Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.659
Peer-review started: February 12, 2021
First decision: March 17, 2021
Revised: March 27, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: June 26, 2021
Processing time: 134 Days and 7.7 Hours
Heat shock proteins (HSPs) are molecular chaperones that protect cells against cellular stresses or injury. However, it has been increasingly recognized that they also play crucial roles in regulating fundamental cellular processes. HSP20 has been implicated in cell proliferation, but conflicting studies have shown that it can either promote or suppress proliferation. The underlying mechanisms by which HSP20 regulates cell proliferation and pluripotency remain unexplored. While the effect of HSP20 on cell proliferation has been recognized, its role in inducing pluripotency in human-induced pluripotent stem cells (iPSCs) has not been addressed.
To evaluate the efficacy of HSP20 overexpression in human iPSCs and evaluate the ability to promote cell proliferation. The purpose of this study was to investigate whether overexpression of HSP20 in iPSCs can increase pluripotency and regeneration.
We used iPSCs, which retain their potential for cell proliferation. HSP20 overexpression effectively enhanced cell proliferation and pluripotency. Overexpression of HSP20 in iPSCs was characterized by immunocytochemistry staining and real-time polymerase chain reaction. We also used cell culture, cell counting, western blotting, and flow cytometry analyses to validate HSP20 overexpression and its mechanism.
This study demonstrated that overexpression of HSP20 can increase the pluripotency in iPSCs. Furthermore, by overexpressing HSP20 in iPSCs, we showed that HSP20 upregulated proliferation markers, induced pluripotent genes, and drove cell proliferation in a sirtuin 1 (SIRT1)-dependent manner. These data have practical applications in the field of stem cell-based therapies where the mass expansion of cells is needed to generate large quantities of stem cell-derived cells for transplantation purposes.
We found that the overexpression of HSP20 enhanced the proliferation of iPSCs in a SIRT1-dependent manner. Herein, we established the distinct crosstalk between HSP20 and SIRT1 in regulating cell proliferation and pluripotency. Our study provides novel insights into the mechanisms controlling cell proliferation that can potentially be exploited to improve the expansion and pluripotency of human iPSCs for cell transplantation therapies. These results suggest that iPSCs overexpressing HSP20 exert regenerative and proliferative effects and may have the potential to improve clinical outcomes.
Core Tip: Heat shock proteins (HSPs) are housekeeper proteins that guard cells against injury and the damage response. HSPs send signals from diseased or damaged cells to the immune system, which activates the body’s defense system leading to the subsequent release of inflammatory signals that recruit other immune cells and kill the pathogen in case of infection or repair the cells in case of damage. HSPs trigger a cascade of events that involve essential proteins to accelerate the repair process or degrade injured proteins as a protection plan to ensure survival. How HSPs respond to stress or injury stimulus is a question of further investigation. Accumulating evidence indicates that HSP20 is associated with cell proliferation. However, the precise mechanisms by which HSP20 promotes stem cell proliferation remain unclear. Herein, we established the distinct crosstalk between HSP20 and sirtuin 1 in regulating cell proliferation. Our study provides a new avenue for drug discovery and treatment of critical diseases.
- Citation: Ullah M, Qian NPM, Yannarelli G, Akbar A. Heat shock protein 20 promotes sirtuin 1-dependent cell proliferation in induced pluripotent stem cells . World J Stem Cells 2021; 13(6): 659-669
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/659.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.659
Heat shock proteins (HSPs) are a highly conserved family of molecular chaperones that serve as ‘defenders’ of cells, protecting them against various forms of intracellular and extracellular stresses and insults[1,2]. Mechanisms underlying the protective action of HSPs consist of repairing the damaged proteins and eliminating unrepairable proteins[1-3]. The ability of cells to repair and regenerate the damaged or dysfunctional proteins is essential for maintaining cellular functions and survival[1-5]. HSPs exist in numerous sizes and localize to different compartments in cells, thereby contributing to a myriad of biological functions[2,4]. In the presence of stress or injury signals, HSPs are synthesized in large quantities and act by physically binding to proteins to ameliorate protein denaturation, misfolding, and aggregation[6,7]. HSPs can also inhibit cell death via apoptosis-dependent and apoptosis-independent pathways[8,9]. Collectively, HSPs aid in cellular repair and recovery as part of a protection plan to ensure cell survival[10-12]. During homeostasis, HSPs can also mediate immune cell functions and immune responses[13-15].
The HSP20/HSPB6 family (small HSPs with molecular weights ranging from 15 to 30 kDa) plays essential but complex roles in health and disease[16,17]. Some studies have shown that HSP20 has significant cardioprotective properties[18]. Overexpressing HSP20 results in improved cardiac outcomes after drug-induced heart injury[19] or even in the diabetic heart[18]. HSP20 is also a vasorelaxant[20], thereby reducing vasospasms and thrombosis associated with vein grafting[21,22]. In addition, HSP20 can reduce levels of oxygen/glucose-deprivation/reperfusion-induced organelle damage and cellular apoptosis, suggesting its neuroprotective capabilities[23].
By contrast, HSP20 can accelerate tumorigenesis by promoting proliferation and migration, and inhibiting apoptosis[24]. Nevertheless, human hepatocellular carcinoma cells overexpressing HSP20 display reduced growth by suppressing nuclear factor kappa B, mitogen-activated protein kinase, and AKT signaling pathways[25,26]. HSP20 is, hitherto, a double-edge sword involved in dual roles in promoting mammalian health and disease.
However, the mechanism by which HSP20 modulates mammalian cell proliferation remains elusive. Sirtuin 1 (SIRT1), a member of the SIRT family of nicotinamide adenosine dinucleotide-dependent deacetylase, induces proliferation and inhibits apoptosis[26,27]. Thus, we hypothesized that HSP20 might regulate cell proliferation by interacting with SIRT1. In this study, we investigated the cytoprotective roles of HSP20 in the high-proliferative human-induced pluripotent stem cells (iPSCs). We found that the overexpression of HSP20 enhanced the proliferation of iPSCs in a SIRT1-dependent manner.
Herein, we established the distinct crosstalk between HSP20 and SIRT1 in regulating cell proliferation. Our study provides new insights into the mechanisms controlling cell proliferation that can potentially be exploited to improve the expansion of human iPSCs for cell transplantation therapies.
The full-length cDNA encoding human HSP20 (GenBank Accession No: AK056951) was amplified from cDNA (Clontech) using forward 5′-GAGATATACATATGGAGATCC CTGTGC-3′ and reverse 5′-GTGCTCGAGTTACTTGGCTGCGGCTGGCGG-3′ primers, with a polymerase chain reaction (PCR) amplification kit (System Biosciences, Palo Alto, CA, United States). The PCR product was purified after electrophoresis on an agarose gel, digested with restriction endonucleases XbaI and BamHI, and inserted into the plasmid vector pCDH (which had been predigested with the same endonucleases). The resulting construct was verified by copGFP expression. Lentiviral constructs (System Biosciences) were based on a fourth-generation lentiviral vector modified with an insert that contained the cytomegalovirus (CMV) promoter driving the expression of HSP20 (Clontech Laboratories, Mountain View, CA, United States) combined with copGFP by P2A sequence.
The fourth-generation lentiviral vectors were generated in the human embryonic kidney 293T/17 cell line (HEK 293T/17) (ATCC® CRL-11268™). Cells were incubated at 37 °C for 6 h, at which point the medium was replaced with fresh medium and returned to 37 °C for 72 h. Lentivirus-containing medium was collected after transfection for 2 d, and cellular debris was cleared by low-speed centrifugation at 1500 × g for 5 min. The collected medium was concentrated with the PEG-it virus precipitation solution (LV810A-1; System Biosciences), and pellets containing viral particles were resuspended with phosphate-buffered saline. Lentiviral titers were measured with the Lenti-X qRT-PCR Titration Kit (Clontech).
The human iPSCs were seeded in fresh culture medium at a density of 1 × 105 cells per well in 6-well plates (three wells in total) as previously described[27]. Before transduction, the cells were incubated for 36 h at 37 °C in a humidified incubator in an atmosphere of 5% CO2. The culture medium was then replaced with 1 mL fresh medium containing 100 μg/mL sterile-filtered protamine sulfate (Sigma-Aldrich, St. Louis, MO, United States). The cells were subsequently infected with a multiplicity of infection (MOI) of 10 for 24 h at 37 °C; this step was repeated with an MOI of 10 for a further 12 h to achieve maximum transduction efficiency. After 36 h and two rounds of transduction, the cells were trypsinized and re-plated at 500 cells/cm2 in T175 cm2 flasks with fresh cell culture medium. Culture media was changed three times a week thereafter. After expansion to about 70% confluency after about 5 d, the cells were further expanded under the same culturing conditions.
Cell proliferation was analyzed by direct cell counting. For the methyl tetrazolium (MTT) assay, the cells were seeded at a density of 1 × 106 viable cells/mL in a 96-well plate. At the indicated time, passage 6 cells were used for the MTT viability assay (Roche, Pleasanton, CA, United States). HSP20-transduced cells and the respective transduced control cells were seeded and cultured in cell culture medium and allowed to adhere to culture plates in 12 h. Next, MTT reagent was added after various time points. Then the viability assay was performed following the manufacturer’s instructions. Five replicates were assayed for each experimental condition.
For RNA isolation, HSP20-transduced iPSCs and controls were separately mixed with TriReagent (Sigma-Aldrich). After 1-bromo-3-chloro-propane (Sigma-Aldrich) was added to all samples, samples were separated via centrifugation (45 min, 13000 g) and the upper phase, which was free of proteins and containing RNA, was collected and mixed with an equal amount of ethanol. Subsequently, samples were processed with the RNeasy Mini Kit (Qiagen, Germantown, MD, United States), according to manufacturer’s recommendations. The quantity and quality of eluted RNA were quantified using NanoDrop (NanoDrop Products, Thermo Fisher Scientific, Waltham, MA, United States).
For qPCR, cDNA was synthesized from 2 μg total RNA by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, United States). TaqMan qPCR was executed in triplicate in 96-well optical plates on the Quantstudio Real-Time PCR system (Applied Biosystems, Foster City, CA, United States). Gene expression assays for all genes were performed with TaqMan probes and primer sets (Applied Biosystems). Quantitative gene expression was analyzed for proliferating cell nuclear antigen (PCNA; Hs00427214_g1), marker of proliferation gene Ki-67 (Ki67; Hs00606991_m1), SIRT1 (SIRT1; Hs01009006_m1), sex determining region Y-box 2 (SOX2; Hs04234836_s1), homeobox transcription factor nanog (Nanog; Hs02387400_g1), Kruppel-like factor 4 (KLF4; Hs00358836_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs99999905_m1). The expression of SIRT1, PCNA, and Ki67 genes was normalized to endogenous GAPDH expression level and calculated with the 2-ΔΔCt formula as the percentage of GAPDH expression.
Cells were homogenized in RIPA buffer (Sigma Aldrich) containing protease inhibitor cocktail, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 2 mmol/L sodium vanadate, 1 mmol/L EDTA, and 1 mmol/L EGTA and centrifuged (15000 × g) for 15 min at 4 ℃. Protein concentrations were quantified using the Pierce BCA assay (Thermo Fisher Scientific). Then 20 μg protein per condition was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (4%-15% Tris-HCL precast gel; Bio-Rad) under reduced conditions. Then proteins were transferred to nitrocellulose filters after separation. Blots were blocked in 5% bovine serum albumin in Tris-buffered saline and Tween 20 for 1 h, and the membranes were incubated overnight (4 ℃) with antibodies against SIRT1 (diluted 1:500; Cell Signaling Technology, Danvers, MA, United States), and β-actin (diluted 1:10000; Abcam, Cambridge, MA, United States).
The statistical analyses were performed using SigmaStat 3.5 software (Systat Software, San Jose, CA, United States), whereas GraphPad Prism 4 was used to generate graphs. The Student’s t-test was used for statistical assessment, and asterisks were assigned in the order aP < 0.05, bP < 0.01, and cP < 0.001 for statistically significant values, whereas exact P values were mentioned for statistically nonsignificant data sets. Error bars in all figures represent the standard error of the mean.
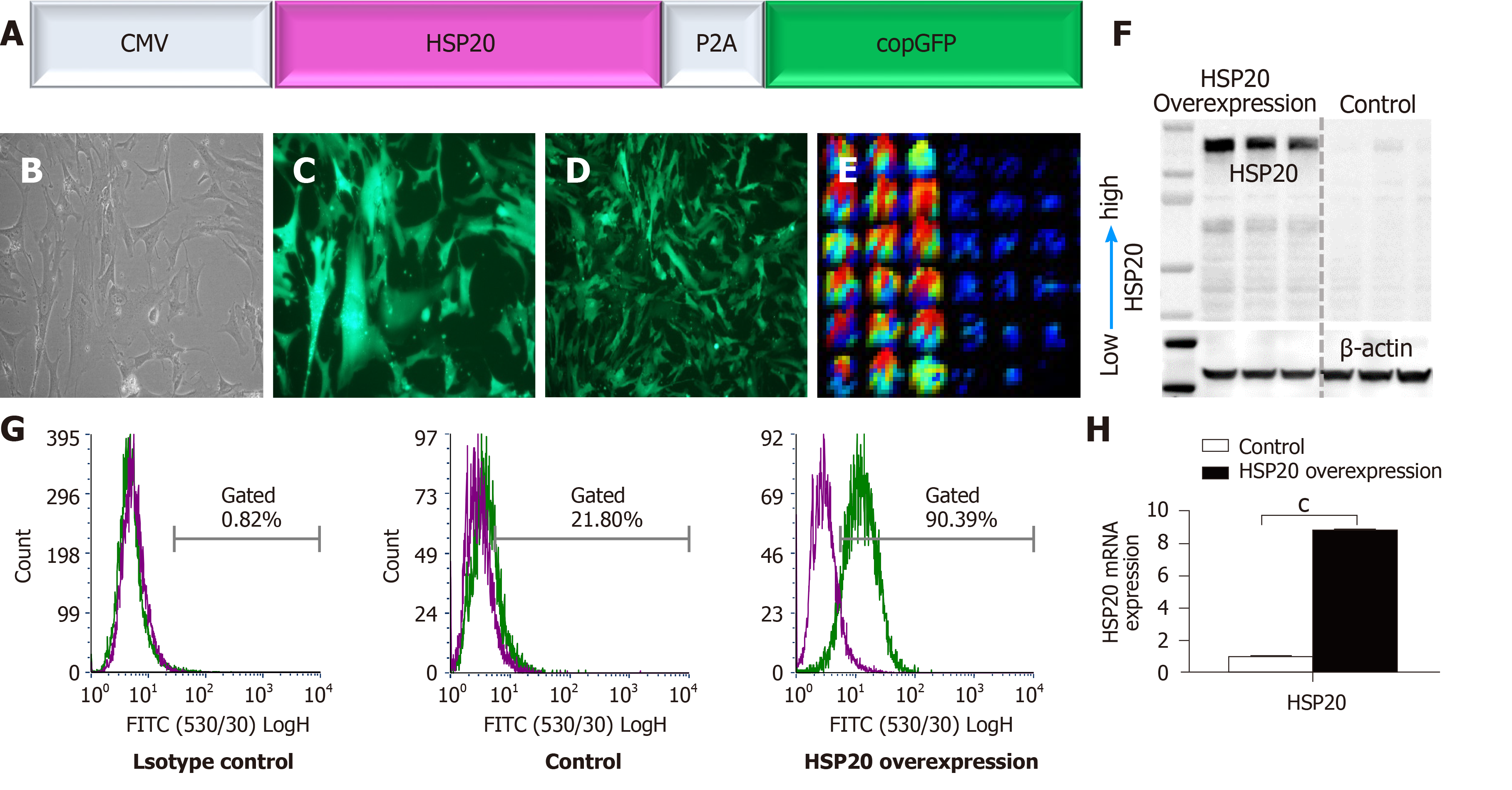
A lentiviral vector construct, pLenti-CMV-HSP20-copGFP (Figure 1A), was constructed in which a CMV promoter drove the expression of HSP20. To track and monitor the expression of HSP20 gene, it was linked to copGFP for easy detection of the transduction of target cells. A lentiviral vector construct, pLenti-CMV-copGFP, was used as an internal control. After packing the construct into lentiviral particles, iPSCs were transduced. The effective transduction of cells was validated through morphology (Figure 1B) and expression of copGFP (Figure 1C and D). iPSCs were transduced with nearly 100% efficiency following infection of suspended cells at an MOI of 20. The expression of HSP20 in transduced iPSCs was confirmed by immunofluorescence enzyme-linked immunoassay (Figure 1E) and western blotting (Figure 1F). HSP20 overexpression in iPSCs was further authenticated by flow cytometry (Figure 1G) and real-time qPCR (Figure 1H). These results confirm the well-documented expression of HSP20 in transduced-iPSCs.
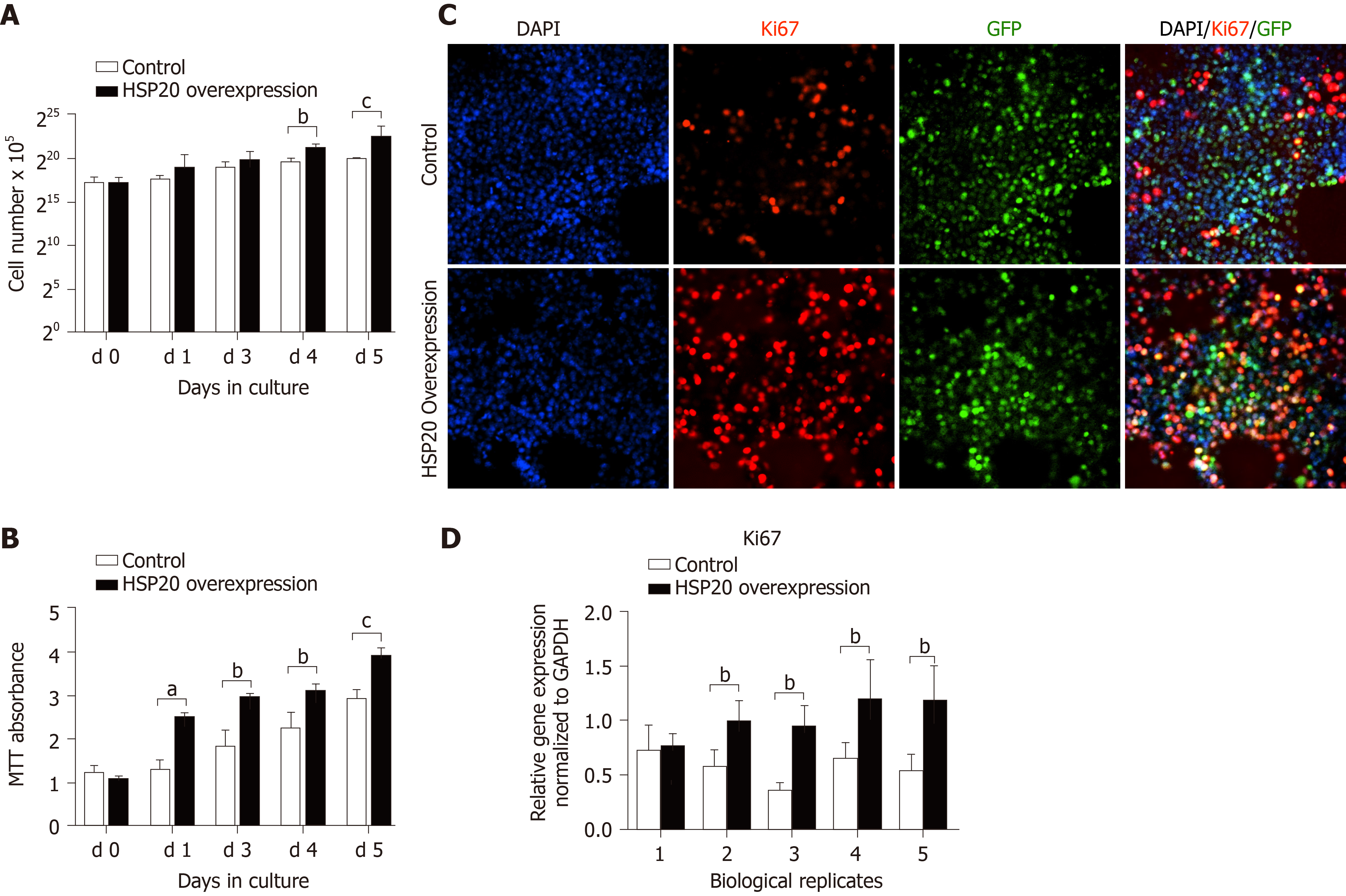
To explore the effect of HSP20 on the proliferative capacity of iPSCs, we assayed cell viability by cell counting and the MTT proliferation assay (Figure 2). An advantage of using iPSCs in this study is that iPSCs are a homogeneous population and easy to transfect. The cells were counted for 6 continuous days, and we observed higher cell counts in HSP20-transduced cells compared to controls (Figure 2A). Our results showed that the cell viability was significantly increased in the HSP20-overexpressing cells. This was further confirmed by the higher metabolization of MTT in these cells (Figure 2B). MTT is used to examine cell viability and proliferation. Therefore, we performed the MTT assay for 4 consecutive weeks to show the increased viability of HSP20-transduced cells when compared to the controls (Figure 2B).
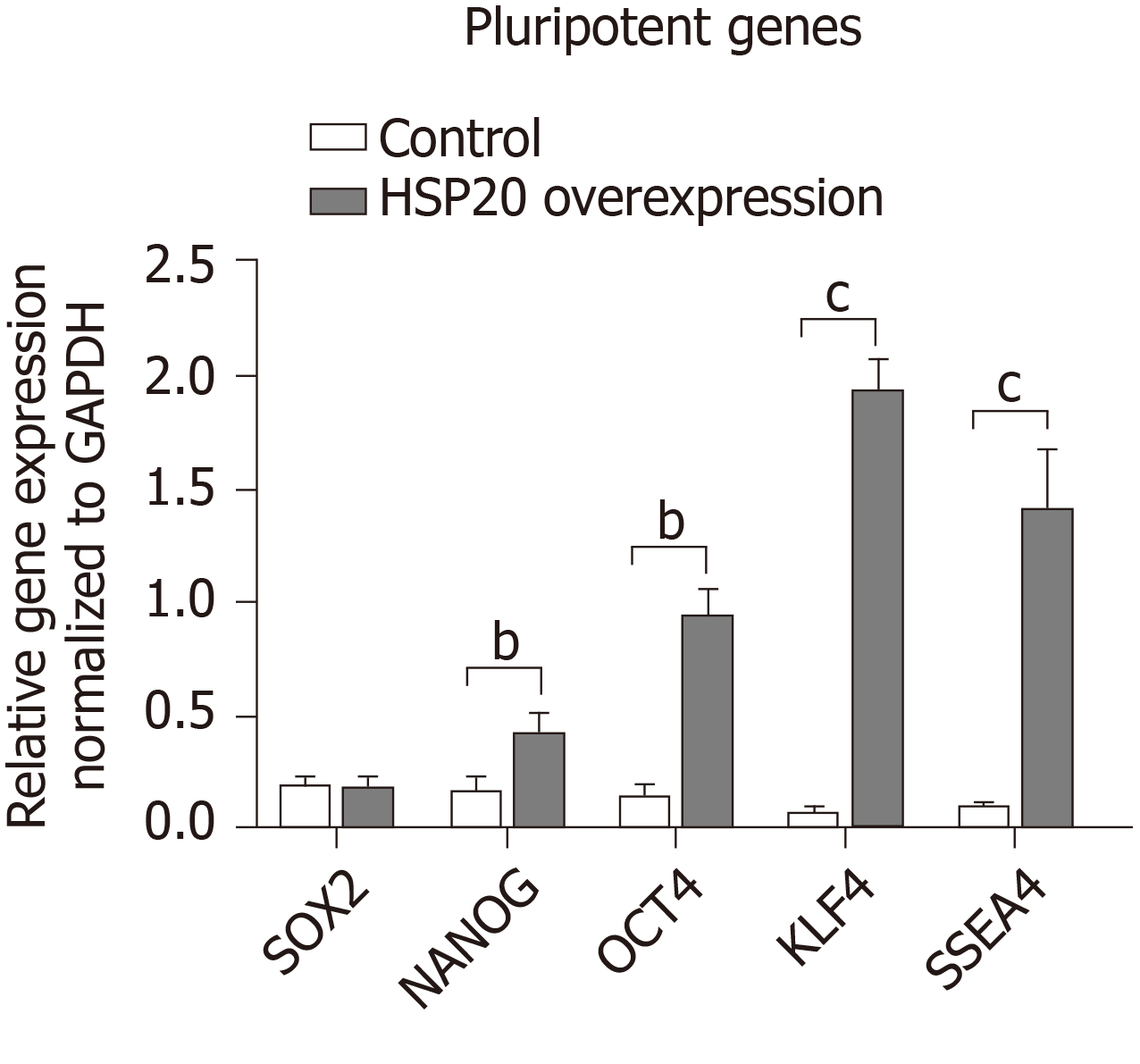
We confirmed that HSP20 was driving cell proliferation by measuring the expression of Ki67, a key proliferation marker, by immunohistochemistry. We found the significantly higher expression of Ki67 in HSP20-overexpressing cells (Figure 2C). Moreover, the mRNA expression of Ki67 was also increased in HSP20-overexpressing cells compared to the controls (Figure 2D). To determine if HSP20 upregulates the expression of PCNA, a key protein in the cell cycle, we performed real-time qPCR for PCNA, and confirmed that HSP20-overexpressing cells have higher PCNA expression. This established a positive association between HSP20 and PCNA (Figure 2D). HSP20 overexpression induced the expression of pluripotent genes (SOX2, NANOG, octamer-binding transcription factor 4, KLF4, and stage-specific embryonic antigen-4) in iPSCs, which resulted in significantly enhanced cell proliferation Figure 3). Thus, HSP20 upregulates pluripotent genes, cell proliferation, and stemness in iPSCs and therefore may be beneficial in tissue repair therapies and regeneration.
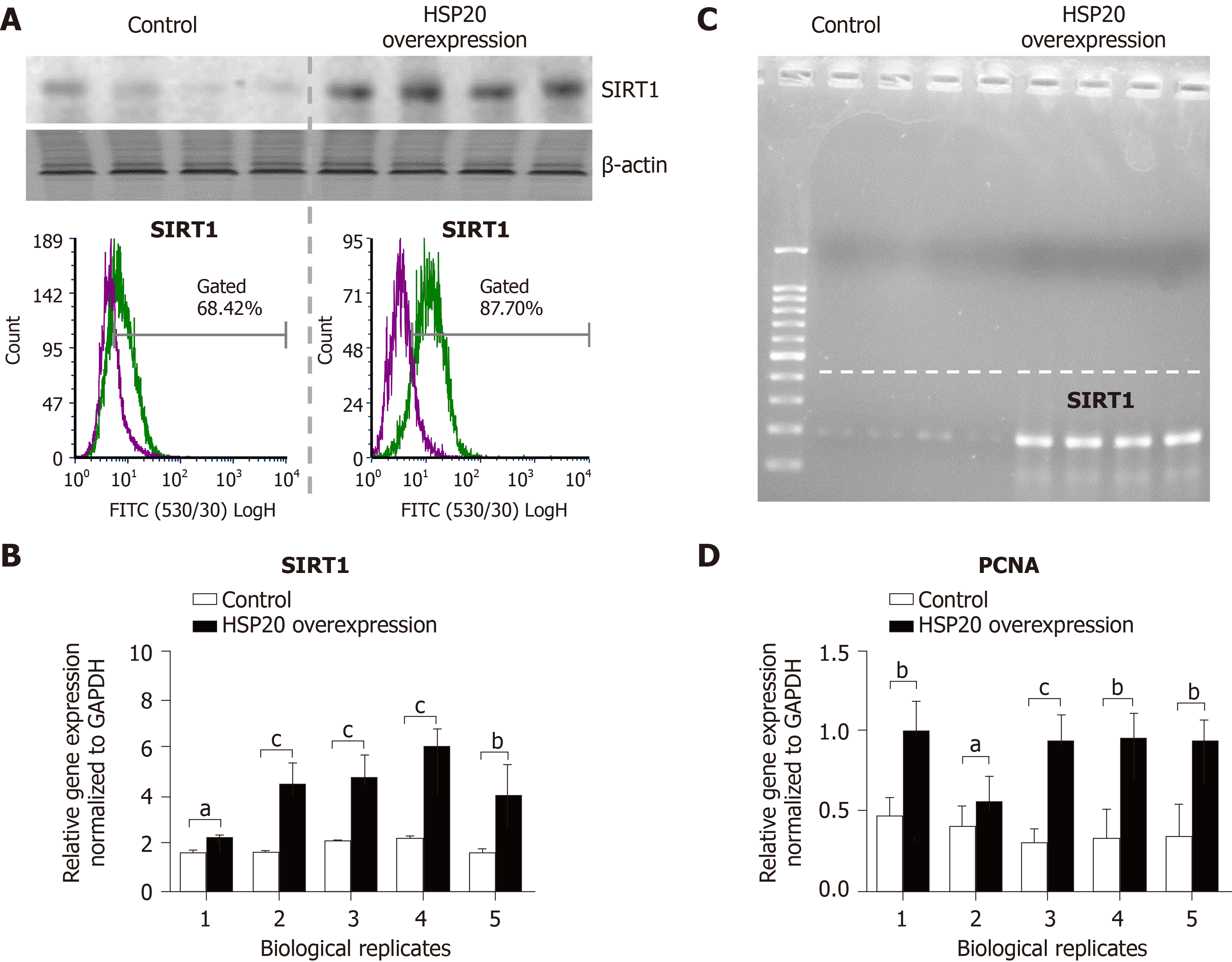
To understand how HSP20 acts to enhance cell proliferation in iPSCs, we evaluated the association of HSP20 with other proteins such as SIRT1, a protein that promotes cell proliferation and stem cell maintenance[22-24]. We found that iPSCs expressing HSP20 showed marked upregulation of SIRT1, as shown by western blotting (Figure 4). We further measured the expression of SIRT1 by flow cytometry and observed significantly higher expression of SIRT1 in HSP20-transduced cells compared to control cells (Figure 4A). Similarly, SIRT1 expression was increased at the mRNA level in HSP20-overexpressing iPSCs (Figure 4B), and its size was validated by agarose gel electrophoresis (Figure 4C). Therefore, we demonstrated that HSP20 stimulates cell proliferation in iPSCs via a SIRT1-dependent pathway. Together, our data suggest that targeting HSP20 in iPSCs opens new avenues of cellular therapies, rejuvenation, and regenerative approaches employing functionally enhanced iPSCs.
In this study, we engineered a lentiviral construct harboring the full-length human HSP20 cDNA and introduced the construct to human iPSCs via viral transfection, thereby generating a system that robustly overexpresses HSP20 in human iPSCs. Human iPSCs serve as important tools for the field of stem cell-based therapies where researchers are looking at large-scale production of stem cell-derived cell types for transplantation and regenerative therapies.
It is apparent that overexpressing HSP20 in human iPSCs resulted in accelerated proliferation through drastic expansion of cell populations (Figure 2A), and increased expression of the key proliferation marker Ki67 at both the mRNA and protein levels (Figure 2C and D). Performing the MTT assay in HSP20-transduced cells confirmed that HSP20 indeed drove cell proliferation in human iPSCs. In addition, we also showed that the overexpression of HSP20 upregulated expression of the cell cycle protein PCNA (Figure 2D). The role played by HSP20 in modulating cell proliferation has not been fully clarified, but studies have corroborated that HSP20 is indeed involved in promoting cell proliferation. However, HSP20 seems to possess the capability to drive or suppress proliferation.
We propose that in the context of human iPSCs, HSP20 exerts pro-proliferative effects via a SIRT-1 dependent pathway. SIRT1 is a deacetylase that removes acetyl groups from proteins and serves as a critical post-translational regulator[28]. The roles undertaken by SIRT1 in cell proliferation, albeit limited, have increasingly been acknowledged. In stem cells, SIRT1 can inactivate tumor suppressor p53[29]. Therefore, it is possible that overexpressing HSP20 in human iPSCs may promote cell proliferation and maintenance of self-renewal capacity through the SIRT1-p53 axis[30,31]. There is also a possibility that the SIRT1-SOX2 axis may be involved in driving cell proliferation[32]. In stark contrast, HSP20 suppressed cell proliferation in the context of cancer cells by inhibiting the phosphoinositide 3-kinase/AKT signaling pathway[26].
Future studies are warranted to establish the precise mechanism by which HSP20 regulates SIRT1 in stem cells, and to determine if the SIRT1-p53 or SIRT1-SOX2 axis triggers cell proliferation. HSP20 unlocks antigenic sites in metastatic cancers by RNA editing technology[33-35]. It will be interesting to dissect the distinct roles played by HSP20 in stem cells and cancer cells. Interaction of HSPs with surface proteins is crucial in fighting coronavirus disease 2019 both as a therapeutic and preventive strategy, and needs further validation[12,36,37]. It will also be interesting to explore if HSP20 physically interacts with SIRT-1 to direct cell proliferation. HSP20 is a protein chaperone that facilitates the nuclear translocation of other proteins[38], while SIRT1 is primarily localized to the cytoplasm and can translocate to the nucleus upon activation[39,40]. Therefore, it is likely that HSP20 binds to SIRT1 and serves as a chaperone for its nuclear translocation, thereby creating a feed-forward loop that upregulates the expression of SIRT1 in stem cells. Additional studies are needed to validate if HSP20 also acts in tandem through SIRT1-p53 to inhibit apoptosis and promote cell survival in stem cells.
In conclusion, the results of this study suggest that HSP20 drives cell proliferation in iPSCs. The overexpression of HSP20 in iPSCs opens new avenues of cellular therapies and regenerative approaches employing functionally enhanced iPSCs. We also, for the first time, illustrate the crosstalk between HSP20 and the protein deacetylase SIRT1. How HSP20 and SIRT1 work together to promote cell proliferation in pluripotent stem cells remains to be further clarified. Our data have practical implications in the area of stem-cell-based replacement therapies, where the goal is to scale up the production of stem cell-derived cells for clinical transplantation. Accelerating stem cell growth by manipulating HSP20 expression may prove a viable solution to aid in the mass production of those tissue-specific cells and cell banking for future use.
The heat shock protein 20 (HSP20) protects cells from cellular damage and dysfunctional enzyme attacks. Therefore, we used an induced pluripotent stem cell (iPSC) model to study the proliferative effects of HSP20, and investigated the effects of HSP20 on cell growth, stemness, pluripotency, and cellular activity.
HSPs make healthy cells stronger by protecting them against stress and injuries, making individuals more resistant to diseases. HSP20 constitutes the first line of protection for cells exposed to stressful/damaged conditions, thereby making it an ideal protein for clinical significance in different diseases.
This study highlighted the more recent findings illustrating the proliferative effects of HSP20 and its potential as a therapeutic agent, with the aim of determining the importance of HSP20 overexpression in iPSCs compared to their control.
We used iPSCs, which retain their potential for cell proliferation. HSP20 overexpression effectively enhanced the proliferation of cells. Overexpression of HSP20 in iPSCs was characterized by immunocytochemistry staining and real-time polymerase chain reaction analysis. We further used cell culture, cell counting, western blotting, and flow cytometry analysis for the validation of HSP20 overexpression and its mechanism.
Our present findings revealed the role of HSP20 in promoting cell proliferation. Our results also showed that the action of HSP20 is dependent on sirtuin 1 (SIRT1).
HSP20 overexpression resulted in significantly better proliferation. These findings provide adequate evidence for mechanistic study of HSP20 role in SIRT1-dependent cell proliferation in iPSCs.
Resolving the detailed cellular and molecular mechanisms underlying cell proliferation is crucial. In this study, we demonstrated the role of iPSCs overexpressing HSP20 in proliferation and stemness to better understand the underlying molecular mechanisms.
Manuscript source: Invited manuscript
Specialty type: Cell biology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Grawish ME, Lei XH, Li T S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX
| 1. | Dubrez L, Causse S, Borges Bonan N, Dumétier B, Garrido C. Heat-shock proteins: chaperoning DNA repair. Oncogene. 2020;39:516-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2982] [Cited by in RCA: 2623] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 3. | Ullah M, Liu DD, Rai S, Dadhania A, Jonnakuti S, Concepcion W, Thakor AS. Reversing Acute Kidney Injury Using Pulsed Focused Ultrasound and MSC Therapy: A Role for HSP-Mediated PI3K/AKT Signaling. Mol Ther Methods Clin Dev. 2020;17:683-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Piippo N, Korhonen E, Hytti M, Skottman H, Kinnunen K, Josifovska N, Petrovski G, Kaarniranta K, Kauppinen A. Hsp90 inhibition as a means to inhibit activation of the NLRP3 inflammasome. Sci Rep. 2018;8:6720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Ullah M, Kodam SP, Mu Q, Akbar A. Microbubbles versus Extracellular Vesicles as Therapeutic Cargo for Targeting Drug Delivery. ACS Nano. 2021;15:3612-3620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Kim HJ, Hwang NR, Lee KJ. Heat shock responses for understanding diseases of protein denaturation. Mol Cells. 2007;23:123-131. [PubMed] |
| 7. | Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94:1167-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Lindquist SL, Kelly JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Wang G, Zhang J, Chen X, Xu H, Heng G, Chen J, Zhao Y, Li J, Ni Y, Zhang Y, Shan J, Qian C. CD9, a potential leukemia stem cell marker, regulates drug resistance and leukemia development in acute myeloid leukemia. Stem Cell Res Ther. 2021;12:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Ho CT, Grousl T, Shatz O, Jawed A, Ruger-Herreros C, Semmelink M, Zahn R, Richter K, Bukau B, Mogk A. Cellular sequestrases maintain basal Hsp70 capacity ensuring balanced proteostasis. Nat Commun. 2019;10:4851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979-5997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 12. | Ullah M. Need for Specialized Therapeutic Stem Cells Banks Equipped with Tumor Regression Enzymes and Anti-Tumor Genes. J Biomed Allied Res. 2020;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003; 362: 469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 14. | Pockley AG, Henderson B. Extracellular cell stress (heat shock) proteins-immune responses and disease: an overview. Philos Trans R Soc Lond B Biol Sci. 2018;373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Koliński T, Marek-Trzonkowska N, Trzonkowski P, Siebert J. Heat shock proteins (HSPs) in the homeostasis of regulatory T cells (Tregs). Cent Eur J Immunol. 2016;41:317-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Reddy VS, Madala SK, Trinath J, Reddy GB. Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress Chaperones. 2018;23:441-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005;15:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, Fan GC. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes. 2016;65:3111-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 19. | Fan GC, Yuan Q, Song G, Wang Y, Chen G, Qian J, Zhou X, Lee YJ, Ashraf M, Kranias EG. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Flynn CR, Komalavilas P, Tessier D, Thresher J, Niederkofler EE, Dreiza CM, Nelson RW, Panitch A, Joshi L, Brophy CM. Transduction of biologically active motifs of the small heat shock-related protein HSP20 leads to relaxation of vascular smooth muscle. FASEB J. 2003;17:1358-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | McLemore EC, Tessier DJ, Flynn CR, Furnish EJ, Komalavilas P, Thresher JS, Joshi L, Stone WM, Fowl RJ, Brophy CM. Transducible recombinant small heat shock-related protein, HSP20, inhibits vasospasm and platelet aggregation. Surgery. 2004;136:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Tessier DJ, Komalavilas P, Liu B, Kent CK, Thresher JS, Dreiza CM, Panitch A, Joshi L, Furnish E, Stone W, Fowl R, Brophy CM. Transduction of peptide analogs of the small heat shock-related protein HSP20 inhibits intimal hyperplasia. J Vasc Surg. 2004;40:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Zhong B, Hu Z, Tan J, Lu T, Lei Q, Chen C, Zeng L. Hsp20 Protects against Oxygen-Glucose Deprivation/Reperfusion-Induced Golgi Fragmentation and Apoptosis through Fas/FasL Pathway. Oxid Med Cell Longev. 2015;2015:606934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Chen S, Huang H, Yao J, Pan L, Ma H. Heat shock protein B6 potently increases non-small cell lung cancer growth. Mol Med Rep. 2014;10:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Matsushima-Nishiwaki R, Adachi S, Yoshioka T, Yasuda E, Yamagishi Y, Matsuura J, Muko M, Iwamura R, Noda T, Toyoda H, Kaneoka Y, Okano Y, Kumada T, Kozawa O. Suppression by heat shock protein 20 of hepatocellular carcinoma cell proliferation via inhibition of the mitogen-activated protein kinases and AKT pathways. J Cell Biochem. 2011;112:3430-3439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Nagasawa T, Matsushima-Nishiwaki R, Yasuda E, Matsuura J, Toyoda H, Kaneoka Y, Kumada T, Kozawa O. Heat shock protein 20 (HSPB6) regulates TNF-α-induced intracellular signaling pathway in human hepatocellular carcinoma cells. Arch Biochem Biophys. 2015;565:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Ullah M, Kuroda Y, Bartosh TJ, Liu F, Zhao Q, Gregory C, Reger R, Xu J, Lee RH, Prockop DJ. iPS-derived MSCs from an expandable bank to deliver a prodrug-converting enzyme that limits growth and metastases of human breast cancers. Cell Death Discov. 2017;3:16064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, Kurosaka M, Kuroda R. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731-2740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 29. | Jang J, Huh YJ, Cho HJ, Lee B, Park J, Hwang DY, Kim DW. SIRT1 Enhances the Survival of Human Embryonic Stem Cells by Promoting DNA Repair. Stem Cell Reports. 2017;9:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Lee JT, Gu W. SIRT1: Regulator of p53 Deacetylation. Genes Cancer. 2013;4:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Ong ALC, Ramasamy TS. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res Rev. 2018;43:64-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 32. | Yoon DS, Choi Y, Jang Y, Lee M, Choi WJ, Kim SH, Lee JW. SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrow-derived mesenchymal stem cells. Stem Cells. 2014;32:3219-3231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Han L, Diao L, Yu S, Xu X, Li J, Zhang R, Yang Y, Werner HMJ, Eterovic AK, Yuan Y, Nair N, Minelli R, Tsang YH, Cheung LWT, Jeong KJ, Roszik J, Ju Z, Woodman SE, Lu Y, Scott KL, Li JB, Mills GB, Liang H. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell. 2015;28:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 34. | Ullah M, Akbar A. Clinical Relevance of RNA Editing to Early Detection of Cancer in Human. Int J Stem Cell Res Ther. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Ullah M, Akbar A, Yannarelli G. Applications of artificial intelligence in, early detection of cancer, clinical diagnosis and personalized medicine. Artif Intell Cancer. 2020;1:39-44. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (4)] |
| 36. | Bakadia BM, He F, Souho T, Lamboni L, Ullah MW, Boni BO, Ahmed AAQ, Mukole BM, Yang G. Prevention and treatment of COVID-19: Focus on interferons, chloroquine/hydroxychloroquine, azithromycin, and vaccine. Biomed Pharmacother. 2021;133:111008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Ullah M. The Pandemic of Novel Coronavirus Disease 2019 (COVID-19): Need for an Immediate Action. J Biomed Sci. 2020;2:301-302. |
| 38. | Sin YY, Martin TP, Wills L, Currie S, Baillie GS. Small heat shock protein 20 (Hsp20) facilitates nuclear import of protein kinase D 1 (PKD1) during cardiac hypertrophy. Cell Commun Signal. 2015;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Yanagisawa S, Baker JR, Vuppusetty C, Koga T, Colley T, Fenwick P, Donnelly LE, Barnes PJ, Ito K. The dynamic shuttling of SIRT1 between cytoplasm and nuclei in bronchial epithelial cells by single and repeated cigarette smoke exposure. PLoS One. 2018;13:e0193921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Pillalamarri N, Abdullah, Ren G, Khan L, Ullah A, Jonnakuti S, Ullah M. Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage. Transl Oncol. 2021;14:101095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |