Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.803
Peer-review started: February 27, 2020
First decision: May 26, 2020
Revised: June 9, 2020
Accepted: July 5, 2020
Article in press: July 5, 2020
Published online: August 26, 2020
Processing time: 180 Days and 20.2 Hours
Intervertebral disc (IVD) degenerative diseases are a common problem in the world, and they cause substantial social and economic burdens for people. The current methods for treating IVD degenerative diseases mainly include surgery and conservative treatment, which cannot fundamentally restore the normal structure of the disc. With continuous research on the mechanism of degeneration and the development of regenerative medicine, rapid progress has been made in the field of regenerative medicine regarding the use of stem cell-derived exosomes, which are active biological substances used in intercellular communication, because they show a strong effect in promoting tissue regeneration. The study of exosomes in the field of IVD degeneration has just begun, and many surprising achievements have been made. This paper mainly reviews the biological characteristics of exosomes and highlights the current status of exosomes in the field of IVD degeneration, as well as future developments regarding exosomes.
Core tip: This article mainly reviews the brief pathological process of disc degeneration and the biological characteristics and functions of exosomes. We highlight the current status and advancement of exosome research in the field of intervertebral disc degeneration, analyze the possible mechanisms, and discuss the future development of exosomes in this field.
- Citation: Hu ZL, Li HY, Chang X, Li YY, Liu CH, Gao XX, Zhai Y, Chen YX, Li CQ. Exosomes derived from stem cells as an emerging therapeutic strategy for intervertebral disc degeneration. World J Stem Cells 2020; 12(8): 803-813
- URL: https://www.wjgnet.com/1948-0210/full/v12/i8/803.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i8.803
Low back pain (LBP) is a common spinal health problem worldwide[1]. In a global systematic review, the mean prevalence of LBP at a given time in the general population was approximately 18%, and the 1-year prevalence was approximately 38%[2]. Therefore, a small reduction in health care or disability rates related to LBP could bring significant social and economic benefits[3,4].
The causes of LBP are complex[5,6], and although there is no direct evidence, IVD degeneration is considered a major cause[5]. The cause of intervertebral disc (IVD) degeneration is still not fully understood, but some factors, such as aging, abnormal mechanical stress, trauma, nutritional deficiencies, and heredity, are considered to be involved in this process[7]. The pathological process of disc degeneration includes the reduction of nucleus pulposus cells (NPCs)[8,9] and extracellular matrix (due to decreased synthesis and increased degradation), aging of the annulus fibrosus, and calcification of cartilage endplates[10].
Current treatments for LBP caused by IVD degeneration include invasive surgery and conservative treatment[11], which are mainly aimed at relieving symptoms rather than changing pathogenic mechanisms. Therefore, there is an urgent need for new therapies that treat disc degeneration by directly addressing causes and mechanisms to retain and/or restore disc structure and mechanical function.
Recently, an increasing number of studies have focused on degenerated disc regeneration, including studies related to bioactive molecular injection[12,13], cell-based therapies[14-16], tissue engineering[17,18], and gene therapy[19,20]. Bioactive molecular injection is a biological therapy utilizing chemical molecules with the effect of recruitment of endogenous stem cells into the IVD or stimulation of their proliferation. Although the short-term effect is acceptable, the long-term maintenance of biological activity has become an unavoidable obstacle for this therapy. Cell-based therapies, as the most attractive method among these studies, involve the injection of extracted cells, such as NPCs or various stem cells, into the disc in vivo to restore IVD homeostasis following the proliferation, differentiation, and immune regulation of the transplanted cells[21]. Although some progress has been made, the complex environment of the degenerated IVD causes a low survival rate of stem cells and makes it difficult to accurately control cell viability and differentiation. Additionally, the sources and safety issues of stem cells need to be considered. Gene therapy refers to modification of genomes to increase the expression of effector genes and promote the continuous production of one or more biologically active factors in the IVD to promote cell proliferation, extracellular matrix production, and inhibition of apoptosis.
Studies on the mechanism of stem cell therapy have provided increasing evidence that the factors that play an important role in these treatments are the exosomes that are secreted by stem cells[22-24]. Exosomes were considered waste products from cells when they were first reported in 1983[25]. Currently, this nanoscale cell vesicle is known to be an important substance in intercellular communication that can transfer biomolecules such as proteins and nucleic acids from parent cells to recipient cells. Their applications in regenerative medicine are also increasing, including in the regeneration of NPCs and the maintenance of disc homeostasis[26-28]. This paper reviews the biological characteristics of exosomes and their research status in the field of disc degeneration, and gives outlook on their future applications in this field.
Exosomes are a type of extracellular vesicle; the other two main types of extracellular vesicles are microvesicles and apoptotic bodies[29,30]. The characteristics of the three main extracellular vesicles are shown in Table 1.
| Types of vesicles | Diameter | Markers | Cargos | Density (g/mL) | Origin | Ref. |
| Exosomes | 30-150 nm | CD63, CD81, CD9, HSP70, Flotillin, TSG101, etc. | mRNA, microRNA, lncRNA, circRNA, DNA lipid, protein, etc. | 1.13-1.18 | Endosomes pathway | [51,74-77] |
| Microvesicles | 50-1000 nm | Integrins, selectins, CD40 ligand | mRNA, microRNA, other non-coding RNA, protein, etc. | 1.16-1.19 | Plasma membrane; outward budding | [51,75] |
| Apoptotic bodies | 500-2000 nm | Phosphatidylserine, genomic DNA | Nuclear fractions, cell organelles, etc. | 1.16-1.28 | Plasma membrane | [47,51,78] |
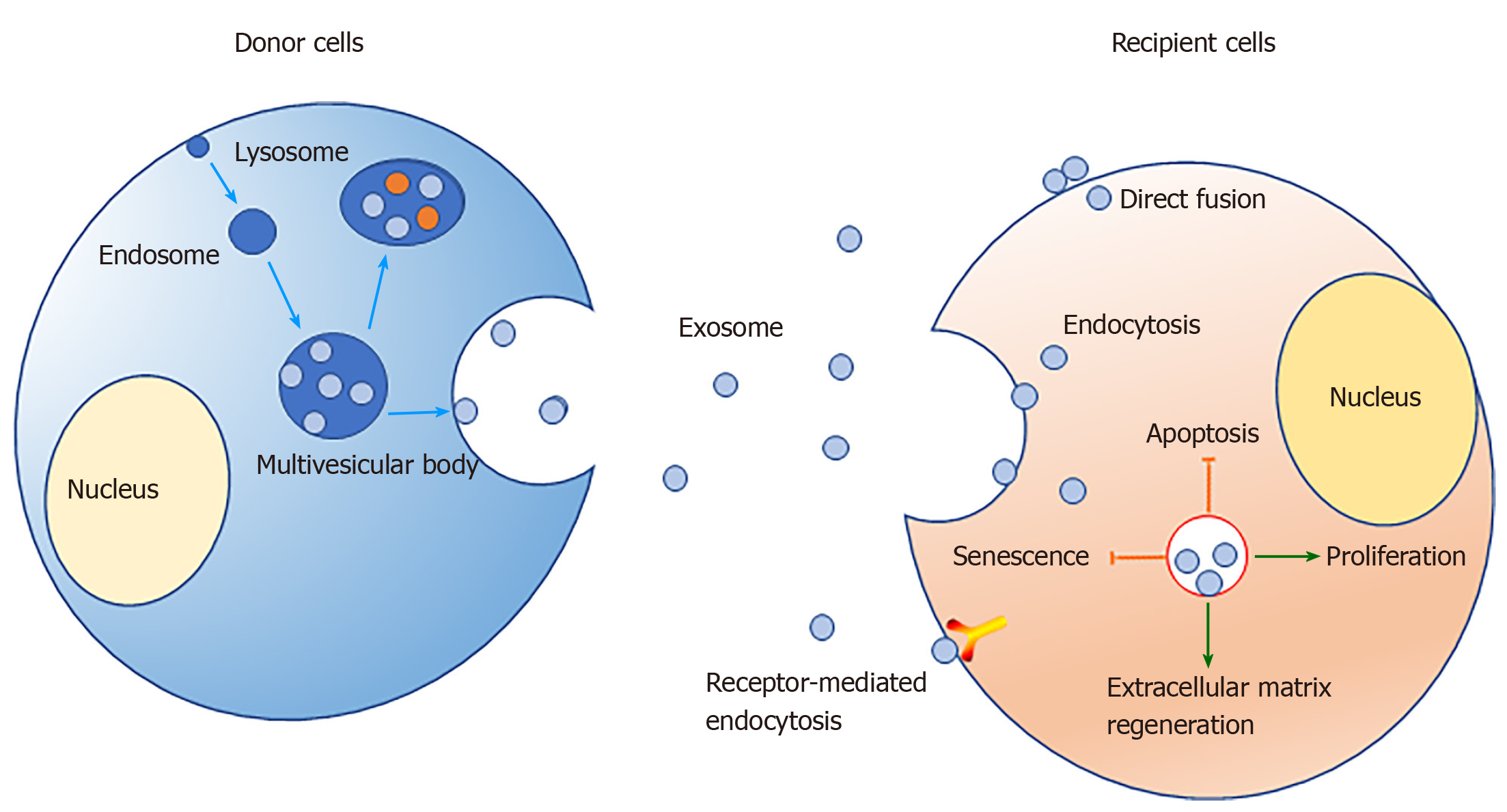
Exosomes have spheroid membranes of a uniform lipid bilayer with diameters of approximately 30-150 nm[31]. They typically can be detected in various body fluids, such as blood[32,33], amniotic fluid[34-36], breast milk[37,38], urine[39,40], synovial fluid[41,42], and saliva[43,44]. They can be transported to corresponding target cells through the body fluids to perform a specific function. In vitro, exosomes also have been isolated from cell culture supernatant[45]. We now know that the mechanism of exosome formation involves the inward invagination of the endosomal membrane pathway. At the first stage, the inward budding of the plasma membrane with receptors leads to the formation of an endosome. Then, small vesicles can be formed by further inward budding of the limiting membrane to form a multivesicular body (MVB) with intraluminal vesicles. The vesicular contents in MVBs are finally degraded when MVBs fuse with the lysosome or are released into the extracellular space[46]. After being released into the extracellular space, exosomes play a biological role when they contact another membrane and are endocytosed into a recipient cell[46,47].
The function of exosomes mainly depends on their contents. Among the components of exosomes, lipids, proteins, and nucleic acids are the three main substances that determine the biological function of exosomes[48,49]. Lipids in exosomes are mainly located in the membrane, including cholesterol, phosphatidylserine, sphingomyelin, etc. In addition to maintaining the biological stability of exosomes, lipids are also involved in biological processes such as the formation and release of exosomes[50]. Exosomes are also rich in a variety of proteins, including cytoskeleton components, tetraspanins, heat-shock proteins, and other types of proteins[51,52]. Among them, ALIX and tetraspanin proteins, such as CD81, CD9, and CD63, are markers of exosomes[51]. However, it is difficult to distinguish exosomes from other extracellular vesicles with overlapping size and density based solely on these markers.
Exosomes usually carry nucleic acids, including mRNAs[53], microRNAs (miRNAs)[54,55], and long noncoding RNAs (lncRNAs)[56]. MiRNAs are a class of endogenous noncoding RNAs found in eukaryotes that have a length of approximately 20-25 nucleotides. Mature miRNAs are produced from longer primary transcripts that undergo a series of nuclease-mediated cleavages; then, the miRNAs are assembled into RNA-induced silencing complexes by complementary base pairing to a target mRNA, which guides degradation of targets or suppresses translation of targets, based on the degree of complementarity[57]. According to the principle of base complementary pairing, a miRNA usually can target hundreds of corresponding genes, which implies that miRNAs carried in exosomes may play an important role in regulating gene transcription in target cells. LncRNAs are a class of RNA molecules longer than 200 bp that do not encode proteins. They are widely involved in the transcription, translation, and posttranslational regulation of genes. LncRNAs can participate in chromatin modification, transcription activation, and transcription interference in cells, or they can act as "bait molecules" that interact with proteins, DNA, and RNA[58,59]. As messengers of intercellular communication, exosomes are secreted by parent cells and taken up by target cells in the following ways: (1) Transmembrane proteins are fused to target cell membranes by binding to receptor proteins; (2) The exosomal membrane fuses directly with the cell membrane, releasing the contents; and (3) Target cells take up exosomes through endocytosis[60,61].
Recently, an increasing number of studies have shown that exosomes derived from stem cells play an important role in restoring tissue homeostasis and promoting tissue regeneration.
Exosomes from bone marrow mesenchymal stem cells (MSCs) can significantly enhance bone regeneration, promote vascular regeneration, and accelerate fracture healing in a rat femur nonunion model[62]. Exosomes from adipose stem cells promote the vascularization of endothelial cells[63]. Human umbilical cord MSC exosomes can promote angiogenesis and repair of second-degree burn wounds of the skin[64]. Exosomes from human stem cells can promote the repair of jaw joints and the synthesis of extracellular matrix in that tissue[24]. Increasing experimental results show the potential regenerative ability of stem cell-derived exosomes through their promotion of cell proliferation, enhancement of angiogenesis, promotion of extracellular matrix homeostasis recovery, inhibition of inflammation, and other unknown effects. Some of these beneficial mechanisms can also be achieved in the repair of disc degeneration.
Stem cell transplantation for treatment of IVD degeneration has made great progress. In vitro and in vivo studies have revealed the great advantages of stem cells as seed cells for cell-based therapies. However, because of the complex and harsh in vivo environment of the IVD, there are obstacles to be overcome by IVD degeneration stem cell therapy approaches[65]. With continued research into stem cell therapies, it has been found that the exosomes secreted by stem cells play an important role in their therapeutic effect[26,66]. Therefore, exosomes have attracted more and more attention in some preclinical studies of promoting IVD regeneration (Table 2).
| Ref. | Experimental objective | Cargo analysis | Animal model | In vitro appraisement | In vivo appraisement | Inhibition test | Research type |
| HBMSCs; Lu et al[26] | To detect the role of exosomes derived from BM-MSCs in NPCs | Not mentioned | None | (1) Promoted proliferation; and (2) Increased synthesis of extracellular matrix and decrease in degradation | None | None | Cell experimentation |
| HBMSC; Cheng et al[28] | To explore the protective effect of MSC-exosomes on NPCs in a cell and rat model | Highly enrichment in miR-21 | SD rat model of IVD degeneration by needle puncture | (1) Decreased apoptosis rate; and (2) Decreased cleaved caspase-3 | (1) IVD degeneration score lower; (2) Decreased apoptosis rate; and (3) Lower histologic score | MiR-21 antagonist enhanced cell apoptosis | Cell and animal experimentation |
| Rat nucleus pulposus; Moen et al[79] | To study the role of extracellular miRNA in lumbar radicular pain | Increased miR-223 | Lewis rat IVD herniation | None | MiR-223 increased after disc herniation | None | Animal experimentation |
| Porcine notochordal cells; Bach et al[80] | To explore the biologic effect of the NCCM-derived EVs on canine and human CLCs from degenerated IVDs in vitro | None | None | Increased glycosaminoglycan (GAG) deposition | None | None | Cell experimentation |
| HBMSCs; Liao et al[27] | To prove that the delivery of MSC-exos could modulate ER stress and inhibit excessive NP cell apoptosis during IDD | None | SD rat model of IVD degeneration by needle puncture | (1) Western blot and TUNEL assays indicated decreased apoptosis rate; and (2) Western blot and qPCR data indicated decreased reticulum stress | (1) Higher DHI; (2) Lower Pfirrmann grade; (3) Lower histological grades; and (4) Decreased apoptosis rate | Akt inhibitor LY294002; ERK inhibitor PD98059 | Cell and animal experimentation |
| C57BL/6 mice BMSCs; Xia et al[69] | To investigate the therapeutic effect of exosomes for use as IVDD therapeutics | None | Rabbit model of IVD degeneration by needle puncture | (1) Decreased apoptosis rate; (2) Western blot and qPCR data indicated recovery of matrix homeostasis; (3) Decreased inflammatory marker expression; (4) Suppressed inflammasome; and (5) Recovery of mitochondrial-related proteins and attenuated mitochondrial dysfunction | (1) Higher DHI; (2) Lower Pfirrmann MRI grade; (3) Lower histological grades; and (4) Decreased apoptosis rate | None | Cell and animal experimentation |
When exosomes derived from bone marrow MSCs were cocultured with NPCs from degenerated IVDs, cell proliferation was significantly accelerated by extending the incubation time with exosomes. Additionally, the expression of the extracellular matrix synthesis and protection genes ACAN, COL2A1, SOX-9, and TIMP-1 increased with incubation time, while the degradation-related genes MMP-1 and MMP-3 were decreased. Therefore, it seems to indicate that MSC-derived exosomes promote the proliferation and extracellular matrix homeostasis of NPCs[26].
Stem cell-derived exosomes not only promote the proliferation of NPCs but also inhibit their apoptosis. In a study by Cheng et al[28], human bone marrow MSCs and fibroblast-derived exosomes were used to treat TNF-α-induced apoptotic NPCs. The cells treated with the exosomes derived from the bone marrow MSC group had a significantly lower apoptotic rate than those of the other groups. In vivo experiments showed that the MSC-derived exosome treatment group had significantly lower Pfirrmann grade, histological grade, and apoptotic rate than the noninjection groups. Another in vitro study also confirmed the anti-apoptotic effect of stem cell exosomes. Liao et al[27] co-incubated exosomes from MSCs with advanced glycation end products-induced NPCs and confirmed that the levels of apoptosis-related markers caspase-3 and caspase-12 decreased significantly. With the increase in exosomal concentration, the declining trend was greater. The above studies confirmed that exosomes have significant anti-apoptotic effects both in vivo and in vitro.
The accumulation of a large number of inflammatory factors and extracellular matrix-degrading enzymes in the IVD is an important cause of NPC apoptosis and loss of the extracellular matrix[67,68]. Xia et al[69] collected the normal nucleus pulposus from trauma patients and the degenerated nucleus pulposus and then screened 150 proteins by gene ontology and KEGG analysis, of which 69 proteins were downregulated and 81 were upregulated. Most of the proteins were associated with inflammatory responses, showing enhanced inflammatory responses in degenerative discs. By adding MSC-derived exosomes to apoptotic NPCs, the expression of IL-1β, iNOS, COX-2, IL-6, MMP3, MMP13, and other inflammation- and extracellular matrix degradation-related enzymes was significantly reduced. In vivo experiments also demonstrated that the exosome injection group had significantly lower MMP13 expression at 2, 4, and 8 wk than the control group[69].
The decrease of viable cells is a key factor in the process of disc degeneration; conversely, in the process of disc regeneration, the recovery of cell numbers is the most important issue. These studies have confirmed that stem cell-derived exosomes could enhance cell proliferation and inhibit apoptosis, especially for stem cells remaining in the disc. Moreover, exosomes could also enhance the expression of the extracellular matrix in NPCs and inhibit the expression of matrix protein degrading enzymes, which is beneficial for maintaining the homeostasis of the extracellular matrix. During IVD degeneration, a large number of cytokines participate in and accelerate the degeneration of the IVD, leading to apoptosis and senescence of NPCs[70]. Exosomes have a significant inhibitory effect on inflammation, which induces the restoration of the microenvironment for the surviving cells and reduces the disturbance of the intracellular environment. Therefore, stem cell-derived exosomes have the potential to treat disc degeneration.
With an increasing understanding of the mechanisms behind disc degeneration and with in-depth studies of exosomes, the application of exosomes in disc degeneration has achieved some new progress. This progress clearly shows the tremendous potential of exosomes in disc repair. However, the exact mechanism of how exosomes affect disc repair is still unclear.
In a study of Cheng et al[28], miRNA array hybridization and data analysis was performed to compare normal NPCs and TNF-induced apoptotic NPCs; five miRNAs (miR-18a, miR-21, miR-106b, miR-217, and miR-26a) were found at significantly lower levels in the TNF-induced NPC group than in the control group. Furthermore, only miR-21 was present in MSC-derived exosomes at higher levels than it was in fibroblast-derived exosomes. MiR-21 also decreased apoptosis and suppressed the expression of PTEN. Based on the above results, the researchers believe that the PTEN-PI3K-Akt pathway is a potential target of exosomal miR-21-mediated apoptosis protection in NPCs. However, they believe that there are still other extracellular vesicles or other components in exosomes that are involved in this procedure[28].
Liao et al[27] proposed another possible mechanism. The endoplasmic reticulum stress-related markers GRP78 and CHOP were significantly increased in degenerated discs, and their expression positively correlated with Pfirrmann classification. Then, exosomes were added to induce NPCs, and the expression of endoplasmic reticulum-related pathways and apoptosis markers was inhibited. This means that exosomes inhibit endoplasmic reticulum stress-mediated apoptosis through AKT and ERK signaling pathways by reducing the levels of CHOP, the key molecule of endoplasmic reticulum stress[27].
In addition to the above studies, Xia et al[69] found that 150 proteins differentially expressed in degenerative discs are closely related to enhanced inflammatory responses. Exosomes can significantly inhibit the inflammatory response of apoptotic NPCs and the formation of inflammatory bodies. The proteins found in bone marrow MSC exosomes mainly recovered the damage to mitochondria in NPCs, restored the normal structure of mitochondria, and reduced oxidative stress in mitochondria. The results indicate that exosomes can play a role in inhibiting disc degeneration by restoring mitochondrial homeostasis and the antioxidative response and inhibiting formation of inflammatory bodies[69].
These potential mechanisms have mainly been studied in terms of promoting extracellular matrix production, inhibiting matrix degradation, promoting an anti-inflammatory response, and inhibiting apoptosis and other aspects of exosome-based promotion of IVD repair. Unfortunately, there is no study on how exosomes promote the proliferation mechanism of NPCs. One of the most important reasons for the degeneration of the disc is the decrease in the number of cells, and how to restore the number of cells in the disc is a key question in treatment. Alternatively, in the process of IVD degeneration, the senescence of NPCs is also an important factor. Some molecules have also been found in exosomes that can inhibit the senescence of cells. Previous research has mainly focused on specific miRNAs in exosome-mediated apoptosis, but are there additional miRNAs in exosomes that promote the proliferation and inhibit the aging of NPCs? Furthermore, how do exosomes inhibit inflammation and promote mitochondrial homeostasis, and how are other molecules in exosomes, including lipids, proteins, mRNAs, and lncRNAs, involved?
The unique double-layered membrane structure of exosomes makes their contents difficult to degrade by various enzymes in body fluids. The unique shape, size, and density range of exosomes, as well as the special molecular markers on their surface, enable their identification and isolation. Animal experiments have confirmed that exosomes are more efficient at delivering effective content into cells and cause a lower immune response in recipients than other methods. By overexpressing miRNAs targeting specific mRNAs in donor cells, exosomes promote cell proliferation, inhibit apoptosis, and promote the production of the extracellular matrix[28]. Engineered exosomes can also be loaded with miRNA synthesized in vitro by electroporation and can then be injected into tissues to achieve therapeutic goals[71]. Exosomes may also be combined with scaffold material to promote IVD regeneration. In a study by Liu et al[72], photoinduced imine crosslinking hydrogel glue combined with stem cell-derived exosomes promoted defective cartilage repair and regeneration[72]. Therefore, an increasing number of studies have shown that exosomes are a promising method for the treatment of disc degeneration. However, there are still many challenges and disadvantages.
First, the physiological environment of the IVD is complex. As the largest avascular tissue in the body, long-term internal high pressure, high permeability, low pH, low nutrition, and low oxygen make it not suitable for cell proliferation[73]. In the degenerated IVD, the complex inflammatory environment, the decrease in the number of cells, and fibrosis may affect in vivo as in vitro results being the same. Additionally, IVD degeneration is a pathological process involving multiple factors, and the exact mechanism has not yet been determined. Therefore, choosing the appropriate exosomes for specific causes is very important.
Second, the exact mechanism of exosomal biogenesis needs to be further investigated. Exosomes are derived from endosomes after cell endocytosis. After processing, they may join multivesicular bodies (MVBs) and may contain proteins, nucleic acids, lipids, cholesterol, and other biologically active molecules; further, they may be secreted by exocytosis or may be encountered by a lysosome and become degraded[29]. This is a complex set of biological process, and more research needs to be done to determine the specific mechanisms. An increasing number of studies have demonstrated that exosome-mediated effects are mainly due to the contents of the exosomes, such as miRNAs, lncRNAs, and other molecules. Understanding how cells assemble these molecules in exosomes will enable additional exosomes to be harvested. The isolation of exosomes also has limitations regardless of the current methods being used, such as ultracentrifugation, ultrafiltration, and chromatography. Therefore, new methods need to be developed to improve the isolation and purity of exosomes.
Moreover, exosomes, as a collection of various biologically active molecules, are also affected by various factors, such as the source of cells, the status of cell growth, the conditions of culture, and even the consistency and reproducibility of their effects. All of those factors need to be considered. As a special carrier in the treatment of diseases, the application of exosomes still faces a series of problems, such as dosage, mode of administration, and evaluation of the efficacy.
Exosomes are attracting increasing attention because of their unique structures and diverse properties. Exosomes have shown favorable possibilities during the repair of IVD, since they can promote the proliferation of NPCs, promote the homeostasis of the extracellular matrix, and inhibit cell apoptosis (Figure 1). However, the detailed mechanisms behind these activities are still unclear, so further research is needed to explore the complex regulation mechanisms, optimize the culture and transplant conditions, and perform more preclinical trials to verify the safety of exosomes.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Oltra E, Tanabe S S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation. 2014;17 Suppl 2:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 353] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 2. | Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 1916] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 3. | Davis MA, Onega T, Weeks WB, Lurie JD. Where the United States spends its spine dollars: expenditures on different ambulatory services for the management of back and neck conditions. Spine (Phila Pa 1976). 2012;37:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Dunn A, Grosse SD, Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Serv Res. 2018;53:175-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 5. | Urits I, Burshtein A, Sharma M, Testa L, Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B, Kaye AD. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr Pain Headache Rep. 2019;23:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 6. | Bikbov MM, Kazakbaeva GM, Zainullin RM, Salavatova VF, Gilmanshin TR, Arslangareeva II, Nikitin NA, Mukhamadieva SR, Yakupova DF, Panda-Jonas S, Khikmatullin RI, Aminev SK, Nuriev IF, Zaynetdinov AF, Uzianbaeva YV, Jonas JB. Prevalence of and factors associated with low Back pain, thoracic spine pain and neck pain in Bashkortostan, Russia: the Ural Eye and Medical Study. BMC Musculoskelet Disord. 2020;21:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Grunhagen T, Shirazi-Adl A, Fairbank JC, Urban JP. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465-477, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 9. | Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 10. | Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 869] [Cited by in RCA: 881] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 11. | Chen BL, Guo JB, Zhang HW, Zhang YJ, Zhu Y, Zhang J, Hu HY, Zheng YL, Wang XQ. Surgical versus non-operative treatment for lumbar disc herniation: a systematic review and meta-analysis. Clin Rehabil. 2018;32:146-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976). 2006;31:742-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976). 2006;31:2909-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Ural IH, Alptekin K, Ketenci A, Solakoglu S, Alpak H, Özyalçın S. Fibroblast Transplantation Results to the Degenerated Rabbit Lumbar Intervertebral Discs. Open Orthop J. 2017;11:404-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Sheyn D, Ben-David S, Tawackoli W, Zhou Z, Salehi K, Bez M, De Mel S, Chan V, Roth J, Avalos P, Giaconi JC, Yameen H, Hazanov L, Seliktar D, Li D, Gazit D, Gazit Z. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics. 2019;9:7506-7524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Jeong JH, Jin ES, Min JK, Jeon SR, Park CS, Kim HS, Choi KH. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology. 2009;59:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Gloria A, Russo T, D'Amora U, Santin M, De Santis R, Ambrosio L. Customised multiphasic nucleus/annulus scaffold for intervertebral disc repair/regeneration. Connect Tissue Res. 2020;61:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Doench I, Torres-Ramos MEW, Montembault A, Nunes de Oliveira P, Halimi C, Viguier E, Heux L, Siadous R, Thiré RMSM, Osorio-Madrazo A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers (Basel). 2018;10:1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Sun W, Zhang K, Liu G, Ding W, Zhao C, Xie Y, Yuan J, Sun X, Li H, Liu C, Tang T, Zhao J. Sox9 gene transfer enhanced regenerative effect of bone marrow mesenchymal stem cells on the degenerated intervertebral disc in a rabbit model. PLoS One. 2014;9:e93570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Ren S, Liu Y, Ma J, Liu Y, Diao Z, Yang D, Zhang X, Xi Y, Hu Y. Treatment of rabbit intervertebral disc degeneration with co-transfection by adeno-associated virus-mediated SOX9 and osteogenic protein-1 double genes in vivo. Int J Mol Med. 2013;32:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Barakat AH, Elwell VA, Lam KS. Stem cell therapy in discogenic back pain. J Spine Surg. 2019;5:561-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, Hara E. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 587] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 23. | Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, Yu Y, Huang H, Hu Y, Yang Z, Yang J, Shen Z. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018;2018:3290372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 400] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 25. | Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1466] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 26. | Lu K, Li HY, Yang K, Wu JL, Cai XW, Zhou Y, Li CQ. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K, Hua W, Zhang Y, Wu X, Yang C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084-4100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 28. | Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, Zhao C, Li H, Li YM, Zhao J. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22:261-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 29. | Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3378] [Cited by in RCA: 3537] [Article Influence: 321.5] [Reference Citation Analysis (0)] |
| 30. | Battistelli M, Falcieri E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology (Basel). 2020;9:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 288] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 31. | Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7:1522236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 32. | Zhang W, Jiang H, Kong Y. Exosomes derived from platelet-rich plasma activate YAP and promote the fibrogenic activity of Müller cells via the PI3K/Akt pathway. Exp Eye Res. 2020;193:107973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Ermakov KV, Bukhvostov AA, Vedenkin AS, Stovbun SV, Dvornikov AS, Kuznetsov DA. Ultrashort ssDNA in Retinoblastoma Patients Blood Plasma Detected by a Novel High Resolution HPLC Technique: a Preliminary Report. Acta Medica (Hradec Kralove). 2019;62:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Tracy SA, Ahmed A, Tigges JC, Ericsson M, Pal AK, Zurakowski D, Fauza DO. A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes: Bone marrow and amniotic fluid. J Pediatr Surg. 2019;54:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Dixon CL, Sheller-Miller S, Saade GR, Fortunato SJ, Lai A, Palma C, Guanzon D, Salomon C, Menon R. Amniotic Fluid Exosome Proteomic Profile Exhibits Unique Pathways of Term and Preterm Labor. Endocrinology. 2018;159:2229-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 36. | Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Miyake H, Lee C, Chusilp S, Bhalla M, Li B, Pitino M, Seo S, O'Connor DL, Pierro A. Human breast milk exosomes attenuate intestinal damage. Pediatr Surg Int. 2020;36:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 38. | Hock A, Miyake H, Li B, Lee C, Ermini L, Koike Y, Chen Y, Määttänen P, Zani A, Pierro A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 39. | Danarto R, Astuti I, Umbas R, Haryana SM. Urine miR-21-5p and miR-200c-3p as potential non-invasive biomarkers in patients with prostate cancer. Turk J Urol. 2020;46:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Duan YR, Chen BP, Chen F, Yang SX, Zhu CY, Ma YL, Li Y, Shi J. Exosomal microRNA-16-5p from human urine-derived stem cells ameliorates diabetic nephropathy through protection of podocyte. J Cell Mol Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 41. | Domenis R, Zanutel R, Caponnetto F, Toffoletto B, Cifù A, Pistis C, Di Benedetto P, Causero A, Pozzi M, Bassini F, Fabris M, Niazi KR, Soon-Shiong P, Curcio F. Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis. Mediators Inflamm. 2017;2017:4814987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, Isales CM, Guldberg RE, Hamrick MW, Fulzele S. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7:2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 43. | Ogawa Y, Miura Y, Harazono A, Kanai-Azuma M, Akimoto Y, Kawakami H, Yamaguchi T, Toda T, Endo T, Tsubuki M, Yanoshita R. Proteomic analysis of two types of exosomes in human whole saliva. Biol Pharm Bull. 2011;34:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 44. | Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 588] [Cited by in RCA: 558] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 45. | Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2443] [Cited by in RCA: 3722] [Article Influence: 195.9] [Reference Citation Analysis (0)] |
| 46. | Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1505] [Cited by in RCA: 1404] [Article Influence: 234.0] [Reference Citation Analysis (0)] |
| 47. | EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2570] [Article Influence: 214.2] [Reference Citation Analysis (0)] |
| 48. | Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1028] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 49. | Conigliaro A, Fontana S, Raimondo S, Alessandro R. Exosomes: Nanocarriers of Biological Messages. Adv Exp Med Biol. 2017;998:23-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60:9-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 51. | Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem Rev. 2018;118:1917-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1146] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 52. | Winczura K, Domanski M, LaCava J. Affinity Proteomic Analysis of the Human Exosome and Its Cofactor Complexes. Methods Mol Biol. 2020;2062:291-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Wei Z, Batagov AO, Schinelli S, Wang J, Wang Y, El Fatimy R, Rabinovsky R, Balaj L, Chen CC, Hochberg F, Carter B, Breakefield XO, Krichevsky AM. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8:1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 386] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 54. | Fu Y, Zhang L, Zhang F, Tang T, Zhou Q, Feng C, Jin Y, Wu Z. Exosome-mediated miR-146a transfer suppresses type I interferon response and facilitates EV71 infection. PLoS Pathog. 2017;13:e1006611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 55. | Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 386] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 56. | Shyu KG, Wang BW, Pan CM, Fang WJ, Lin CM. Hyperbaric oxygen boosts long noncoding RNA MALAT1 exosome secretion to suppress microRNA-92a expression in therapeutic angiogenesis. Int J Cardiol. 2019;274:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Tafrihi M, Hasheminasab E. MiRNAs: Biology, Biogenesis, their Web-based Tools, and Databases. Microrna. 2019;8:4-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 58. | Khorkova O, Hsiao J, Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 2015;87:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 59. | Li X, Wu Z, Fu X, Han W. lncRNAs: insights into their function and mechanics in underlying disorders. Mutat Res Rev Mutat Res. 2014;762:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 60. | Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, Li J, Zhang G, Huang J, Lin Z, Xiong N, Wang T. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016;2016:7653489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 61. | Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1462] [Cited by in RCA: 1399] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 62. | Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W, Wang H, Liu H, Zhou H, Chen Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 63. | An Y, Zhao J, Nie F, Qin Z, Xue H, Wang G, Li D. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci Rep. 2019;9:12861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 64. | Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 65. | Wuertz K, Godburn K, Neidlinger-Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine (Phila Pa 1976). 2008;33:1843-1849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 66. | Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 488] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 67. | Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473:1903-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 68. | Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1254] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 69. | Xia C, Zeng Z, Fang B, Tao M, Gu C, Zheng L, Wang Y, Shi Y, Fang C, Mei S, Chen Q, Zhao J, Lin X, Fan S, Jin Y, Chen P. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 70. | Patil P, Niedernhofer LJ, Robbins PD, Lee J, Sowa G, Vo N. Cellular senescence in intervertebral disc aging and degeneration. Curr Mol Biol Rep. 2018;4:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 71. | Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, Sun B, Chen B, Xiao Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 433] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 72. | Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, Zhu L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430-4438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 73. | Gruber HE, Ingram JA, Norton HJ, Hanley EN. Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine (Phila Pa 1976). 2007;32:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 74. | Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J, Chen D. Exosome-Induced Regulation in Inflammatory Bowel Disease. Front Immunol. 2019;10:1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 75. | Deng H, Sun C, Sun Y, Li H, Yang L, Wu D, Gao Q, Jiang X. Lipid, Protein, and MicroRNA Composition Within Mesenchymal Stem Cell-Derived Exosomes. Cell Reprogram. 2018;20:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 76. | Jan AT, Rahman S, Khan S, Tasduq SA, Choi I. Biology, Pathophysiological Role, and Clinical Implications of Exosomes: A Critical Appraisal. Cells. 2019;8:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 77. | Huo C, Li Y, Qiao Z, Shang Z, Cao C, Hong Y, Xiao H. [Proteomics analysis of serum exosomes and its application in osteoporosis]. Se Pu. 2019;37:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 1052] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 79. | Moen A, Jacobsen D, Phuyal S, Legfeldt A, Haugen F, Røe C, Gjerstad J. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J Transl Med. 2017;15:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Bach F, Libregts S, Creemers L, Meij B, Ito K, Wauben M, Tryfonidou M. Notochordal-cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Oncotarget. 2017;8:88845-88856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |