Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.605
Peer-review started: January 15, 2021
First decision: February 14, 2021
Revised: February 24, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: June 26, 2021
Processing time: 161 Days and 12.4 Hours
Inflammatory periodontal disease known as periodontitis is one of the most common conditions that affect human teeth and often leads to tooth loss. Due to the complexity of the periodontium, which is composed of several tissues, its regeneration and subsequent return to a homeostatic state is challenging with the therapies currently available. Cellular therapy is increasingly becoming an alternative in regenerative medicine/dentistry, especially therapies using mesenchymal stem cells, as they can be isolated from a myriad of tissues. Periodontal ligament stem cells (PDLSCs) are probably the most adequate to be used as a cell source with the aim of regenerating the periodontium. Biological insights have also highlighted PDLSCs as promising immunomodulator agents. In this review, we explore the state of knowledge regarding the properties of PDLSCs, as well as their therapeutic potential, describing current and future clinical applications based on tissue engineering techniques.
Core Tip: Periodontal ligament stem cells (PDLSCs) have been studied for their potential to regenerate not only the periodontal complex but also other dental and non-dental tissues. We herein discuss the general features of PDLSCs, and their potential for immunomodulatory, and regenerative therapy.
- Citation: Queiroz A, Albuquerque-Souza E, Gasparoni LM, França BN, Pelissari C, Trierveiler M, Holzhausen M. Therapeutic potential of periodontal ligament stem cells. World J Stem Cells 2021; 13(6): 605-618
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/605.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.605
Mesenchymal stem cells (MSCs) can be isolated from different tissues and, in culture, show characteristics such as: fibroblast-like morphology, plastic adherent properties, and the ability to form colonies and to differentiate into osteogenic, adipogenic, and chondrogenic lineages[1]. Other known features of MSCs include their migratory activity, immunomodulatory capacity, and paracrine effects on other cell types[2-4]. Despite these common aspects, different sources of MSCs present peculiarities related to the tissue from which they are isolated. By considering these source-dependent characteristics, dental stem cells are likely to be more suitable for regeneration in dentistry. It is also important to take into account that dental tissues samples are easily obtained during procedures of relative low invasiveness.
The first dental MSCs to be isolated and well-characterized were dental pulp stem cells (DPSCs)[5]. Subsequently, other tissue sources were discovered, including the pulp of deciduous teeth[6], periodontal ligament[7], apical papilla[8], dental follicle[9], alveolar bone[10], dental germ[11], and gingiva[12].
Periodontal ligament stem cells (PDLSCs), also known as periodontal ligament mesenchymal cells, are a unique cell population that are easily obtained and exhibit important characteristics of MSCs, such as self-renewal, multipotency, and immunomo
Pre-clinical studies, carried out using different in vivo models, as well as pilot studies and clinical trials published so far, show promising results regarding the effectiveness of PDLSCs in regeneration of the periodontal complex and the safety of their use in humans[15-17]. In this review, we address the current knowledge regarding the properties of PDLSCs as well as their therapeutic potential, describing current and future clinical applications based on tissue engineering techniques.
The periodontium is a complex set of tissues composed of the gingiva, periodontal ligament, cementum, and alveolar bone. The periodontal ligament is a unique specialized connective tissue responsible for anchoring the teeth to the alveolar bone, providing them with the necessary fixation and protection against the forces of the masticatory system. Thus, it guarantees homeostasis and the other functions performed by this system[18,19]. By considering this complexity, the regeneration of lost or damaged periodontal tissues due to periodontitis remains a challenge for the currently available therapeutics. To date, periodontitis is a complex chronic inflammatory disease that is the result of a dysregulated immune response to a dysbiotic biofilm with the presence of pathogens such as Porphyromonas gingivalis (P. gingivalis). This causes destruction of the supporting periodontal tissues (i.e., periodontal ligament, cementum, and alveolar bone) and eventually loss of the tooth.
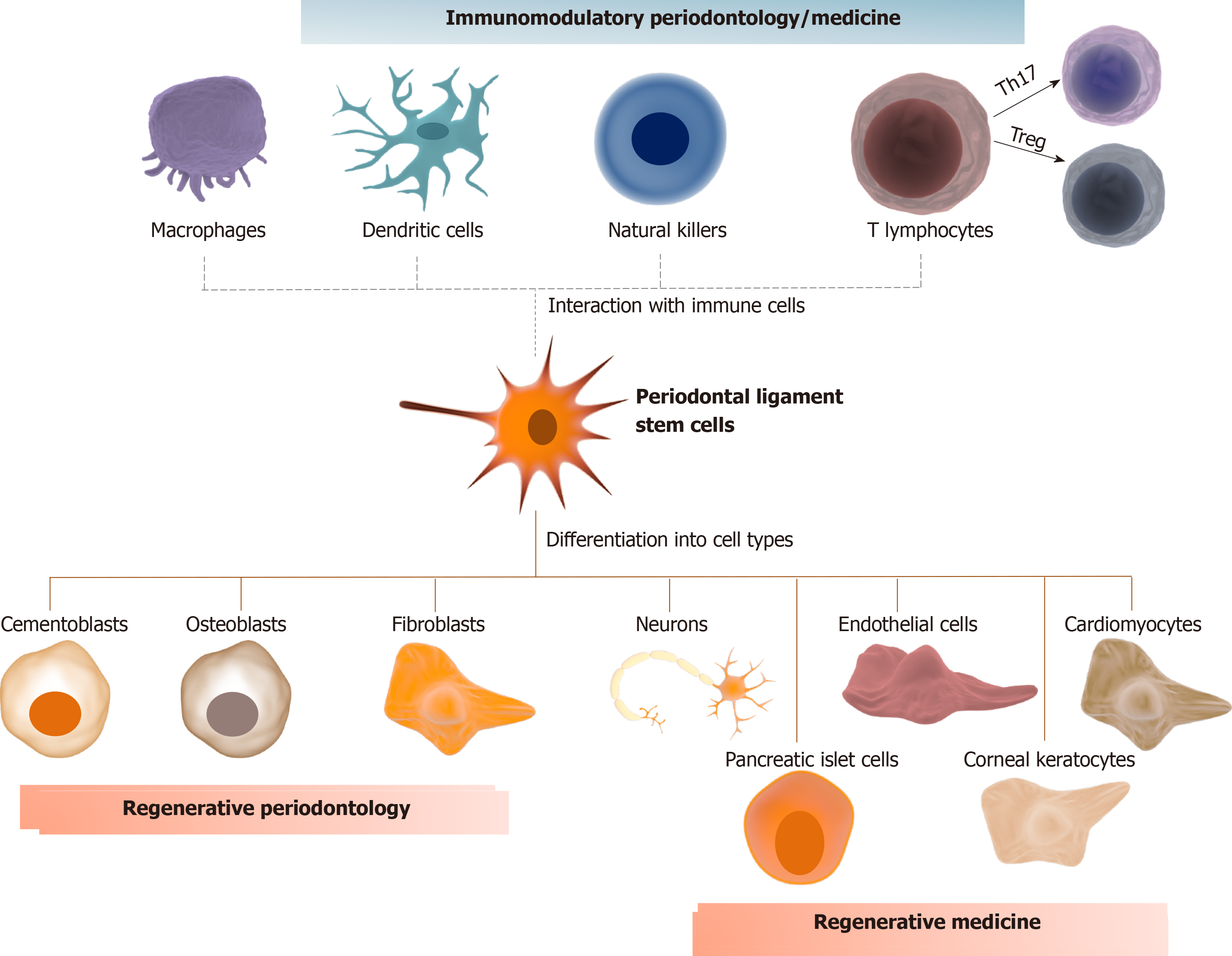
PDLSCs seem to have a fundamental role as progenitor cells and coordinate the events related to regeneration of the periodontal tissues[20]. They are heterogeneous, clonogenic, highly proliferative, and multipotent cells capable of differentiating into osteoblasts, cementoblasts, chondrocytes, and adipocytes[7,21,22]. Not surprisingly, they promote neoformation of periodontal tissues, including periodontal ligament fibers similar to Sharpey’s fibers, bone and cementum[13,23,24]. Osteoblasts, cementoblasts and fibroblasts are the main cell types responsible for tissue homeostasis of the alveolar bone, cementum, and periodontal ligament, respectively. From this comes the importance of evaluating the role of PDLSCs as a source of differentiation for these cells[25].
Currently, there is no specific cell surface marker that can discriminate PDLSCs from other constitutive periodontal cells. Instead, a combination of markers can be used to identify and isolate them, as they correspond to a small fraction of cells in the heterogeneous cell population of the periodontal ligament[7]. The minimum criteria for isolating PDLSCs include positivity for MSCs surface markers, such as CD90/Thy-1, CD73, CD105 and CD166/VCAM-1[24]. Other markers known to be expressed by MSCs are also positive on PDLSCs, including CD44[26-28], CD10[29], CD13[27,29], CD29[26,27], CD26[30], STRO-3[28], and STRO-4/HSP90[26]. In addition, PDLSCs are negative for endothelial (CD31) and hematopoietic markers (CD45, CD34, CD14, CD11b, CD79α, CD19), as well as for molecules related to antigen presentation (HLA-DR, CD40, CD54, CD80, CD86)[27-29].
In one of the first studies that characterized PDLSCs, it was observed that they express the pericyte markers STRO-1 and CD146, and the typical tendon antigen Scleraxis[7]. It was further observed that PDLSCs express other markers shared by pericytes and stem cells as well, including NG2 and CD140b/platelet-derived growth factor receptor β[31]. Pericytes are cells that surround endothelial cells in the microvasculature, and MSCs are believed to reside in these perivascular niches[32].
PDLSCs also express the following neural crest derived cell markers: Nestin[33,34], β-tubulin III[35], CD271/p75NTR[33,34], SLUG[33] and SOX10[33,34]. Due to these features, the induction of the neural phenotype in PDLSCs can be an interesting and effective method for future neuroregenerative therapies. In fact, a combination of surface markers for CD51/CD140α, CD271/STRO-1, and CD271/CD146 could help to isolate populations of PDLSCs with a large range of potential for cell differentiation[36].
The pluripotent embryonic stem cells markers NANOG[30,34,37], OCT-4[30,34,37,38], SOX2[34,37,38], SSEA-1[30,37], SSEA-3[37], SSEA-4[30,37], TRA-1-60, and TRA-1-81[37] were also reported to be expressed by PDLSCs, which may indicate an even more undifferentiated state of these cells. In a recent study, we reported that about 10% of PDLSCs could present double positivity for SOX2 and OCT-4[38], two transcription factors important for the pluripotency of embryonic stem cells, but which are also detected in somatic stem cells, such as those derived from the periodontal ligament[39].
By isolating stem cells from the periodontal ligament for the first time, Seo et al[7] demonstrated a population of multipotent MSCs that could perform adipogenic differentiation and acquire an osteoblast-cementoblast-like phenotype. Subsequently, it was actually proved that PDLSCs could differentiate into osteoblasts, chondrocytes, and adipocytes under appropriate culture conditions[21].
The fibroblastic activity of PDLSCs has been demonstrated. When stimulated with appropriate growth factors, these cells express fibrogenic-like genes [e.g., Collagen-1 (COL1), COL3, fibroblast-specific protein 1, Periodontal-ligament-associated protein 1], and Elastin and exhibit a strong immunofluorescence label for fibronectin. In fact, the stimulus with connective tissue growth factor increases the synthesis of collagen by PDLSCs, which points to their potential to differentiate into periodontal ligament fiber-forming cells[40].
Primary cultures containing fibroblasts derived from periodontal ligament fibers with a phenotype of undifferentiated mesenchymal cells also expressed endothelial cell markers when cultured in medium containing fibroblast growth factor (FGF) and heparin. This single cell-derived culture was able to form structures similar to blood vessels by using a three dimensional (3D) type 1 collagen scaffold. This angiogenic potential was associated with activation of the PI3K signaling cascade[41]. The angiogenic differentiation of CD105+-enriched PDLSCs can be reached using endothelial growth medium-2, and down-regulation of neuropilin-2 is observed in this process, which further indicates the role of this molecule for the angiogenic differentiation of PDLSCs[42].
Since cells isolated from the periodontal ligament also express neural crest markers, it was proposed that they could differentiate into neural lineages[33,34]. Under cultivation in a neurogenic induction medium, cells derived from the periodontal ligament underwent morphological changes similar to neurons. PDLSCs transplanted into the brains of adult mice were able to survive, migrate, and differentiate into cells with a neural-like phenotype[43]. Recently, PDLSCs grown in appropriate media exhibited cellular and nuclear morphology similar to rodent brain cells. They were able to develop domains similar to axon branches and dendrites, which was further confirmed by positive staining for β-tubulin III and F-actin. In addition, morphological analysis demonstrated that these cells could connect to each other through synaptic-like interactions and expressed related proteins, such as synaptophysin and synapsin-1[44].
With a view to the future use of these cells in neuroregenerative medicine, Fortino et al[35] differentiated PDLSCs to glial and neuron-like cells by treating them with specific growth factors (EGF and βFGF). In addition, the transdifferentiation of PDLSCs in retinal ganglia cells was followed by the regulation of microRNAs argued to control neural differentiation, especially miR-132[45,46]. However, the exact mechanisms involved in the neural differentiation of PDLSCs are still unknown, and neuroinduction protocols still need to be improved.
Populations of PDLCs positive for well-known markers of embryonic stem cells and neural crest markers also expressed genes related to cardiomyogenesis after treatment with low concentrations of hydrogen peroxide[33]. Upon discovering this potential, neural crest stem cells residing in the periodontal ligament were able to express cardiomyocyte markers, and their contraction activity was observed after induction by pulsed infrared radiation[47]. The potential for differentiating PDLSCs into pancreatic islet-like cells has also been reported. These cells could release insulin in response to glucose stimulus[48].
Regarding the factors that can affect the differentiation potential of PDLSCs, a lower osteogenic capacity was observed in PDLSCs grown in medium containing P. gingivalis-derived lipopolysaccharide (LPS), an important pathogen associated with the etiopathogenesis of periodontal diseases. A reduced activity of alkaline phosphatase and in the number of calcified deposits, as well as down-regulation of COL1 and Osteocalcin genes were observed[49]. Similar results were found under Escherichia coli-LPS stimulus[50], which was further argued to be associated with the activation of Toll-like receptor 4 (TLR-4) and its downstream NF-κB cascade[51].
On this basis, we recently showed that inflammatory conditions in periodontal tissues also alter the expression of typical pluripotent embryonic stem cell markers in PDLSCs, as well as their proliferative activity, ability to differentiate into osteoblasts and cementoblasts, and their expression of periodontal ligament related markers (periostin, tenomodulin, and α-SMA). If a typical pro-inflammatory environment with the presence of cytokines such as interleukin (IL)-1β and tumor necrosis factor-α would reduce these properties, the induction of a pro-resolving environment with the synthesis of specialized lipid mediators such as maresin and resolvin would act by improving these properties on PDLSCs[38]. Thus, although future studies are necessary, it is known that periodontal disease conditions can have the potential to affect the regenerative capacity of PDLSCs.
The different methods for culturing and isolating PDLSCs were also analyzed for their ability to affect the differentiation potential of these cells. The use of the outgrowth instead the enzymatic digestion method to isolate PDLSCs was shown to be more suitable for inducing cementogenesis. Better results for this method were observed in terms of the formation of mineralized deposits similar to cementum in both in vitro and in vivo experiments, and the expression of cementoblast-like genes[52]. Additionally, the in vitro expansion of PDLSCs cultures also induces morphological changes, by bursting their myofibroblastic phenotype, which up-regulates their contractile activity and reduces the expression of NANOG, SOX2 and OCT-4, factors associated with pluripotency of embryonic stem cells, but that are also expressed in MSCs[53].
There is still insufficient evidence of differences in the potential for differentiating PDLSCs due to donor populations related characteristics. To date, PDLSCs isolated from deciduous teeth exhibited a greater expression of genes related to adipogenic differentiation compared to those from permanent teeth[54]. In a later study, PDLSCs from either deciduous or permanent dentitions presented similar in vitro adipogenic and osteogenic differentiation[55]. By analyzing the donor-age impact, it was observed that PDLSCs from elderly populations exhibited less osteogenic activity, and a relationship between age and expression of osteogenesis-related genes (i.e. Osteocalcin, COL1, and Runx2) was established[56].
Over the years, immunomodulation has been presented as a typical characteristic of mesenchymal stem cell-like populations. The absence of the expression of costimulatory factors such as major histocompatibility complex class II antigen, CD40, and CD80, have been highlighted as responsible for the low immunogenicity of MSCs[4,57]. Further evidence demonstrated the mechanisms activated by mesenchymal cells from periodontal tissues that could result in modulation of the host response[28,58-60]. Therefore, in addition to their low immunogenicity/compatibility, MSCs perform their function by suppressing the function of a range of immune response cells and by improving immune regulatory functions. As a result of these activities, MSCs became a prominent alternative for the immune therapy of chronic inflammatory disorders, such as periodontitis.
Overall, MSCs reveal their potential by inducing phenotypic changes in several cell types, including dendritic cells (DCs), T and B lymphocytes, monocytes/macrophages, and natural killer cells[61-64]. Although the ability of periodontal ligament cells to perform such functions remains under review, recent findings point to promising correlated activities of MSCs from this tissue. How MSCs exert their immune-regulatory properties is still a matter of debate, albeit both soluble factors/secretome and cell-cell contact were described as mechanisms implicated to mediate such functions. To date, living, apoptotic, and dead MSCs, as well as their secretome consisting of a diverse range of cytokines, chemokines, growth factors, and extrace
Supporting a cell-cell interaction effect, PDLSCs co-cultured with human monocyte-derived DCs under stimulation with P. gingivalis-derived LPS, a keystone pathogen in the etiopathogenesis of periodontitis, down-regulated the expression of non-classical major histocompatibility complex glycoprotein CD1b on DCs. This, in turn, resulted in defective proliferation of T lymphocytes[60]. Indeed, the proliferative index of CD4+ T cells can be considerably reduced in the presence of PDLSCs, as well as a decrease in the ratio of CD4 + CD25 high/CD4 + CD25 low T cells within mitogen-stimulated peripheral blood mononuclear cells (PBMNCs) can be observed. Alongside these effects, PDLSCs when co-stimulated with LPS up-regulated COX-2 and IL-6 through downstream activation of extracellular signal-regulated kinase 1/2, and reduced the frequency of CD14+ cells within PBMNCs[50]. However, the supernatant of CD105-enriched PDLSCs challenged with P. gingivalis total protein extract increased neutrophil recruitment in vitro, suggesting secretome-mediated signaling as important via paracrine immune regulation[67]. Similarly, PDLSCs exhibited non-cell contact or secretome dependent suppression activity on the rate of monocyte proliferation in co-culture experiments via secretion of metabolites such as indoleamine 2, 3-dioxygenase, hepatocyte, and transforming growth factors (TGF-β)[28].
Indoleamine 2, 3-dioxygenase has been identified as an important molecule for the immunomodulation process triggered by MSCs in the typical microenvironment of chronic inflammatory diseases, particularly periodontitis[4,28,63,68]. When PDLSCs were co-stimulated with (interferon) INF-γ and TLRs agonists, an increase in the expression of this enzyme as well as IL-6, CXCL8, and MCP-1 was observed[69]. Nevertheless, the effect of these cells is not only dependent on cytokines and chemokines release. Recently, it was demonstrated that PDLSCs reduced apoptosis and stimulated the microbicidal activity of human neutrophils, via both cell-cell interactions and paracrine mechanisms. This effect could be related to the synthesis of specialized pro-resolving lipid mediators, e.g., resolvins, protectins, maresins, and lipoxins, which are major molecules responsible for orchestrating the resolution phase of inflammation and the return to tissue homeostasis[59].
However, the immunomodulatory capacity of MSCs seems to be dependent on the conditions of the source tissue. MSCs have been reported as important regulators of the T cell phenotype by inducing an increase in the proportion of T regulatory (Treg) populations (FOXP3+ lymphocytes) over typical pro-inflammatory Th17 subsets (RORγT+ lymphocytes)[70]. Notwithstanding, compared to healthy cells, PDLSCs collected from inflamed periodontal sites present less regulatory properties, reducing Treg differentiation as well as its signature cytokine IL-10. The suppressive effect on Th17 differentiation and its signature cytokine IL-17 was also reduced in PDLSCs collected from periodontally healthy individuals compared to those from periodontitis affected patients[58]. Thus, it is also important to keep in mind such biological differences in the future therapeutic application of these cell types.
MSCs present tissue-specific functional differences[71,72] that may be related to the composition of the secretome of these cells, which as reported above plays an important role in their regulatory properties. Not surprisingly, mass-spectrometry analysis of PDLSCs metabolites showed differences in the endogenous level of 5-lysophosphatidylcholines and 3-lysophosphatidylethanolamines, which can discriminate these cells from MSCs from other sources, such as adipose tissue and salivary glands[73]. These differences are also age-dependent as PDLSCs from deciduous teeth have a higher expression of cytokines that regulate the host immunity (e.g., interleukins I, II, and IV) and secreted proteins e.g., matrix metalloproteinases and cullins) responsible for tissue degradation and catalytic activities when compared to those from permanent teeth[74]. In fact, the lack of clear-cut assays that help to clarify the heterogeneity of MSCs populations is a further challenge within a translational therapeutic perspective.
Alongside the natural potential of PDLSCs to be used in regulating the host response in periodontitis, more recently MSCs of dental origin have also been mentioned as possible sources for cell therapy in other cases of inflammatory imbalance-related disorders, such as those affecting the brain (e.g., Alzheimer's disease, Parkinson's disease) and gut (e.g., Crohn’s disease, colitis)[75]. Recently, PDLSCs were used to control colitis in an in vivo model, and the authors concluded that their immunomodulatory activity was mediated by a group of deoxyribonucleic acid demethylases of the ten-eleven translocation family (Tet1 and Tet 2)[76].
Cell therapy with MSCs, taking immunomodulation as the perspective, is still in its infancy, especially with respect to those from periodontal origin, and further studies need to be delineated so that the mechanisms by which these cells exert such activity are unraveled. Doubts still exist as to whether these cells would actually need to be injected into patients, or whether it is their products that may exert more appropriate host response regulation, which could overcome common risks associated with this therapy such as ectopic differentiation and tumor growth promotion. It is necessary to overcome discrepancies between studies that apply different isolation methods, cell culture, expansion conditions and cryopreservation of PDLSCs. In addition, it appears that preconditioning these cells prior to treatment may improve their immunoregulatory functions, as suggested in a previous study with PDLSCs under INF-γ stimuli[69]. Of note, MSCs are thought to be immunoprivileged, but repeated infusion of mismatched MSCs may also induce alloimmunization[77]. Hence, more mechanistic investigations and more caution still need to be taken into account before the clinical application of this therapy, which despite the challenges, seems promising for immunomodulation.
Current approaches to treatment of periodontitis limit disease progression, but periodontal regeneration of the dental supporting tissues (alveolar bone, cementum and periodontal ligament) cannot be predictably achieved[78,79]. In the early 1990s, Tissue Engineering was proposed as a field that would apply the principles of human biology and engineering to the development of functional substitutes for damaged tissues. This strategy should be based on three strategies: (1) cells; (2) scaffolds; and (3) signaling molecules[80].
In 2004, Seo et al[7] investigated the ability of PDLSCs during tissue regeneration and found that PDLSCs were able to generate structures similar to the cementum/ periodontal ligament in vivo. This study suggested the important role of PDLSCs in periodontal regeneration[7]. Since then, many studies have evaluated the regeneration of periodontal tissue by combining PDLSCs in scaffolds and signaling molecules. PDLSCs exhibit a multipotent differentiation capacity and are good candidates for use in tissue engineering due to their potential to promote regeneration of dental and non-dental tissues.
Scaffolds have a key role in periodontal regeneration. The ideal scaffold has the following properties: (1) Ability to recruit or retain cells; (2) Biocompatibility; and (3) Biodegradation. In this sense, countless attempts have been made to find an ideal scaffold, that is, a material engineered to support desirable cellular interactions and contribute to the formation of new functional tissues. In such structures, specific mediators are responsible for mediating signaling transduction between cells. Such molecules include growth factors which bind to surface receptors that modulate target cells and accordingly improve the regenerative potential of PDLSCs sheet engineering[81]. Parallel experiments demonstrated that PDLSC sheets were able to generate structures similar to cementum and periodontal ligament-connective tissue when transplanted in a hydroxyapatite/tricalcium phosphate matrix to immunocompromised mice[82]. The potential of a nanohydroxyapatite-chitosan scaffold combined with PDLSCs in bone regeneration was also demonstrated in a calvarial bone repair model[83].
Within the molecules artificially used to mimic biological processes, FGF is a mediator that promotes regeneration of the periodontium. The application of TGFβ1 subsequent to growth under FGF stimulus for the differentiation of PDLSCs into fibroblastic cells was reported to accelerate the generation of a functional periodontium[84]. Furthermore, a combination of platelet-rich plasma and PDLSCs sheets suggested that this stimulus could increase the production of extracellular matrix and affect cellular behavior[85]. Another study evaluated the effects of human PDLSCs sheets potentiated by an enamel matrix derivative and showed that they had better mineralization capacity in vitro[86]. Our group has also been working on the incorporation of specific molecules for transmembrane receptors, as we have shown that the activation of the protease-activated receptor type 1 enhances the osteogenic activity of PDLSCs[87].
New scaffold technologies have also emerged. A better understanding of the mechanisms related to growth factors and the advent of these new technologies that can be combined with the cells highlight the potential of PDLSCs to be applied for future periodontal regeneration[81].
Tissue engineering using PDLSCs can also assist in the neoformation of periodontal tissues on the titanium surface of dental implants, facilitating their fixation and providing better mechanical and immunological properties in patients with a previous history of tooth loss treated by implant replacement therapy. Studies have reported that the use of PDLSCs sheets on the surface of titanium implants stimulate the formation of cementum-like and periodontal ligament-like tissues, both in xenogenic and autologous models[53,88].
In addition, the properties of PDLSCs, e.g., immunomodulation, homeostasis, and remodeling of the periodontal ligament, allow these cells to play an important role in tooth movement during orthodontic treatments. PDLSCs are involved in the control of compression and tension forces caused by the orthodontic movement, as well as in the periodontal ligament recovery process when such forces are removed[89-91]. PDLSCs isolated from healthy individuals and those from periodontitis responded differently to static mechanical stress, indicating that the microenvironment of periodontitis directly influences the activity of these cells, making them more susceptible to greater mechanical loads[90,92].
Besides the use of PDLSCs in dental therapies, studies have proposed their use as a source of autologous cells in patients with non-odontogenic conditions, especially considering that their isolation is easier compared to other MSCs[92]. Aimed at their use in patients with multiple sclerosis, human PDLSCs obtained from both, healthy and multiple sclerosis patients, were evaluated and showed similar immunophenotypic, proliferative and differentiating properties, which suggests that they may be effective in personalized therapies for such conditions[93]. Also, PDLSCs have the potential to be used in corneal regenerative therapy. It was demonstrated that they are able to differentiate into corneal stromal keratocyte-like cells. To date, dental MSCs and the corneal stromal keratocytes share a common embryological origin, which is the cranial neural crest[94].
Hematopoietic stem cells have been widely used in humans for several decades, mainly for the treatment of neoplasms and other hematolymphoid disorders. These were the first stem cells described and characterized, and the efficacy and safety of these populations have already been shown by countless in vitro and in vivo studies[95]. In contrast, the description of MSCs is more recent albeit in preclinical studies and clinical trials involving these cells. Clinical studies using dental MSCs are at a less advanced stage than those involving several other stem cells, which can be explained by the lack of well described methods to obtain these cells, and efficient and safe protocols for their use in humans[96].
A search on the ClinicalTrials.gov portal website for registered clinical trials using PDLSCs transplantation for regenerative therapies in humans resulted in only two studies that fit these criteria. A phase I and already concluded trial (NIH Clinical Trial Registration Number: NCT01357785), evaluated 35 patients with periodontitis over a 12-mo follow-up period. Autologous PDLSCs sheets were transplanted into intraosseous defects in association with lyophilized bone, and it was concluded that, despite the good results regarding the absence of adverse effects on patients, there was no statistical difference in relation to the reduction of probing depth (PD)[15]. To date, PD is one of the main outcomes evaluated in regenerative therapy for periodontitis. The second study, a phase I and II clinical trial, was registered in 2010 and remains with unknown status so far. Its objective was to transplant PDLSCs from healthy patients into 80 adults (40-50 years) with periodontitis (NCT01082822).
Of note, a pilot clinical study evaluated three patients with periodontitis treated with autologous PDLSCs. A significant reduction in PD and improvement in clinical attachment level were observed in the patients who underwent cell therapy. No adverse effects of this therapy were found[16].
In another study registered with the UMIN Clinical Trials Registry, autologous transplantation of PDLSCs in 10 patients resulted in improvement of PD, clinical attachment level, and radiographic bone height (alveolar bone regeneration). PDLSCs sheets were transplanted in association with β-tricalcium phosphate granules into severe alveolar bone defects. During the 3 and 6-mo follow-up, no adverse effects were noted[97].
Furthermore, it is worth mentioning that there are also ongoing and finalized clinical trials aiming to regenerate periodontal tissues with stem cells from other sources, such as DPSCs (NCT02523651; NCT0338687), bone marrow MSCs (NCT00221130; NCT02449005), and adipose-derived stem cells (NCT04270006). Gingival MSCs are the cell source in two clinical trials that aimed to treat periodontal defects using these cells in collagen (NCT03137979) and β-tricalcium phosphate (NCT03638154) scaffolds.
In summary, the regenerative and therapeutic potential of PDLSCs seems to be auspicious from a biological perspective. Clinical studies with these cells are already a reality in regenerative periodontology. No clinical evidence of their application with specific use for immunomodulatory periodontology/medicine has been reported so far. Despite some promising clinical results and biological insights herein discussed (summarized Figure 1), several gaps need to be filled before adopting a protocol for their clinical use in patients with periodontitis or other inflammatory conditions. In this sense, we draw attention to the fact that most studies associate PDLSCs with osteoinductive/osteoconductive materials. These materials, especially those synthetic and xenogenic, can affect therapeutic efficacy and safety by reducing mechanical properties, impairing bone remodeling, and inducing an immunological reaction[98]. Standardization of the bone defects in periodontitis affected patients, as well as a larger number of individuals allocation and multicenter trials are also necessary.
In addition, tissue-dependent factors are critical to successful cell therapy with PDLSCs, as the effectiveness of their autologous transplantation also depends on variables such as the number of isolated cells, the microenvironment from which they were collected (the presence of inflammation is a known limitation), the general health status of the patient, and the differentiation pathway after transplantation[99]. Basic research in this field still has a long way to go. Defining better protocols for cultivating, storing, expanding, and differentiating PDLSCs is the challenge to ensure safety, reproducibility, and cost-effectiveness of clinical applications.
Manuscript source: Invited manuscript
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ortiz-Sanchez E S-Editor: Zhang L L-Editor: Webster JR P-Editor: Xing YX
| 1. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1368] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 2. | Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016;1416:123-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 299] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 3. | Kot M, Musiał-Wysocka A, Lasota M, Ulman A, Majka M. Secretion, migration and adhesion as key processes in the therapeutic activity of mesenchymal stem cells. Acta Biochim Pol. 2019;66:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Wada N, Gronthos S, Bartold PM. Immunomodulatory effects of stem cells. Periodontol 2000. 2013;63:198-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3349] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 6. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 1975] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 7. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2371] [Cited by in RCA: 2496] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 8. | Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 891] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 9. | Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 619] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 10. | Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K, Miyazaki K, Shimizu M, Bhawal UK, Tsuji K, Nakamura K, Kato Y. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, Kawase M, Go MJ, Adachi H, Yokota Y, Kirita T, Ohgushi H. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787-7798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 567] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 13. | Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 437] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 14. | Trubiani O, Pizzicannella J, Caputi S, Marchisio M, Mazzon E, Paganelli R, Paganelli A, Diomede F. Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives. Stem Cells Dev. 2019;28:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 15. | Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, Lu H, Chu Q, Xu J, Yu Y, Wu RX, Yin Y, Shi S, Jin Y. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 16. | Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Tassi SA, Sergio NZ, Misawa MYO, Villar CC. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J Periodontal Res. 2017;52:793-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontol 2000. 2006;40:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. 1997;13:20-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 379] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 20. | Han J, Menicanin D, Gronthos S, Bartold PM. Stem cells, tissue engineering and periodontal regeneration. Aust Dent J. 2014;59 Suppl 1:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 22. | Xu J, Wang W, Kapila Y, Lotz J, Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18:487-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Flores MG, Yashiro R, Washio K, Yamato M, Okano T, Ishikawa I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J Clin Periodontol. 2008;35:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Suaid FF, Ribeiro FV, Gomes TR, Silvério KG, Carvalho MD, Nociti FH Jr, Casati MZ, Sallum EA. Autologous periodontal ligament cells in the treatment of Class III furcation defects: a study in dogs. J Clin Periodontol. 2012;39:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Bartold PM, Gronthos S. Standardization of Criteria Defining Periodontal Ligament Stem Cells. J Dent Res. 2017;96:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, Gronthos S. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev. 2014;23:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Trubiani O, Di Primio R, Traini T, Pizzicannella J, Scarano A, Piattelli A, Caputi S. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol. 2005;18:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 29. | Hakki SS, Bozkurt B, Hakki EE, Kayis SA, Turac G, Yilmaz I, Karaoz E. Bone morphogenetic protein-2, -6, and -7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. J Biomed Mater Res B Appl Biomater. 2014;102:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Trubiani O, Zalzal SF, Paganelli R, Marchisio M, Giancola R, Pizzicannella J, Bühring HJ, Piattelli M, Caputi S, Nanci A. Expression profile of the embryonic markers nanog, OCT-4, SSEA-1, SSEA-4, and frizzled-9 receptor in human periodontal ligament mesenchymal stem cells. J Cell Physiol. 2010;225:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Komaki M. Pericytes in the Periodontal Ligament. Adv Exp Med Biol. 2019;1122:169-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Crisan M, Corselli M, Chen WC, Péault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851-2860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 33. | Huang CY, Pelaez D, Dominguez-Bendala J, Garcia-Godoy F, Cheung HS. Plasticity of stem cells derived from adult periodontal ligament. Regen Med. 2009;4:809-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Pelaez D, Huang CY, Cheung HS. Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev. 2013;22:2906-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Fortino VR, Chen RS, Pelaez D, Cheung HS. Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol. 2014;229:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Alvarez R, Lee HL, Wang CY, Hong C. Characterization of the osteogenic potential of mesenchymal stem cells from human periodontal ligament based on cell surface markers. Int J Oral Sci. 2015;7:213-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Kawanabe N, Murata S, Murakami K, Ishihara Y, Hayano S, Kurosaka H, Kamioka H, Takano-Yamamoto T, Yamashiro T. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation. 2010;79:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Albuquerque-Souza E, Schulte F, Chen T, Hardt M, Hasturk H, Van Dyke TE, Holzhausen M, Kantarci A. Maresin-1 and Resolvin E1 Promote Regenerative Properties of Periodontal Ligament Stem Cells Under Inflammatory Conditions. Front Immunol. 2020;11:585530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Peng Z, Liu L, Zhang W, Wei X. Pluripotency of Dental Pulp Cells and Periodontal Ligament Cells Was Enhanced through Cell-Cell Communication via STAT3/Oct-4/Sox2 Signaling. Stem Cells Int. 2021;2021:8898506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Liu J, Zhao Z, Ruan J, Weir MD, Ma T, Ren K, Schneider A, Oates TW, Li A, Zhao L, Xu HHK. Stem cells in the periodontal ligament differentiated into osteogenic, fibrogenic and cementogenic lineages for the regeneration of the periodontal complex. J Dent. 2020;92:103259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Okubo N, Ishisaki A, Iizuka T, Tamura M, Kitagawa Y. Vascular cell-like potential of undifferentiated ligament fibroblasts to construct vascular cell-specific marker-positive blood vessel structures in a PI3K activation-dependent manner. J Vasc Res. 2010;47:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Amorim BR, Silvério KG, Casati MZ, Sallum EA, Kantovitz KR, Nociti FH Jr. Neuropilin Controls Endothelial Differentiation by Mesenchymal Stem Cells From the Periodontal Ligament. J Periodontol. 2016;87:e138-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Bueno C, Ramirez C, Rodríguez-Lozano FJ, Tabarés-Seisdedos R, Rodenas M, Moraleda JM, Jones JR, Martinez S. Human adult periodontal ligament-derived cells integrate and differentiate after implantation into the adult mammalian brain. Cell Transplant. 2013;22:2017-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Bueno C, Martínez-Morga M, Martínez S. Non-proliferative neurogenesis in human periodontal ligament stem cells. Sci Rep. 2019;9:18038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Ng TK, Yung JS, Choy KW, Cao D, Leung CK, Cheung HS, Pang CP. Transdifferentiation of periodontal ligament-derived stem cells into retinal ganglion-like cells and its microRNA signature. Sci Rep. 2015;5:16429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Ng TK, Yang Q, Fortino VR, Lai NY, Carballosa CM, Greenberg JM, Choy KW, Pelaez D, Pang CP, Cheung HS. MicroRNA-132 directs human periodontal ligament-derived neural crest stem cell neural differentiation. J Tissue Eng Regen Med. 2019;13:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Greenberg JM, Lumbreras V, Pelaez D, Rajguru SM, Cheung HS. Neural Crest Stem Cells Can Differentiate to a Cardiomyogenic Lineage with an Ability to Contract in Response to Pulsed Infrared Stimulation. Tissue Eng Part C Methods. 2016;22:982-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Lee JS, An SY, Kwon IK, Heo JS. Transdifferentiation of human periodontal ligament stem cells into pancreatic cell lineage. Cell Biochem Funct. 2014;32:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch Oral Biol. 2014;59:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 50. | Kukolj T, Trivanović D, Djordjević IO, Mojsilović S, Krstić J, Obradović H, Janković S, Santibanez JF, Jauković A, Bugarski D. Lipopolysaccharide can modify differentiation and immunomodulatory potential of periodontal ligament stem cells via ERK1,2 signaling. J Cell Physiol. 2018;233:447-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Li C, Li B, Dong Z, Gao L, He X, Liao L, Hu C, Wang Q, Jin Y. Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through Toll-like receptor 4 mediated nuclear factor κB pathway. Stem Cell Res Ther. 2014;5:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 52. | Shinagawa-Ohama R, Mochizuki M, Tamaki Y, Suda N, Nakahara T. Heterogeneous Human Periodontal Ligament-Committed Progenitor and Stem Cell Populations Exhibit a Unique Cementogenic Property Under In Vitro and In Vivo Conditions. Stem Cells Dev. 2017;26:632-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Iwasaki K, Komaki M, Akazawa K, Nagata M, Yokoyama N, Watabe T, Morita I. Spontaneous differentiation of periodontal ligament stem cells into myofibroblast during ex vivo expansion. J Cell Physiol. 2019;234:20377-20391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Silvério KG, Rodrigues TL, Coletta RD, Benevides L, Da Silva JS, Casati MZ, Sallum EA, Nociti FH Jr. Mesenchymal stem cell properties of periodontal ligament cells from deciduous and permanent teeth. J Periodontol. 2010;81:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Song JS, Kim SO, Kim SH, Choi HJ, Son HK, Jung HS, Kim CS, Lee JH. In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Eng Part A. 2012;18:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Zheng W, Wang S, Ma D, Tang L, Duan Y, Jin Y. Loss of proliferation and differentiation capacity of aged human periodontal ligament stem cells and rejuvenation by exposure to the young extrinsic environment. Tissue Eng Part A. 2009;15:2363-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 58. | Liu D, Xu J, Liu O, Fan Z, Liu Y, Wang F, Ding G, Wei F, Zhang C, Wang S. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J Clin Periodontol. 2012;39:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 59. | Cianci E, Recchiuti A, Trubiani O, Diomede F, Marchisio M, Miscia S, Colas RA, Dalli J, Serhan CN, Romano M. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem Cells Transl Med. 2016;5:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Shin C, Kim M, Han JA, Choi B, Hwang D, Do Y, Yun JH. Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J Periodontal Res. 2017;52:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E (2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1919] [Cited by in RCA: 1814] [Article Influence: 113.4] [Reference Citation Analysis (1)] |
| 62. | Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, Jorgensen C, Noël D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells. 2016;34:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 63. | Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 64. | Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 65. | Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 66. | Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol. 2019;10:1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 479] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 67. | Misawa MYO, Silvério Ruiz KG, Nociti FH Jr, Albiero ML, Saito MT, Nóbrega Stipp R, Condino-Neto A, Holzhausen M, Palombo H, Villar CC. Periodontal ligament-derived mesenchymal stem cells modulate neutrophil responses via paracrine mechanisms. J Periodontol. 2019;90:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | DelaRosa O, Lombardo E, Beraza A, Mancheño-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, García L, Abad JL, Trigueros C, Delgado M, Büscher D. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 69. | Andrukhov O, Hong JS, Andrukhova O, Blufstein A, Moritz A, Rausch-Fan X. Response of human periodontal ligament stem cells to IFN-γ and TLR-agonists. Sci Rep. 2017;7:12856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, Dahlke MH. Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J Immunol. 2014;193:4988-4999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Vasandan AB, Shankar SR, Prasad P, Sowmya Jahnavi V, Bhonde RR, Jyothi Prasanna S. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med. 2014;18:344-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | Kim JH, Jo CH, Kim HR, Hwang YI. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018;2018:8429042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 73. | Lee SJ, Yi T, Ahn SH, Lim DK, Kim SN, Lee HJ, Cho YK, Lim JY, Sung JH, Yun JH, Lim J, Song SU, Kwon SW. Comparative study on metabolite level in tissue-specific human mesenchymal stem cells by an ultra-performance liquid chromatography quadrupole time of flight mass spectrometry. Anal Chim Acta. 2018;1024:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Kim K, Jeon M, Lee HS, Park JC, Moon SJ, Kim SO, Cho SW, Song JS. Comparative analysis of secretory factors from permanent- and deciduous-teeth periodontal ligament cells. Arch Oral Biol. 2016;71:65-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Földes A, Kádár K, Kerémi B, Zsembery Á, Gyires K, S Zádori Z, Varga G. Mesenchymal Stem Cells of Dental Origin-Their Potential for Antiinflammatory and Regenerative Actions in Brain and Gut Damage. Curr Neuropharmacol. 2016;14:914-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Yu T, Liu D, Zhang T, Zhou Y, Shi S, Yang R. Inhibition of Tet1- and Tet2-mediated DNA demethylation promotes immunomodulation of periodontal ligament stem cells. Cell Death Dis. 2019;10:780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 520] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 78. | Du J, Li M. Functions of Periostin in dental tissues and its role in periodontal tissues' regeneration. Cell Mol Life Sci. 2017;74:4279-4286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, Ivanovski S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv Healthc Mater. 2018;7:e1800457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 80. | Langer R, Vacanti J. Advances in tissue engineering. J Pediatr Surg. 2016;51:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 81. | Song IS, Han YS, Lee J-H, Um S, Kim HY, Seo BM. Periodontal Ligament Stem Cells for Periodontal Regeneration. Curr Oral Heal Rep. 2015;2:236-244. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 83. | Ge S, Zhao N, Wang L, Yu M, Liu H, Song A, Huang J, Wang G, Yang P. Bone repair by periodontal ligament stem cellseeded nanohydroxyapatite-chitosan scaffold. Int J Nanomedicine. 2012;7:5405-5414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 84. | Kono K, Maeda H, Fujii S, Tomokiyo A, Yamamoto N, Wada N, Monnouchi S, Teramatsu Y, Hamano S, Koori K, Akamine A. Exposure to transforming growth factor-β1 after basic fibroblast growth factor promotes the fibroblastic differentiation of human periodontal ligament stem/progenitor cell lines. Cell Tissue Res. 2013;352:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Xu Q, Li B, Yuan L, Dong Z, Zhang H, Wang H, Sun J, Ge S, Jin Y. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J Tissue Eng Regen Med. 2017;11:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 86. | Wang Z, Feng Z, Wu G, Bai S, Dong Y, Zhao Y. In vitro studies on human periodontal ligament stem cell sheets enhanced by enamel matrix derivative. Colloids Surf B Biointerfaces. 2016;141:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Rovai ES, Ambrósio LMB, de França BN, de Oliveira LR, Gasparoni LM, Sipert CR, Holzhausen M. Protease-Activated Receptor Type 1 Activation Enhances Osteogenic Activity in Human Periodontal Ligament Stem Cells. Stem Cells Int. 2019;2019:6857386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Washio K, Tsutsumi Y, Tsumanuma Y, Yano K, Srithanyarat SS, Takagi R, Ichinose S, Meinzer W, Yamato M, Okano T, Hanawa T, Ishikawa I. In Vivo Periodontium Formation Around Titanium Implants Using Periodontal Ligament Cell Sheet. Tissue Eng Part A. 2018;24:1273-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Feng L, Yang R, Liu D, Wang X, Song Y, Cao H, He D, Gan Y, Kou X, Zhou Y. PDL Progenitor-Mediated PDL Recovery Contributes to Orthodontic Relapse. J Dent Res. 2016;95:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Huang H, Yang R, Zhou YH. Mechanobiology of Periodontal Ligament Stem Cells in Orthodontic Tooth Movement. Stem Cells Int. 2018;2018:6531216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 91. | Zhang E, Zhu C, Yang J, Sun H, Zhang X, Li S, Wang Y, Sun L, Yao F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2016;58:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 92. | Liu J, Li Q, Liu S, Gao J, Qin W, Song Y, Jin Z. Periodontal Ligament Stem Cells in the Periodontitis Microenvironment Are Sensitive to Static Mechanical Strain. Stem Cells Int. 2017;2017:1380851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 93. | Diomede F, Rajan TS, D'Aurora M, Bramanti P, Merciaro I, Marchisio M, Gatta V, Mazzon E, Trubiani O. Stemness Characteristics of Periodontal Ligament Stem Cells from Donors and Multiple Sclerosis Patients: A Comparative Study. Stem Cells Int. 2017;2017:1606125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Yam GH, Teo EP, Setiawan M, Lovatt MJ, Yusoff NZBM, Fuest M, Goh BT, Mehta JS. Postnatal periodontal ligament as a novel adult stem cell source for regenerative corneal cell therapy. J Cell Mol Med. 2018;22:3119-3132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 95. | Müller AM, Huppertz S, Henschler R. Hematopoietic Stem Cells in Regenerative Medicine: Astray or on the Path? Transfus Med Hemother. 2016;43:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 96. | Yamada Y, Nakamura-Yamada S, Kusano K, Baba S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int J Mol Sci. 2019;20:1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 97. | Iwata T, Yamato M, Washio K, Yoshida T, Tsumanuma Y, Yamada A, Onizuka S, Izumi Y, Ando T, Okano T, Ishikawa I. Periodontal regeneration with autologous periodontal ligament-derived cell sheets - A safety and efficacy study in ten patients. Regen Ther. 2018;9:38-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 98. | Sanz M, Dahlin C, Apatzidou D, Artzi Z, Bozic D, Calciolari E, De Bruyn H, Dommisch H, Donos N, Eickholz P, Ellingsen JE, Haugen HJ, Herrera D, Lambert F, Layrolle P, Montero E, Mustafa K, Omar O, Schliephake H. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J Clin Periodontol. 2019;46 Suppl 21:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 99. | Zhang J, Huang X, Wang H, Liu X, Zhang T, Wang Y, Hu D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |