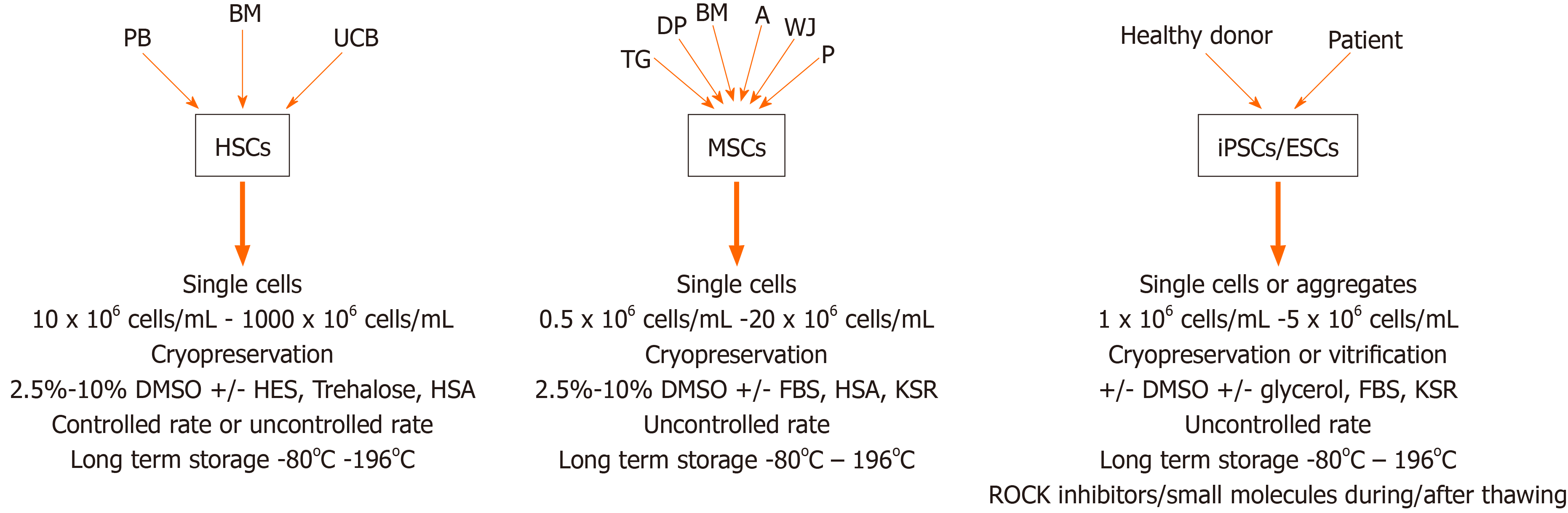
Hematopoietic stem cell transplantation (HSCT) is used for the treatment of various malignant and non-malignant diseases affecting the hematopoietic and immune system as well as for the treatment of a variety of inborn errors of metabolism[55]. HSC products derived from bone marrow (BM), peripheral blood or umbilical cord blood (UCB) are usually stored for a brief period that may range from a few days to months but may increase up to several years, depending on the disease state of the patient and treatment schedule[56]. Banking of HSC transplants is becoming increasingly important because of the possibility to use previously stored material even years after collection. In addition, storage of UCB for personal (private banking) or transplantation purposes (biobanks) is becoming increasingly popular and may require banking for up to several decades. For this reason, it is critically important that HSCs retain their potential during the freezing, banking and thawing[57]. HSCs can be stored unprocessed at +4 °C or room temperature for approximately 72 h after collection without massive apoptosis, cell death or loss of stem cell function. Within this time period, they can be transported and engrafted without any problems, but additional protocols may be required for longer storage[22,58,59]. Freezing the cells extends their shelf life greatly and increases the safety of HSC therapy by providing time to perform quality controls (microbiologically) and product testing (HSC content, colony assay, CD34+ enumeration). Despite these benefits, cryopreservation of HSCs poses several challenges, most notably a decrease in cell viability after thawing and side effects in patients due to the CPAs used[60]. An overview of current protocols used for cryopreservation of HSCs has been provided in Table 1.
| HSC source | Storage period and temperature | Cryopreservation | Viability post freezing | Engraftment in days | Results | Ref. |
| < 600 x 106 cells/mL autologous PBSC | 5-15 yr, -150 °C | 10% DMSO and 23.3% Plasma Lyte A | 66.4% | 12 | Viable CD34+ cells or CFU-GM is a reliable predictor of rapid engraftment | [13] |
| < 300 x 106 cells/mL autologous PBSC | < 6 mo, -80 °C | 3.5% DMSO, 1% HSA and 2.5% HES | 72% | 14 | Low DMSO conc allows successful engraftment and reduces toxicity (8%); Similar engraftment after combination of DMSO with or without HES and HSA | [115] |
| < 100 x 106 cells/ mL autologous PBSC | < 6 mo, -80 °C | 5% or 10% DMSO, autologous plasma, 5% ACD | 85% | 14 | 19.1% infusion-related toxicity in the 10% DMSO group vs 6.8% in the 5% DMSO group, lowering DMSO results in reduction in infusion toxicity and lower costs with a similar hematopoietic reconstitution | [116] |
| Autologous PBSC | < 11 yr, -80 °C | 3.5% DMSO + 1% HSA and 2.5% HES vs 6% DMSO + 6% HES | no significant change | 11-12 | Uncontrolled-rate freezing and cryopreservation with 5% DMSO/HES at −80 °C supports hematopoietic reconstitution comparable to that of controlled-rate freezing and liquid nitrogen storage | [117] |
| < 4000 x 106 cells/mL autologous PBSC | 1-98 wk, -80 °C | 3.5% DMSO, 2.5% HES and 1% HSA | 60.8% | 11-20 | Reduction in DMSO concentration decreases transfusion-related adverse events. PBPCs cryopreserved in low DMSO/HES/HSA at -80°C allow successful engraftment | [24] |
| 50 x 106 cells/mL autologous PBSC and BM | PB: 35 mo (26-78); BM 16 mo (27-71), -90 C | 5% DMSO, 6% HES and 4% HSA in RPMI1640 | 93% | DMSO-associated toxicity during infusion, storage of HSCs at -90°C in DMSO/HES/HSA did not cause loss of cell numbers, viability, and clonogenic activity | [118] | |
| Autologous PBSC | Controlled rate freezing at -186 °C | 5% or 10% DMSO and 6% HES | 10-20 | Two patients who received components cryopreserved with DMSO alone experienced serious neurological toxicity, none of the recipients who received components frozen in DMSO/HES experienced serious infusion-related toxicity, better hematopoietic recovery in presence of HES independent of DMSO concentration | [14] | |
| 100 x 106 cells/mL – 200 x 106 cells/mL autologous PBSC | 5-6 yr, controlled rate freezing at -160 °C | 2%-10% DMSO, 10% ACD | 73% with 5% DMSO | 10-14 | Cryopreservation using 5% instead of 10% DMSO improves CD34 + cell and leukocyte viability, but has only minor effects on supernatant levels of leukocyte- and platelet-derived soluble mediators | [61] |
| 75 x 106 cells/mL - 250 x 106 cells/mLautologous PBSC | 32-180 d, controlled rate freezing, -196 °C | 5% or 10% DMSO | 84%-95% | 10-14 | The use of 5% instead of 10% DMSO was associated with a decrease in side effects, cryopreservation with 5% DMSO followed by storage in nitrogen is a simple, highly standardized, and safe procedure for cryopreservation of autologous stem cell graft | [119] |
| UCB | 1-2 mo, uncontrolled vs controlled rate freezing at -90 °C | 5% or 10% DMSO | Uncontrolled 84.2%; controlled 92.5% | Best recovery of UCB cells when controlled-rate freezing and 5% DMSO were combined | [120] | |
| 15 x 106 cells/mL UCB | > 2 wk, controlled rate freezing at -170 C | 5%, 10% or 20% DMSO and 2% HSA or autologous plasma | 89% | Optimal conditions for cryopreservation were 10% DMSO and 2% HSA with fast addition and removal of DMSO | [121] | |
| 800 x 106 cells/mL UCB | 10 yr, controlled rate freezing at -196 °C | 10% DMSO and 5% Dextran | 83.7% | Long term storage of UCB units does not affect the quality of the HSCs | [122] | |
| Autologous BM | 4 mo, -80 °C | 5% DMSO and 6% HES | 82.2% | 21 | BM cells can be rapidly and inexpensively cryopreserved in DMSO/ HES, without need for rate-controlled freezing or storage in liquid nitrogen | [123] |
| 20 x 106 cells/mL BM or 17 x 106 cells/mL PBSC | Controlled rate freezing at -196 °C | 10% DMSO or 0.25-1 mol/L TH with or without 0.25 IU/mL insulin (I) | DMSO: 33%TH: 32%; TH/I: 30% | DMSO-cryopreserved cells exhibited the best median viability-rate after thawing. Comparable results could be achieved with trehalose 0.5 mol/L with/without insulin | [45] | |
| 200 x 106 cells/mL autologous BM or PBSC | BM: 11.8 yr vs PB: 33 d controlled rate freezing at -196 °C | 10% Medium 199 , 80% autologous plasma and 10% DMSO | BM: 81.5%; PBSC: 68.0% | BM can be cryopreserved for more than a decade without apparent loss of progenitor activity in comparison to short-term cryopreserved PBSC | [124] |
Throughout the years, DMSO has been the CPA of choice in most studies. It has been tested at different concentrations, ranging from 2.5% to 10% with variable results. Since DMSO is highly hyperosmotic, rapid infusion of the cryopreserved cells into the isosmotic blood system may cause osmotic damage, excessive cell expansion and decreased cell viability. This in turn may cause immediate side effects but can also affect engraftment in the long term[14,22]. Generally, lower doses of DMSO provided less toxicity, but in some cases, this was accompanied by a decrease in cell viability. Nevertheless, observed effects and side-effects of DMSO may differ widely between the protocols used due to the addition of other supplements (HES, HSA, Trehalose), cell dose (ranging from 15 x 106 cells/mL-4000 x 106 cells/mL), cell source (peripheral blood/BM/UCB), use of controlled rate or uncontrolled rate freezing, duration of storage (< 1 wk to > 1 decade) and the temperature used for long-term storage (-80 °C to -196 °C). To reduce the toxic effects of DMSO-cryopreserved HSCs during transplantation, it has been opted to divide the infusions into multiple portions, given at intervals of several hours or days, or alternatively to concentrate further HSC grafts to reduce cryopreservation volume and DMSO content[61]. In addition, alternatives such as different CPAs to reduce or replace DMSO for cryopreservation[14,62] or complete removal of DMSO prior to infusion[63,64] are being investigated. Even though a concentration of 10% DMSO in HSC cryopreservation is widely accepted as the cryopreservation medium of choice[65,66], similar or even more successful results have been obtained using percentages of DMSO as low as 2.5%-5%, with or without the addition of HES. Using these protocols similar engraftment was observed but with less toxicity[14,67,68]. Use of trehalose in combination with DMSO in UCB-derived HSC freezing has been shown to increase survival and cell differentiation capacity of HSCs in comparison to HSCs frozen without trehalose[53]. Direct comparison of trehalose and DMSO for cryopreservation of BM-HSCs showed no differences on viability between both groups[45]. Similarly, in NOD-SCID mice, the use of low amounts of DMSO (5%) and trehalose (5%) to reduce the toxic effects of DMSO showed a positive effect on HSC survival and engraftment after transplantation[69]. When BM-derived HSCs were frozen using a combination of 7.5% DMSO and 4% HSA, cells displayed high viability and sustained engraftment[70]. Studies using combinations of DMSO with dextran-40 showed increased HSC viability and functionality in comparison to the DMSO only group[71]. In conclusion, a lower concentration of DMSO and addition of a non-toxic second CPA or supplement, such as HSA and trehalose, decreases toxicity related to DMSO, while maintaining high HSC viability and sustaining engraftment.
Multipotent mesenchymal stem/stromal cells (MSCs) can be isolated from many tissues, including the bone marrow (BM-MSC), adipose tissue (adipose tissue derived stem cell), umbilical cord Wharton Jelly (Wharton Jelly-MSC), placenta (placenta-MSC), tooth germ (tooth germ MSC) or dental pulp (dental pulp stem cell) and many other connective tissues[72,73]. MSCs can differentiate into cells from several mesenchymal lineages, including but not limited to osteoblasts, adipocytes and chondrocytes[74,75]. MSCs are highly positive for cell surface molecules like CD29, CD44, CD73, CD90 and CD105[76]. They hold great potential for clinical application due to their capacity for regeneration of damaged or injured tissues, migration to sites of injury and regulation (usually suppression) of local and generalized immune responses. In order to obtain a sufficient amount of MSCs for clinical application, cells are often profoundly expanded in culture. Since MSCs themselves do not express HLA-DR, the cells are considered immunologically inert and expanded MSCs from unrelated, third-party donors can be used for treatment of a variety of diseases, ranging from graft vs host disease to severe acute respiratory distress syndromes[77,78]. These characteristics make MSCs ideal for ready, off-the-shelf treatments but require significant expansion and long-term cryopreservation[79-81]. Similar to the protocols developed for freezing of HSCs, a variety of freezing solutions and protocols has been tested for cryopreservation of MSCs (Table 2). Similar to freezing protocols used for HSCs, MSC freezing media generally consists of a basic medium [alpha-modified minimal essential medium, Dulbecco's Modified Eagle's Medium (DMEM) or advanced DMEM], supplemented with 3%-10% DMSO. In most studies expression of MSC surface markers (CD29, CD44, CD73, CD90, CD105 and/or CD166) was assessed before and after cryopreservation, and in almost all cases, MSC phenotype was not affected by cryopreservation, with overall expression levels > 90%. Cell viability ranged from 60% to 95% when fetal bovine serum (FBS) was used in addition to DMSO. In the presence of 10% DMSO, viability was typically very high (80% to 100%) after thawing, regardless of the duration of the freezing period[81-84].
| MSC source, passage | Culture medium | Storage period and temperature | Cryopreservation | Viability | Phenotype | Results | Ref. |
| BM-MSC/P3 | MEM, 15% FBS, 1% P/S, 1% L-glutamin | 7 wk at -196 °C | 90% FBS and 10% DMSO | Osteogenic and adipogenic differentiation, high expression of CD44, CD73, CD90 and CD105 | No effects of freezing on function, differentiation and phenotype of the cells | ||
| 1 x 106BM-MSC/P3, P4, P8, P13, P18 | MEM, 10% FBS, 1% P/S, 1% L-glutamin | 12 mo, controlled rate freezing at -80 °C | 30% FBS, 60% MEM and 10% DMSO | 85%-100% | Chondrogenic, adipogenic, neurogenic differentiation, no difference in expression of cell surface markers between passages | No differences in phenotype or differentiation between different cryopreserved MSCs from different passages | [82] |
| 0.5 x 106/mL; BM-MSC | MSC growth medium, 10% FBS | 1-5 mo, controlled rate freezing at -196 °C vs 4 d at 4 °C | Freezing medium (FM): 10% DMSO, 10% FBS, MSC growth medium, 30% BSA vs CryoStor (CS) animal component free freezing medium with 2%, 5% or 10% DMSO vs storage in HypoThermosol-FRS medium (HTS-FRS) at 4°C | FM 10% DMSO: 102.8%; CS 2% DMSO: 91.7%; CS 5% DMSO: 95.6%; CS 10% DMSO: 95.4%; HTS-FRS: 85.0% (rapid loss of viability after > 6 d) | Osteogenic differentiation, high expression of CD44, CD90, CD105, CD166, loss of expression of CD9 after hypothermic storage | No difference in differentiation or phenotype before and after freezing; HTS-FRS preserved MSC marker expression, proliferation and osteogenic differentiation after storage for at least 4 d | [81] |
| 1 x 106/mL; BM-MSC | MEM, 10% FBS, 1% P/S | 7 wk at -196 °C | 10% DMSO ± 10% or 90% FBS, 7.5% DMSO, 2.5% PEG ± 2% BSA, 5% DMSO, 5% PEG, 5% DMSO, 2% PEG, 3% Trehalose ± 2% BSA, 2.5% DMSO, 7.5% PEG ± 2% BSA, 10% Propanediol, 2%BSA, 7.5% Propanediol 2%BSA, 2.5% PEG | Highest viability with 7.5% DMSO, 2.5% PEG and 2% BSA: 82.9% ± 4.3% vs 10% DMSO: 82.7% ± 3.7% | Adipogenic, osteogenic and chondrogenic differentiation | In comparison to 10% DMSO, best results with 7.5% DMSO, 2.5% PEG and 2% BSA. In presence of and 2% BSA also good results with 5% DMSO, 5% PEG or 7.5% propanediol with 2.5% PEG | [84] |
| BM-MSC/P1-6 | MEM, 10% Human Serum, 1% L-glutamine, 1% P/S | 1 yr at -196 °C | MEM, 40% Human Serum, 5% DMSO | Osteogenic, adipogenic and myogenic differentiation, before and after thawing high expression of CD73, CD90 and CD105, no expression of CD16, CD34, CD45 and HLA-DR | Cryopreserved MSCs show slightly lower proliferation rate, no differences in differentiation, senescence markers, CFU-F or karyotype between frozen and fresh cells | [89] | |
| 5 x 105/mL; BM-MSC/P1 | MEM, 15% FBS, 1% P/S | < 6 mo vs 33-37 mo | CELLBANKER cryopreservation medium (contains serum and DMSO) | 90% | Osteogenic differentiation, both fresh and cryopreserved MSCs were negative for CD14, CD34, CD45 and HLA-DR and positive for CD29 and CD105 | No difference in osteogenic potential between fresh and cryopreserved cells. Long-term cryopreserved MSCs retained high osteogenic potential, no difference in phenotype | [86] |
| 1 x 106/mL; WJ-MSC | ADMEM, 10% FBS, 1% P/S, 1% L-glutamine | 3 mo, controlled rate freezing at -196 °C | A: ADMEM, 10% PVP ± 10% FBS, B: ADMEM, 10% FBS, 0.05 mol/L glucose, 0.05 mol/L sucrose, 1.5 mol/L ethylene glycol ± 10% FBS, C: ADMEM, 10% DMSO ± 10% FBS | A: 62.9% ± 0.4%; A without FBS: 6.8% ± 0.2%; B: 72.2% ± 0.23%; C: 81.2% ± 0.6% | Adipogenic and osteogenic differentiation, both fresh and cryopreserved MSCs were negative for CD34 and CD45 and positive for CD73, CD90 and CD105 | Complete elimination of FBS in cryoprotectants resulted in drastic reduction in cell viability. Cryopreservation did not alter basic stem cell characteristics, plasticity and multipotency, except for proliferation rate | [83] |
| 1 x 106/mL; tgMSC | DMEM, 10% FBS, 1% P/S/A | 1 d or 6 mo, freezing at -196 °C | 20 μg/mL NaB, 20% FBS, 1% P/S/A , 10%, 7%, 5%, 3% or 0% DMSO | First cycle: > 90%; Second cycle: > 70%; Third cycle: > 80%; Fourth cycle: > 80% | Osteogenic, chondrogenic, and adipogenic differentiation, high expression of CD29 and CD73, medium expression of CD90, CD105 and CD166, no expression of CD14, CD45, CD34 | < 5% DMSO in freezing medium resulted in increased cell death, NaB improved cellular viability after freeze-thaw cycles, addition of NaB to the freezing medium did not affect differentiation capacity of MSCs | [85] |
| 5 x 105/mL ADSC/P2 | DMEM-LG, 10% FBS | 2 wk, freezing at -196 °C | 0.9% NaCl containing 10% DMSO HSA, HS, KSR or 90% FBS | DMSO + 9%; HSA: 78.0%; DMSO + 90%; HS: 72.4%; DMSO + 90%; KSR: 77.0%; DMSO + 90%; FBS: 78.5%; DMSO alone: 19.6% | No differences in adipogenic, osteogenic, and chondrogenic differentiation, gene expression of CD73, CD90, CD105, CD106, CD166, SCF, REX1 and NANOG. All ADSCs were positive for surface expression of CD44, CD73, CD90, CD105, CD166 and HLA-ABC and negative for CD31, CD34 and HLA-DR | ADSCs frozen with HSA, HS, or KSR showed similar growth kinetics as cells frozen with FBS. Multilineage differentiation of ADSCs did not differ between groups | [88] |
| 1 x 106/mL DPSC/P5-7 | MEM, 15% FBS, 1% P/S/A, 100 uM L- ascorbic acid 2-phosphate | 1 wk, freezing with Mr. Frosty (NMF) vs magnetic freezing (MF) | Serum-free cryopreservation medium (SFM) containing 3% DMSO, SFM + 10% DMSO, FBS + 3% DMSO, FBS + 10% DMSO | SFM + 3%; DMSO: 75%; SFM + 10%; DMSO: 78%; FBS + 3%; DMSO: 70%; FBS + 10%; DMSO: 73% | CD29, CD44 and STRO-1 expression did not differ between the NMF and the MF groups, whereas levels of CD73, CD90, CD146 and CD166 in the MF group increased compared to the NMF group. | DPSC viability using MF was significantly superior to that of the NMF using 2%–10% DMSO; Post-thaw MF-DPSCs expressed MSC markers and showed osteogenic and adipogenic differentiation similar to fresh DPSCs | [90] |
| ESC-derived MSC | MEM, 10% FBS, 1% NEAA | Controlled rate freezing at 196 C | Sucrose, glycerol, creatine (SGC) and sucrose/ glycerol/isoleucine (SGI) solutions were incubated for 1h before freezing, Sucrose, mannitol, creatine (SMC) solutions were incubated for 2 h before freezing | SGI>SGC>SMC | Osteogenic and chondrogenic differentiation, all groups were positive for CD73, CD90 and CD105, and negative for CD45 | Osmolyte-based cryopreservation formulations retain MSC post-thaw viability, cell surface markers expression, proliferation, and osteochondral differentiation potential | [31] |
While there was no significant difference between 2% and 10% DMSO in terms of viability after a 1 mo freezing period, a significant portion of the cells frozen in presence of 2% DMSO died after long-term cryopreservation[81]. Therefore, in order to reduce the toxicity related to DMSO, either the percentage of DMSO was reduced or secondary CPAs (trehalose, sucrose, boron) were added to the freezing media[83-85]. Alternatively, high molecular weight macromolecules, such as FBS, polyethylene glycol (PEG) or polyvinylpyrrolidone were added as secondary CPAs to the freezing media[83,84,86]. However, since FBS contains animal components, cell products may contain remnants of FBS despite post-thaw washing that may trigger adverse (immune) reactions when used in a clinical setting[87]. Therefore, animal component free media, such as Cryostor, have been developed as an alternative to standard freezing medium formulations[81]. Studies using adipose tissue-derived MSCs frozen with 10% DMSO, 0.9% NaCl and human serum, HSA or knockout serum replacement (KSR)[88] revealed that all FBS replacements supported a similar multilineage differentiation potential, expression of cell surface markers and gene expression of stem cell markers, indicating that these may be good alternatives for clinical use. Carnevale et al[89] used 5% DMSO and human serum instead of FBS for cryopreservation of BM-MSCs and found no differences in terms of differentiation or phenotype. Cryopreservation of BM-MSCs using 7.5% DMSO, supplemented with 2.5% PEG and 2% BSA or even 5% DMSO, supplemented with 5% PEG and 2% BSA were shown to be almost as good as 10% DMSO in terms of viability and similar in terms of differentiation[84]. Comparison of mixed osmolyte solutions, consisting of sucrose/glycerol/creatine and sucrose/glycerol/isoleucine with standard DMSO containing freezing media further showed the potential of these type of cryopreservation solutions by improving post-thawing function of MSCs[31].
For research purposes often non-controlled, simple isopropanol-jacketed freezing containers (such as the Mr. Frosty from NALGENE) are used. Using this system, temperature in cryovials decreases approximately 1 C/min[89,90]. In contrast, for clinical use, temperature controlled freezing devices are often preferred. Lee et al[90] used a programmed freezer with a magnetic field to freeze human dental pulp MSCs. Using the magnetic freezing procedure, the researchers were able to decrease the level of DMSO to 3% without a significant difference in cell viability. Using the magnetic field freezer “Cells Alive System” (CAS) rat BM-MSCs were frozen in serum-free freezing medium (10% DMSO, 5% Albumin, 0.2% D-Glucose, 0.6% NaCl, 0.03% glutamine, 0.2%NaHCO3)[91]. After 3 years, viability and in vivo bone formation in the CAS group was significantly higher than that in cells stored in a non-programmed or non-magnetic freezer (87.7% and 48.5%, respectively). These data show the potential for use of alternative freezing systems for cryopreservation of MSCs as well as the use of secondary CPAs that decrease the need for DMSO. Most clinical trials use MSCs from related donors rather than off-the-shelf products. These MSCs are often directly after expansion infused into the patients. However, considering the increasing requirement for readily available MSC products, MSC culture and cryopreservation protocols under good manufacturing practice conditions will need to be revisited and low DMSO protocols that are optimized for clinical use and support MSC function in the absence of animal components remain to be developed.
Whereas studies on HSCs have been the focus of stem cell research since the 1960s-70s, studies assessing the role and function of MSCs have intensified since the 1990s. Since 2006, a substantial portion of the focus within the stem cell field has moved steadily towards the use of the new kid on the block, i.e. induced pluripotent stem cells (iPSC). iPSCs are stem cells with embryonic stem cell (ESC)-like properties, but lack the ethical issues involved with the use of ESCs. This is related to the fact that iPSCs are artificially generated from somatic cells by forced overexpression of the pluripotency transcription factors OCT4, SOX2, KLF4 and c-Myc[92,93]. New protocols using different combinations of transcription factors, including NANOG and LIN28[94] and others, devoid of oncogenic potential, as well as different methods for transfer (e.g., integrating lentiviral vectors, non-integrating sendai based vectors, episomal vectors, direct mRNA transfer, etc.)[95] have not affected the characteristics of the derived iPSCs: iPSCs have unlimited self-renewal capacity and the ability to differentiate into cells from all three germ layers (endoderm, mesoderm, ectoderm). iPSCs thus provide the tools to study early developmental biology in vitro and can be used for disease modeling and drug discovery. In addition, patient-derived iPSCs offer the opportunity to study the pathophysiology of diseases that could not be studied previously and can be used for the development of personalized medicine. All these features further stimulated iPSCs to become an important source of stem cells, and biobanks for storage of healthy and patient-derived iPSCs have now been established in many countries. However, efficient banking requires cell production facilities where cells can be expanded, maintained and cryopreserved under optimal conditions to ensure protection of iPSC characteristics and properties for weeks to years. In contrast to the cryopreservation protocols developed for HSCs and MSCs, current protocols for cryopreservation of iPSCs have focused on different issues, including freezing of cells in small aggregates vs single cell freezing in the presence of absence of DMSO[96-99], cell freezing using vitrification or different combinations of CPAs[100-102], cell recovery after cryopreservation using small molecules, such as the Rho kinase (ROCK) inhibitor Y-27632[103-105] and development of animal-component free formulations of culture and cryopreservation media using KSR instead of serum[106-108] (Table 3).
| Source of cell | Storage periode and temperature | Cryopreservation | Viability | Parameters | Results | Ref. |
| 1.5 x 106-2 x 106 hiPSC line UMN PCBC16iPS | Controlled rate; -196 °C | NEAA, sucrose, glycerol, isoleucine and albumin in a P188 in HBSS vs 7.5% DMSO; Aggregates vs single cells | Viability, adherence and intracellular ice formation | P188 was found here to not only inhibit ice formation significantly but also soften the solid-liquid interface of ice and increase the distance between adjacent ice crystals; The cryoprotective effects of the DMSO- free CPA cocktail could be capitalized only with the optimized composition. Deviation from the optimum may result in less desirable outcomes | [96,97] | |
| H9 hESC and hiPSC | 3-6 d,controlled rate; -80 °C | 10% DMSO, 10% EG, 10% PG, 10% glycerol, clumps vs single cells; ROCK inhibitor after thawing | EG-DMSO> PG>>glycerol | Toxicity of CPAs, expression of NANOG by hiPSCs | Freezing single cell iPSCs in the presence of a ROCK inhibitor and EG and programmable freezing drastically improved the yield of iPSCs in comparison to standard freezing in clumps without ROCK inhibitor | [98] |
| 1-2x106 hiPSC | -196 °C | A: 10% DMSO/90% FBS; B: 10% DMSO/90% KSR; C: 10% DMSO/ESC medium + 20%KSR + ROCK inhibitor; Single cells | A: 90%; B: 70%; C: 70% | Viability, karyotype, expression of pluripotency markers TRA-1-60, TRA-1-81, Oct4, SSEA-3, and SSEA-4, embryoid body formation, neuronal differentiation, colony formation | Addition of ROCK inhibitor to pre- and post-thaw culture media increased survival rate, hiPSCs retained typical morphology, stable karyotype, expression of pluripotency markers and the potential to differentiate into derivatives of all three germ layers after long-term culture | [103,105,108] |
| hiPSC | -196 °C | 10% DMSO in KO DMEM, 20% KSR, 1% NEAA, 1% L-glutamine, 0.2% b-mercaptoethanol, 1% antibiotic/ antimycotic and 8 ng/mL bFGF; ROCK inhibitor after thawing; Single cells | Colony number and size | ROCK inhibitor Y-27632 significantly improves the recovery of cryopreserved human iPS cells and their growth upon subculture | [104] | |
| hiPSC line 253G4 and 201B2 | 7 d, Vitrification in; -196 °C | VS2E vitrification solution (40% EG, 10% PEG in Euro-Collins medium), DAP213 vitrification solution (1.2% DMSO, 22% PG, 5.9% acetamide); Single cells | VS2E>DAP213 | Proliferation, expression of pluripotency markers Oct3/4, SSEA4, ALP, pluripotency in teratoma assay | Higher recovery rate of hiPSCs with DMSO and serum-free VS2E vitrification medium, cells after vitrification expressed Oct-3/4 and SSEA-4 and alkaline phosphatase and retained their pluripotency | [114] |
Using Raman spectroscopy to assess intracellular ice formation in iPSCs during cooling, Li et al[96] showed that iPSC aggregates are more sensitive to supercooling than single iPSCs in suspension due to the decreased water permeability of iPSCs in aggregates vs single cells. They also showed a greater variation in DMSO concentration across the aggregates than in single cells, suggesting that the size of the aggregates may hinder equal diffusion of the cryoprotectant to the cells. They also found that iPSC aggregates frozen in an optimized solution consisting of non-essential amino acids, sucrose, glycerol, isoleucine and albumin dissolved in a buffer made of poloxamer 188 (P188) in Hank’s Balanced Saline Solution, did not exhibit the same sensitivity to undercooling as those frozen in non-optimized solutions or those containing 7.5% DMSO[97]. In addition, cryopreservation of iPSCs in aggregates requires a significantly modified freezing technique, where iPSC aggregates are first incubated at room temperature for 30 min to 1 h before freezing to allow sufficient internalization of the CPAs[97], in contrast to freezing with DMSO, which usually requires working at low temperatures (4 °C) and rapid mixing of cells.
Miyamoto et al[100] compared the efficacy of a variety of different cryopreservation media on an established murine iPSC line. These media consisted of control 10% DMSO formulations to reduced DMSO solutions, glycerol-containing solutions, combinations of DMSO and glycerol and commercially available cryopreservation media (CELLBANKER 1, 1+, 2 and STEM-CELLBANKER) and were used to freeze mouse iPSCs in suspension. Comparison of viability, proliferation and multipotency after long-term freezing of iPSCs in these media showed optimal results with the serum-free formulations of CELLBANKER (CELLBANKER 2 and STEM-CELLBANKER)[100]. However, the precise formulations of these freezing media is proprietary, Hank’s Balanced Saline Solution and the researchers did not mention whether the STEM-CELLBANKER formulation used contained DMSO. Katkov et al[98] compared freezing of iPSCs in aggregates and as single cells using different CPAs including DMSO, ethylene glycol (EG), propylene glycol and glycerol. After extensive comparison, they found that freezing in aggregates resulted in favorable iPSC recovery after thawing. In addition, toxicity tests revealed that EG was not only less toxic than DMSO, it also supported better maintenance of pluripotency than propylene glycol or glycerol[98].
The use of KSR as a serum replacement has shown promising results and is another step in the development of animal component-free cryopreservation solutions. In combination with 10% DMSO, KSR has been used at concentrations of 25%-90% to freeze effectively iPSCs, ESCs and iPSC-derived cells with high post-thaw viability[105,106,108,109]. Inhibition of Rho kinase activity with ROCK inhibitors has shown favorable outcomes after freezing of both ESCs and iPSCs, and although not added during cryopreservation itself, it promotes both plating and cloning efficiency[104,105,108,110,111] by preventing apoptosis of detached cells[112]. Since addition of ROCK inhibitors up to 5 d after thawing still promotes colony formation, and since the effects of ROCK inhibition appear to be reversible, it has been also been suggested that ROCK inhibitors may relieve cellular stress[104].
Similar to studies in MSCs, the effects of magnetic fields on iPSC recovery after freezing have been assessed. Using the CAS researchers showed improved survival after thawing of iPSCs, but no effect on proliferation, gene expression and multilineage differentiation[113]. Reubinoff et al[101] previously showed that vitrification of both ESCs and iPSCs is feasible, using precooled freezing medium consisting of 90% FBS and 10% DMSO and a cooling rate of 1 C/min. ESC aggregates were preincubated in 80% DMEM, 10% DMSO and 10% EG and then placed into small 1-2 mL droplets containing 60% DMEM, 20% DMSO, 20% EG and 0.5 mol/L sucrose. All vitrified ESC aggregates recovered upon thawing and gave rise to colonies after plating. However, vitrified colonies were significantly smaller and showed increased differentiation compared with control colonies. Nevertheless, colonies generally recovered within 1-2 d of cell culture. Using a similar method for iPSCs, but using a DMSO and serum-free medium based on 40% EG and 10% PEG, Nishigaki et al[114] obtained a higher recovery rate of iPSCs than with a vitrification solution containing DMSO and serum.
The universally used cryoprotectant DMSO has been associated with in vitro and in vivo toxicity and has been shown to affect many cellular processes through dysregulation of gene expression and changes in DNA methylation. Despite studies showing that DMSO affects cell characteristics including differentiation potential, DMSO remains to be the CPA of choice both in a research setting and in the clinics. Many different protocols have been developed for different types of stem cells and a broad range of alternatives to DMSO have been shown to hold promise for use as a CPA (Figure 2). These alternatives include such molecules as trehalose, sucrose, EG, PEG and many more. It is obvious that a single protocol that can be used for all types of stem cells is not feasible, but the enormous amount of available alternatives should make it possible to adapt and optimize DMSO-free and animal component and serum-free cryopreservation solutions adapted for different types of stem cells in the foreseeable future.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Gene and Cellular Therapy, No. 69786; International Society of Stem Cell Research, No. 52502.
Specialty type: Cell biology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L, Schenke M S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 373] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Jang TH, Park SC, Yang JH, Kim JY, Seok JH, Park US, Choi CW, Lee SR, Han J. Cryopreservation and its clinical applications. Integr Med Res. 2017;6:12-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 3. | Porcu E. Oocyte freezing. Semin Reprod Med. 2001;19:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Fahy GM, Wowk B. Principles of Ice-Free Cryopreservation by Vitrification. Methods Mol Biol. 2021;2180:27-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313:573-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1017] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Huang H, Zhao G, Zhang Y, Xu J, Toth TL, He X. Predehydration and Ice Seeding in the Presence of Trehalose Enable Cell Cryopreservation. ACS Biomater Sci Eng. 2017;3:1758-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2007;368:39-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Gao D, Critser JK. Mechanisms of cryoinjury in living cells. ILAR J. 2000;41:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Rowley SD, Bensinger WI, Gooley TA, Buckner CD. Effect of cell concentration on bone marrow and peripheral blood stem cell cryopreservation. Blood. 1994;83:2731-2736. [PubMed] |
| 10. | Agca Y. Cryopreservation of oocyte and ovarian tissue. ILAR J. 2000;41:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Miller RH, Mazur P. Survival of frozen-thawed human red cells as a function of cooling and warming velocities. Cryobiology. 1976;13:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 70] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Ketheesan N, Whiteman C, Malczewski AB, Hirst RG, La Brooy JT. Effect of cryopreservation on the immunogenicity of umbilical cord blood cells. Transfus Apher Sci. 2004;30:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Yang H, Acker JP, Cabuhat M, Letcher B, Larratt L, McGann LE. Association of post-thaw viable CD34+ cells and CFU-GM with time to hematopoietic engraftment. Bone Marrow Transplant. 2005;35:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Rowley SD, Feng Z, Chen L, Holmberg L, Heimfeld S, MacLeod B, Bensinger WI. A randomized phase III clinical trial of autologous blood stem cell transplantation comparing cryopreservation using dimethylsulfoxide vs dimethylsulfoxide with hydroxyethylstarch. Bone Marrow Transplant. 2003;31:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Bhattacharya S. Cryoprotectants and their usage in cryopreservation process. In: Bozkurt Y Cryopreservation biotechnology in biomedical and biological sciences. Intechopen, 2018: 7-20. |
| 16. | Berz D, McCormack EM, Winer ES, Colvin GA, Quesenberry PJ. Cryopreservation of hematopoietic stem cells. Am J Hematol. 2007;82:463-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183:1394-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 626] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | Rowley SD. Hematopoietic stem cell processing and cryopreservation. J Clin Apher. 1992;7:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 19. | Cavas M, Beltrán D, Navarro JF. Behavioural effects of dimethyl sulfoxide (DMSO): changes in sleep architecture in rats. Toxicol Lett. 2005;157:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Pal R, Mamidi MK, Das AK, Bhonde R. Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation potential of human embryonic stem cells. Arch Toxicol. 2012;86:651-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Lin CK, Kalunta CI, Chen FS, Nguyen TT, Kaptein JS, Lad PM. Dimethyl sulfoxide suppresses apoptosis in Burkitt's lymphoma cells. Exp Cell Res. 1995;216:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014;49:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 23. | Rubin LF. Toxicologic update of dimethyl sulfoxide. Ann N Y Acad Sci. 1983;411:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Halle P, Tournilhac O, Knopinska-Posluszny W, Kanold J, Gembara P, Boiret N, Rapatel C, Berger M, Travade P, Angielski S, Bonhomme J, Deméocq F. Uncontrolled-rate freezing and storage at -80 degrees C, with only 3.5-percent DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. Transfusion. 2001;41:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Okamoto Y, Takaue Y, Saito S, Shimizu T, Suzue T, Abe T, Sato J, Hirao A, Watanabe T, Kawano Y. Toxicities associated with cryopreserved and thawed peripheral blood stem cell autografts in children with active cancer. Transfusion. 1993;33:578-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Rowley SD, Feng Z, Yadock D, Holmberg L, Macleod B, Heimfeld S. Post-thaw removal of DMSO does not completely abrogate infusional toxicity or the need for pre-infusion histamine blockade. Cytotherapy. 1999;1:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Stroncek DF, Fautsch SK, Lasky LC, Hurd DD, Ramsay NK, McCullough J. Adverse reactions in patients transfused with cryopreserved marrow. Transfusion. 1991;31:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 119] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Kollerup Madsen B, Hilscher M, Zetner D, Rosenberg J. Adverse reactions of dimethyl sulfoxide in humans: a systematic review. F1000Res. 2018;7:1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Otrock ZK, Sempek DS, Carey S, Grossman BJ. Adverse events of cryopreserved hematopoietic stem cell infusions in adults: a single-center observational study. Transfusion. 2017;57:1522-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 30. | Verheijen M, Lienhard M, Schrooders Y, Clayton O, Nudischer R, Boerno S, Timmermann B, Selevsek N, Schlapbach R, Gmuender H, Gotta S, Geraedts J, Herwig R, Kleinjans J, Caiment F. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci Rep. 2019;9:4641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 31. | Pollock K, Samsonraj RM, Dudakovic A, Thaler R, Stumbras A, McKenna DH, Dosa PI, van Wijnen AJ, Hubel A. Improved Post-Thaw Function and Epigenetic Changes in Mesenchymal Stromal Cells Cryopreserved Using Multicomponent Osmolyte Solutions. Stem Cells Dev. 2017;26:828-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Adler S, Pellizzer C, Paparella M, Hartung T, Bremer S. The effects of solvents on embryonic stem cell differentiation. Toxicol In Vitro. 2006;20:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Smith AU, Polge C, Smiles J. Microscopic observation of living cells during freezing and thawing. J R Microsc Soc. 1951;71:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1368] [Cited by in RCA: 1107] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 35. | Rowley SD. Hematopoietic stem cell cryopreservation: a review of current techniques. J Hematother. 1992;1:233-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Mazur P, Kleinhans FW. Relationship between intracellular ice formation in oocytes of the mouse and Xenopus and the physical state of the external medium--a revisit. Cryobiology. 2008;56:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Stolzing A, Naaldijk Y, Fedorova V, Sethe S. Hydroxyethylstarch in cryopreservation - mechanisms, benefits and problems. Transfus Apher Sci. 2012;46:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Bakaltcheva I, Ganong JP, Holtz BL, Peat RA, Reid T. Effects of high-molecular-weight cryoprotectants on platelets and the coagulation system. Cryobiology. 2000;40:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | McGann LE. Differing actions of penetrating and nonpenetrating cryoprotective agents. Cryobiology. 1978;15:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Takahashi T, Hirsh A, Erbe E, Williams RJ. Mechanism of cryoprotection by extracellular polymeric solutes. Biophys J. 1988;54:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Jain NK, Roy I. Trehalose and protein stability. Curr Protoc Protein Sci. 2010;Chapter 4:Unit 4.9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17R-27R. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1308] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 43. | Behm CA. The role of trehalose in the physiology of nematodes. Int J Parasitol. 1997;27:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Trevelyan WE, Harrison JS. Studies on yeast metabolism. 5. The trehalose content of baker's yeast during anaerobic fermentation. Biochem J. 1956;62:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Scheinkönig C, Kappicht S, Kolb HJ, Schleuning M. Adoption of long-term cultures to evaluate the cryoprotective potential of trehalose for freezing hematopoietic stem cells. Bone Marrow Transplant. 2004;34:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Dockal M, Carter DC, Rüker F. Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J Biol Chem. 2000;275:3042-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 367] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 47. | Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology. 2010;62:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 48. | Horváthy DB, Simon M, Schwarz CM, Masteling M, Vácz G, Hornyák I, Lacza Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. Biofactors. 2017;43:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Heinze T, Liebert T, Heublein B, Hornig S. Functional polymers based on dextran. In: Klemm D Polysaccharides ii. Berlin, Heidelberg: Springer Berlin Heidelberg, 2006: 199-291. |
| 50. | Masuelli MA. Dextrans in aqueous solution. Experimental review on intrinsic viscosity measurements and temperature effect. J Polymer Biopolymer Physics Chem. 2013;1: 13-21. [DOI] [Full Text] |
| 51. | Ljungström KG, Renck H, Strandberg K, Hedin H, Richter W, Widerlöv E. Adverse reactions to dextran in Sweden 1970-1979. Acta Chir Scand. 1983;149:253-262. [PubMed] |
| 52. | Pellerin-Mendes C, Million L, Marchand-Arvier M, Labrude P, Vigneron C. In vitro study of the protective effect of trehalose and dextran during freezing of human red blood cells in liquid nitrogen. Cryobiology. 1997;35:173-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Chen G, Yue A, Ruan Z, Yin Y, Wang R, Ren Y, Zhu L. Comparison of the Effects of Different Cryoprotectants on Stem Cells from Umbilical Cord Blood. Stem Cells Int. 2016;2016:1396783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | O'Neill HC, Nikoloska M, Ho H, Doshi A, Maalouf W. Improved cryopreservation of spermatozoa using vitrification: comparison of cryoprotectants and a novel device for long-term storage. J Assist Reprod Genet. 2019;36:1713-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Gratwohl A, Baldomero H, Gratwohl M, Aljurf M, Bouzas LF, Horowitz M, Kodera Y, Lipton J, Iida M, Pasquini MC, Passweg J, Szer J, Madrigal A, Frauendorfer K, Niederwieser D; Worldwide Network of Blood and Marrow Transplantation (WBMT). Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a Global Observational Study. Haematologica. 2013;98:1282-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Shima T, Iwasaki H, Yamauchi T, Kadowaki M, Kiyosuke M, Mochimaru T, Takenaka K, Miyamoto T, Akashi K, Teshima T. Preserved in vivo reconstitution ability of PBSCs cryopreserved for a decade at -80 °C. Bone Marrow Transplant. 2015;50:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Kubiak A, Matuszak P, Bembnista E, Kozlowska-Skrzypczak M. Banking of Hematopoietic Stem Cells: Influence of Storage Time on Their Quality Parameters. Transplant Proc. 2016;48:1806-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Weinberg RS. Cryopreservation techniques and freezing solutions. In: Schwartz J and Shaz BH Best practices in processing and storage for hematopoietic cell transplantation. Cham: Springer International Publishing, 2018: 63-72. |
| 59. | Aerts-Kaya FSF, Visser TP, Pervin B, Mammadova A, Özyüncü Ö, Wagemaker G, Uçkan-Çetinkaya FD. SUL-109 Protects Hematopoietic Stem Cells from Apoptosis Induced by Short-Term Hypothermic Preservation and Maintains Their Engraftment Potential. Biol Blood Marrow Transplant. 2020;26:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Hornberger K, Yu G, McKenna D, Hubel A. Cryopreservation of Hematopoietic Stem Cells: Emerging Assays, Cryoprotectant Agents, and Technology to Improve Outcomes. Transfus Med Hemother. 2019;46:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 61. | Akkök CA, Holte MR, Tangen JM, Ostenstad B, Bruserud O. Hematopoietic engraftment of dimethyl sulfoxide-depleted autologous peripheral blood progenitor cells. Transfusion. 2009;49:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Hayakawa J, Joyal EG, Gildner JF, Washington KN, Phang OA, Uchida N, Hsieh MM, Tisdale JF. 5% dimethyl sulfoxide (DMSO) and pentastarch improves cryopreservation of cord blood cells over 10% DMSO. Transfusion. 2010;50:2158-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Hirata Y, Kishino K, Onozaki F, Nakaki Y, Fujiwara S, Yamamoto C, Sato K, Matsuyama T, Ozaki K, Mori M, Ozawa K, Muroi K. Use of cryoprotectant-depleted allogeneic peripheral blood stem cells for transplantation. Hematology. 2011;16:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Aerts-Kaya F, Koca G, Sharafi P, Sayla FÇ, Uçkan-Çetinkaya D, Özdemir E. Automated washing of long-term cryopreserved peripheral blood stem cells promotes cell viability and preserves CD34+ cell numbers. Bone Marrow Transplant. 2018;53:1225-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci U S A. 2003;100:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Campos L, Roubi N, Guyotat D. Definition of optimal conditions for collection and cryopreservation of umbilical cord hematopoietic cells. Cryobiology. 1995;32:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Abrahamsen JF, Rusten L, Bakken AM, Bruserud Ø. Better preservation of early hematopoietic progenitor cells when human peripheral blood progenitor cells are cryopreserved with 5 percent dimethylsulfoxide instead of 10 percent dimethylsulfoxide. Transfusion. 2004;44:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood. 1990;75:781-786. [PubMed] |
| 69. | Zhang XB, Li K, Yau KH, Tsang KS, Fok TF, Li CK, Lee SM, Yuen PM. Trehalose ameliorates the cryopreservation of cord blood in a preclinical system and increases the recovery of CFUs, long-term culture-initiating cells, and nonobese diabetic-SCID repopulating cells. Transfusion. 2003;43:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Gorin NC, Lopez M, Laporte JP, Quittet P, Lesage S, Lemoine F, Berenson RJ, Isnard F, Grande M, Stachowiak J. Preparation and successful engraftment of purified CD34+ bone marrow progenitor cells in patients with non-Hodgkin's lymphoma. Blood. 1995;85:1647-1654. [PubMed] |
| 71. | Stylianou J, Vowels M, Hadfield K. Novel cryoprotectant significantly improves the post-thaw recovery and quality of HSC from CB. Cytotherapy. 2006;8:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248-269. [PubMed] |
| 73. | Sarıkaya A, Aydın G, Özyüncü Ö, Şahin E, Uçkan-Çetinkaya D, Aerts-Kaya F. Comparison of immune modulatory properties of human multipotent mesenchymal stromal cells derived from bone marrow and placenta. Biotech Histochem. 2021;1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15195] [Article Influence: 584.4] [Reference Citation Analysis (0)] |
| 75. | Ulum B, Teker HT, Sarikaya A, Balta G, Kuskonmaz B, Uckan-Cetinkaya D, Aerts-Kaya F. Bone marrow mesenchymal stem cell donors with a high body mass index display elevated endoplasmic reticulum stress and are functionally impaired. J Cell Physiol. 2018;233:8429-8436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12672] [Article Influence: 704.0] [Reference Citation Analysis (2)] |
| 77. | von Bonin M, Stölzel F, Goedecke A, Richter K, Wuschek N, Hölig K, Platzbecker U, Illmer T, Schaich M, Schetelig J, Kiani A, Ordemann R, Ehninger G, Schmitz M, Bornhäuser M. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 78. | Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 79. | Kastrup J, Haack-Sørensen M, Juhl M, Harary Søndergaard R, Follin B, Drozd Lund L, Mønsted Johansen E, Ali Qayyum A, Bruun Mathiasen A, Jørgensen E, Helqvist S, Jørgen Elberg J, Bruunsgaard H, Ekblond A. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl Med. 2017;6:1963-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 80. | Poh KK, Sperry E, Young RG, Freyman T, Barringhaus KG, Thompson CA. Repeated direct endomyocardial transplantation of allogeneic mesenchymal stem cells: safety of a high dose, "off-the-shelf", cellular cardiomyoplasty strategy. Int J Cardiol. 2007;117:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Xiang Y, Zheng Q, Jia B, Huang G, Xie C, Pan J, Wang J. Ex vivo expansion, adipogenesis and neurogenesis of cryopreserved human bone marrow mesenchymal stem cells. Cell Biol Int. 2007;31:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Shivakumar SB, Bharti D, Jang SJ, Hwang SC, Park JK, Shin JK, Byun JH, Park BW, Rho GJ. Cryopreservation of Human Wharton's Jelly-derived Mesenchymal Stem Cells Following Controlled Rate Freezing Protocol Using Different Cryoprotectants; A Comparative Study. Int J Stem Cells. 2015;8:155-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Liu Y, Xu X, Ma X, Martin-Rendon E, Watt S, Cui Z. Cryopreservation of human bone marrow-derived mesenchymal stem cells with reduced dimethylsulfoxide and well-defined freezing solutions. Biotechnol Prog. 2010;26:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Demirci S, Doğan A, Şişli B, Sahin F. Boron increases the cell viability of mesenchymal stem cells after long-term cryopreservation. Cryobiology. 2014;68:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Kotobuki N, Hirose M, Machida H, Katou Y, Muraki K, Takakura Y, Ohgushi H. Viability and osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells. Tissue Eng. 2005;11:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Mackensen A, Dräger R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 88. | Park S, Lee DR, Nam JS, Ahn CW, Kim H. Fetal bovine serum-free cryopreservation methods for clinical banking of human adipose-derived stem cells. Cryobiology. 2018;81:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Carnevale G, Pisciotta A, Riccio M, De Biasi S, Gibellini L, Ferrari A, La Sala GB, Bruzzesi G, Cossarizza A, de Pol A. Optimized Cryopreservation and Banking of Human Bone-Marrow Fragments and Stem Cells. Biopreserv Biobank. 2016;14:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Lee SY, Huang GW, Shiung JN, Huang YH, Jeng JH, Kuo TF, Yang JC, Yang WC. Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs. 2012;196:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Kojima SI, Kaku M, Kawata T, Motokawa M, Sumi H, Shikata H, Abonti TH, Kojima ST, Yamamoto T, Tanne K, Tanimoto K. Cranial suture-like gap and bone regeneration after transplantation of cryopreserved MSCs by use of a programmed freezer with magnetic field in rats. Cryobiology. 2015;70:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18178] [Article Influence: 956.7] [Reference Citation Analysis (0)] |
| 93. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14305] [Article Influence: 841.5] [Reference Citation Analysis (0)] |
| 94. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7589] [Cited by in RCA: 7242] [Article Influence: 402.3] [Reference Citation Analysis (0)] |
| 95. | Malik N, Rao MS. A review of the methods for human iPSC derivation. Methods Mol Biol. 2013;997:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 96. | Li R, Yu G, Azarin SM, Hubel A. Freezing Responses in DMSO-Based Cryopreservation of Human iPS Cells: Aggregates Versus Single Cells. Tissue Eng Part C Methods. 2018;24:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 97. | Li R, Hornberger K, Dutton JR, Hubel A. Cryopreservation of Human iPS Cell Aggregates in a DMSO-Free Solution-An Optimization and Comparative Study. Front Bioeng Biotechnol. 2020;8:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 98. | Katkov II, Kan NG, Cimadamore F, Nelson B, Snyder EY, Terskikh AV. DMSO-Free Programmed Cryopreservation of Fully Dissociated and Adherent Human Induced Pluripotent Stem Cells. Stem Cells Int. 2011;2011:981606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Sart S, Ma T, Li Y. Cryopreservation of pluripotent stem cell aggregates in defined protein-free formulation. Biotechnol Prog. 2013;29:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Miyamoto Y, Noguchi H, Yukawa H, Oishi K, Matsushita K, Iwata H, Hayashi S. Cryopreservation of Induced Pluripotent Stem Cells. Cell Med. 2012;3:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 223] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 102. | Yuan Y, Yang Y, Tian Y, Park J, Dai A, Roberts RM, Liu Y, Han X. Efficient long-term cryopreservation of pluripotent stem cells at -80 °C. Sci Rep. 2016;6:34476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 103. | Baharvand H, Salekdeh GH, Taei A, Mollamohammadi S. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat Protoc. 2010;5:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 104. | Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 105. | Mollamohammadi S, Taei A, Pakzad M, Totonchi M, Seifinejad A, Masoudi N, Baharvand H. A simple and efficient cryopreservation method for feeder-free dissociated human induced pluripotent stem cells and human embryonic stem cells. Hum Reprod. 2009;24:2468-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 106. | Wagner K, Welch D. Cryopreserving and recovering of human iPS cells using complete Knockout Serum Replacement feeder-free medium. J Vis Exp. 2010;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 107. | Wagner K, Welch D. Feeder-free adaptation, culture and passaging of human IPS cells using complete Knockout Serum Replacement feeder-free medium. J Vis Exp. 2010;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 108. | Baharvand H, Totonchi M, Taei A, Seifinejad A, Aghdami N, Salekdeh GH. Human-induced pluripotent stem cells: derivation, propagation, and freezing in serum- and feeder layer-free culture conditions. Methods Mol Biol. 2010;584:425-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | van den Brink L, Brandão KO, Yiangou L, Mol MPH, Grandela C, Mummery CL, Verkerk AO, Davis RP. Cryopreservation of human pluripotent stem cell-derived cardiomyocytes is not detrimental to their molecular and functional properties. Stem Cell Res. 2020;43:101698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 111. | Li X, Meng G, Krawetz R, Liu S, Rancourt DE. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 2008;17:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1566] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 113. | Nishiyama Y, Iwanami A, Kohyama J, Itakura G, Kawabata S, Sugai K, Nishimura S, Kashiwagi R, Yasutake K, Isoda M, Matsumoto M, Nakamura M, Okano H. Safe and efficient method for cryopreservation of human induced pluripotent stem cell-derived neural stem and progenitor cells by a programmed freezer with a magnetic field. Neurosci Res. 2016;107:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Nishigaki T, Teramura Y, Nasu A, Takada K, Toguchida J, Iwata H. Highly efficient cryopreservation of human induced pluripotent stem cells using a dimethyl sulfoxide-free solution. Int J Dev Biol. 2011;55:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Calvet L, Cabrespine A, Boiret-Dupré N, Merlin E, Paillard C, Berger M, Bay JO, Tournilhac O, Halle P. Hematologic, immunologic reconstitution, and outcome of 342 autologous peripheral blood stem cell transplantations after cryopreservation in a -80°C mechanical freezer and preserved less than 6 mo. Transfusion. 2013;53:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 116. | Galmes A, Gutiérrez A, Sampol A, Canaro M, Morey M, Iglesias J, Matamoros N, Duran MA, Novo A, Bea MD, Galán P, Balansat J, Martínez J, Bargay J, Besalduch J. Long-term hematological reconstitution and clinical evaluation of autologous peripheral blood stem cell transplantation after cryopreservation of cells with 5% and 10% dimethylsulfoxide at -80 degrees C in a mechanical freezer. Haematologica. 2007;92:986-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Detry G, Calvet L, Straetmans N, Cabrespine A, Ravoet C, Bay JO, Petre H, Paillard C, Husson B, Merlin E, Boon-Falleur L, Tournilhac O, Delannoy A, Halle P. Impact of uncontrolled freezing and long-term storage of peripheral blood stem cells at - 80 °C on haematopoietic recovery after autologous transplantation. Report from two centres. Bone Marrow Transplant. 2014;49:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 118. | Ayello J, Semidei-Pomales M, Preti R, Hesdorffer C, Reiss RF. Effects of long-term storage at -90 degrees C of bone marrow and PBPC on cell recovery, viability, and clonogenic potential. J Hematother. 1998;7:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Akkök CA, Liseth K, Nesthus I, Løkeland T, Tefre K, Bruserud O, Abrahamsen JF. Autologous peripheral blood progenitor cells cryopreserved with 5 and 10 percent dimethyl sulfoxide alone give comparable hematopoietic reconstitution after transplantation. Transfusion. 2008;48:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | Skoric D, Balint B, Petakov M, Sindjic M, Rodic P. Collection strategies and cryopreservation of umbilical cord blood. Transfus Med. 2007;17:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Meyer TP, Hofmann B, Zaisserer J, Jacobs VR, Fuchs B, Rapp S, Weinauer F, Burkhart J. Analysis and cryopreservation of hematopoietic stem and progenitor cells from umbilical cord blood. Cytotherapy. 2006;8:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 122. | Yamamoto S, Ikeda H, Toyama D, Hayashi M, Akiyama K, Suzuki M, Tanaka Y, Watanabe T, Fujimoto Y, Hosaki I, Nishihira H, Isoyama K. Quality of long-term cryopreserved umbilical cord blood units for hematopoietic cell transplantation. Int J Hematol. 2011;93:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 123. | Stiff PJ, Koester AR, Weidner MK, Dvorak K, Fisher RI. Autologous bone marrow transplantation using unfractionated cells cryopreserved in dimethylsulfoxide and hydroxyethyl starch without controlled-rate freezing. Blood. 1987;70:974-978. [PubMed] |
| 124. | Donnenberg AD, Koch EK, Griffin DL, Stanczak HM, Kiss JE, Carlos TM, Buchbarker DM, Yeager AM. Viability of cryopreserved BM progenitor cells stored for more than a decade. Cytotherapy. 2002;4:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Antebi B, Asher AM, Rodriguez LA 2nd, Moore RK, Mohammadipoor A, Cancio LC. Cryopreserved mesenchymal stem cells regain functional potency following a 24-h acclimation period. J Transl Med. 2019;17:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |