Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.841
Peer-review started: February 25, 2020
First decision: May 26, 2020
Revised: July 17, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: August 26, 2020
Processing time: 182 Days and 16.1 Hours
Cigarette smoking (CS) is the most common method of consuming tobacco. Deleterious effects on bone integrity, increased incidence of fractures, and delayed fracture healing are all associated with CS. Over 150 of the 6500 molecular species contained in cigarette smoke and identified as toxic compounds are inhaled by CS and, via the bloodstream, reach the skeletal system. New technologies designed to develop a reduced-risk alternative for smokers are based on electronic nicotine delivery systems, such as e-cigarettes and tobacco heating systems (THS). THS are designed to heat tobacco instead of burning it, thereby reducing the levels of harmful toxic compounds released.
To examine the effects of THS on osteoprogenitor cell viability and function compared to conventional CS.
Human immortalized mesenchymal stem cells (n = 3) and primary human pre-osteoblasts isolated from cancellous bone samples from BG Unfall Klinik Tübingen (n = 5) were osteogenically differentiated in vitro with aqueous extracts generated from either the THS 2.4 “IQOS” or conventional “Marlboro” cigarettes for up to 21 d. Cell viability was analyzed using resazurin conversion assay (mitochondrial activity) and calcein-AM staining (esterase activity). Osteogenic differentiation and bone cell function were evaluated using alkaline phosphatase (AP) activity, while matrix formation was analyzed through alizarin red staining. Primary cilia structure was examined by acetylated α-tubulin immunofluorescent staining. Free radical production was evaluated with 2’,7’-dichlorofluorescein-diacetate assay.
Our data clearly show that THS is significantly less toxic to bone cells than CS when analyzed by mitochondrial and esterase activity (P < 0.001). No significant differences in cytotoxicity between the diverse flavors of THS were observed. Harmful effects from THS on bone cell function were observed only at very high, non-physiological concentrations. In contrast, extracts from conventional cigarettes significantly reduced the AP activity (by two-fold) and matrix mineralization (four-fold) at low concentrations. Additionally, morphologic analysis of primary cilia revealed no significant changes in the length of the organelle involved in osteogenesis of osteoprogenitor cells, nor in the number of ciliated cells following THS treatment. Assessment of free radical production demonstrated that THS induced significantly less oxidative stress than conventional CS in osteoprogenitor cells.
THS was significantly less harmful to osteoprogenitor cells during osteogenesis than conventional CS. Additional studies are required to confirm whether THS is a better alternative for smokers to improve delays in bone healing following fracture.
Core tip: Aqueous extracts (AE) generated with tobacco heating systems (THS) showed no differences in suspended particles compared to the cell culture medium. This finding supports previous studies demonstrating reduced levels of harmful constituents reported in THS AE in comparison to conventional cigarettes AE. The time to consume one unit (stick/cigarette) was longer for THS than for a conventional cigarette. The pH from both AE fractions was similar to the cell culture medium. Following a single exposure, THS AE was significantly less toxic to bone-forming cells and osteoprogenitor cells than conventional cigarettes AE. The cytotoxicity observed following THS exposure was associated with very high, non-physiological concentrations. No significant differences in cytotoxicity were observed between different flavors of THS AE. Moreover, THS AE displayed less impact on osteogenic differentiation of osteoprogenitor cells and the function of bone-forming cells when compared to conventional cigarettes AE. Finally, compared to conventional cigarettes AE, THS AE induced lower levels of oxidative stress due to the reduced level of harmful constituents, resulting in less damage to primary cilia structure and reduced impact on osteogenic differentiation.
- Citation: Aspera-Werz RH, Ehnert S, Müller M, Zhu S, Chen T, Weng W, Jacoby J, Nussler AK. Assessment of tobacco heating system 2.4 on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts compared to conventional cigarettes. World J Stem Cells 2020; 12(8): 841-856
- URL: https://www.wjgnet.com/1948-0210/full/v12/i8/841.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i8.841
Cigarette smoking (CS) is the most popular way to consume tobacco, and is one of the leading causes of preventable death worldwide[1]. Of the current estimated one billion smokers, 6 million die per year due to harmful substances that arise when tobacco is burned and become distributed throughout the body via the bloodstream, thereby affecting several organs[2,3].
Detrimental effects of CS also manifest in the musculoskeletal system[4,5]. Recent evidence demonstrated that CS could lead to an imbalance in bone turnover mechanisms, leading to osteoporosis, osteoarthritis, and fracture[6-8]. Moreover, CS increases the risk of delayed fracture healing[9], non-union[10], complication rate[11], and leads to more extended hospital stays[12-14].
Tobacco combusted at about 800 °C generates approximately 6500 molecular species, more than 150 of which have been identified as toxic compounds[3,15-17]. However, it remains unknown which of these compounds are involved in the impaired bone homeostasis observed in smokers. Our previous results, as well as other publications, have demonstrated that the most pharmacologically active component, nicotine, and its first metabolite, cotinine are not the main factors responsible for the adverse effects observed in bone-forming cells[18-20].
Interestingly, it has been demonstrated that oxidative stress induced by compounds produced during conventional cigarette combustion may be one of the factors responsible for the impaired osteogenic differentiation of bone-forming cells and osteogenic precursors cells[18,21-25].
Quitting conventional CS is the most efficient way to significantly reduce the harmful effects of cigarette smoke on human health[11]; unfortunately, quitting smoking is not always a viable alternative for many smokers (i.e., those which cannot, wish not, or fail to quit)[26]. Many attempts have been made to replace cigarettes with smoke-free nicotine replacement therapies (e.g., nicotine patches, sprays, or chewing gums). However, these replacement products tend to fail in smokers because, although they deliver nicotine, the smoking ritual is absent. Therefore, new approaches have been on developing reduced-risk alternatives for smokers that maintain the smoking ritual, while providing the same levels of nicotine as conventional cigarettes with less harmful constituents.
For this purpose, electronic nicotine delivery systems (ENDS), including e-cigarettes or tobacco heating systems (THS), focus on heating rather than combustion to reduce the generation of harmful constituents. E-cigarettes heat liquids based on propylene glycol, glycerin, flavor, and selectively nicotine into an aerosol that is inhaled. Instead of burning tobacco, THS heat tobacco rolled up in a stick form up to 350 °C (avoiding combustion and formation of ashes). In contrast to E-cigarettes, THS contain tobacco and convey the feeling of smoking a conventional cigarette.
Several studies have demonstrated reduced levels of toxic and harmful compounds from ENDS[27-29]. However, effects on cell toxicity and function have shown controversial results[30-34]. To our knowledge, the effects of THS compared to conventional cigarettes on skeletal tissue and bone-forming cells has not previously been explored.
Therefore, the present study aimed to evaluate the effect of THS on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts, as well as to directly compare THS and conventional cigarette combustion on bone cells.
Cell Culture Medium and supplements were purchased from Life Technologies (Darmstadt, Germany). Chemicals were obtained from Sigma (Munich, Germany). Tobacco heating system 2.4 "IQOS®" and sticks (three commercially available flavors; bronce, amber and yellow) were provided by Philip Morris (Germany).
Human osteoblasts (hOBs) were isolated from cancellous bone samples from BG Unfallklinik Tübingen. A consent form was obtained from all participants included in the study. hOBs isolation as well as all following experiments were performed in accordance with the 1964 Declaration of Helsinki and accordance with the ethical vote (538/2016BO2) approved by the ethics committee of the medical faculty of the Eberhard-Karls-Universität and University clinic Tübingen. The donors’ average age was 73.2 ± 4.3 years (1 male and 4 female). Bone fragments were collected from cancellous bone by mechanical disruption and washed with PBS to remove residual blood. Cancellous bone fragments were digested with 0.07%w/v Collagenase II (Serva, Heidelberg, Germany) in PBS at 37 °C for one hour. Following washing with PBS, released hOB were cultured in MEM/Ham’s F12, 5%v/v FCS, 100 U/mL penicillin and 100 mg/mL streptomycin, 50 µmol/L L-ascorbate-2-phosphate, 50 µmol/L β-glycerol-phosphate in a water-saturated atmosphere of 5% CO2 at 37 °C. Medium change was performed every 5 d. To induce osteogenic differentiation, cells in passage 2 were seeded at a density 1.3 × 105 cells/cm2 and treated with MEM/Ham’s F12, 1%v/v FCS, 2 mmol/L L-glutamine, 200 µmol/L L-ascorbate-2-phosphate, 10 mmol/L β-glycerol-phosphate, 25 mmol/L HEPES, 1.5 mmol/L CaCl2, 100 nmol/L dexamethasone. For experiments, several concentrations of AE of conventional cigarettes or THS were added to the differentiation medium. Twice a week, the medium was changed during osteogenic differentiation, which was sustained for 21 d[35].
Human immortalized bone marrow mesenchymal stem cells (SCP-1 cells, provided by Dr. Matthias Schieker[36]) were cultured in Minimum Essential Medium Eagle alpha (MEM α) supplemented with 5%v/v fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin, in a water-saturated atmosphere of 5% CO2 at 37 °C. Medium change was performed every 5 d. Osteogenic differentiation of SCP-1 cells seeded at a density 1 × 105 cells/cm2 was induced with MEM α medium containing 1%v/v FCS, 100 U/mL penicillin, 100 mg/mL streptomycin, 200 μmol/L L-ascorbate-2-phosphate, 10 mmol/L β-glycerol-phosphate, 25 mmol/L HEPES, 1.5 mmol/L CaCl2, and 100 nmol/L dexamethasone. For experiments, several concentrations of AE of conventional cigarettes or THS were added to the differentiation medium. The medium was changed twice a week during osteogenic differentiation, which was sustained for 21 d[18].
AE were generated according to the standard of Health Canada smoking regime which better represent the human smoking behavior[37]. For conventional cigarettes (Marlboro, Philip Morris, New Your City, NY, United States) AE preparation, a blocked filter cigarette was placed in a standard gas washing bottle, subjected to a negative pressure by using a peristaltic pump. One cigarette was bubbled through 25 mL of cell culture medium at a rate of 2 puff/min, each puff lasting for 2 s and puff volume 58.89 ± 4.54 mL. For THS AE preparation, a stick connected with the device THS 2.4 "IQOS®" was placed in a standard gas washing bottle and proceeded as described before. The freshly prepared AE was sterile filtered (0.22 μm filter) before use and freshly prepared for every exposure. The concentration of AE was determined and standardized by its optical density at 320 nm (OD320). An OD320 of 0.61 ± 0.08 or 0.25 ± 0.01 was considered 4 × 10-1 puff/mL AE of conventional cigarettes or THS, respectively.
SCP-1 cells (n = 3) and hOBs (n = 5) were seeded in culture medium at a concentration of 1 × 105 or 1.3 × 105 cells/cm2, respectively. After attachment, cells were washed with PBS and stimulated with AE from conventional cigarettes or THS in concentrations between 4 × 10-1–4 × 10-5 puffs/mL in differentiation medium. Untreated cells were considered as control. The medium was changed twice a week with fresh AE during osteogenic differentiation, which was sustained for 21 d.
Cell viability was indirectly measured by resazurin conversion assay (mitochondrial activity). Briefly, cells were incubated with 0.0025%w/v resazurin in PBS for 30 min at 37 °C. The resulting Resorufin fluorescence was measured (excitation = 544 nm/emission = 590 nm) with a plate reader and corrected to the background. Changes in resazurin conversion are displayed relative to untreated cells[18,25]. The EC50 was calculated using the EC50 calculator tool of the AAT Bioquest webpage (http://www.aatbio.com/tools/ec50-calculator).
Cell viability was determined by intracellular esterase activity with Calcein-AM staining (permeable non-fluorescent dye which is converted to a green fluorescent dye by esterases). Cells stimulated with AE according to the experimental setup were washed with PBS and were incubated with calcein-AM (2 µmol/L), and Hoechst 33342 (1:1000 in PBS) at 37 °C for 30 min. Cell images were taken (Epifluorescence: EVOS FL, life technologies, Darmstadt, Germany) after washing with PBS[25,38].
Osteoblast function was evaluated by AP activity (early osteogenic marker). Cells were incubated with AP reaction buffer (0.2%w/v 4-nitrophenyl-phosphate, 50 mmol/L glycine, 1 mmol/L MgCl2, 100 mmol/L TRIS, pH = 10.5) for 40 min at 37 °C. Formed 4-nitrophenol was determined photometrically (λ = 405 nm) with a plate reader, corrected to the background and normalized to relative cell numbers. Changes in AP activity are displayed relative to untreated cells[18,25].
The cell number was determined by sulforhodamine B (SRB) staining (total protein content). Cells were fixed with ice-cold ethanol for one hour at -20 °C. After washing with tap water, cells were stained with 0.4%w/v SRB (in 1% acetic acid) for 30 min at ambient temperature. Unbound SRB was removed by washing with 1% acetic acid. Bound SRB was resolved with 10 mmol/L unbuffered TRIS solution (pH = 10.5). Resulting SRB staining was quantified photometrically (λ = 565 nm) with a plate reader and corrected to the background[18].
Calcium deposition was measured by Alizarin red staining (late osteogenic marker). Cells were fixed with ice-cold ethanol for one hour at -20 °C. After washing with tap water, cells were stained with 0.5%w/v Alizarin Red solution (pH = 4.0) for 30 min at ambient temperature. Unbound Alizarin red was removed by washing with tap water. The resulting staining (red) was assessed microscopically. Bound Alizarin red was resolved with 10%w/v cetylpyridinium chloride. Resolved Alizarin Red was quantified photometrically (λ = 562 nm) with a plate reader and corrected to the background. Changes in matrix mineralization are displayed relative to untreated cells[18,25].
To measure the formation of reactive oxygen species (ROS), 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) fluorescent probe was used. Briefly, cells were washed with PBS and incubated with 10 μmol/L DCFH-DA in serum-free culture medium for 25 min at 37 °C. After washing twice with PBS, cells were stimulated with AE according to the experimental setup. As a positive control, 0.01%v/v (882 μmol/L) H2O2 was used. The fluorescence intensity, representing levels of O2−, H2O2, HO, and ONOO−, was quantified after 0, 5, 10, and 15 min of incubation using a plate reader (excitation = 485 nm/emission = 520 nm). The slope of the linear part of the curve, resembling the product formation rate, was calculated. Cellular localization of the fluorescence was confirmed by fluorescence microscopy[18,25].
Primary cilia length was determined by acetylated α-tubulin immunofluorescence staining. After washing with PBS, cells were fixed with 4%w/v paraformaldehyde for 10 min at room temperate. Briefly, cells were washed and permeabilised with 0.2%v/v Triton-X-100 solution for 20 min at room temperature, followed by treatment with 2%v/v paraformaldehyde for 10 min at room temperature. Unspecific binding sites were blocked (5%w/v BSA in PBS) for one hour at room temperature, followed by incubation with anti-acetylated α-tubulin antibody SC-23,950 (1:100 in PBS, Santa Cruz, Heidelberg, Germany) overnight at 4 °C. After washing, cells were incubated with ALEXA-488 labelled secondary antibody (1:2000 in DPBS, Life Technologies, Darmstadt, Germany) for 2 h at room temperature. Nuclei were stained with Hoechst 33342 (1:1000). Images were taken with an epifluorescence microscope (EVOS FL, life technologies, Darmstadt, Germany), and primary cilia length was analyzed using the ImageJ software (Version 1.5, NIH, Bethesda, MD, United States) (line tool) based on the maximum intensity projection method[39,40].
Results are presented as mean ± SE. Each experiment was performed three independent times for SCP-1 cells or with five donors for hOBs (biological replicates, n = 3; n = 5 respectively) measured as triplicate or more (technical replicates, n ≥ 3). Statistical analyses were performed using the GraphPad Prism Software (Version 5, El Camino Real, United States). Data sets were compared by the nonparametric Mann Whitney test U-test (two single groups) or the Kruskal-Wallis H test (multiple groups) followed by Dunn’s multiple comparison test. α = 0.05 was set as the maximum type I error rate. aP < 0.05, bP < 0.01, cP < 0.001 are used to classify P values in comparisons with untreated cells within AE concentration; eP < 0.05, fP < 0.01, gP < 0.001 are used to classify P values for comparisons between AE from conventional cigarette and AE from THS system within AE concentration. The statistical methods of this study were reviewed by Johann Jacoby from Institute for Clinical Epidemiology and Applied Biometry at the University of Tübingen.
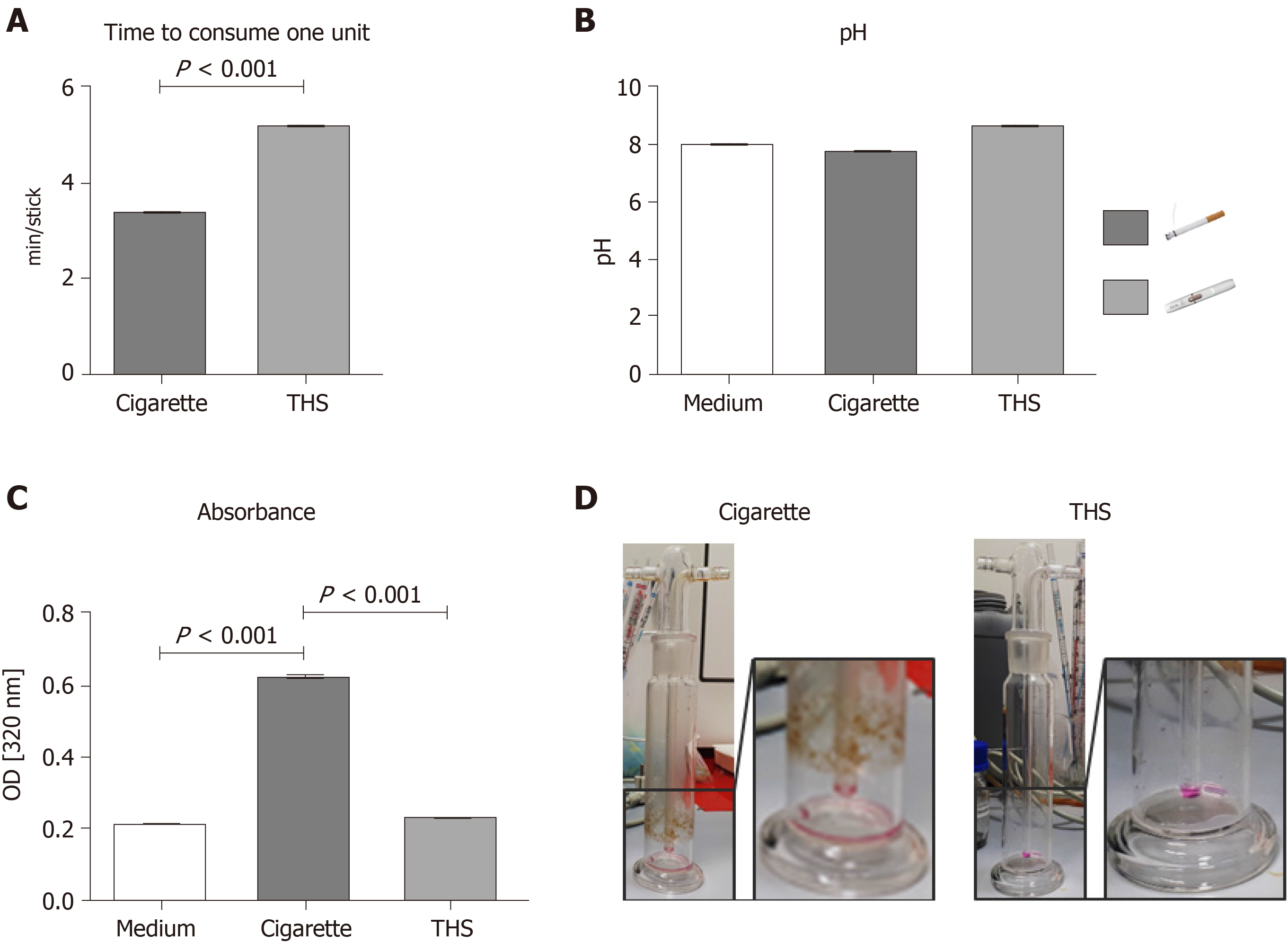
In order to compare the AE generated with THS and conventional cigarettes, the time to consume one unit, the pH, and the absorbance at 320 nm were measured after AE generation. The time to consume one unit was 1.6 minutes longer for THS than for a conventional cigarette, a difference that was significant (Figure 1A). The pH of the AE varied: Kruskal-Wallis χ2df=2 = 8.9027, P = 0.0116. In particular, the pH of the AE generated from the THS (Median = 8.17) was higher than that generated from conventional cigarettes (Median = 7.70, Figure 1B). Interestingly, the absorbance at 320 nm of the AE produced from conventional cigarettes was two-fold higher than the AE produced from THS (Figure 1C), suggesting that the AE generated with regular cigarettes possessed significantly increased turbidity (presence of suspended particles) compared to AE generated using THS. AE generated with THS showed no significant differences in suspended particulates compared to the cell culture medium. This result was corroborated by increases in the amount of particles found in the gas washing bottle after conventional cigarette AE generation compared to THS AE generation (Figure 1D).
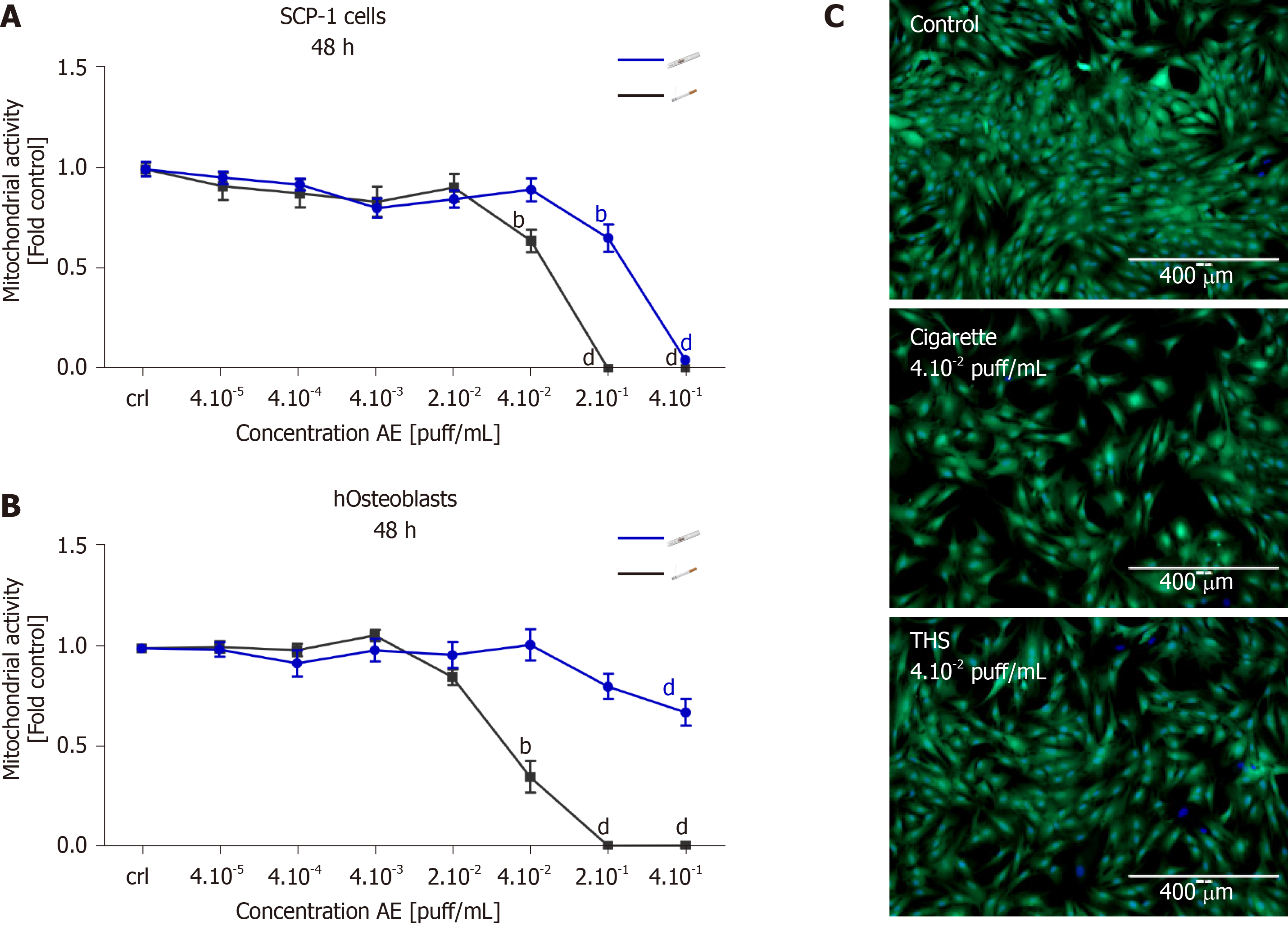
In order to evaluate the impact of THS on MSCs and the viability of bone-forming cells, SCP-1 cells and hOBs were exposed to fresh AE generated using either conventional cigarettes or THS. After 48 h, cell viability was evaluated by resazurin conversion and esterase activity. The use of THS produced a significant reduction in SCP-1 cells' metabolic activity with concentrations of 2 × 10-1 and 4 × 10-1 puff/mL when compared to control cells (Figure 2A). However, for conventional cigarettes AE, similar adverse effects on SCP-1 cell viability were observed with concentrations of 2 × 10-1, 4 × 10-1 and 4 × 10-2 puff/mL (Figure 2A). We detected a significant decrease of SCP-1 cell viability by approximately 30% for THS and conventional cigarettes compared to control, however concentrations from THS were five times higher than the conventional cigarettes (Figure 2A). Esterase activity (measured with calcein-AM) confirmed the previous results, as 4 × 10-2 puff/mL AE from conventional cigarettes produced detrimental effects on SCP-1 cell viability compared to AE generated from THS (Figure 2C). Cytotoxicity from THS was decreased in hOBs when compared to MSCs. Interestingly, AE from conventional cigarettes produced a stronger effect on the viability of hOBs compared to MSCs (Figure 2A, B). A significant reduction to 35% viable cells was observed with AE from conventional cigarettes compared to untreated hOBs (Figure 2B). THS only showed a significant decrease to 67% viable cells for the highest concentration evaluated (4 × 10-1 puff/mL) (Figure 2B). From these results, we concluded that THS AE was significantly less toxic to bone-forming cells and osteoprogenitor cells than AE from conventional cigarettes following a single exposure.
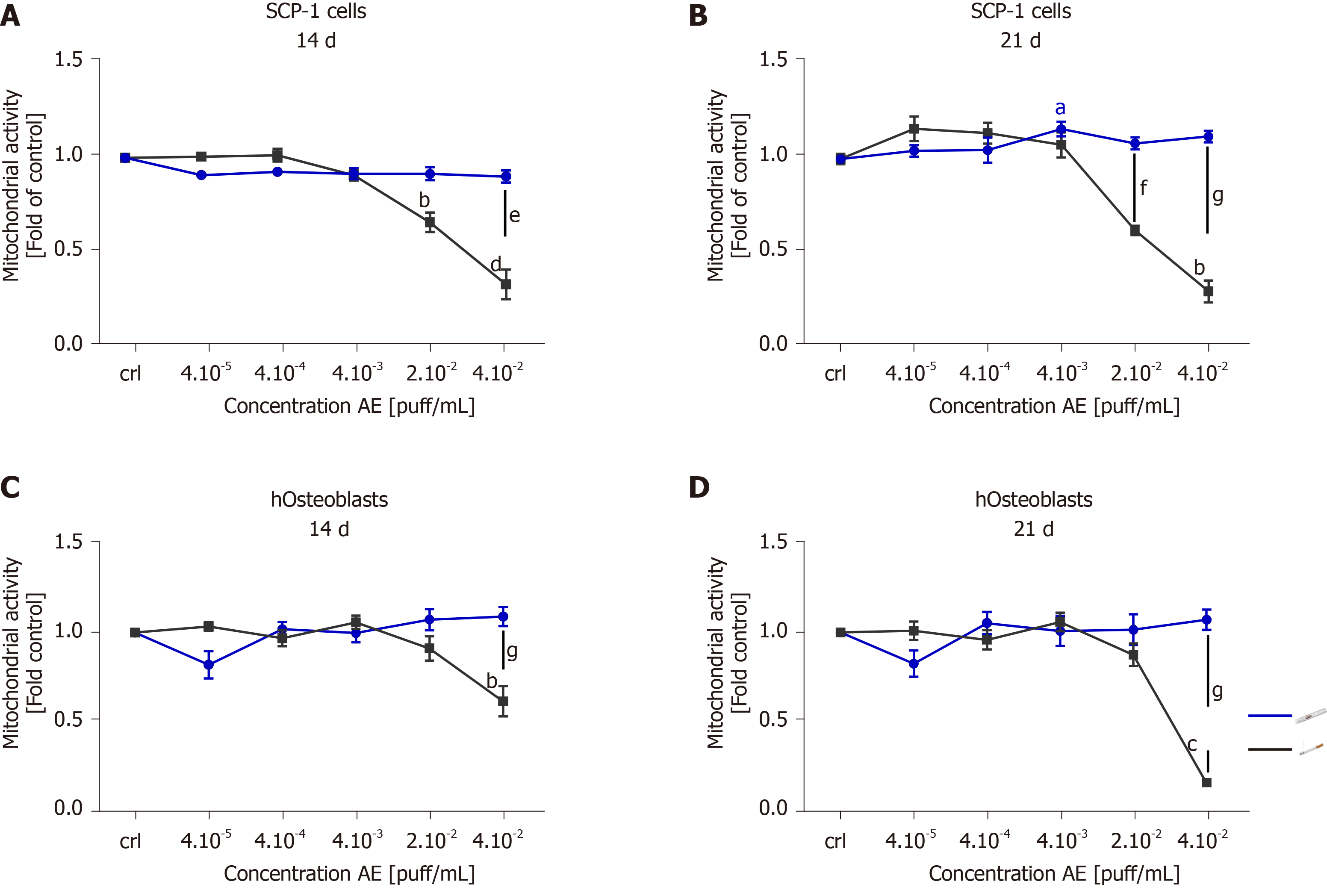
Chronic smokers consume cigarettes on a regular basis over long periods of time. Therefore, smokers are in continuous and repeated contact with the toxic compounds present in cigarette smoke. The toxic components of cigarette smoke are distributed throughout the bloodstream to the entire body, producing adverse effects on several different tissues and cells, including the bone-forming cells. To better understand the cytotoxicity of tobacco alternative products on MSCs and hOBs, we evaluated the effects of chronic exposure. SCP-1 cells and hOBs were osteogenically differentiated with AE from conventional cigarettes and THS. The concentrations of AE ranged from 4 × 10-5 puff/mL to 4 × 10-2 puff/mL. These concentrations were chosen based on previous results obtained from the single exposure experiment (Figure 2). The concentrations 2 × 10-1 puff/mL and 4 × 10-1 puff/mL were not used for further experiments due to the cytotoxic effects observed from the single exposure (Figure 2). Following either 14 or 21 d of osteogenic differentiation, the metabolic status of the cells was evaluated using resazurin conversion assay (Figure 3). Chronic exposure to THS AE resulted in no significant changes in SCP-1 cell viability after either 14 or 21 days of osteogenic differentiation when compared to untreated cells (Figure 3A, B). As expected, conventional cigarette AE significantly reduced cell viability by 50% using a concentration of 2 × 10-2 puff/mL after 14 and 21 d of exposure compared to control cells (Figure 3A, B). Conventional cigarette AE significantly increased cytotoxicity using concentrations of 4 × 10-2 puff/mL and 2 × 10-2 puff/mL on SCP-1 cells after 14 and 21 d of chronic exposure, respectively (Figure 3A, B). Similarly, THS AE did not show cytotoxic effects in hOBs exposed to concentrations up to 4 × 10-2 puff/mL after either 14 or 21 d of chronic exposure (Figure 3C, D). However, conventional cigarette AE significantly decreased hOBs viability following exposure to a concentration of 4 × 10-2 puff/mL (Figure 3C, D). Chronic exposure to 4 × 10-2 puff/mL conventional cigarette AE resulted in a significant decrease in hOBs mitochondrial activity in comparison to THS exposed to the same concentration (Figure 3C, D). These results were confirmed by the EC50 calculated for THS and conventional cigarette AE. In the case of THS AE, an EC50 of 0.19 puff/mL and > 0.04 puff/mL were calculated for SCP-1 cells and hOBs, respectively. The EC50 of conventional cigarettes was lower in SCP-1 cells (0.02 puff/mL) and hOBs (0.03 puff/mL). Thus, THS showed less cytotoxic effects than conventional cigarettes on bone cells chronically exposed to AE. Aditionally, MSCs were more sensitive to AE adverse effects that hOBs.
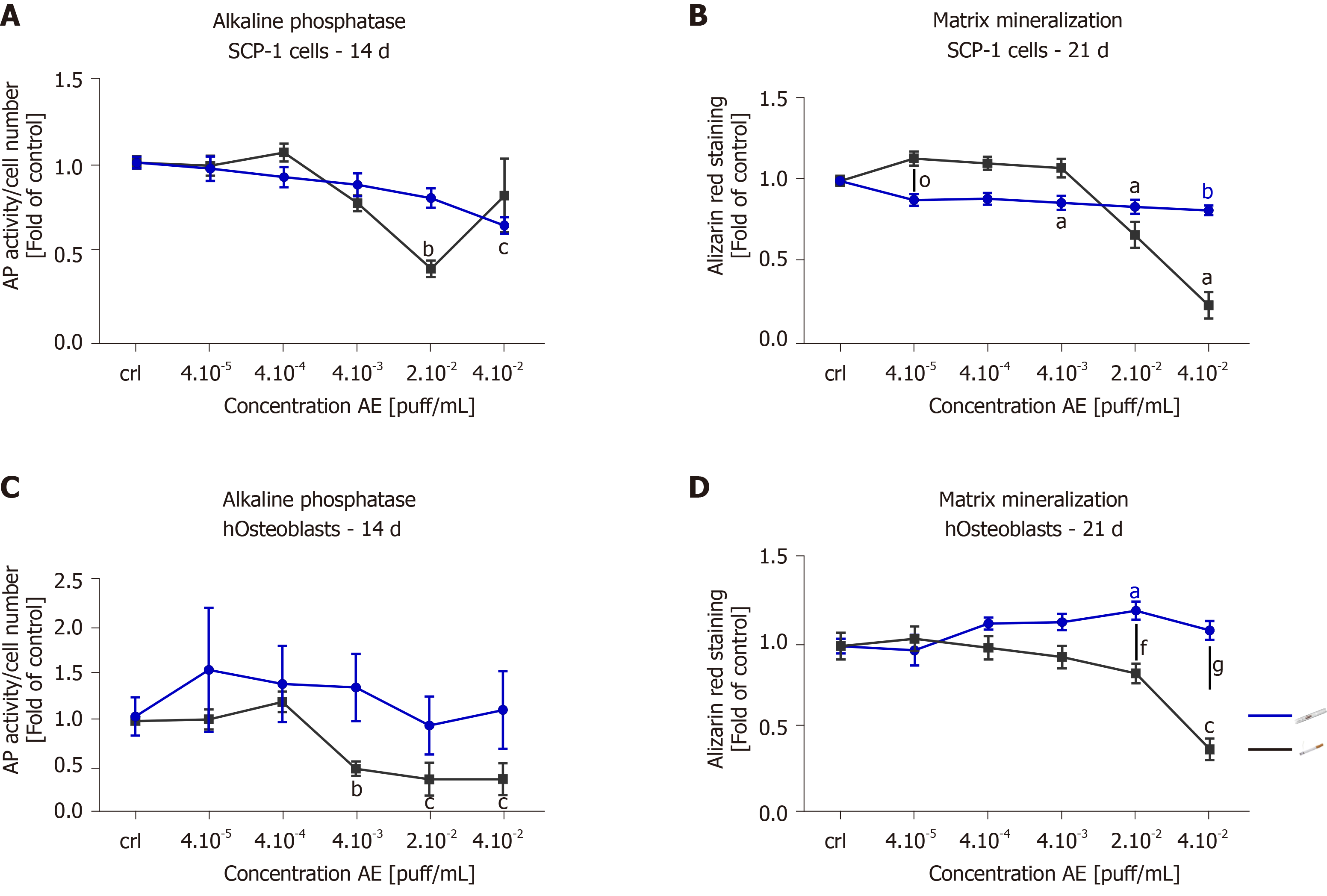
Since AE from THS produced less cytotoxic effects than AE from conventional cigarettes, we were interested in whether THS would affect osteogenic differentiation of MSCs and hOBs function. The potential of SCP-1 cells to differentiate into osteoblasts and hOBs function were evaluated using AP activity (an early marker of osteogenic differentiation[41]) and calcium deposition (a late marker of osteogenic differentiation[41]) after 14 and 21 d, respectively. As expected, 2 × 10-2 puff/mL conventional cigarette AE significantly decreased AP activity three-fold in SCP-1 cells when compared to untreated cells following 14 d (Figure 4A). In contrast, increased AP activity was observed in SCP-1 cells treated with 4 × 10-2 puff/mL AE from conventional cigarettes (Figure 4A). This increase in AP activity could be due to the detrimental effects on SCP-1 cell viability observed after treatment with 4 × 10-2 puff/mL AE from conventional cigarettes (Figure 2A). Nevertheless, THS AE produced significant reductions in the AP activity of SCP-1 cells by 1.6 times that of untreated cells, however the concentration of THS AE was twice as high as that of conventional cigarette AE (Figure 4A). Regarding matrix production, THS decreased calcium deposition in a dose-independent manner up to 20% (Figure 4B). However, conventional cigarette AE significantly decreased calcium deposition at concentrations of 2 × 10-2 and 4 × 10-2 puff/mL (Figure 4B). Due to the role of AP and matrix formation as early and late markers of osteogenic differentiation respectively, these results indicate that THS had less impact on osteogenic differentiation in SCP-1 cells compared to conventional cigarettes. Repeated exposure of hOBs to THS AE did not affect AP activity (Figure 4C). Conventional cigarettes AE significantly decreased AP activity in hOBs in a dose-dependent manner using concentrations higher than 4 × 10-3 puff/mL (Figure 4C). THS did not significantly affect calcium deposition in hOBs using any of the concentrations evaluated (Figure 4D). Only conventional cigarette AE significantly decreased calcium deposition in hOBs using concentrations of 2 × 10-2 and 4 × 10-2 puff/mL in comparison to THS (Figure 4D), suggesting that THS has less impact on hOBs function compared to conventional cigarettes.
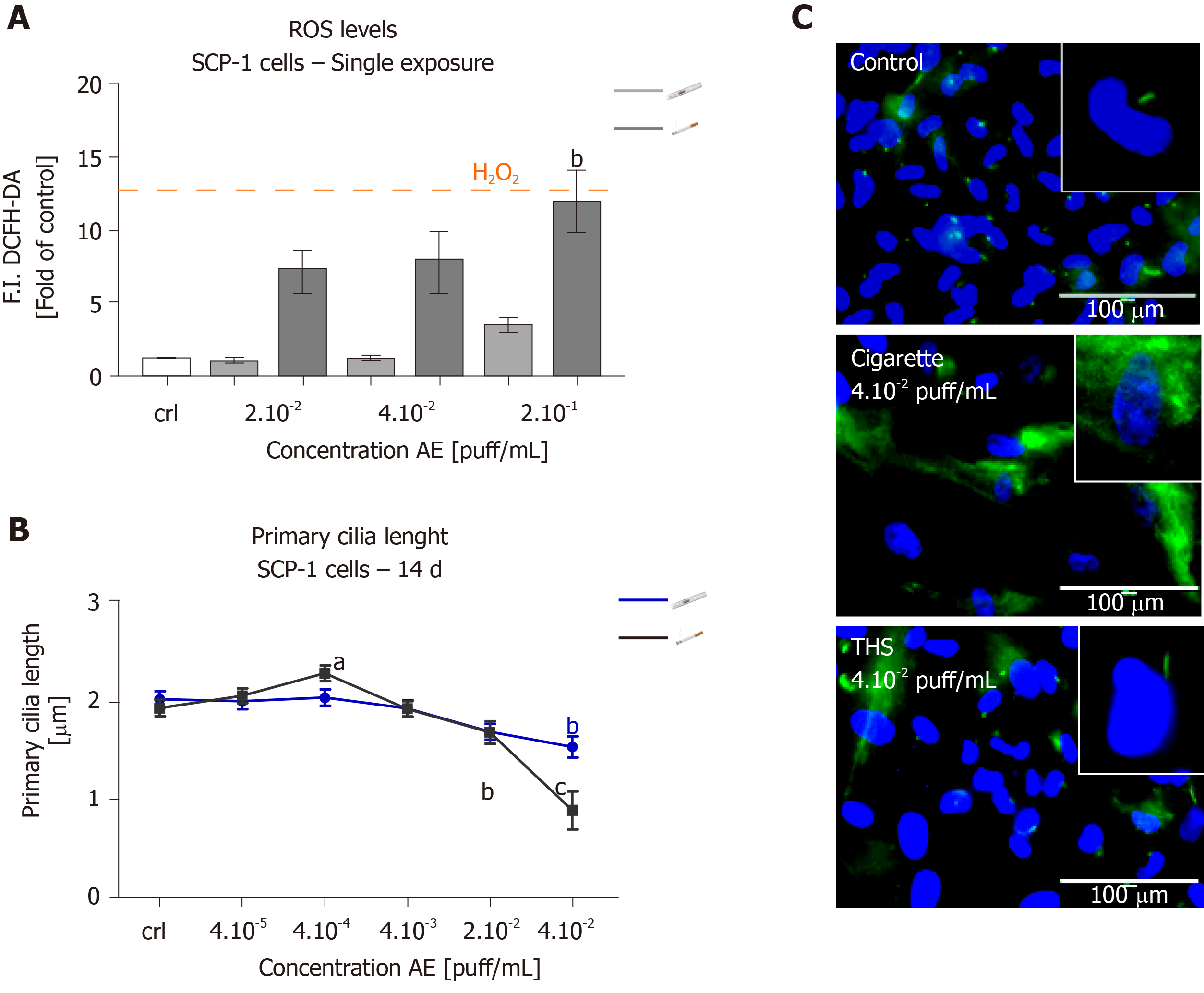
Since it has previously been demonstrated that oxidative stress induced by the compounds contained in CS causes primary cilia structure disruption, which in turn impairs osteogenic differentiation of SCP-1 cells[18,25]; we were interested in evaluating the levels of ROS produced by SCP-1 cells as well as primary cilia structure integrity following THS exposure. As expected, conventional cigarettes AE significantly increased ROS levels by ten-fold in SCP-1 cells using a concentration of 2 × 10-1 puff/mL (Figure 5A). This increase in oxidative stress seemed to be dose-dependent. Surprisingly, THS showed no significant increase in ROS levels in SCP-1 cells (Figure 5A). Only a slight (not significant) increase in ROS levels was observed in SCP-1 cells treated with the highest concentration of THS evaluated (Figure 5A). Additionally, THS did not affect the length of the microtubule-based organelles, primary cilia (involved in the initiation and maintenance of MSCs osteogenic differentiation[42,43]) in SCP-1 cells after 14 d of chronic exposure for any of the concentrations evaluated (Figure 5B, C). However, primary cilia structure of SCP-1 cells exposed to conventional cigarettes showed significant reductions in length by 50% using a concentration of 4 × 10-1 puff/mL (Figure 5B, C). Representative immunofluorescence staining pictures of acetylated α-tubulin in SCP-1 cells differentiated with either conventional cigarette AE or THS AE at a concentration of 4 × 10-1 puff/mL, or untreated, are shown in Figure 5C. In summary, THS induced less oxidative stress due to reduced levels of harmful constituents, and did not impair primary cilia structure, thereby producing less impact on MSCs osteogenic differentiation than conventional cigarettes.
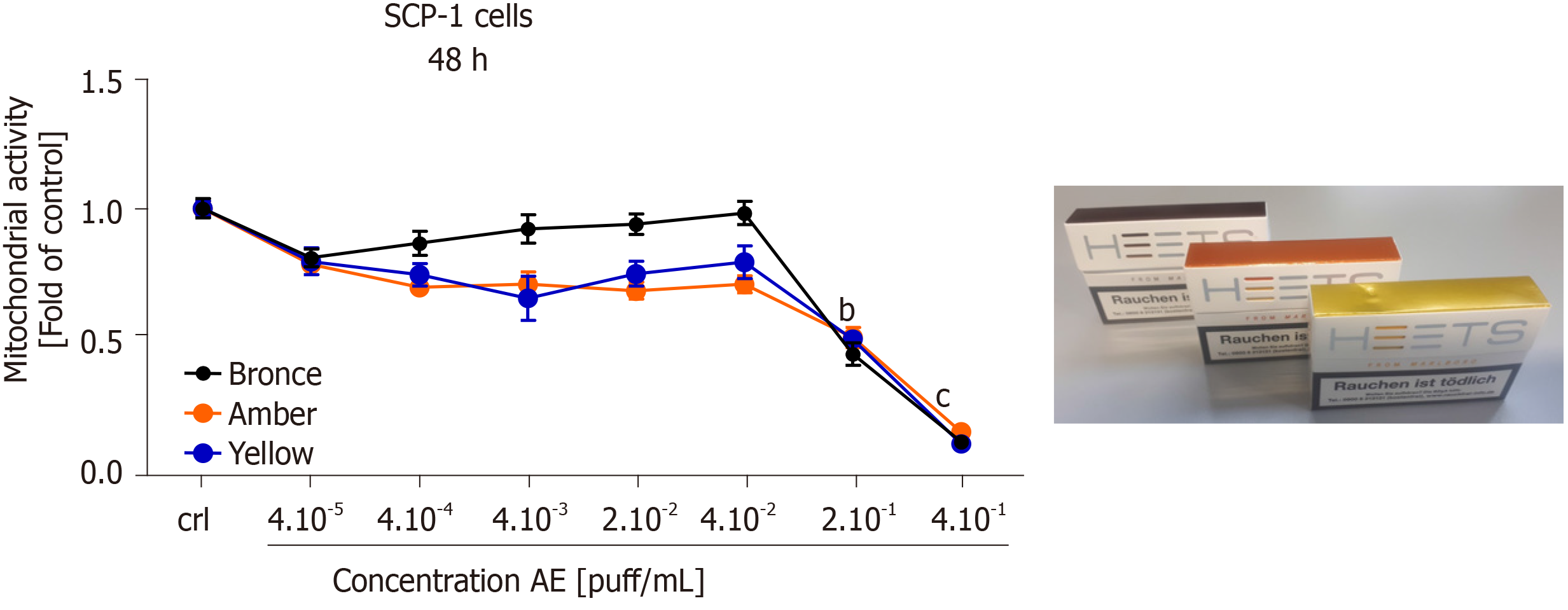
Our previous results showed that THS AE was less harmful than conventional cigarettes on MSCs osteogenic differentiation, as well as hOBs function. Three THS-flavored sticks (bronze - mocha and dried fruit flavor, amber - wood and nut flavor, and yellow - herbal flavor) were selected for further cytotoxicity screening. Dose-responses of mitochondrial activity in SCP-1 cells exposed to selected THS-flavored AE are shown in Figure 6. No significant differences in cell viability were detected after a 48 h exposure to all AE tested with a concentration up to 4 × 10-2 puff/mL compared to untreated cells. It is essential to highlight that a significant decrease in cell viability was observed with concentrations higher or equal to 2 × 10-1 puff/mL with all flavored THS AE evaluated (Figure 6), thus confirming the results shown in Figure 2A. These results suggest that the cytotoxicity observed following THS treatment may be associated with exposure to very high, non-physiological concentrations, and was not associated with flavor.
While the detrimental effects of conventional cigarettes on the skeletal system have been extensively reported[4,5], the effects of THS on bone health remain unknown. Smoking conventional cigarettes disturbs bone homeostasis, resulting in an imbalance of bone-forming and bone-resorbing cells, resulting in loss of bone mass and an increased risk of osteoporosis and fracture[6,7,9-11]. The detrimental impact of conventional cigarettes on bone function raises the need to develop new alternatives for smokers. ENDS, including e-cigarettes or THS, focus on heating rather than combustion, in order to reduce the generation of harmful constituents. Therefore, ENDS provide the same levels of nicotine as conventional cigarettes and maintain the smoking ritual. E-cigarettes heat liquids based on propylene glycol, glycerin, flavor and (optionally) nicotine into an aerosol that is then inhaled. THS, in contrast, heat tobacco rolled up in a stick form up to 350 °C instead of burning it.
A better understanding of the effects of THS on the skeletal system is required. Previous in vitro studies demonstrated that the detrimental effects of cigarette smoke on bone homeostasis could take place independently of nicotine[18-20]. The effects appear to be dependent on oxidative stress induced by constituents present in the cigarette smoke[18,21,23,25,44].
The results presented in this study have demonstrated that the time to consume one unit was significantly longer for THS than conventional cigarettes, thus the smoking experience was longer for THS. Additionally, pH did not significantly change between the different AE fractions and the cell culture medium, validating that this was not the reason for the stronger cytotoxicity observed from conventional cigarette AE. Interestingly, AE produced from THS showed no substantial differences in suspended particulates compared to the cell culture medium. Conversely, AE generated with conventional cigarettes had a significantly increased turbidity. This result was in line with previous studies that have demonstrated that THS delivers less harmful constituents than conventional cigarettes[27,28].
Our analysis demonstrated a reduced impact on MSCs and hOBs viability following treatment with THS AE compared to conventional cigarettes after a single exposure. Moreover, different THS flavors were not related to the cytotoxic effects on bone cells. More sustained adverse effects were observed in response to chronic exposure for 21 d to conventional cigarette AE than to THS AE. This result was supported by reduced cell viability, reduced AP activity, and less mineralized matrix formation measured in bone-forming cells and bone precursor cells. This demonstrated a significantly lower influence by THS AE on the differentiation of MSCs and hOBs function, supporting previous publications regarding decreases in detrimental effects by THS in lung tissue and the cardiovascular system evaluated in vivo and in vitro when compared to 3R4F cigarette combustion[30,31,45,46].
It was demonstrated that oxidative stress induced by the compounds contained in conventional cigarette combustion could be one of the responsible factors for the impaired osteogenic differentiation of bone-forming cells and precursors cells, due to an imbalance in the anti-oxidative system that negatively affects the function of the anti-oxidative enzymes[18,21-25]. Interesting, Munakata et al[32] demonstrated that THS induced less oxidative stress than conventional cigarettes in bronchial epithelial cells. Our results also show that THS AE did not increase the level of ROS production in MSCs. Nevertheless, previous results demonstrated that nicotine and cotinine inhibit catalase and glutathione reductase enzymatic activity, affecting the function of the cells' antioxidant system[18]. This fact alludes that using ENDS may be a less harmful alternative for smokers that are orthopedic patients with increased oxidative stress levels (due to the trauma and associated surgery).
In our study, we observed no detrimental effect of THS AE on MSCs primary cilia structure. Since primary cilia play an essential role in the initiation of osteogenic differentiation in MSCs, as well as in the maintenance of the differentiated status of the cells[42,47], we conclude that the minor effect of THS on bone-forming cells and progenitor cells is due to decreased oxidative stress, which conserved the primary cilia structure, in contrast to the previous results reported with conventional cigarettes[18,25,39].
A major limitation in this study that could be addressed in future research is that this study focused on the effect of THS AE only in MSCs and hOBs. As several cell types are involved in bone homeostasis, THS could potentially influence the function of other cells, such as immune cells, osteoclasts, or osteocytes. Co-culture systems may provide a better alternative for screening the effects of ENDS on bone metabolism and predicting cytotoxicity in bone tissue. Furthermore, the addition of cytokines, normally increased after fracture, to a co-culture system could better represent the fracture healing process. By combining a more complex setup with different cells types and an inflammatory environment expected after fracture, disturbance in factors that influence the healing could be analyzed under ENDS exposure (e.g., TGF-β1, MSCs chemoattractant affected conventional cigarette AE[39])
Additionally, our system could be improved by including other cell types, e.g., endothelial cells or immune cells, to represent better the molecular species that finally cross the bloodstream. Additionally, multiple interconnected cell culture systems representing functionalities of different organs, could represent exposure of bone-forming cells closer to humans. In this system, the paracrine interaction among different organs could be instigated as well as the potential toxicity of or side effects of the additional metabolites of ENDS coming from different tissues (e.g., lung, liver, etc.) could be evaluated. However, it should be consider that increasing the complexity of the model also decreases the analytical methods available for characterization.
This research serves as one of the first in vitro studies to demonstrate reductions in the harmful effects on bone-forming cells and bone progenitor cells treated with THS compared to conventional cigarettes. THS could be a potential alternative for smokers to maintain appropriate bone homeostasis and delay development of secondary osteoporosis, which consequently would reduce health system costs. However, more studies are required to confirm if THS could be a viable alternative for smokers to maintain appropriate bone homeostasis and improve delays in bone healing.
Cigarette smoking (CS) is the most common method of consuming tobacco. Deleterious effects on bone integrity, increased incidence of fractures, and delayed fracture healing are all associated with CS. Tobacco combusted at about 800 °C generates approximately 6500 molecular species, more than 150 of which have been identified as toxic compounds. New approaches have been on developing reduced-risk alternatives for smokers that maintain the smoking ritual, while providing the same levels of nicotine as conventional cigarettes with less harmful constituents.
New technologies designed to develop a reduced-risk alternative for smokers are based on electronic nicotine delivery systems, such as e-cigarettes and tobacco heating systems (THS). Instead of burning tobacco, THS heat tobacco rolled up in a stick form up to 350 °C (avoiding combustion and formation of ashes). THS contain tobacco and convey the feeling of smoking a conventional cigarette. Several studies have demonstrated reduced levels of toxic and harmful compounds from electronic nicotine delivery systems.
The present study aim to examine the effects of THS on osteoprogenitor cell viability and function compared to conventional CS.
Human immortalized mesenchymal stem cells and primary human pre-osteoblasts isolated from cancellous bone samples were osteogenically differentiated with aqueous extracts generated from either the THS 2.4 “IQOS” or conventional “Marlboro” cigarettes for up to 21 d. Cell viability was analyzed using resazurin conversion assay (mitochondrial activity) and calcein-AM staining (esterase activity). Osteogenic differentiation and bone cell function were evaluated using alkaline phosphatase (AP) activity, while matrix formation was analyzed through alizarin red staining. Primary cilia structure was examined by acetylated α-tubulin immunofluorescent staining. Free radical production was evaluated with 2′,7′-dichlorofluorescein-diacetate assay.
THS is significantly less toxic to bone cells than CS when analyzed by mitochondrial and esterase activity (P < 0.001). No significant differences in cytotoxicity between the diverse flavors of THS were observed. Harmful effects from THS on bone cell function were observed only at non-physiological concentrations. In contrast, conventional cigarettes significantly reduced the AP activity (by two-fold) and matrix mineralization (four-fold) at low concentrations. Moreover, morphologic analysis of primary cilia revealed no significant changes in the length of the organelle involved in osteogenesis of osteoprogenitor cells, nor in the number of ciliated cells following THS treatment. Assessment of free radical production demonstrated that THS induced significantly less oxidative stress than conventional CS in osteoprogenitor cells.
The present study demonstrate reductions in the harmful effects on bone-forming cells and bone progenitor cells treated with THS compared to conventional cigarettes.
THS could be a potential alternative for smokers to maintain appropriate bone homeostasis and delay development of secondary osteoporosis, which consequently would reduce health system costs.
We would like to Dr. Alexander Nussbaum for providing the THS 2.4 system "IQOS®" device and sticks, Bianca Braun for their excellent technical assistance, Dr. Julia Hoeng, Dr. Bjorn Titz, Dr. Anita Iskandar and Shoaib Majeed (M.Eng.) for the interesting discussions.
Manuscript source: Invited manuscript
Specialty type: Toxicology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Durán Alonso MB, Hassan A, Kode JA, Park JB S-Editor: Ma YJ L-Editor: A P-Editor: Xing YX
| 1. | Kahnert S, Pötschke-Langer M, Kahnert S, Viarisio V, Heidt C, Schunk S, Mons U, Fode K. Tabakatlas deutschland 2015. Pabst Science Publishers. 2015;. |
| 2. | GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1275] [Cited by in RCA: 1227] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 3. | Cooke M. The chemical components of tobacco and tobacco smoke. Chromatographia. 2010;71:977-977. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Cusano NE. Skeletal Effects of Smoking. Curr Osteoporos Rep. 2015;13:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Wong PK, Christie JJ, Wark JD. The effects of smoking on bone health. Clin Sci (Lond). 2007;113:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 630] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 8. | Amin S, Niu J, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, LaValley MP, Genant HK, Felson DT. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Ann Rheum Dis. 2007;66:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury. 2001;32:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Scolaro JA, Schenker ML, Yannascoli S, Baldwin K, Mehta S, Ahn J. Cigarette smoking increases complications following fracture: a systematic review. J Bone Joint Surg Am. 2014;96:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Mills E, Eyawo O, Lockhart I, Kelly S, Wu P, Ebbert JO. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med. 2011;124:144-154.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 12. | Abate M, Vanni D, Pantalone A, Salini V. Cigarette smoking and musculoskeletal disorders. Muscles Ligaments Tendons J. 2013;3:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Singh JA, Schleck C, Harmsen WS, Jacob AK, Warner DO, Lewallen DG. Current tobacco use is associated with higher rates of implant revision and deep infection after total hip or knee arthroplasty: A prospective cohort study. BMC Med. 2015;13:283. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Ehnert S, Aspera-Werz RH, Ihle C, Trost M, Zirn B, Flesch I, Schroter S, Relja B, Nussler AK. Smoking dependent alterations in bone formation and inflammation represent major risk factors for complications following total joint arthroplasty. J Clin Med. 2019;8:406. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab. 2009;27:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Pappas RS. Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics. 2011;3:1181-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | U.S. Food and Drug Administration. Harmful and potentially harmful constituents in tobacco products and tobacco smoke: Established list. 2012. Available from: https://www.fda.gov/tobacco-products. |
| 18. | Aspera-Werz RH, Ehnert S, Heid D, Zhu S, Chen T, Braun B, Sreekumar V, Arnscheidt C, Nussler AK. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid Med Cell Longev. 2018;2018:3172480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Daffner SD, Waugh S, Norman TL, Mukherjee N, France JC. Nicotine Increases Osteoblast Activity of Induced Bone Marrow Stromal Cells in a Dose-Dependent Manner: An in vitro Cell Culture Experiment. Global Spine J. 2012;2:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Kim BS, Kim SJ, Kim HJ, Lee SJ, Park YJ, Lee J, You HK. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012;90:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Braun KF, Ehnert S, Freude T, Egaña JT, Schenck TL, Buchholz A, Schmitt A, Siebenlist S, Schyschka L, Neumaier M, Stöckle U, Nussler AK. Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes HO-1 and SOD-1. ScientificWorldJournal. 2011;11:2348-2357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Ehnert S, Braun KF, Buchholz A, Freude T, Egaña JT, Schenck TL, Schyschka L, Neumaier M, Döbele S, Stöckle U, Nussler AK. Diallyl-disulphide is the effective ingredient of garlic oil that protects primary human osteoblasts from damage due to cigarette smoke. Food Chem. 2012;132:724-729. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Ehnert S, Stefan D, Friedrich BK, Britta B, Valeska H, Mario H, Tomas EJ, Ulrich S, Thomas F, Klaus NA. N-acetylcyteine and flavonoid rich diet: The protective effect of 15 different antioxidants on cigarette smoke-damaged primary human osteoblasts. Adv Biosci Biotechnol. 2012;3:1129-1139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Holzer N, Braun KF, Ehnert S, Egaña JT, Schenck TL, Buchholz A, Schyschka L, Neumaier M, Benzing S, Stöckle U, Freude T, Nussler AK. Green tea protects human osteoblasts from cigarette smoke-induced injury: possible clinical implication. Langenbecks Arch Surg. 2012;397:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Sreekumar V, Aspera-Werz R, Ehnert S, Strobel J, Tendulkar G, Heid D, Schreiner A, Arnscheidt C, Nussler AK. Resveratrol protects primary cilia integrity of human mesenchymal stem cells from cigarette smoke to improve osteogenic differentiation in vitro. Arch Toxicol. 2018;92:1525-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Centers for Disease Control and Prevention (CDC). Quitting smoking among adults--United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513-1519. [PubMed] |
| 27. | Haziza C, de La Bourdonnaye G, Donelli A, Poux V, Skiada D, Weitkunat R, Baker G, Picavet P, Lüdicke F. Reduction in Exposure to Selected Harmful and Potentially Harmful Constituents Approaching Those Observed Upon Smoking Abstinence in Smokers Switching to the Menthol Tobacco Heating System 2.2 for 3 Months (Part 1). Nicotine Tob Res. 2020;22:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Li X, Luo Y, Jiang X, Zhang H, Zhu F, Hu S, Hou H, Hu Q, Pang Y. Chemical Analysis and Simulated Pyrolysis of Tobacco Heating System 2.2 Compared to Conventional Cigarettes. Nicotine Tob Res. 2019;21:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 29. | Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, Proctor C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem Res Toxicol. 2016;29:1662-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 30. | Davis B, To V, Talbot P. Comparison of cytotoxicity of IQOS aerosols to smoke from Marlboro Red and 3R4F reference cigarettes. Toxicol In Vitro. 2019;61:104652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Phillips B, Szostak J, Titz B, Schlage WK, Guedj E, Leroy P, Vuillaume G, Martin F, Buettner A, Elamin A, Sewer A, Sierro N, Choukrallah MA, Schneider T, Ivanov NV, Teng C, Tung CK, Lim WT, Yeo YS, Vanscheeuwijck P, Peitsch MC, Hoeng J. A six-month systems toxicology inhalation/cessation study in ApoE-/- mice to investigate cardiovascular and respiratory exposure effects of modified risk tobacco products, CHTP 1.2 and THS 2.2, compared with conventional cigarettes. Food Chem Toxicol. 2019;126:113-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Munakata S, Ishimori K, Kitamura N, Ishikawa S, Takanami Y, Ito S. Oxidative stress responses in human bronchial epithelial cells exposed to cigarette smoke and vapor from tobacco- and nicotine-containing products. Regul Toxicol Pharmacol. 2018;99:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Bozier J, Zakarya R, Chapman DG, Oliver BGG. How harmless are E-cigarettes? Effects in the pulmonary system. Curr Opin Pulm Med. 2020;26:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? BMJ. 2019;366:l5275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 317] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 35. | Ehnert S, Linnemann C, Aspera-Werz RH, Bykova D, Biermann S, Fecht L, De Zwart PM, Nussler AK, Stuby F. Immune Cell Induced Migration of Osteoprogenitor Cells Is Mediated by TGF-β Dependent Upregulation of NOX4 and Activation of Focal Adhesion Kinase. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Böcker W, Yin Z, Drosse I, Haasters F, Rossmann O, Wierer M, Popov C, Locher M, Mutschler W, Docheva D, Schieker M. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J Cell Mol Med. 2008;12:1347-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | ISO and health canada intense smoking parameters - Part 2: Examination of factors contributing to variability in the routine measurement of TPM, water and NFDPM smoke yields of cigarettes. ISO, 2015. Available from: https://www.iso.org/obp/ui/#iso:std:iso:tr:19478:-2:ed-1:v1:en. |
| 38. | Häussling V, Deninger S, Vidoni L, Rinderknecht H, Ruoß M, Arnscheidt C, Athanasopulu K, Kemkemer R, Nussler AK, Ehnert S. Impact of Four Protein Additives in Cryogels on Osteogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering (Basel). 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Aspera-Werz RH, Chen T, Ehnert S, Zhu S, Fröhlich T, Nussler AK. Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Ehnert S, Sreekumar V, Aspera-Werz RH, Sajadian SO, Wintermeyer E, Sandmann GH, Bahrs C, Hengstler JG, Godoy P, Nussler AK. TGF-β1 impairs mechanosensation of human osteoblasts via HDAC6-mediated shortening and distortion of primary cilia. J Mol Med (Berl). 2017;95:653-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 471] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 42. | Tummala P, Arnsdorf EJ, Jacobs CR. The Role of Primary Cilia in Mesenchymal Stem Cell Differentiation: A Pivotal Switch in Guiding Lineage Commitment. Cell Mol Bioeng. 2010;3:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Anderson CT, Castillo AB, Brugmann SA, Helms JA, Jacobs CR, Stearns T. Primary cilia: cellular sensors for the skeleton. Anat Rec (Hoboken). 2008;291:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478-L488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 45. | Sewer A, Kogel U, Talikka M, Wong ET, Martin F, Xiang Y, Guedj E, Ivanov NV, Hoeng J, Peitsch MC. Evaluation of the Tobacco Heating System 2.2 (THS2.2). Part 5: microRNA expression from a 90-day rat inhalation study indicates that exposure to THS2.2 aerosol causes reduced effects on lung tissue compared with cigarette smoke. Regul Toxicol Pharmacol. 2016;81 Suppl 2:S82-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Haswell LE, Corke S, Verrastro I, Baxter A, Banerjee A, Adamson J, Jaunky T, Proctor C, Gaça M, Minet E. In vitro RNA-seq-based toxicogenomics assessment shows reduced biological effect of tobacco heating products when compared to cigarette smoke. Sci Rep. 2018;8:1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | James AW. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica (Cairo). 2013;2013:684736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (0)] |