Published online Sep 26, 2020. doi: 10.4252/wjsc.v12.i9.938
Peer-review started: March 3, 2020
First decision: June 7, 2020
Revised: June 18, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: September 26, 2020
Processing time: 202 Days and 13.3 Hours
In recent years, several studies have reported positive outcomes of cell-based therapies despite insufficient engraftment of transplanted cells. These findings have created a huge interest in the regenerative potential of paracrine factors released from transplanted stem or progenitor cells. Interestingly, this notion has also led scientists to question the role of proteins in the secretome produced by cells, tissues or organisms under certain conditions or at a particular time of regenerative therapy. Further studies have revealed that the secretomes derived from different cell types contain paracrine factors that could help to prevent apoptosis and induce proliferation of cells residing within the tissues of affected organs. This could also facilitate the migration of immune, progenitor and stem cells within the body to the site of inflammation. Of these different paracrine factors present within the secretome, researchers have given proper consideration to stromal cell-derived factor-1 (SDF1) that plays a vital role in tissue-specific migration of the cells needed for regeneration. Recently researchers recognized that SDF1 could facilitate site-specific migration of cells by regulating SDF1-CXCR4 and/or HMGB1-SDF1-CXCR4 pathways which is vital for tissue regeneration. Hence in this study, we have attempted to describe the role of different types of cells within the body in facilitating regeneration while emphasizing the HMGB1-SDF1-CXCR4 pathway that orchestrates the migration of cells to the site where regeneration is needed.
Core tip: In the last few decades, cell-based regenerative therapy has received considerable attention for the treatment of degenerative diseases or the regeneration of injured organs. However, poor cell retention is considered a major drawback associated with the short-term regenerative benefits. Furthermore, the short-term regenerative benefits are linked to paracrine factors secreted by the transplanted stem cells. To improve regenerative outcomes, researchers have identified the role of stromal cell-derived factor-1 (SDF1) as a key chemotactic factor that can facilitate site-specific migration and retention of transplanted cells, and stem or progenitor cells within the body by activating the SDF1-CXCR4 or HMGB1-SFD1-CXCR4 pathways.
- Citation: Haque N, Fareez IM, Fong LF, Mandal C, Abu Kasim NH, Kacharaju KR, Soesilawati P. Role of the CXCR4-SDF1-HMGB1 pathway in the directional migration of cells and regeneration of affected organs. World J Stem Cells 2020; 12(9): 938-951
- URL: https://www.wjgnet.com/1948-0210/full/v12/i9/938.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i9.938
Over the past decades, non-communicable diseases, especially degenerative diseases are becoming more prevalent worldwide, which also contributes to major morbidity. During the past two decades, stem cell-based regenerative therapy has been considered hopeful in addressing the unmet needs of treating degenerative diseases[1].
Among the different tools of regenerative medicine “stem cells” are considered the most promising due to their self-renewal capability and multi-differentiation potential. However, recent studies have shown that the positive outcomes of different types of stem cell-based regenerative therapies are not directly correlated to the engraftment of transplanted cells[2,3]. These findings have created a huge interest in the regenerative potential of paracrine factors and have led scientists to reveal the regenerative potential of proteins in the secretomes. Further studies have revealed the mitogenic, angiogenic, anti-apoptotic, anti-scarring and chemoattractant features of secretomes or cell culture supernatants that make them a potential tool for regenerative therapy[1,4]. Furthermore, the regenerative potential of the secretome from adult stem cells[5], freshly isolated healthy peripheral blood mononuclear cells (PBMC)[6] and apoptosis-induced PBMC[7,8] has been acknowledged by several researchers. Secretomes from stem and progenitor cells have been found to be favorable for regenerating tissues or treating several disorders including neuronal disorders[9], vascular diseases[10] and cutaneous wounds[11]. The growing evidence on the role of paracrine factors (cytokines, chemokines and growth factors) in the regeneration of affected organs has led to the introduction of cell culture supernatants or secretomes as a new therapeutic tool of regenerative medicine.
Regeneration is a complex process and several types of cells namely lymphocytes, monocytes, neutrophils, endothelial progenitor cells (EPCs), hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and tissue resident stem cells are involved in the process of regeneration. Recent studies reported that stromal cell-derived factor-1α (SDF1α) present in the secretome increases proliferation, viability, migration and homing of stem and progenitor cells; helps lymphoid tissue development and differentiation; and inhibits apoptosis of cells[4,12]. All these features are highly important for regeneration of damaged organs[13]. Furthermore, SDF1 and C-X-C-X-C chemokine receptor type 4 (CXCR-4) play a vital role in the high mobility group box 1 (HMGB1)-mediated inflammatory cell recruitment to the site of damaged tissue which is also vital for tissue repair and regeneration[14]. Hence, in this review we have attempted to describe the role of different types of cells in regeneration while emphasizing activation of the HMGB1-SDF1-CXCR4 pathway which is considered a key pathway that regulates the directional migration of all cells and facilitates the process of regeneration.
PBMC are widely used in preclinical research and applications in vaccine trials, source of biomarkers in various infectious and chronic diseases, and are a useful tool in studying various aspects of pathology and biology in vitro[15]. In addition, PBMC are an easily accessible source of different types of adult stem and progenitor cells, such as HSCs, MSCs, osteoclast precursor cells, and EPCs[16]. Due to the content of different types of adult stem cells, in a favorable microenvironment the potential to differentiate into several tissue specific cells including mature blood cells, endothelial cells, hepatocytes, cardiomyocytes, smooth muscle cells, epithelial cells, neural cells, osteoblasts, osteoclasts, and myofibroblasts has been shown[16-18]. Furthermore, compared to bone marrow (BM) or other multipotent cells sources, the isolation of PBMC is less invasive. However, a series of standard procedures for PBMC collection, isolation, cryopreservation and preparation are crucial for their use in cell-based regenerative therapy[15]. PBMC contain terminally differentiated immune cells, namely monocytes and lymphocytes that also a play vital role in tissue remodeling and regeneration[19-21].
Monocytes that contribute approximately 4%-10% of leukocytes in our bloodstream are highly plastic in nature[22]. Monocytes and macrophages are the largest types of white blood cells and are involved in inflammation and elimination of harmful foreign substances[23,24]. As part of the innate immunity they are involved in tissue homeostasis and facilitate wound healing by removing apoptotic and necrotic cells[24].
In regenerative tissues, macrophages are highly plastic and play a decisive role in tissue repair and regeneration[25]. In response to injury and subsequent healing, macrophages are capable of polarization towards a spectrum of phenotypes. Based on the environmental cues and molecular mediators, these cells will differentiate into either pro-inflammatory type I macrophage (M1) or anti-inflammatory type II macrophage (M2) phenotypes[25-27].
Studies have reported that M1 macrophages infiltrate tissues at the earlier stages of acute injury to promote the clearance of necrotic cells or tissue debris. Moreover, following activation M1 macrophages secrete a wide range of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, IL-12, IL-18, IL-23, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-1[28]. Whereas, M2 macrophages appear within the injured tissue at later stages and release high amounts of anti-inflammatory paracrine factors such as IL-10 and transforming growth factor-β (TGF-β)[25,29]. They are also capable of secreting extracellular matrix (ECM) remodeling components such as fibronectin, osteopontin, fibrin cross-linker transglutaminase and promote tissue healing[30,31]. Although there are controversies regarding the sequential presence of the two different macrophages within tissues, this is because of the dynamic shift in macrophage polarization or the recruitment of new monocytes which do not invalidate the role of macrophages in tissue regeneration.
Among the different types of lymphocytes, the regulatory T-cells (Treg) are involved in the repair and regeneration of affected tissues and organ systems. Following injury, Treg are recruited to the site to regulate inflammation and modulate the process of regeneration[32]. Following the initiation of inflammation, Treg inhibit recruitment of neutrophils by secreting IL-10 which in turn helps to minimize the secretion of inflammatory cytokines namely IL-1β, IL-6, interferon (IFN)-γ, and TNF-α. Moreover, Treg induce apoptosis of neutrophils and clear debris by activation of M1 macrophages. In addition, they play a vital role in macrophage polarization towards the M2 phenotype by secreting anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 which eventually support tissue repair and regeneration[32,33]. However, the regenerative function of Treg follows a tissue specific manner. For instance, in skeletal muscle, Treg infiltrate the tissues in response to IL-33 following activation of the M1 population and removal of necrotic tissues by these cells. Following infiltration, Treg inhibit M1-mediated inflammation and shift the polarization of macrophages towards the M2 population[32]. Whereas, in heart tissues, recruitment of the higher number of Treg induces polarization of macrophages towards the M2 phenotype that help to inhibit inflammation, excessive matrix degradation, and adverse remodeling which eventually reduce ventricular ruptures and increases the rate of survival[34].
Neutrophils are the most abundantly found white blood cells in the human peripheral circulation and contribute approximately 50%-70% to all circulatory white blood cells. They are the first leukocyte population recruited to the site of injury and regulate the process of tissue regeneration positively or negatively[32]. However, the role of neutrophils in the process of regeneration is microenvironment-dependent and context-specific.
For example, in the case of skeletal muscle injury, neutrophils impair the restoration and function of muscles by releasing hypochlorous acid, nicotinamide adenine dinucleotide phosphate oxidase, and other cytokines[32,35,36]. Downregulation of lung regeneration following ischemia-reperfusion by neutrophils has also been reported[37]. It is noteworthy that neutrophils have also shown positive effects on the repair of lung epithelium and nerve cells[38,39].
Apart from HSCs, other adult stem cells or tissue-specific progenitor cells such as MSCs, EPCs, mammary stem cells, intestinal stem cells, and neural stem cells are found in adult tissues[40]. Tissue-specific stem cells maintain tissue homeostasis, while MSCs can differentiate into a variety of cell types. MSCs, in particular, have promising cell sources, as they can be harvested from various sources, such as BM, umbilical cord (UC), adipose tissue, and dental tissues[41-43]. Unlike embryonic stem cells (ES cells or ESCs), which are pluripotent, MSCs are multipotent cells which possess limited differentiation potential. Nevertheless, their potential to differentiate into osteoblasts and osteocytes is very well known. There is also accumulating evidence regarding their robust potential in tissue healing and regenerative medicine, in both preclinical and clinical studies[44-46]. According to a recent PubMed search conducted on November 2019, there were 110 MSC-based human clinical trials exploring the safety and efficacy of stem cells for tissue healing and the treatment of degenerative diseases. However, most of these trials were phase I and phase II, or a mixture of phase I/II studies. Whereas, phase III or phase II/III trials which investigate the long-term safety of MSC-based therapies prior to full establishment of MSCs in clinical practice are poorly documented. Thus, BM-derived MSCs (BMSCs) have been the most studied stem cells in cell therapy and tissue repair for the last 5 years, due to their multi-lineage differentiation potential[47].
However, it is worthwhile noting that different MSC populations exhibit tissue-specific characteristics such as the expression of specific cell surface markers and transcription factors. In response to injury signals, these MSCs can potentially migrate from their niche to reach target tissues through vessel walls in the peripheral circulation[48]. Many studies have been conducted to investigate both the chemical and mechanical factors that influence the homing mechanism and engraftment of MSCs into local areas of damaged sites. The chemical factors that affect the trafficking process are the presence of a variety of chemokines, growth factors and cytokines, whereas the mechanical factors involved in the process include ECM stiffness, vascular cyclic stretching and hemodynamic forces or shear stress on the vessel walls[49]. These factors make up the vital characteristics of MSCs and result in their promising effect in tissue healing and differentiation.
The first characteristic of MSCs is their multi-lineage differentiation potential. MSCs are capable of differentiating into several mesoderm lineages, including adipogenic, osteogenic, chondrogenic and myogenic lineages, depending on the multitude of stimuli and inhibitors present in the tissue microenvironment[50]. The micro-environment plays an important role in the activation or downregulation of transcription factors that regulate the expression of genes responsible for the induction and progression of tissue-specific differentiation[51]. MSCs can also generate neural cells in the ectodermal layer, and hepatic cells and pancreatic cells in the endodermal layer[52]. The study by Chen et al[53] was among the first to explore the ability of MSCs to differentiate into functional islet-like cells that might play an important role in the future treatment of diabetes. MSCs cultured in stiff scaffolds can easily differentiate into osteoblasts, and showed the potential for myogenic, adipogenic and neurogenic differentiation, respectively, but with a decrease in elasticity. Recently, Jung et al[54] demonstrated that ECM proteins in 3D composites were able to trigger differentiation of BMSCs into mesodermal lineages with enhanced adipogenic differentiation and IL-6 expression compared to that in 2D ECM proteins.
Secondly, MSCs are capable of dynamic interactions with their microenvironment and secrete a wide variety of paracrine factors that are required for tissue recovery or wound healing. Several studies refuted the hypothesis that direct trans-differentiation or cell fusion of MSCs was the principal mechanism underlying their therapeutic action in tissue regeneration[55]. Indeed, MSCs transplantation regulated released factors in experimental models of tissue injury, which was largely associated with suppression of immune and inflammatory reactions, inhibition of apoptosis, and enhancement of cell proliferation and angiogenesis, thereby promoting regeneration of the tissue[56]. Apart of MSCs-mediated secretion of these paracrine and autocrine factors, extracellular vesicles such as exosomes and microvesicles may also regulate these functional roles[57,58]. The MSC-mediated factors released at high levels include the following: (1) Growth factors and their receptors [i.e., granulocyte-macrophage colony-stimulating factor (GM-CSF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), insulin-like growth factor-binding proteins (IGFBP3, IGFBP4, IGFBP7) and bone morphogenetic protein 2 (BMP-2)]; (2) Extracellular matrix remodelers/mediators [i.e., periostin, fibronectin, collagen, TIMP metallopeptidase inhibitor 2 (TIMP-2), metalloproteinase inhibitors, and decorin]; and (3) Immune system signaling regulatory proteins (i.e., TGF-β, MCP-1, IL-6, and IL-8)[49,59]. Various studies have demonstrated that the released pro-inflammatory cytokines up-regulate the efficacy of MSC-mediated immunomodulation and functional improvement in microvascular injury[60], inflammatory liver disease[61], osteoarthritis[62], spinal cord injury[63], brain cancer[64], ischemic limb regeneration[65], and asthmatic[66] models. Taken together, it is well accepted that the combination of MSCs with these trophic factors can modulate their behavior during inflammation and tissue injury. Research should now focus on the strategies to manipulate and modulate the secretion of these molecules in the infused or implanted MSCs microenvironment to enhance their functional role[1].
Finally, MSCs exhibit immunomodulatory properties[67-69]. The immunomodulatory properties of MSCs proved effective in treating various immune disorders in both in vivo and human studies. MSCs modulate the functions of almost all cells of both the innate and adaptive immune systems and induce an anti-inflammatory phenotype[59]. MSCs interact with a variety of immune cells and have the capacity to inhibit the excessive response of B cells, T cells, macrophages, dendritic cells, and natural killer cells[68]. Nevertheless, the underlying molecular and cellular mechanisms behind MSC-mediated immunomodulation have not been fully elucidated. MSCs have been shown to modulate the immune response by secreting soluble factors [e.g., IL-6, M-CSF, IL-10, TGF-β, HGF, and prostaglandin E2 (PGE2)] in the presence of adhesion molecules [i.e., vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1, and lymphocyte function-associated antigen (LFA)-3][70-72]. Through a synergy of cell contact-dependent mechanisms and these soluble factors, MSCs are able to initiate the T-cell interactions that play a prominent role in their immunomodulatory potential[71,73]. Furthermore, anti-inflammatory monocytes/macrophages and Tregs are also important in MSC-mediated immunosuppression[69,74]. Studies also linked the low immunogenic properties of MSCs to the lower level of expression of major histocompatibility complex (MHC) class I antigens, and lack of MHC class II and co-stimulatory molecules such as CD80, CD86, and CD40[75,76]. Although the immunomodulatory effect of MSCs is hypothesized to be via MSC-secreted cytokines in many studies, most studies documented that MSCs act differently depending on the local microenvironment and the presence of inflammatory cytokines during the pre-treatment of MSCs. An understanding of the immune suppressive role of MSCs would enhance prospective clinical applications of these cells.
Thus, the fate of MSCs is vastly influenced by their environment which includes mechanical or physical stimulation, growth factors, cell density, and cell-cell attachment or interactions. However, this multipotency of MSCs could also be due to another reason which has been widely discussed. In fact, a debate is currently ongoing regarding the ‘stem cell’ status of MSCs[77]. It is postulated that MSCs are purely specific adult stem cells, which contradicts findings that MSCs are a diverse mixture of many specific lineage progenitor cells. However, these shortcomings provide a good reason for the continuous research on MSCs in stem-cell based therapy.
Progenitors and MSCs migrate and initiate the homing mechanism in response to inflammatory signaling molecules and corresponding receptors around the injured tissue. MSCs are therapeutically capable of reaching and homing to sites of inflammation by various routes such as intravenous (IV), intra-arterial (IA), intraparenchymal, intracoronary (IC) local administration and into the subarachnoid and epidural spaces[48]. From the systemic circulation, MSCs migrate specifically to damaged tissue sites and exert their functional effects locally under a variety of pathologic conditions. Luger et al[78] demonstrated that intravenously administered fluorescent and radiolabeled MSCs homed to regions of myocardial injury to suppress the progressive deterioration in left ventricular function and adverse remodeling in mice, and it is thought to be a feasible and effective therapeutic strategy for the treatment of patients with large infarcts and ischemic cardiomyopathy. MSCs homing involves various chemokines and their receptors (i.e., SDF1, CCL5, CXCR4, CXCR5, CXCR6, CCR2, CCR3, and CCR4), matrix metalloproteinases (MMPs) [MMP-2 and membrane type 1 MMP (MT1-MMP)], receptor tyrosine kinase dependent growth factors [e.g., hepatocyte growth factor-Mesenchymal Epithelial Transition Factor (c-Met) proto-oncogene/receptor tyrosine kinase (HGF/c-Met) axes, platelet-derived growth factor (PDGF) and insulin-like growth factor 1 (IGF-1)] and some other adhesion molecules (i.e., integrin β1, integrin α4, and VCAM)[79-82]. These homing signals are released by injured cells and/or respondent immune cells. Besides these homing signals, other molecules are implicated in different steps of the homing process such as PGE2 and hematopoietic cell E-/L-selectin ligand (HCELL) that are functionally involved in cell migration to the injured tissue[83]. These factors could be a feasible strategy to facilitate therapeutic delivery of MSCs to targeted injured tissue.
Of the different chemokines and chemokine-mediated pathways, the SDF1-CXCR4 and HMGB1-SDF1-CXCR4 axis have received considerable attention due to their potential in-site specific directional migration of stem and progenitor cells. The role of HMGB1-SDF1-CXCR4 in regeneration of injured tissues or organs is discussed further below.
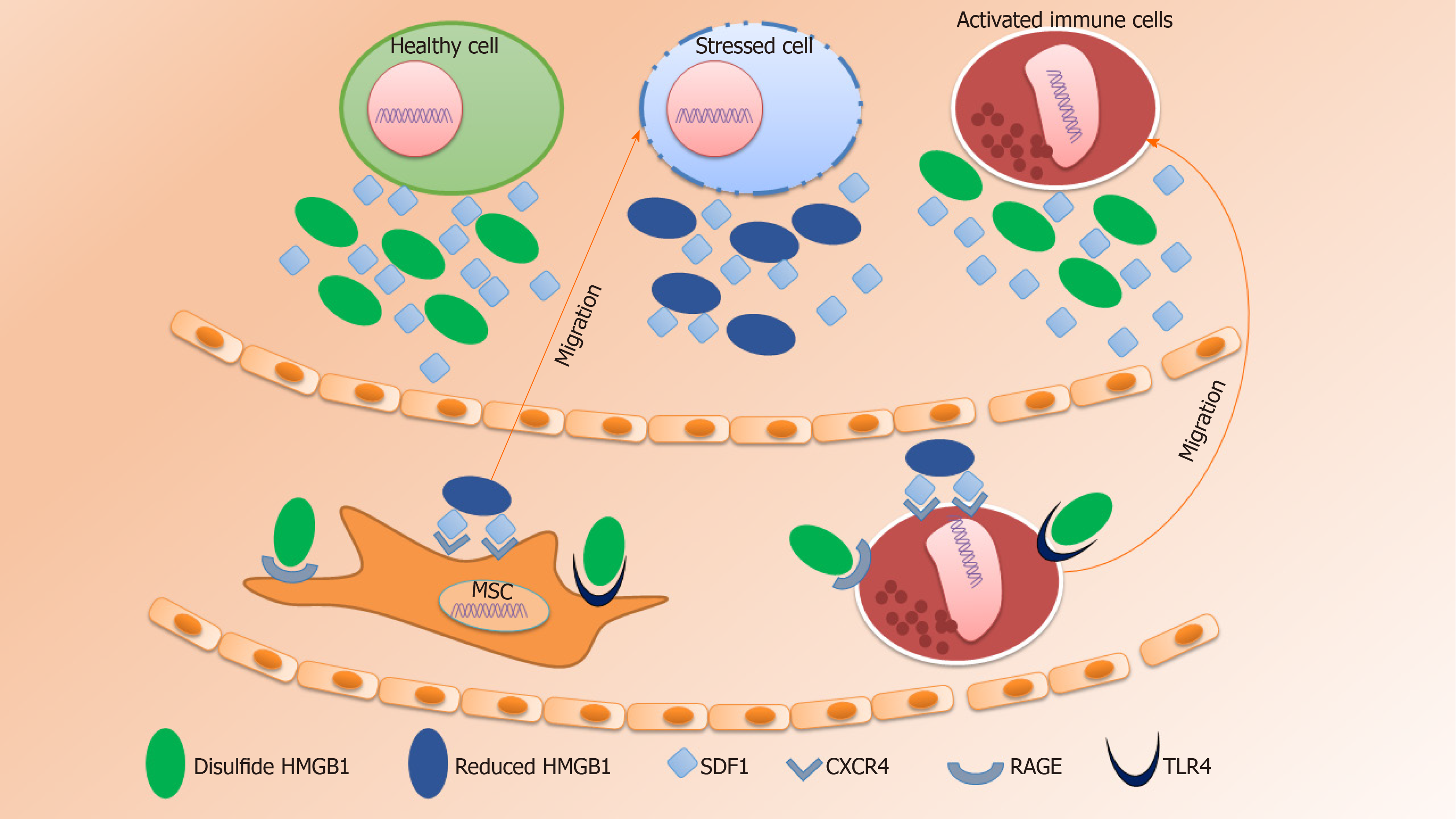
HMGB1 protein is a highly conserved non-histone nuclear protein that binds to DNA and regulates the expression of genes and the chromosomal architecture[84]. Extracellular HMGB1 is actively secreted from activated or stressed immune cells, while passively secreted from necrotic tissues[85,86]. Following secretion into the extracellular space, HMGB1 exerts chemotactic activity or acts as a damage-associated molecular pattern molecule[87]. Indeed, the overall signaling mechanism by HMGB1 interacting with target cells needs to be elucidated for future therapeutic intervention[88].
Wound healing is a complex process that involves the ECM, cytokines, growth factors and several types of cells. The steps involved in the process of wound healing include hemostasis, inflammation, cell migration and proliferation, wound contraction, and remodeling[89,90]. During the inflammatory phase, vasodilation followed by early vasoconstriction which is mediated by histamine, leukotrienes, and prostaglandins, increases capillary permeability and cell migration into the wound site[91]. Neutrophils are the first among the infiltrated cells to the site of injury followed by monocytes and lymphocytes. Initiation of leukocyte migration is mediated by several autocrine and paracrine factors. In addition, proteases are involved in the elimination of denatured ECM components. Following infiltration into the site of injury, monocytes transform into macrophages and clear debris from the area, release cytokines and growth factors, such as FGF, TGF-β, PDGF, and EGF that help to initiate the formation of granulation tissue[92]. HMGB1 also acts as an important chemotactic factor that regulates the directional migration of monocytes and neutrophils[93]. Following injury or inflammation, HMGB1 is released into the extracellular space and triggers the secretion of TNF, IL-1α, IL-6, and IL-8 from monocytes, macrophages and neutrophils[94-96].
Upon interaction of HMGB1 with the advanced glycation products (RAGE), toll-like receptor (TLR) 2, TLR4, and TLR9, activate pro-inflammatory responses thereby facilitating cell migration and the release of pro-inflammatory cytokines (Figure 1)[97-99]. In 2018, Xue et al[100] showed that the HMGB1/RAGE axis mediated migration of neural stem cells (NSCs) by the formation of filopodia which was further linked to the activation of RAGE/Rac and CDC42 or the RAGE/MAPK signaling cascade.
SDF1α, known as C-X-C motif chemokine 12 (CXCL12), is a chemotactic factor encoded by the CXCL12 gene on chromosome 10[101]. Studies have reported the therapeutic potential of the SDF1-CXCR4 axis in tissue regeneration. SDF1 is capable of activation, mobilization, homing and retention of HSCs, MSCs and several progenitor cells[80,102-104]. SDF1 is able to bind to CXCR4 and CXCR7. However, the SDF1-CXCR4 axis induces the homing process by regulating the cellular secretion and cell adhesion molecules, while SDF1-CXCR7 is involved in angiogenesis and tumor development[105].
Principally, the binding of chemokine SDF1 to the chemokine receptor CXCR4 plays an important role in homeostatic regulation of leukocyte trafficking, hematopoiesis, organogenesis, cell differentiation and tissue regeneration in response to other molecules that are involved in triggering inflammation[14,106]. The mechanism of MSCs mobilization mediated by HMGB1 is analogous to the recruitment of inflammatory cells to injured tissues for leukocyte trafficking and homing (Figure 1). As mentioned above, HMGB1 acts as a damage-associated molecular pattern which is released either from necrotic cells or by secretion from activated immune cells, hepatocytes, enterocytes, and possibly several other types of cells under distress[107]. Stress conditions that promote HMGB1 secretion include hypoxia[108], lethal irradiation[109], treatment with specific antitumor drugs[110] or through regulation of autophagy[111]. HMGB1-induced cell migration requires both IκB kinase (IKK)-β and IKKα-dependent nuclear factor-κB (NF-κB) activation. IKKβ-mediated activation of NF-κB maintains expression of RAGE, while continuous production of SDF1 is ensured by IKKα-dependent NF-κB activation[112,113]. Moreover, HMGB1 induces both physical and functional interactions between molecules that prevent the degradation of SDF1[114].
In 2012, Kew et al[115] proposed that the SDF1-CXCR4 axis works as a co-receptor signal for RAGE receptor-dependent HMGB1 migration responses. Furthermore, SDF1 binding to CXCR4 can also induce CXCR4-TCR heterodimerization, which in turn can enhance gene transcription, cytokine production, increased calcium ion concentrations, and could facilitate cell migration. However, it is possible that the SDF1-CXCR4 axis might have other indirect effects on the regulation of cell migration such as enhancing HMGB1 binding to RAGE, which require further investigation.
There is ample evidence of the capability of stem cells to regulate numerous growth factors, cytokines and chemokines. Chemokines, specifically, regulate cell locomotion and integrin function by binding to seven transmembrane domain receptors coupled to G-protein-coupled receptors (GPCR)s, which are heterotrimeric GTP-binding proteins, that are differentially expressed in various cell types[48,107]. In addition, there is always a need to assess the consequences of the combined activity of these chemokines and other inflammatory molecules to control appropriate tissue distribution of distinct leukocyte subsets under normal and pathological conditions. One of the interesting insights in stem cell research is the effect of such paracrine factors in the HMGB1-SDF1-CXCR4 signaling pathway during tissue regeneration.
Extracellular HMGB1 can interact with different molecules to dictate their biologic effects. The role of HMGB1 as a chemokine or cytokine is determined by its oxidative state (Figure 1). The role of extracellular HMGB1 to promote cell migration was first reported in smooth muscle cells in 2010[116]. Similar involvement was also reported in different cell types in the same year by Rauvala and Rouhiainen[117]. Studies showed that HMGB1-induced cell migration requires the formation of a heterocomplex with SDF1 and further binding with CXCR4, and not with RAGE, TLR2, or TLR4. Furthermore, it was also reported that HMGB1 does not affect migration by other chemokines such as CXCL8, CCL2, CCL7, CCL19, and CCL21[14].
The bonding chemistry between SDF1 and HMGB1 was analyzed by NMR chemical shift mapping and revealed that most of the amino acids present in SDF1 have the ability to bind HMGB1 or its individual HMG boxes[14]. Each HMGB1 molecule has two HMG boxes and thereby can attach two SDF1 molecules at a time. Interestingly, the first few N-terminal residues of the SDF1 molecule do not attach to the HMGB1 molecule and remain free. These free residues can access deep inside the CXCR4 transmembrane domain to initiate signaling cascades[118]. As the HMGB1-SDF1 heterocomplex can present two SDF1 ligand molecules to dimers of the CXCR4 receptor, this heterocomplex would be more efficient than SDF1 alone in inducing cellular migration[119]. Alternatively, the HMGB1-SDF1 heterocomplex may help to unlock the CXCR4 binding site to promote SDF1 binding, or help lock in SDF1 into the CXCR4 transmembrane domain by providing direct HMGB1-CXCR4 contacts.
HMGB1 induces changes in SDF1 residues that are responsible for the activation of CXCR4, the SDF1 receptor. An analysis using fluorescence resonance energy transfer (FRET) demonstrated that there are different conformational rearrangements of CXCR4 homodimers triggered by SDF1 alone or in complex with HMGB1[14]. It has also been hypothesized that the formation of a heterocomplex between HMGB1 and SDF1 acts through CXCR4 which promotes the recruitment of monocytes to the injury site[14]. The interaction of locally produced SDF1 and its receptor CXCR4 expressed on the surface of MSCs plays an important role in the homing of transplanted cells. The binding of SDF1 to both CXCR4 and CXCR7 is also responsible for the production of paracrine mediators, including VEGF, IGF-1, β-FGF and HGF that exert mitogenic, pro-angiogenic, anti-apoptotic, and anti-inflammatory effects[120]. Hypoxia has been shown to enhance the expression of both SDF1 receptors, CXCR4 and CXCR7, in MSCs. Liu et al[121] demonstrated that SDF1α is upregulated in ischemic kidneys during reduced oxygen tension. Hypoxia induces expression of CXCR4 and CXCR7 while promoting the role of both SDF1 receptors for enhanced migration, adhesion and survival of hypoxia preconditioned (HP)-MSCs and thus improves homing of systemically delivered MSCs to the ischemic kidney. In addition, in normal culture-expanded MSCs, CXCR4 expression will alleviate progressively and thus could affect its ability to migrate toward the SDF1 gradient in the ischemic tissue.
The intracellular signaling cascades have not yet been clearly demonstrated. In the case of SDF1-CXCR4 bonding, activation and coordination of focal adhesion kinase (FAK) and phosphoinositide 3-kinase (PI3K) were reported in the migration of human dental pulp stem cells[122]. Subsequently, increased β-catenin expression by phosphorylation of protein kinase B (Akt) at ser473 that inhibits the activation of glycogen synthase kinase 3 beta (GSK3β) was also reported. All these results indicate that the SDF1-CXCR4 axis activates the FAK/PI3K/Akt and GSK3β/β-catenin pathways that could facilitate the migration of human dental pulp stem cells. Whereas in the HMGB1-SDF1-CXCR4 axis, elevated extracellular signal-regulated kinase (ERK) phosphorylation and Ca2+ release from stores were reported[14]. Elevated ERK phosphorylation was observed in the presence of the SDF1-HMGB1 heterocomplex but not observed in the presence of SDF1 and HMGB1 alone. In the presence of HMGB1 a suboptimal SDF1 concentration was reported with a rapid increase in intracellular Ca2+.
Until now, the migration and retention of transplanted cells have been considered a major drawback of cell-based regenerative therapy. SDF1 and its receptor CXCR4 play an important role in maintaining homeostasis by facilitating the homing of progenitor or other adult multipotent stem cells in the BM and regulating their mobilization into peripheral tissues during injury or stress. Studies have shown the potential of the SDF1-CXCR4 axis and/or HMGB1-SDF1-CXCR4 signaling pathways in regulating the process of directional migration followed by retention which are vital for the regeneration of injured tissues or organs. In addition, these pathways could play a major role in regulating the inflammatory conditions at the site of injury. Further studies concentrating on these pathways could make cell-based regenerative therapy more efficient and fruitful.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Federation of Biotechnology; and Bangladesh Association of Biotechnology Graduates.
Specialty type: Cell and tissue engineering
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huang YC, Politi L, Tang JM S-Editor: Yan JP L-Editor: Webster JR P-Editor: Xing YX
| 1. | Haque N, Abdullah BJJ, BJJ, Kasim NHA. Secretome: Pharmaceuticals for Cell-Free Regenerative Therapy. In: Pham PV. Stem Cell Drugs - A New Generation of Biopharmaceuticals. Cham: Springer International Publishing; 2018: 17-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Crivelli B, Chlapanidas T, Perteghella S, Lucarelli E, Pascucci L, Brini AT, Ferrero I, Marazzi M, Pessina A, Torre ML; Italian Mesenchymal Stem Cell Group (GISM). Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J Control Release. 2017;262:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Boido M, Ghibaudi M, Gentile P, Favaro E, Fusaro R, Tonda-Turo C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci Rep. 2019;9:6402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Haque N, Kasim NHA, Kassim NLA, Rahman MT. Autologous serum supplement favours in vitro regenerative paracrine factors synthesis. Cell Prolif. 2017;50:e12354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 439] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 6. | Hoetzenecker K, Zimmermann M, Hoetzenecker W, Schweiger T, Kollmann D, Mildner M, Hegedus B, Mitterbauer A, Hacker S, Birner P, Gabriel C, Gyöngyösi M, Blyszczuk P, Eriksson U, Ankersmit HJ. Mononuclear cell secretome protects from experimental autoimmune myocarditis. Eur Heart J. 2015;36:676-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Hoetzenecker K, Assinger A, Lichtenauer M, Mildner M, Schweiger T, Starlinger P, Jakab A, Berényi E, Pavo N, Zimmermann M, Gabriel C, Plass C, Gyöngyösi M, Volf I, Ankersmit HJ. Secretome of apoptotic peripheral blood cells (APOSEC) attenuates microvascular obstruction in a porcine closed chest reperfused acute myocardial infarction model: role of platelet aggregation and vasodilation. Basic Res Cardiol. 2012;107:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Altmann P, Mildner M, Haider T, Traxler D, Beer L, Ristl R, Golabi B, Gabriel C, Leutmezer F, Ankersmit HJ. Secretomes of apoptotic mononuclear cells ameliorate neurological damage in rats with focal ischemia. F1000Res. 2014;3:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Pires AO, Neves-Carvalho A, Sousa N, Salgado AJ. The Secretome of Bone Marrow and Wharton Jelly Derived Mesenchymal Stem Cells Induces Differentiation and Neurite Outgrowth in SH-SY5Y Cells. Stem Cells Int. 2014;2014:438352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Dao M, Tate CC, McGrogan M, Case CC. Comparing the angiogenic potency of naïve marrow stromal cells and Notch-transfected marrow stromal cells. J Transl Med. 2013;11:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Mildner M, Hacker S, Haider T, Gschwandtner M, Werba G, Barresi C, Zimmermann M, Golabi B, Tschachler E, Ankersmit HJ. Secretome of peripheral blood mononuclear cells enhances wound healing. PLoS One. 2013;8:e60103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | Abduelmula A, Huang R, Pu Q, Tamamura H, Morosan-Puopolo G, Brand-Saberi B. SDF-1 controls the muscle and blood vessel formation of the somite. Int J Dev Biol. 2016;60:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 15. | Betsou F, Gaignaux A, Ammerlaan W, Norris PJ, Stone M. Biospecimen Science of Blood for Peripheral Blood Mononuclear Cell (PBMC) Functional Applications. Curr Pathobiol Rep. 2019;7:17-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Zhang M, Huang B. The multi-differentiation potential of peripheral blood mononuclear cells. Stem Cell Res Ther. 2012;3:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Hopper N, Wardale J, Brooks R, Power J, Rushton N, Henson F. Peripheral Blood Mononuclear Cells Enhance Cartilage Repair in in vivo Osteochondral Defect Model. PLoS One. 2015;10:e0133937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Xian B, Zhang Y, Peng Y, Huang J, Li W, Wang W, Zhang M, Li K, Zhang H, Zhao M, Liu X, Huang B. Adult Human Peripheral Blood Mononuclear Cells Are Capable of Producing Neurocyte or Photoreceptor-Like Cells That Survive in Mouse Eyes After Preinduction With Neonatal Retina. Stem Cells Transl Med. 2016;5:1515-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Wang X, Balaji S, Steen EH, Li H, Rae MM, Blum AJ, Miao Q, Butte MJ, Bollyky PL, Keswani SG. T Lymphocytes Attenuate Dermal Scarring by Regulating Inflammation, Neovascularization, and Extracellular Matrix Remodeling. Adv Wound Care (New Rochelle). 2019;8:527-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2865] [Cited by in RCA: 2957] [Article Influence: 328.6] [Reference Citation Analysis (0)] |
| 21. | Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood). 2016;241:1084-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 22. | Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 758] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 23. | Chiu S, Bharat A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr Opin Organ Transplant. 2016;21:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3531] [Cited by in RCA: 3826] [Article Influence: 201.4] [Reference Citation Analysis (0)] |
| 25. | Atri C, Guerfali FZ, Laouini D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci. 2018;19:1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 929] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 26. | Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7233] [Cited by in RCA: 6860] [Article Influence: 403.5] [Reference Citation Analysis (0)] |
| 27. | Gu B, Kaneko T, Zaw SYM, Sone PP, Murano H, Sueyama Y, Zaw ZCT, Okiji T. Macrophage populations show an M1-to-M2 transition in an experimental model of coronal pulp tissue engineering with mesenchymal stem cells. Int Endod J. 2019;52:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72:1303-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 29. | Rigamonti E, Zordan P, Sciorati C, Rovere-Querini P, Brunelli S. Macrophage plasticity in skeletal muscle repair. Biomed Res Int. 2014;2014:560629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Gratchev A, Kzhyshkowska J, Köthe K, Muller-Molinet I, Kannookadan S, Utikal J, Goerdt S. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018;9:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 930] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 32. | Li J, Tan J, Martino MM, Lui KO. Regulatory T-Cells: Potential Regulator of Tissue Repair and Regeneration. Front Immunol. 2018;9:585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 33. | Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446-19451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 671] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 34. | Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 616] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 35. | Toumi H, F'guyer S, Best TM. The role of neutrophils in injury and repair following muscle stretch. J Anat. 2006;208:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol. 2005;562:899-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137:695-702; discussion 702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Lindborg JA, Mack M, Zigmond RE. Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration. J Neurosci. 2017;37:10258-10277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 39. | Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, Kahn M, Henson PM, Colgan SP, Downey GP. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA. 2011;108:15990-15995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | Saez B, Yusuf RZ, Scadden DT. Harnessing the Biology of Stem Cells' Niche. Biology and Engineering of Stem Cell Niches: Elsevier; 2017: 15-31. [DOI] [Full Text] |
| 41. | Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 42. | Haque N, Abu Kasim NH. Pooled Human Serum Increases Regenerative Potential of In Vitro Expanded Stem Cells from Human Extracted Deciduous Teeth. Adv Exp Med Biol. 2018;1083:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Haque N, Widera D, Abu Kasim NH. Stem Cells from Human Extracted Deciduous Teeth Expanded in Foetal Bovine and Human Sera Express Different Paracrine Factors After Exposure to Freshly Prepared Human Serum. Adv Exp Med Biol. 2019;1084:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 45. | Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1010] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 46. | Gu W, Hong X, Potter C, Qu A, Xu Q. Mesenchymal stem cells and vascular regeneration. Microcirculation. 2017;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 47. | Shi Z, Wang Q, Jiang D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J Transl Med. 2019;17:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 48. | Nitzsche F, Müller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells. 2017;35:1446-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 49. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 661] [Article Influence: 110.2] [Reference Citation Analysis (36)] |
| 50. | Visweswaran M, Pohl S, Arfuso F, Newsholme P, Dilley R, Pervaiz S, Dharmarajan A. Multi-lineage differentiation of mesenchymal stem cells - To Wnt, or not Wnt. Int J Biochem Cell Biol. 2015;68:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Almalki SG, Llamas Valle Y, Agrawal DK. MMP-2 and MMP-14 Silencing Inhibits VEGFR2 Cleavage and Induces the Differentiation of Porcine Adipose-Derived Mesenchymal Stem Cells to Endothelial Cells. Stem Cells Transl Med. 2017;6:1385-1398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Zhang Y, Khan D, Delling J, Tobiasch E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. ScientificWorldJournal. 2012;2012:793823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 207] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Jung JP, Bache-Wiig MK, Provenzano PP, Ogle BM. Heterogeneous Differentiation of Human Mesenchymal Stem Cells in 3D Extracellular Matrix Composites. Biores Open Access. 2016;5:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Gruh I, Martin U. Transdifferentiation of stem cells: a critical view. Adv Biochem Eng Biotechnol. 2009;114:73-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Bassi ÊJ, de Almeida DC, Moraes-Vieira PM, Câmara NO. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev Rep. 2012;8:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 57. | Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front Physiol. 2016;7:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 58. | Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125-R134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 718] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 59. | Haque N, Khan IM, Abu Kasim NH. Survival and immunomodulation of stem cells from human extracted deciduous teeth expanded in pooled human and foetal bovine sera. Cytokine. 2019;120:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Chang PY, Zhang BY, Cui S, Qu C, Shao LH, Xu TK, Qu YQ, Dong LH, Wang J. MSC-derived cytokines repair radiation-induced intra-villi microvascular injury. Oncotarget. 2017;8:87821-87836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | de Witte SFH, Merino AM, Franquesa M, Strini T, van Zoggel JAA, Korevaar SS, Luk F, Gargesha M, O'Flynn L, Roy D, Elliman SJ, Newsome PN, Baan CC, Hoogduijn MJ. Cytokine treatment optimises the immunotherapeutic effects of umbilical cord-derived MSC for treatment of inflammatory liver disease. Stem Cell Res Ther. 2017;8:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 62. | Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 63. | Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noël A, Brook G, Schoenen J, Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;8:e69515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 64. | Motaln H, Turnsek TL. Cytokines play a key role in communication between mesenchymal stem cells and brain cancer cells. Protein Pept Lett. 2015;22:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Bhang SH, Cho SW, Lim JM, Kang JM, Lee TJ, Yang HS, Song YS, Park MH, Kim HS, Yoo KJ, Jang Y, Langer R, Anderson DG, Kim BS. Locally delivered growth factor enhances the angiogenic efficacy of adipose-derived stromal cells transplanted to ischemic limbs. Stem Cells. 2009;27:1976-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Zhang LB, He M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2019;54:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Yaochite JN, de Lima KW, Caliari-Oliveira C, Palma PV, Couri CE, Simões BP, Covas DT, Voltarelli JC, Oliveira MC, Donadi EA, Malmegrim KC. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res Ther. 2016;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Palomares Cabeza V, Hoogduijn MJ, Kraaijeveld R, Franquesa M, Witte-Bouma J, Wolvius EB, Farrell E, Brama PAJ. Pediatric Mesenchymal Stem Cells Exhibit Immunomodulatory Properties Toward Allogeneic T and B Cells Under Inflammatory Conditions. Front Bioeng Biotechnol. 2019;7:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Haque N, Ramasamy TS, Kasim NHA. Mechanisms of Mesenchymal Stem Cells for Autoimmune Disease Treatment. In: Pham PV. Stem Cell Transplantation for Autoimmune Diseases and Inflammation. Cham: Springer International Publishing; 2019: 27-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Chen X, Wang S, Cao W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell Immunol. 2018;326:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 72. | Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Kao WW. Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. Ocul Surf. 2016;14:121-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 73. | Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy. 2016;18:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 74. | Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in 'innate tolerance'? Immunology. 2012;137:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722-3729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1165] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 76. | Romieu-Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 77. | Bhartiya D. The need to revisit the definition of mesenchymal and adult stem cells based on their functional attributes. Stem Cell Res Ther. 2018;9:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, Waksman R, Epstein SE. Intravenously Delivered Mesenchymal Stem Cells: Systemic Anti-Inflammatory Effects Improve Left Ventricular Dysfunction in Acute Myocardial Infarction and Ischemic Cardiomyopathy. Circ Res. 2017;120:1598-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 79. | Chen W, Zhou D, Li YR, Liu JX, Wang XM, Zhu F, Xu KL. [Effect of Lentiviral Vector-Mediated CXCR4 Gene Overexpression on Mesenchymal Stem Cell Homing Capacity]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26:1543-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 80. | Jin W, Liang X, Brooks A, Futrega K, Liu X, Doran MR, Simpson MJ, Roberts MS, Wang H. Modelling of the SDF-1/CXCR4 regulated in vivo homing of therapeutic mesenchymal stem/stromal cells in mice. PeerJ. 2018;6:e6072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 81. | Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Hocking AM. The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds. Adv Wound Care (New Rochelle). 2015;4:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 83. | Sackstein R. The biology of CD44 and HCELL in hematopoiesis: the 'step 2-bypass pathway' and other emerging perspectives. Curr Opin Hematol. 2011;18:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Martinotti S, Patrone M, Ranzato E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015;4:101-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 85. | Yang H, Wang H, Chavan SS, Andersson U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol Med. 2015;21 Suppl 1:S6-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 86. | Raucci A, Di Maggio S, Scavello F, D'Ambrosio A, Bianchi ME, Capogrossi MC. The Janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci. 2019;76:211-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 87. | Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T, Ishizaka A, Ogawa S. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res. 2009;81:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 88. | Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002;72:1084-1091. [PubMed] |
| 89. | Frangogiannis NG. Fibroblast-Extracellular Matrix Interactions in Tissue Fibrosis. Curr Pathobiol Rep. 2016;4:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 568] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 91. | Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 92. | Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 93. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2703] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 94. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1100] [Cited by in RCA: 1173] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 95. | Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 397] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 96. | Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870-C879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 348] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 97. | Palumbo R, De Marchis F, Pusterla T, Conti A, Alessio M, Bianchi ME. Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol. 2009;86:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 99. | Yang J, Chen L, Yang J, Ding J, Rong H, Dong W, Li X. High mobility group box-1 induces migration of vascular smooth muscle cells via TLR4-dependent PI3K/Akt pathway activation. Mol Biol Rep. 2012;39:3361-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 100. | Xue X, Chen X, Fan W, Wang G, Zhang L, Chen Z, Liu P, Liu M, Zhao J. High-mobility group box 1 facilitates migration of neural stem cells via receptor for advanced glycation end products signaling pathway. Sci Rep. 2018;8:4513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 463] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 102. | Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 896] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 103. | Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, Laughlin MJ, Ratajczak J. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Cheng M, Qin G. Progenitor cell mobilization and recruitment: SDF-1, CXCR4, α4-integrin, and c-kit. Prog Mol Biol Transl Sci. 2012;111:243-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 105. | Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 941] [Cited by in RCA: 1027] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 106. | Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 724] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 107. | Gauley J, Pisetsky DS. The translocation of HMGB1 during cell activation and cell death. Autoimmunity. 2009;42:299-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Tang Z, Jiang M, Ou-Yang Z, Wu H, Dong S, Hei M. High mobility group box 1 protein (HMGB1) as biomarker in hypoxia-induced persistent pulmonary hypertension of the newborn: a clinical and in vivo pilot study. Int J Med Sci. 2019;16:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Zhao Y, Yi J, Tao L, Huang G, Chu X, Song H, Chen L. Wnt signaling induces radioresistance through upregulating HMGB1 in esophageal squamous cell carcinoma. Cell Death Dis. 2018;9:433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 110. | Yadav SS, Kumar M, Varshney A, Yadava PK. KLF4 sensitizes the colon cancer cell HCT-15 to cisplatin by altering the expression of HMGB1 and hTERT. Life Sci. 2019;220:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 111. | Foglio E, Pellegrini L, Germani A, Russo MA, Limana F. HMGB1-mediated apoptosis and autophagy in ischemic heart diseases. Vasc Biol. 2019;1:H89-H96. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 112. | Penzo M, Habiel DM, Ramadass M, Kew RR, Marcu KB. Cell migration to CXCL12 requires simultaneous IKKα and IKKβ-dependent NF-κB signaling. Biochim Biophys Acta. 2014;1843:1796-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Penzo M, Molteni R, Suda T, Samaniego S, Raucci A, Habiel DM, Miller F, Jiang HP, Li J, Pardi R, Palumbo R, Olivotto E, Kew RR, Bianchi ME, Marcu KB. Inhibitor of NF-kappa B kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis [corrected]. J Immunol. 2010;184:4497-4509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 114. | Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, Bianchi ME, Cossu G, Manfredi AA, Brunelli S, Rovere-Querini P. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 115. | Kew RR, Penzo M, Habiel DM, Marcu KB. The IKKα-dependent NF-κB p52/RelB noncanonical pathway is essential to sustain a CXCL12 autocrine loop in cells migrating in response to HMGB1. J Immunol. 2012;188:2380-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 116. | Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 379] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 117. | Rauvala H, Rouhiainen A. Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta. 2010;1799:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 118. | Kofuku Y, Yoshiura C, Ueda T, Terasawa H, Hirai T, Tominaga S, Hirose M, Maeda Y, Takahashi H, Terashima Y, Matsushima K, Shimada I. Structural basis of the interaction between chemokine stromal cell-derived factor-1/CXCL12 and its G-protein-coupled receptor CXCR4. J Biol Chem. 2009;284:35240-35250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 119. | Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 120. | Lee G, Espirito Santo AI, Zwingenberger S, Cai L, Vogl T, Feldmann M, Horwood NJ, Chan JK, Nanchahal J. Fully reduced HMGB1 accelerates the regeneration of multiple tissues by transitioning stem cells to GAlert. Proc Natl Acad Sci USA. 2018;115:E4463-E4472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 121. | Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7:e34608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 122. | Li M, Sun X, Ma L, Jin L, Zhang W, Xiao M, Yu Q. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Sci Rep. 2017;7:40161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 123. | Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 124. | Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from Cell Death to New Life. Front Immunol. 2015;6:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 125. | Zhao W, Jin K, Li J, Qiu X, Li S. Delivery of stromal cell-derived factor 1α for in situ tissue regeneration. J Biol Eng. 2017;11:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |