Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.721
Peer-review started: May 6, 2020
First decision: May 15, 2020
Revised: May 21, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: August 26, 2020
Processing time: 111 Days and 23.9 Hours
Coronavirus disease-2019 (COVID-19) has affected more than 200 countries worldwide. This disease has hugely affected healthcare systems as well as the economy to an extent never seen before. To date, COVID-19 infection has led to about 165000 deaths in 150 countries. At present, there is no specific drug or efficient treatment for this disease. In this analysis based on evidential relationships of the biological characteristics of MSCs, especially umbilical cord (UC)-derived MSCs as well as the first clinical trial using MSCs for COVID-19 treatment, we discuss the use of UC-MSCs to improve the symptoms of COVID-19 in patients.
Core tip: Based on the biological characteristics of mesenchymal stem cells (MSCs), especially umbilical cord (UC)-derived MSCs, and the first clinical trial using MSCs for coronavirus disease-2019 (COVID-19) treatment, we discuss the use of UC-MSCs to improve COVID-19 symptoms. UC-MSCs are suitable therapeutic candidates for COVID-19. Indeed, they are the strongest immunomodulatory MSCs and exhibit low immunogenicity compared with bone marrow-derived MSCs, adipose tissue-derived MSCs, and other MSCs. UC-MSCs are easily collected and expanded in vitro with minimal ethical concerns. In summary, UC-MSCs can be used to improve symptoms in patients with severe COVID-19 by suppressing inflammation and stimulating wound healing.
- Citation: Pham PV, Vu NB. Off-the-shelf mesenchymal stem cells from human umbilical cord tissue can significantly improve symptoms in COVID-19 patients: An analysis of evidential relations. World J Stem Cells 2020; 12(8): 721-730
- URL: https://www.wjgnet.com/1948-0210/full/v12/i8/721.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i8.721
Coronaviruses express spike glycoprotein on their surfaces, which facilitates binding and entry into host cells via angiotensin I-converting enzyme 2 (ACE2) receptor expressed on host cells[1,2]. Although this receptor exists in almost all cells of the human body, it is highly expressed in alveolar type II (AT2) cells, the capillary endothelium, endothelial cells, and smooth muscle cells[3]. Therefore, all of these cells can be infected by a novel coronavirus (nCoV).
Similar to other viral infectious diseases, virus-infected cells are recognized and killed by the immune system of patients. With multiple affected organs, the host immune system is activated to kill the virus and virus-infected cells through activation of immune cells, such as natural killer (NK) cells, which produce a large number of inflammatory factors. This so-called severe cytokine storm entails the release of cytokines such as interleukin 2 (IL-2), IL-6, IL-7, granulocyte-colony stimulating factor (G-CSF), interferon-inducible protein of 10 kD, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-alpha (MIP-1A), and tumor necrosis factor alpha (TNF-α). In addition to affecting the virus and virus-infected cells, these immune cells and their cytokines have an extreme effect on normal cells, causing edema, dysfunction of air exchange, acute respiratory distress syndrome (ARDS), and acute cardiac injury. As a result, there are multiple injuries in multiple organs and tissues. Moreover, these injured tissues and organs can be infected by other microorganisms. After entry of coronavirus into ACE2 receptor-expressing cells, multiple organs are injured in patients, which subsequently leads to death. Examinations of peripheral blood from coronavirus disease-2019 (COVID-19) patients have revealed increases of inflammatory factors such as IL-6, IL-10, and TNF-α[4]. In intensive care unit patients, the plasma levels of IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A, and TNF-α also increase[5].
It appears that the death of nCoV-infected patients is related to the injury and failure of multiple organs, which is triggered by the host immune system. The positive feedback regulation of the immune system not only allows attack of the virus and virus-infected cells but can also destroys healthy cells, leading to severe injury in some vital organs such as the lungs, heart, liver, and kidneys. In two reports about the clinical features of COVID-19 patients in Wuhan, China, COVID-19 was found to cause complications such as ARDS, arrhythmia, shock[6], severe kidney injury, acute cardiac injury, liver dysfunction, and secondary infection[7].
Current treatments for COVID-19 are mainly focused on symptomatic and respiratory support. Almost all patients receive oxygen therapy and rescue treatment with convalescent plasma, and in some critical cases, immunoglobulin G is applied. In addition to some anti-viral drugs, chloroquine has emerged as a repurposed drug with a great potential to treat COVID-19[8,9]. Indeed, chloroquine inhibits the replication of several viruses[10-12] and has immunomodulatory effects such as suppressing the production and release of TNF-α and IL-6[13]. Although the use of chloroquine/ hydroxychloroquine may be promising, their use should be restricted to clinical trials. Indeed, to date, there have been 17 clinical trials regarding the use of chloroquine/hydroxychloroquine for COVID-19 worldwide, but none have been completed[14]. Thus, there is insufficient evidence to recommend them for routine treatment of COVID-19.
Mesenchymal stem cells (MSCs) are the most common stem cells in the human body. They exist in almost all tissues and organs, especially bone marrow and adipose tissue[15,16]. These cells have been isolated from umbilical cord tissue[17-19] and umbilical cord blood[20,21]. They are characterized by their shape when adhered onto the surface of a tissue culture vessel and the expression profile of markers (positive for cluster of differentiation 44 [CD44], CD73, CD90, and CD105, while negative for CD14, CD34, CD45, and human leukocyte antigen DR [HLA-DR]). They are also unique in their potential to differentiate into mesodermal cells including adipocytes, osteoblasts, and chondrocytes[22,23].
MSCs have been used clinically to treat more than 10 different diseases[24,25]. Importantly, MSC transplantations have been approved in some countries to treat various inflammatory diseases[26,27]. In Canada, since 2012, bone marrow-derived MSCs have been approved for graft-versus-host disease (GVHD) treatment (Prochymal)[28,29]. Also in that year, umbilical cord blood-derived MSCs (UC-MSCs) were used in South Korea to treat knee osteoarthritis (Cartistem)[30]. Recently, the Japanese government has approved HS Temcell, which contains allogeneic MSCs from bone marrow for GVHD treatment[31,32]. In Europe, adipose-derived MSCs have been approved for Crohn’s disease[33]. Indeed, there have been many products related to clinical MSC transplantation, which have been used or are being investigated in clinical trials in several countries[34].
MSC transplantation for pulmonary diseases has also been carried out with positive outcomes[35,36]. In our recent publication, we showed that transplantation of UC-MSCs significantly improved symptoms of chronic obstructive pulmonary disease (COPD) in late-stage patients[37].
Lastly, a clinical trial by Leng et al[38] (2020) showed that transplantation of MSCs was a safe and effective treatment for patients with COVID-19, especially critically severe patients. Seven patients in the study were cured or showed significant improvement of pulmonary functions with symptoms of COVID-19, but without observed adverse effects. Interestingly, C-reactive protein (CRP) and TNF-α were decreased, and cytokine-secreting immune cells, such as CXCR3+CD4+ T cells, CXCR3+CD8+ T cells, and CXCR3+ NK cells, disappeared after 3-6 d of treatment[38].
Although the role of MSCs in killing nCoV has not been reported, some published studies have shown that MSCs attack bacteria and viruses[39-44]. In 2009, Gonzalez et al[39] reported that MSC transplantation reduces the number of bacterial colony-forming units in the blood, liver, spleen, and peritoneal fluid of septic mice. The mechanism of the anti-bacterial activity of MSCs remains unclear. Some reports have suggested that MSCs enhance phagocytosis of monocytes[40,41]. An increase of C5a complement was found by Krasnodembskaya et al[41] (2012). The anti-bacterial activity is also enhanced by triggering the phagocytic index of macrophages by transferring MSC-derived mitochondria to macrophages[42,43].
The patent WO2010053350A1 from Erasmus University Medical Center in Rotterdam , Netherlands, describes methods to produce concentrated MSC-conditioned medium (C-MSC-CM) that retains potent anti-viral activity. C-MSC-CM inhibits hepatitis C virus (HCV) and hepatitis B virus replication. Recent studies have suggested that MSCs also display anti-viral activities. For example, the study by Qian et al[44] (2016) was the first to demonstrate that MSCs inhibit HCV infection via their exosomes. The authors showed that exosomes from MSCs contained functional miRNAs, mainly let-7f, miR-145, miR-199a, and miR-221. These microRNAs possess binding sites for HCV RNA.
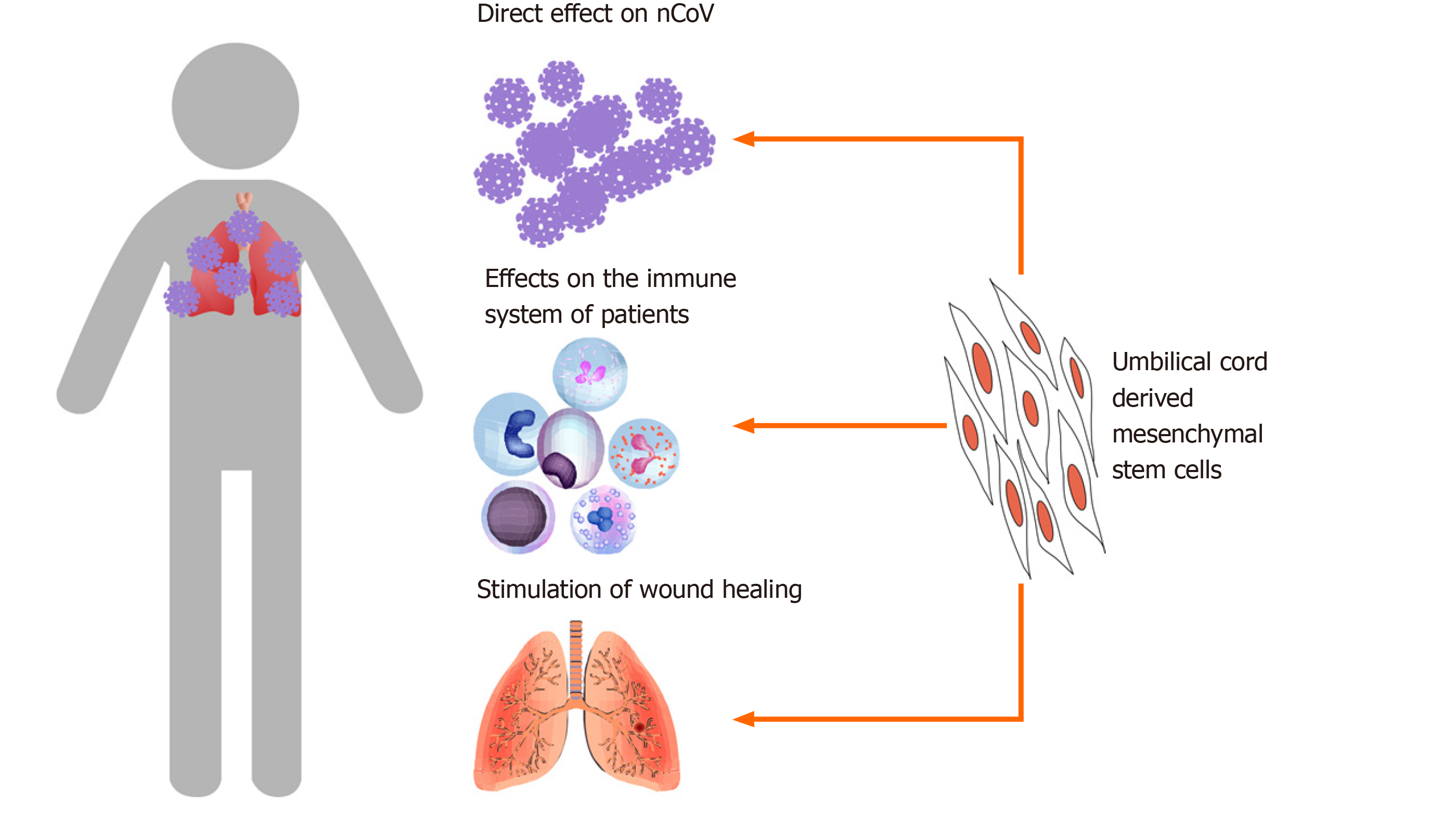
The primary mechanism by which MSCs improve the symptoms of COVID-19 is related to their immunomodulation potential (Figure 1). MSCs efficiently regulate inflammation by suppression of effector immune cells, upregulation of regulatory T cells, and inhibition of T-cell activation[45-47]. Therefore, MSCs have great potential to treat immune diseases, especially acute and chronic inflammation[48-51].
In the report by Leng et al[38] (2020), the levels of CRP and TNF-α were both reduced in treated COVID-19 patients. These observations are similar to those previously published on MSC transplantation for immunological diseases[52,53]. MSCs also inhibit the maturation of T cells and prevent them from becoming too active[54,55]. This may be why some cytokine-secreting immune cells decrease significantly in treated COVID-19 patients.
Indeed, under in vitro conditions, MSCs have been observed to inhibit the production of TNF-α by immune cells. Zheng et al[56] (2008) reported that allogenic MSCs inhibit both CD4+ and CD8+ T cells from producing interferon gamma (IFN-γ) and TNF-α. Another study showed that MSCs inhibit macrophages from producing proinflammatory cytokines such as IFN-γ, IL-6, and TNF-α[57].
Similar to sepsis and septic shock, patients infected with COVID-19 experience a severe cytokine storm that is harmful and ultimately leads to death. Preclinical trials in rodents showed that MSC transplantation improves sepsis and septic shock[58-61]. In a meta-analysis, Lalu et al[62] (2016) analyzed 20 controlled comparison experiments (980 animals from 18 publications) of in vivo sepsis models and found that MSC transplantation significantly reduced mortality under a range of experimental conditions. Some current clinical trials have reported that MSC transplantation is safe in severe sepsis patients[63].
Several tissues and organs affected by activated immune cells and severe cytokine storms can be injured. MSCs contribute to stimulating the wound healing process. In preclinical and clinical trials, MSC administration facilitated healing a range of injuries. Some publications have shown that MSC transplantation triggers lung injury healing. In models of radiation-induced lung injury in rats and mice, MSC transplantation significantly reduced pulmonary radiation fibrosis[64], reduced serum levels of IL-1, IL-6, and TNF-α[65], and inhibited fibrosis[66].
The roles of MSCs in wound healing appear to be related to the growth factors that they produce and secrete into the medium. These factors include epidermal growth factor, fibroblast growth factor, and insulin-like growth factor. All of these growth factors strongly stimulate wound healing. Moreover, MSCs promote wound healing via their exosomes (extracellular vesicles) that are secreted and targeted to injured tissues. These growth factors and exosomes produced by MSCs enable MSCs to exert their anti-apoptosis effects, rescuing apoptotic cells from traumatic exposure to hypoxia, chemicals/acidity, and mechanical damage or radiation[67-69].
Although MSCs provide benefits for COVID-19 patients via these possible mechanisms, the treatment efficacy of COVID-19 by MSC transplantation is variable among patients because of the complexity of COVID-19. The stage of COVID-19 may be the main issue affecting treatment efficacy. MSCs appear to be effective to control inflammation and the cytokine storm by immunomodulation. This effect also reduces the immune response to virus-infected cells and bacteria. Therefore, the risk of bacterial infection can be increased in MSC-transplanted COVID-19 patients. The rejection response of recipients versus transplanted allogenic MSCs should also be considered and monitored. Theoretically, MSCs do not express HLA. Hence, they can be used in allogenic transplantation. However, expression of HLA can be upregulated after MSCs are transplanted into patients and differentiate into specialized cells. Indeed, chronic rejection of transplanted allogenic MSCs decreases the treatment efficacy and causes slight fever for a long time, even after successful treatment of COVID-19.
The treatment efficacy also depends on several factors included the MSC type, quality of MSCs, and dose of MSCs. MSCs can be obtained from various tissues for clinical treatment, but MSCs from different sources usually exhibit different biological characteristics, especially immunomodulation, as well as angiogenic characteristics. These characteristics play important roles in COVID-19 treatment. The therapeutic characteristics can also be affected by the biological status, especially the senescent status, tissue donation, and culture conditions for in vitro expansion. Indeed, some studies have shown that immunomodulation of MSCs is reduced significantly in the senescent phase and in MSCs derived from older individuals. In vitro expansion conditions significantly affect MSC immunomodulation. Chen et al[70] (2017) showed that MSCs expanded in a 3D-stirred tank bioreactor/microcarrier culture system display better immunomodulation than those expanded in flasks. Some recent studies have shown MSCs expanded in a platelet lysate have a superior capacity to suppress the proliferation of allogeneic lymphocytes than those expanded in fetal bovine serum[71,72]. Kabat et al[73] (2020) analyzed 914 MSC trials reported through 2018, concerning the MSC dose for transplantation. They found that the minimal effective dose (MED) of MSCs ranged from 70 to 190 million MSCs/patient/dose. Lower or higher intravenous doses of MSC transplantation were less effective. Indeed, the dose of MSCs directly affected the immunomodulation response in recipients. At a lower dose of MSCs than the MED, the immunomodulation response was insufficient to induce therapeutic effects in patients. However, at higher doses, MSCs caused too much immunomodulation that increased side effects. Therefore, determining the optimal dose of MSCs for transplantation is important for effective treatment with the lowest side effects.
Although MSCs can be obtained from several tissue sources, it appears that UC-MSCs are optimal to treat COVID-19. The first reason is that transplantation of UC-MSCs is safe and effective in humans for sepsis and chronic inflammation. He et al[63] (2018) reported no serious adverse events associated with infusion of UC-MSCs in 15 patients. At a high dose of UC-MSCs (3 × 106 cells/kg), the allogenic UC-MSCs were also found to be safe and well tolerated in the 15 patients with severe sepsis. Additionally, in chronic inflammation such as COPD, we confirmed that infusion of allogenic UC-MSCs is safe[37].
UC-MSCs have great potential and suitability for COVID-19 treatment because of their useful properties. A publication in 2019 demonstrated that MSCs from Wharton’s Jelly (a part of the UC) improve bacterial clearance and survival in sepsis mouse models, whereas bone marrow-derived MSCs did not have these effects[74]. Several studies have suggested that UC-MSCs perform their immunomodulation better than bone marrow-derived MSCs and adipose-derived MSCs[75,76]. This therapeutic effect is obtained by the activatable status of UC-MSCs. Selich et al[77] (2019) demonstrated that UC-MSCs are activatable for immunomodulation, whereas bone marrow-derived MSCs[78,79] and adipose-tissue derived MSCs[80] are activated by TNF-α[80], IFN-γ[79], and lymphocyte extracts[78] to trigger immunomodulation. UC-MSCs also express higher levels of immunomodulatory surface proteins, such as CD200, CD273, and CD274, and cytokines such as IL-1β, IL-8, leukemia inhibitory factor, and TGF-β2 compared with bone marrow-derived MSCs[81], which makes them the strongest immunomodulatory MSCs.
UC-MSCs are suitable sources of allogenic MSCs with low immunogenicity and high yield manufacturing. In 2018, Kim et al[76] compared the immunological characteristics of MSCs derived from the periodontal ligament, umbilical cord, and adipose tissue. They found that UC-MSCs expressed minimal levels of HLA-DR and HLA-ABC after activation by IFN-γ[76] compared with adipose tissue- and periodontal ligament-derived MSCs. Li et al[82] (2018) also obtained similar findings in which UC-MSCs displayed the strongest immunomodulatory ability compared with MSCs from exfoliated deciduous teeth, bone marrow, and gingival tissues. After treatment with IFN-γ, UC-MSCs expressed HLA-DR at a low level compared with those from other sources, whereas bone marrow-derived MSCs expressed HLA-DR at the highest level compared with those from other sources[82]. High expression of HLA-DR or HLA-ABC is a major obstacle for allogenic transplantation.
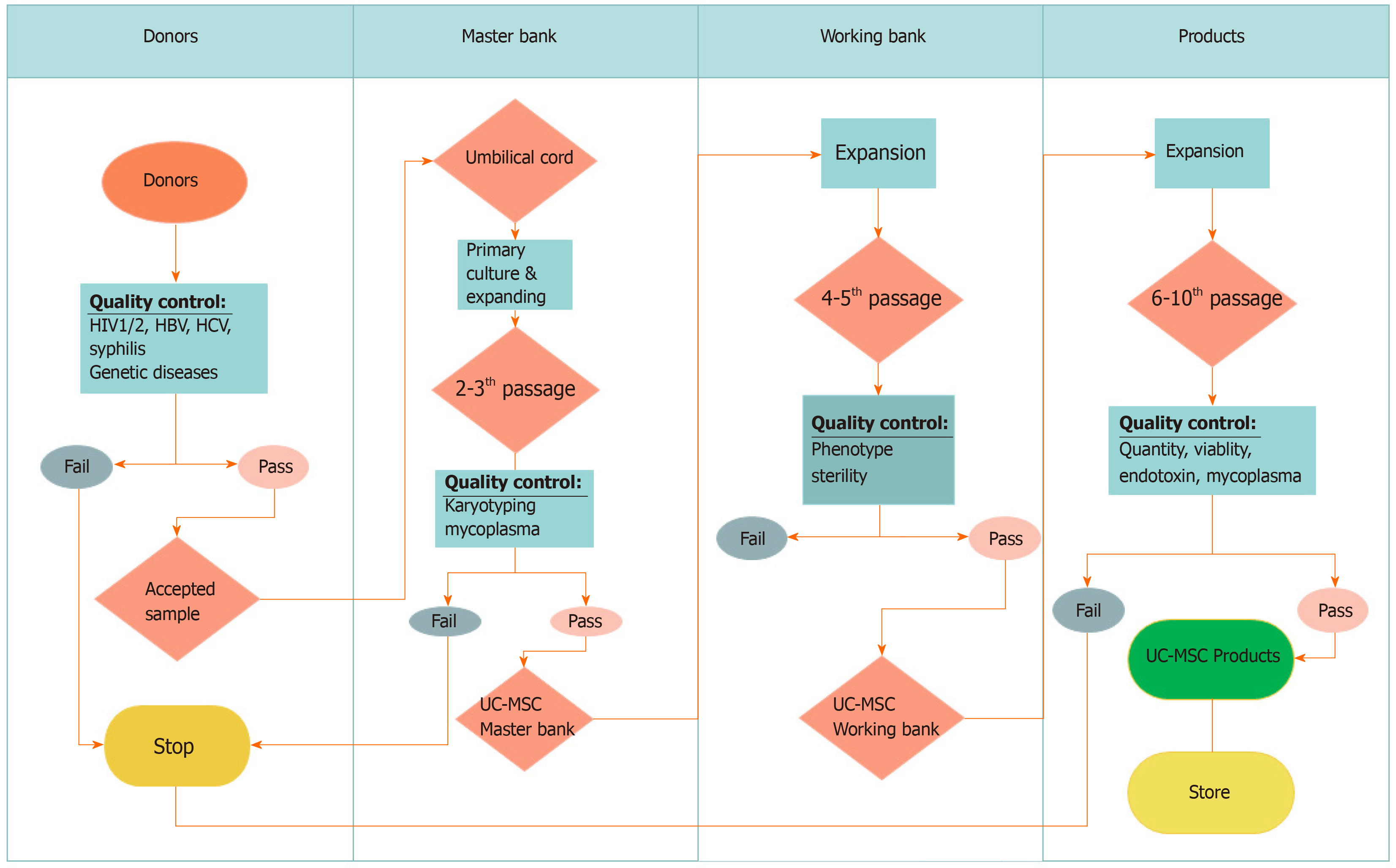
Moreover, UC-MSCs are easily collected and expanded in vitro with minimal ethical concerns. UC-MSCs also grow faster than adipose tissue- and periodontal ligament-derived MSCs[76]. UC-MSCs can be obtained easily from the umbilical cord, according to GMP-compliant conditions for clinical usage[19,37] with established high yield manufacturing[83] (Figure 2). These characteristics suggest that UC-MSCs are suitable MSC therapeutic candidates for COVID-19.
Clinically, patients with COVID-19 have two phases of immunoresponses after nCoV infection. The first phase is the incubation and non-severe stages in which the immunoresponse of patients is triggered to eliminate the virus. The second phase occurs when the immune system fails to eliminate the severe acute respiratory syndrome coronavirus 2, which will cause the severe stage because of severely damaged lungs. These differences in the immunoresponse suggest that different approaches are needed to treat COVID-19 at incubation and severe stages.
In the first stage, some strategies related to boosting the patient’s immune system may provide good outcomes, whereas lung inflammation control is important for severe-stage patients. Xu et al[84] (2020) reported that lung inflammation is the main cause of life-threatening respiratory disorders. UC-MSC transplantation that suppresses inflammation and manages symptoms appears to be effective to treat severe-stage COVID-19 patients.
COVID-19 patients should be confirmed by a real-time reverse transcription polymerase chain reaction assay and classified as the severe stage of COVID-19 that released by National Health Commission of China included respiratory distress, RR ≥ 30/min; oxygen saturation ≤ 93% at rest; arterial partial pressure of oxygen/fraction of inspiration O2 ≤ 300 mmHg, 1 mmHg = 0.133 kPa[38].
Based on the above analyses, severe-stage COVID-19 patients would be transfused intravenously with 1 million thawed off-the-shelf UC-MSCs per kilogram of body weight. Inflammatory markers, including cytokines (CRP and TNF-α) and cytokine-secreting immune cells (CXCR3+CD4+ T cells, CXCR3+CD8+ T cells, and CXCR3+ NK cells) should be monitored everyday post-stem cell transplantation. The UC-MSC transplantation should be repeated with the same dose of off-the-shelf UC-MSCs after 2 wk. Decreases of inflammatory cytokine concentrations in peripheral blood are a good indicator of reduced inflammation in combination with other improved symptoms. Other therapies should be maintained during UC-MSC transplantation until COVID-19 symptoms clearly improve.
COVID-19 has spread rapidly across the globe. The World Health Organization officially declared COVID-19 as a public health emergency of international concern. In addition to anti-viral drugs and oxygen therapy, MSC transplantation is a therapeutic option for the treatment of COVID-19. Based on the related evidence of COVID-19, the biology of UC-MSCs, and the initial results of a clinical trial using MSCs for COVID-19 treatment, we believe that off-the-shelf UC-MSC transplantation may be an additional therapy to improve treatment efficacy, especially in critically ill COVID-19 patients.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership(s) in Professional Societies: International Society of Cell and Gene Therapy; and International Society of Stem Cell Research.
Specialty type: Cell and tissue engineering
Country/Territory of origin: Viet Nam
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen CM, Elhamid SA, Fatkhudinov TK, Tanabe S S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX
| 1. | Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3747] [Cited by in RCA: 3308] [Article Influence: 551.3] [Reference Citation Analysis (0)] |
| 2. | Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 558] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 3. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 4. | Gao G, Chen L, Huang C. Anti-cancer drug discovery: update and comparisons in yeast, Drosophila, and zebrafish. Curr Mol Pharmacol. 2014;7:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30122] [Article Influence: 6024.4] [Reference Citation Analysis (3)] |
| 6. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 7. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18201] [Article Influence: 3640.2] [Reference Citation Analysis (0)] |
| 8. | Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 1602] [Article Influence: 320.4] [Reference Citation Analysis (0)] |
| 9. | Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55:105932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 546] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 10. | Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 840] [Cited by in RCA: 837] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 11. | Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 657] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 12. | Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55:105938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 674] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 13. | Jang CH, Choi JH, Byun MS, Jue DM. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford). 2006;45:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Palmeira VA, Costa LB, Perez LG, Ribeiro VT, Lanza K, Silva ACSE. Do we have enough evidence to use chloroquine/hydroxychloroquine as a public health panacea for COVID-19? Clinics (Sao Paulo). 2020;75:e1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 608] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 16. | Stewart MC, Stewart AA. Mesenchymal stem cells: characteristics, sources, and mechanisms of action. Vet Clin North Am Equine Pract. 2011;27:243-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Han YF, Tao R, Sun TJ, Chai JK, Xu G, Liu J. Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology. 2013;65:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 19. | Pham PV, Truong NC, Le PT, Tran TD, Vu NB, Bui KH, Phan NK. Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications. Cell Tissue Bank. 2016;17:289-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Pham PV, Vu NB, Pham VM, Truong NH, Pham TL, Dang LT, Nguyen TT, Bui AN, Phan NK. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med. 2014;12:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Fujii S, Miura Y, Iwasa M, Yoshioka S, Fujishiro A, Sugino N, Kaneko H, Nakagawa Y, Hirai H, Takaori-Kondo A, Ichinohe T, Maekawa T. Isolation of mesenchymal stromal/stem cells from cryopreserved umbilical cord blood cells. J Clin Exp Hematop. 2017;57:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 23. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1374] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 24. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1032] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 25. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1208] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 26. | Pham PV. Stem cell drugs: The next generation of pharmaceutical products. Biomed Res Ther. 2016;3:857-871. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Vu NB, Le PT-B, Truong NC, Pham PV. Off-the-shelf mesenchymal stem cell technology. Stem cell drugs-a new generation of biopharmaceuticals. Springer. 2018;119-141. |
| 28. | Reicin C, McMahon E, Chung C. Stem cell therapy regulation in canada: Implications of the prochymal approval. Westlaw J. 2012;28:1-4. |
| 29. | Rattue P. Prochymal-first stem cell drug approved. Medical News Today. 2012;22. |
| 30. | Song JS, Hong KT, Kim NM, Jung JY, Park HS, Lee SH, Cho YJ, Kim SJ. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regen Ther. 2020;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Konishi A, Sakushima K, Isobe S, Sato D. First Approval of Regenerative Medical Products under the PMD Act in Japan. Cell Stem Cell. 2016;18:434-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Najima Y, Ohashi K. Mesenchymal stem cells: The first approved stem cell drug in japan. Journal of Hematopoietic Cell Transplantation. 2017;6:125-132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Sheridan C. First off-the-shelf mesenchymal stem cell therapy nears European approval. Nat Biotechnol. 2018;36:212-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Pham PV, Nguyen HT, Vu NB. Evolution of stem cell products in medicine: Future of off-the-shelf products. Stem cell drugs-a new generation of biopharmaceuticals. Stem Cell Drugs-A New Generation of Biopharmaceuticals. 2018;93-118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Harrell CR, Sadikot R, Pascual J, Fellabaum C, Jankovic MG, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019;2019:4236973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med. 2020;14:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 37. | Le Thi Bich P, Nguyen Thi H, Dang Ngo Chau H, Phan Van T, Do Q, Dong Khac H, Le Van D, Nguyen Huy L, Mai Cong K, Ta Ba T, Do Minh T, Vu Bich N, Truong Chau N, Van Pham P. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res Ther. 2020;11:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 39. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 40. | Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 536] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 41. | Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003-L1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 42. | Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O'Kane CM, Krasnodembskaya AD. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells. 2016;34:2210-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 398] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 43. | Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, Krasnodembskaya AD. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 566] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 44. | Qian X, Xu C, Fang S, Zhao P, Wang Y, Liu H, Yuan W, Qi Z. Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl Med. 2016;5:1190-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 391] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 46. | Yarygin KN, Lupatov AY, Sukhikh GT. Modulation of Immune Responses by Mesenchymal Stromal Cells. Bull Exp Biol Med. 2016;161:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta. 2017;59:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Ansboro S, Roelofs AJ, De Bari C. Mesenchymal stem cells for the management of rheumatoid arthritis: immune modulation, repair or both? Curr Opin Rheumatol. 2017;29:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 49. | Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 810] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 50. | Liang J, Zhang H, Kong W, Deng W, Wang D, Feng X, Zhao C, Hua B, Wang H, Sun L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 51. | Lu X, Wang X, Nian H, Yang D, Wei R. Mesenchymal stem cells for treating autoimmune dacryoadenitis. Stem Cell Res Ther. 2017;8:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Gu X, Yu X, Zhao C, Duan P, Zhao T, Liu Y, Li S, Yang Z, Li Y, Qian C, Yin Z, Wang Y. Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell Physiol Biochem. 2018;49:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Karamini A, Bakopoulou A, Andreadis D, Gkiouras K, Kritis A. Therapeutic Potential of Mesenchymal Stromal Stem Cells in Rheumatoid Arthritis: a Systematic Review of In Vivo Studies. Stem Cell Rev Rep. 2020;16:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Niu J, Yue W, Le-Le Z, Bin L, Hu X. Mesenchymal stem cells inhibit T cell activation by releasing TGF-β1 from TGF-β1/GARP complex. Oncotarget. 2017;8:99784-99800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Laing AG, Fanelli G, Ramirez-Valdez A, Lechler RI, Lombardi G, Sharpe PT. Mesenchymal stem cells inhibit T-cell function through conserved induction of cellular stress. PLoS One. 2019;14:e0213170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 56. | Zheng ZH, Li XY, Ding J, Jia JF, Zhu P. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 58. | Pedrazza L, Lunardelli A, Luft C, Cruz CU, de Mesquita FC, Bitencourt S, Nunes FB, de Oliveira JR. Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflamm Res. 2014;63:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Rocheteau P, Chatre L, Briand D, Mebarki M, Jouvion G, Bardon J, Crochemore C, Serrani P, Lecci PP, Latil M, Matot B, Carlier PG, Latronico N, Huchet C, Lafoux A, Sharshar T, Ricchetti M, Chrétien F. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun. 2015;6:10145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 60. | Rojas M, Cárdenes N, Kocyildirim E, Tedrow JR, Cáceres E, Deans R, Ting A, Bermúdez C. Human adult bone marrow-derived stem cells decrease severity of lipopolysaccharide-induced acute respiratory distress syndrome in sheep. Stem Cell Res Ther. 2014;5:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Zhao X, Liu D, Gong W, Zhao G, Liu L, Yang L, Hou Y. The toll-like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem Cells. 2014;32:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 62. | Lalu MM, Sullivan KJ, Mei SH, Moher D, Straus A, Fergusson DA, Stewart DJ, Jazi M, MacLeod M, Winston B, Marshall J, Hutton B, Walley KR, McIntyre L. Evaluating mesenchymal stem cell therapy for sepsis with preclinical meta-analyses prior to initiating a first-in-human trial. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | He X, Ai S, Guo W, Yang Y, Wang Z, Jiang D, Xu X. Umbilical cord-derived mesenchymal stem (stromal) cells for treatment of severe sepsis: aphase 1 clinical trial. Transl Res. 2018;199:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Wei L, Zhang J, Yang ZL, You H. Extracellular superoxide dismutase increased the therapeutic potential of human mesenchymal stromal cells in radiation pulmonary fibrosis. Cytotherapy. 2017;19:586-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Jiang X, Jiang X, Qu C, Chang P, Zhang C, Qu Y, Liu Y. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy. 2015;17:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Wang H, Yang YF, Zhao L, Xiao FJ, Zhang QW, Wen ML, Wu CT, Peng RY, Wang LS. Hepatocyte growth factor gene-modified mesenchymal stem cells reduce radiation-induced lung injury. Hum Gene Ther. 2013;24:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Cselenyák A, Pankotai E, Horváth EM, Kiss L, Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 68. | Li N, Sarojini H, An J, Wang E. Prosaposin in the secretome of marrow stroma-derived neural progenitor cells protects neural cells from apoptotic death. J Neurochem. 2010;112:1527-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Kim SY, Lee JH, Kim HJ, Park MK, Huh JW, Ro JY, Oh YM, Lee SD, Lee YS. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol. 2012;302:L891-L908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Chen C, Tseng P, Lo W, Wang F, Lee C. Comparing the impact of 3d bioreactor and 2d culture system on immunomodulation potency of warton's jelly derived-msc. Cytotherapy. 2017;19:S186. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Kandoi S, L PK, Patra B, Vidyasekar P, Sivanesan D, S V, K R, Verma RS. Evaluation of platelet lysate as a substitute for FBS in explant and enzymatic isolation methods of human umbilical cord MSCs. Sci Rep. 2018;8:12439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Naskou MC, Sumner SM, Chocallo A, Kemelmakher H, Thoresen M, Copland I, Galipeau J, Peroni JF. Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 74. | Laroye C, Boufenzer A, Jolly L, Cunat L, Alauzet C, Merlin JL, Yguel C, Bensoussan D, Reppel L, Gibot S. Bone marrow vs Wharton's jelly mesenchymal stem cells in experimental sepsis: a comparative study. Stem Cell Res Ther. 2019;10:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Van Pham P, Vu NB, Phan NK. Umbilical cord-derived stem cells (modulatisttm) show strong immunomodulation capacity compared to adipose tissue-derived or bone marrow-derived mesenchymal stem cells. Biomed Res Ther. 2016;3:687-696. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Kim JH, Jo CH, Kim HR, Hwang YI. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018;2018:8429042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 77. | Selich A, Zimmermann K, Tenspolde M, Dittrich-Breiholz O, von Kaisenberg C, Schambach A, Rothe M. Umbilical cord as a long-term source of activatable mesenchymal stromal cells for immunomodulation. Stem Cell Res Ther. 2019;10:285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Saldaña L, Bensiamar F, Vallés G, Mancebo FJ, García-Rey E, Vilaboa N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther. 2019;10:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 79. | Cuerquis J, Romieu-Mourez R, François M, Routy JP, Young YK, Zhao J, Eliopoulos N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy. 2014;16:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 80. | Kwon YW, Heo SC, Jeong GO, Yoon JW, Mo WM, Lee MJ, Jang IH, Kwon SM, Lee JS, Kim JH. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta. 2013;1832:2136-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 81. | Bárcia RN, Santos JM, Filipe M, Teixeira M, Martins JP, Almeida J, Água-Doce A, Almeida SC, Varela A, Pohl S, Dittmar KE, Calado S, Simões SI, Gaspar MM, Cruz ME, Lindenmaier W, Graça L, Cruz H, Cruz PE. What Makes Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Superior Immunomodulators When Compared to Bone Marrow Derived Mesenchymal Stromal Cells? Stem Cells Int. 2015;2015:583984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 82. | Li J, Xu SQ, Zhao YM, Yu S, Ge LH, Xu BH. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol Med Rep. 2018;18:4969-4977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Mennan C, Garcia J, Roberts S, Hulme C, Wright K. A comprehensive characterisation of large-scale expanded human bone marrow and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019;10:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 84. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5785] [Article Influence: 1157.0] [Reference Citation Analysis (2)] |