Published online Jun 26, 2020. doi: 10.4252/wjsc.v12.i6.514
Peer-review started: February 21, 2020
First decision: March 30, 2020
Revised: April 24, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: June 26, 2020
Processing time: 125 Days and 2.7 Hours
High tibial osteotomy (HTO) is a well-established method for the treatment of medial compartment osteoarthritis of the knee with varus deformity. However, HTO alone cannot adequately repair the arthritic joint, necessitating cartilage regeneration therapy. Cartilage regeneration procedures with concomitant HTO are used to improve the clinical outcome in patients with varus deformity.
To evaluate cartilage regeneration after implantation of allogenic human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) with concomitant HTO.
Data for patients who underwent implantation of hUCB-MSCs with concomitant HTO were evaluated. The patients included in this study were over 40 years old, had a varus deformity of more than 5°, and a full-thickness International Cartilage Repair Society (ICRS) grade IV articular cartilage lesion of more than 4 cm2 in the medial compartment of the knee. All patients underwent second-look arthroscopy during hardware removal. Cartilage regeneration was evaluated macroscopically using the ICRS grading system in second-look arthroscopy. We also assessed the effects of patient characteristics, such as trochlear lesions, age, and lesion size, using patient medical records.
A total of 125 patients were included in the study, with an average age of 58.3 ± 6.8 years (range: 43-74 years old); 95 (76%) were female and 30 (24%) were male. The average hip-knee-ankle (HKA) angle for measuring varus deformity was 7.6° ± 2.4° (range: 5.0-14.2°). In second-look arthroscopy, the status of medial femoral condyle (MFC) cartilage was as follows: 73 (58.4%) patients with ICRS grade I, 37 (29.6%) with ICRS grade II, and 15 (12%) with ICRS grade III. No patients were staged with ICRS grade IV. Additionally, the scores [except International Knee Documentation Committee (IKDC) at 1 year] of the ICRS grade I group improved more significantly than those of the ICRS grade II and III groups.
Implantation of hUCB-MSCs with concomitant HTO is an effective treatment for patients with medial compartment osteoarthritis and varus deformity. Regeneration of cartilage improves the clinical outcomes for the patients.
Core tip: This is the first study to evaluate clinical outcomes and cartilage regeneration via second-look arthroscopy after implantation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) with concomitant high tibial osteotomy (HTO) for treatment of osteoarthritic knee with varus deformity. HTO treatment of medial compartment osteoarthritis of the knee with varus deformity alone does not sufficiently repair arthritic joints. However, HTO decreases pressure in the medial compartment, providing an environment in which damaged cartilage can be regenerated via implantation of allogenic hUCB-MSCs. hUCB-MSC implantation with HTO is an effective treatment for patients with osteoarthritis of the knee with varus deformity, leading to improved clinical outcomes.
- Citation: Song JS, Hong KT, Kong CG, Kim NM, Jung JY, Park HS, Kim YJ, Chang KB, Kim SJ. High tibial osteotomy with human umbilical cord blood-derived mesenchymal stem cells implantation for knee cartilage regeneration. World J Stem Cells 2020; 12(6): 514-526
- URL: https://www.wjgnet.com/1948-0210/full/v12/i6/514.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i6.514
Increased load bearing on the medial compartment of the knee joint in varus deformity, abnormally activates chondrocytes, osteoblasts, and synoviocytes. These cells, then, aberrantly secrete several inflammatory-response proteins and matrix-degrading enzymes, thereby contributing to the gradual progression of medial compartment osteoarthritis (MCOA)[1-4]. High tibial osteotomy (HTO), a well-established method for treating MCOA, provides an environment in which damaged cartilage can be regenerated via decreased medial compartment pressure[5-7]. HTO alone offers excellent short- and mid-term outcomes, however, these outcomes tend to deteriorate over time[6-10]. Cartilage regeneration procedures, such as microfracture (MFx) and autologous chondrocyte implantation (ACI) with concomitant HTO, may improve long-term outcomes in this patient population[11-17]. Although MFx and ACI are widely used for cartilage regeneration, they are not suitable for osteoarthritis (OA) therapy[18-20].
Injection or implantation of mesenchymal stem cells (MSCs) with concomitant HTO has been reported to regenerate cartilage in MCOA[21-24]. MSCs can be obtained from the bone marrow (BM), synovium, adipose tissue, and umbilical cord. These cells possess anti-inflammatory, anti-apoptotic, and anti-fibrotic properties. Moreover, MSCs facilitate chondrogenesis, demonstrate a remarkable safety profile without tumorigenecity, and have been shown to improve clinical outcomes in patients with OA[25-31]. Among the variously sourced MSCs, human umbilical cord blood-derived MSCs (hUCB-MSCs) have shown superior cartilage repair without bone formation or degeneration of the repaired cartilage[32-34]. hUCB-MSCs are additionally advantageous because of their high expansion capacity, non-invasive harvesting, and hypo-immunogenicity. Moreover, as hUCB-MSCs are an allogeneic cell source, they are produced as an off-the-shelf product, and can supply sufficiently high numbers of pure stem cells with respect to the cartilage defect area being treated[35-37]. However, reports on the clinical application of hUCB-MSCs are scarce, and no studies have examined the use of hUCB-MSCs with concomitant HTO[38,39].
To demonstrate whether regenerated cartilage affects clinical outcomes, herein we retrospectively evaluated clinical outcomes and cartilage regeneration via second-look arthroscopy following implantation of hUCB-MSCs with concomitant HTO. In addition, we investigated whether patient characteristics, such as articular cartilage lesions on the patellofemoral joint, age, and cartilage defect size, influence clinical outcomes.
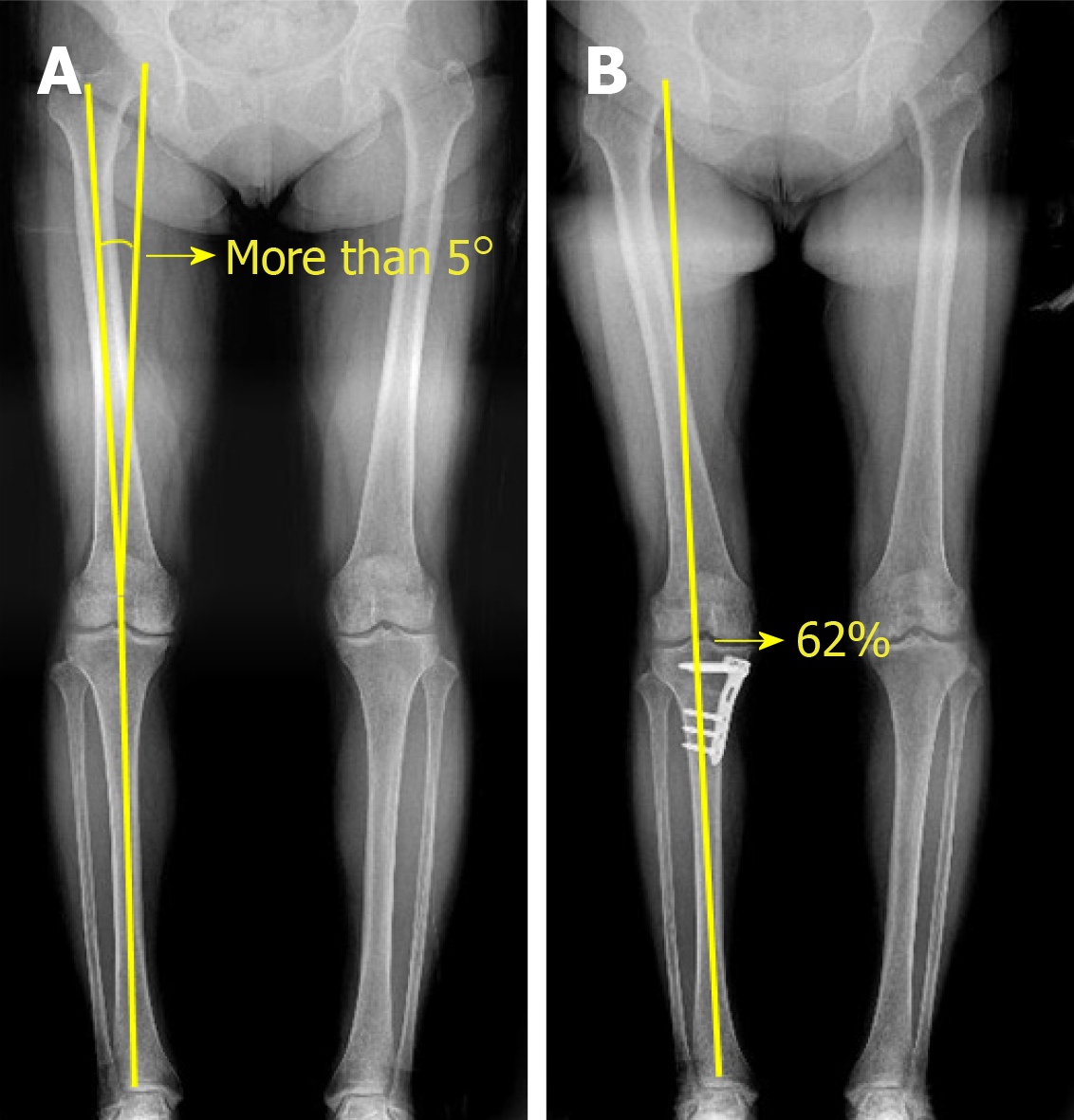
We retrospectively reviewed the medical records of patients who underwent second-look arthroscopy during hardware removal after receiving implantation of hUCB-MSCs with concomitant HTO for the treatment of MCOA between January 2014 and November 2016. The study protocol was approved by the institutional review board of Korea Ministry of Health and Welfare (2019-3100-003). The patients included in this study were over 40 years old, and had a varus deformity of more than 5° and a full-thickness International Cartilage Repair Society (ICRS) grade IV articular cartilage lesion of more than 4 cm2 in the medial compartment of the knee[40] (Figures 1 and 2A). Patients with grade IV OA of the medial compartment (identified by radiological assessment according to Kellgren and Lawrence system[41]), knee ligament injuries, metabolic arthritis, joint infections, and articular cartilage lesions at the lateral compartment were excluded. Herein, we evaluated clinical outcomes 3 years post-surgery and assessed cartilage regeneration via second-look arthroscopy. Effects of regenerated cartilage on clinical outcomes were evaluated after classification according to the ICRS grading system. We also assessed the effects of patient characteristics such as patient age, presence of a trochlear lesion, and lesion size of medial femoral condyle (MFC) from the patient's medical records.
CARTISTEM® (Medipost, Seongnam-si, Gyeonggi-do, South Korea), an off-the-shelf medicinal product for cartilage regeneration, was used in the study. This product, which consists of 1.5 mL hUCB-MSCs (7.5 × 106 cells/vial) and 4% hyaluronic acid (HA) hydrogel, was approved for cartilage regeneration by the Korea Food and Drug Administration in January 2012. The therapeutic dose is 500 μL/cm2 as specified in the manufacturer's instructions. Preoperatively, the cartilage defect size was measured by magnetic resonance imaging (MRI), and the therapeutic dose was determined. After combining hUCB-MSCs with 4% HA hydrogel using a spatula, the mixture was transferred into a 5-mL syringe for implantation into the defect.
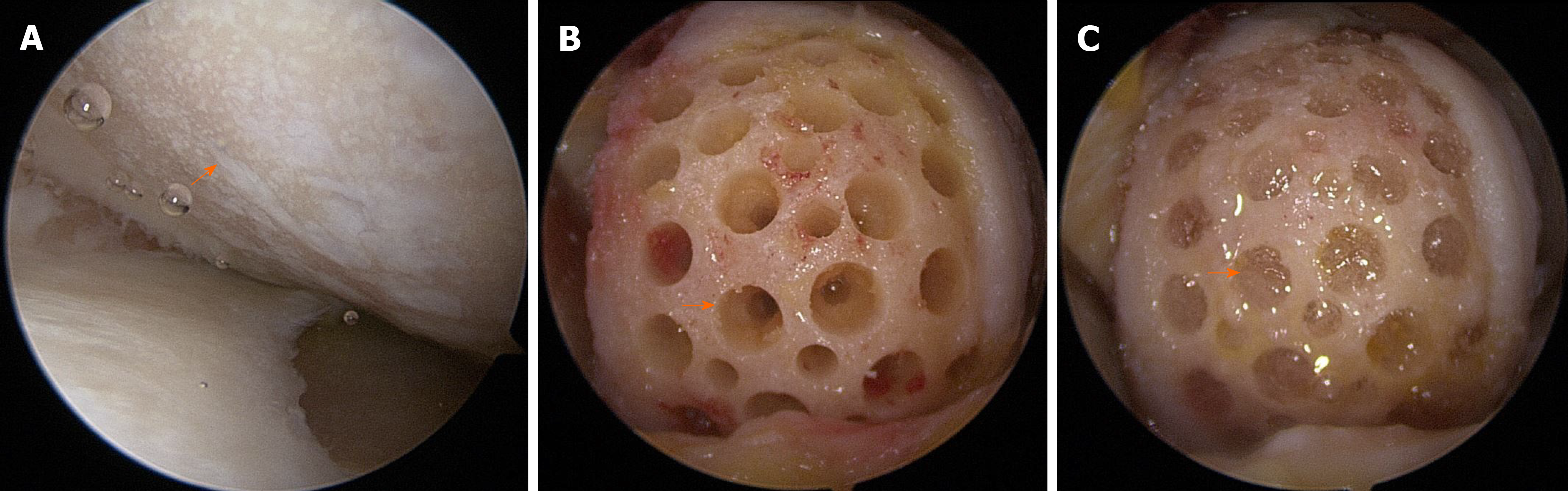
All surgical procedures, including diagnostic arthroscopy, synovectomy, excision of degenerative menisci tears, microfracture, and HTO, were performed by a single surgeon. After completion of the arthroscopic procedure, the fluid was washed out, and arthroscopic instruments were removed from the joint. A 5-7 cm longitudinal incision was made on the medial parapatellar area, and the medial femoral condyle (MFC) was exposed by dissecting the medial patellofemoral ligament and joint capsule. The damaged cartilage was removed using a curette, and sclerotic bone on the surface of the femoral condyle was removed using a burr. For implantation of hUCB-MSCs, multiple holes (4 mm in diameter and 4 mm in depth) were made in cartilage defects, and the space between the holes was drilled using a 2-mm-thick Kirschner wire. Irrigation was used to remove intra-articular debris. The hUCB-MSC and HA hydrogel mixture was then implanted into the holes and articular surface (Figure 3A-C). After implantation of hUCB-MSC, open-wedge HTO was performed using an anatomical locking metal-block plate (Ohtofix; Ohtomedial CO. Ltd., Goyang-si, South Korea)[42]. All knees underwent uniplanar osteotomy aiming to correct the mechanical axis to approximately 62% lateral to the tibial plateau[43] (Figure 2B). After surgery, patients were encouraged to perform isometric quadricep/hamstring exercise and straight leg-raises, however, knee flexion was limited to 90° for 4 wk. Partial weight-bearing began after 4 wk, and full weight-bearing was permitted at week 6.
The clinical outcomes of all patients were evaluated preoperatively, as well as at 1 year, 2 years, and 3 years postoperatively. The guidelines of the International Knee Documentation Committee (IKDC) were used to evaluate knee function and sport activity[44], while the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score was used to evaluate OA[45]. Scores obtained using visual analog scales (VAS) were also used to assess pain.
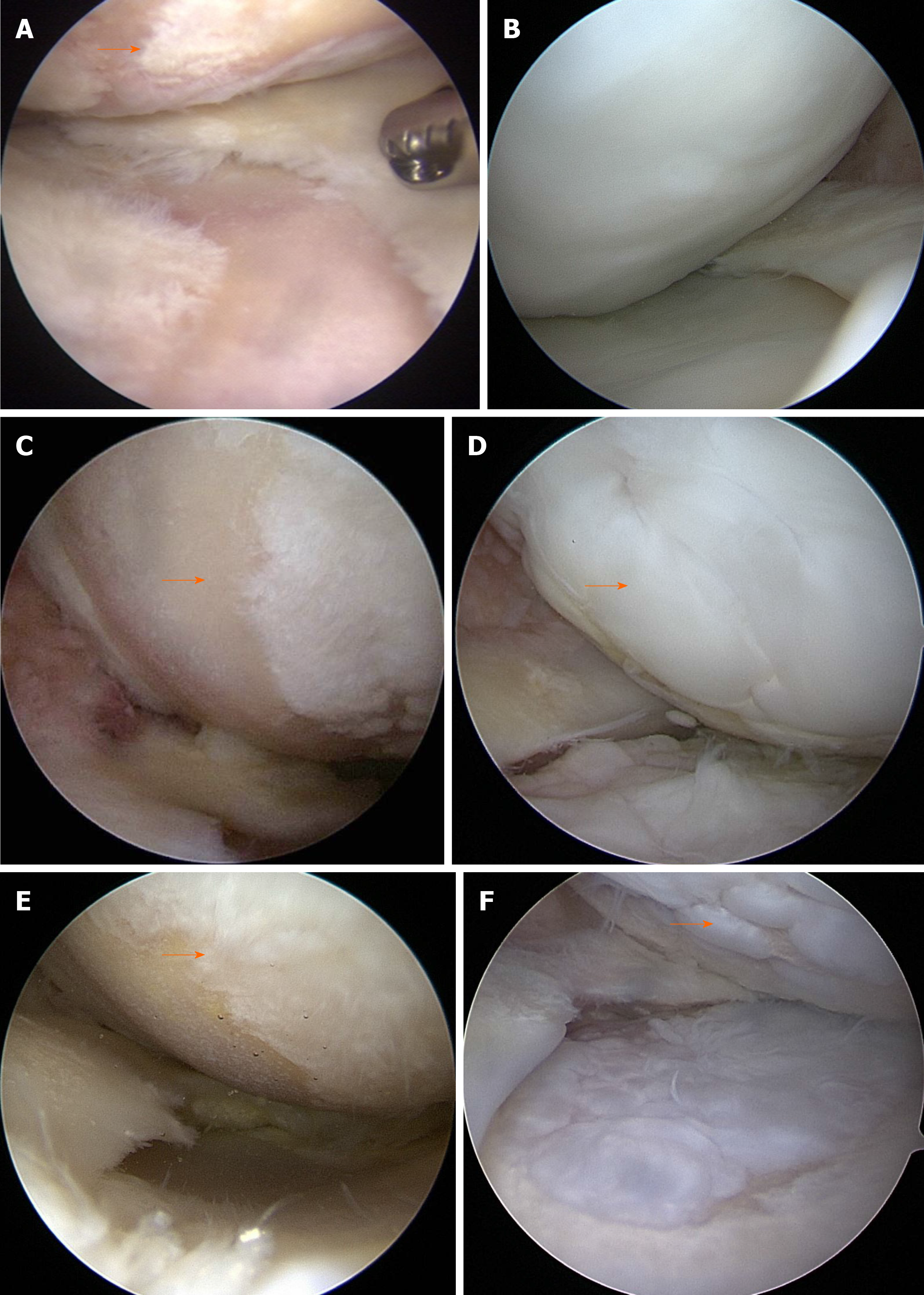
All patients underwent second-look arthroscopy during hardware removal. Cartilage regeneration was evaluated macroscopically using the ICRS grading system in second-look arthroscopy[40]. According to the ICRS grading system, grade I is considered normal, grade II considered nearly normal, grade III abnormal, and grade 4 severely abnormal.
Statistical analyses were performed using SPSS version 23.0 (SPSS, Chicago, IL, United States) with significance defined as P < 0.05. All data are presented as the mean ± standard deviation. IKDC, WOMAC, and VAS scores were applied as the primary dependent variables in clinical outcomes. Wilcoxon signed-rank test was performed to compare the preoperative and postoperative state of articular cartilage in the patient cohort. Kruskal-Wallis test was performed to compare three or more variables. Mann-Whitney U test with Bonferroni adjustment was used for post-hoc comparison. Mann-Whitney U test was used to compare cartilage regeneration in patients with trochlear lesions vs patients without trochlear lesions. Simple regression analysis was performed to identify the effects of age and lesion size on clinical outcomes. The statistical methods used in this study were reviewed by Dr. Young Ju Kim from the Department of Statistics at the Catholic University of Korea.
In this study, 125 patients with an average age of 58.3 ± 6.8 years (range: 43-74 years old) were included, of whom 95 (76%) were female and 30 (24%) were male. The average body mass index (BMI) was 25.6 ± 2.7 kg/m2 (range: 19.2-35.5 kg/m2) and average hip-knee-ankle (HKA) angle for measuring varus deformity was 7.6° ± 2.4° (range: 5.0°-14.2°). Seventy-three (58.4%) patients had trochlear lesions, while the remaining 52 (41.6%) did not (Table 1).
| Patients | n = 125 |
| Age, yr | 58.3 ± 6.8 |
| Sex, female/male | 95 (76%)/30 (24%) |
| Lesion size, cm2 | 6.9 ± 2 |
| HKA angle, degree | 7.6 ± 2.4 |
| Trochlear lesion | |
| With lesion | 73 |
| Without lesion | 52 |
| Second look arthroscopic findings | |
| ICRS grade I | 73 |
| ICRS grade II | 37 |
| ICRS grade III | 15 |
Postoperative second-look arthroscopy with hardware removal was performed at 20.2 ± 6.5 mo (range: 8-38 mo) post-surgery. In second-look arthroscopy, the MFC cartilage status was as follows: 73 (58.4%) patients with ICRS grade I, 37 (29.6%) with ICRS grade II, and 15 (12%) with ICRS grade III. No patients had ICRS grade IV (Figure 4A-F) (Table 1).
Clinical results were analyzed by classifying patient groups according to the ICRS grading system. In the ICRS grade I group, the preoperative IKDC score was 28.4 ± 7.4 (range: 10.3–43.7) and increased to 59.3 ± 8.7 (range: 40.8–82.8), 64.3 ± 9.2 (range: 44.8-90.8), and 68.1 ± 10.8 (range: 40.2–90.8) at 1-, 2-, and 3-year follow-up, respectively (P < 0.001 for both 1- and 2-year follow-up; P = 0.001 for the final follow-up). The WOMAC score decreased from 45.1 ± 11 (range: 22-79) preoperatively to 11.0 ± 6.9 (range: 2–39), 8.4 ± 6.0 (range: 0-28), and 6.5 ± 6.0 (range: 0-33) at 1-, 2-, and 3-year follow-up, respectively (P < 0.001 for both 1- and 2-year follow-up; P = 0.003 at 3-year follow-up). The VAS score also decreased from 7.6 ± 1.4 (range: 4–10) preoperatively to 2.1 ± 1.7 (range: 0–7), 1.5 ± 1.4 (range: 0-6), and 1.1 ± 1.5 (range: 0-33) at 1-, 2-, and 3-year follow-up, respectively (P < 0.001 at both 1- and 2-year follow-up; P = 0.004 at 3-year follow-up).
All clinical outcomes in the ICRS grade I group improved significantly over time. In the ICRS grade II group, the IKDC score increased from 30.1 ± 7.2 (range: 16.1-54.0) preoperatively to 52.4 ± 10.7 (range: 29.9-70.1), 58.6 ± 11.1 (range: 40.5-82.8), and 61.0 ± 11.3 (range: 43-90.8) at 1-, 2-, and 3-year follow-up, respectively (P < 0.001 for both 1- and 2-year follow-up; P = 0.063 for 3-year follow-up). The WOMAC score decreased from 41.9 ± 9.2 (range: 30-64) preoperatively to 16.8 ± 8.5 (range: 5-40), 13.4 ± 8.2 (range: 1–39), and 10.5 ± 5.6 (range: 0-22) at 1-, 2-, and 3-year follow-up, respectively (P < 0.001 for 1-year follow-up; P = 0.001 for 2-year follow-up; P = 0.002 for 3-year follow-up). The VAS score decreased from 7.5 ± 1.1 (range: 6-10) preoperatively to 3.0 ± 1.6 (range: 0–7), 2.7 ± 1.8 (range: 0–8), and 2.0 ± 1.4 (range: 0-4) at 1- (P < 0.001), 2- (P= 0.229), and 3-year (P = 0.019) follow-up, respectively. In the ICRS grade III group, the IKDC score increased from 29.2 ± 8.1 (range: 11.4-43.6) preoperatively to 54.8 ± 7.1 (range: 45.2–70.1), 55.0 ± 8.0 (range: 40.4-75.9), and 59.3 ± 5.8 (range: 45.8–72.4) at 1-, 2-, and 3-year follow-up, respectively (P = 0.001, P = 0.842, and P = 0.047, respectively). The WOMAC score decreased from 44.3 ± 12.2 (range: 29–76) preoperatively to 17.6 ± 5.1 (range: 10–26), 17.3 ± 7.4 (range: 4–30), and 12.6 ± 8.3 (range: 1–28) at 1-, 2-, and 3-year follow-up, respectively (P = 0.001, P = 0.607, and P = 0.018, respectively). The VAS score decreased from 7.7 ± 0.8 (range: 6–9) preoperatively to 3.3 ± 1.2 (range: 1–6), 3.1 ± 1.2 (range: 1–5), and 2.6 ± 0.9 (range: 1–4) at 1-, 2-, and 3-year follow-up, respectively (P = 0.001, P = 0.658, and P = 0.103, respectively).
Preoperative scores showed no significant differences among the groups of patients (IKDC, WOMAC, and VAS; P = 0.610, P = 0.275, and P = 0.817, respectively). However, postoperative scores showed significant differences among patient groups at all the time points of follow-up (IKDC score: P = 0.005 at 1 year, and P < 0.001 at 2 and 3 years; WOMAC score: P < 0.001 at all follow-up time points; VAS score: P = 0.002 at 1 year, P < 0.001 at 2 and 3 years). Post hoc analysis revealed that except for IKDC at 1 year, all scores in the ICRS grade I group improved more than those of the ICRS grade II and III groups; IKDC scores of the ICRS grade II group did not differ significantly from those of the ICRS grade III group. The IKDC score of the ICRS grade I group differed significantly from that of the ICRS grade II group at 1-year follow-up; however, IKDC scores of other groups did not differ significantly at 1-year follow-up (Table 2).
| ICRS grade I (n = 73) | ICRS grade II (n = 37) | ICRS grade III (n = 15) | P value1 | Post hoc5 | |
| IKDC | |||||
| Preoperative | 28.4 ± 7.4 | 30.1 ± 7.2 | 29.2 ± 8.1 | 0.610 | |
| 1 yr | 59.3 ± 8.7 | 52.4 ± 10.7 | 54.8 ± 7.1 | 0.005 | I > II = III,I = III |
| 2 yr | 64.3 ± 9.2 | 58.6 ± 11.1 | 55.0 ± 8.0 | < 0.001 | I > II = III |
| 3 yr | 68.1 ± 10.8 | 61.0 ± 11.3 | 59.3 ± 5.8 | < 0.001 | I > II = III |
| P value2 | < 0.001 | < 0.001 | 0.001 | ||
| P value3 | < 0.001 | < 0.001 | 0.842 | ||
| P value4 | 0.001 | 0.063 | 0.047 | ||
| WOMAC | |||||
| Preoperative | 45.1 ± 11 | 41.9 ± 9.2 | 44.3 ± 12.2 | 0.275 | |
| 1 yr | 11.0 ± 6.9 | 16.8 ± 8.5 | 17.6 ± 5.1 | < 0.001 | I > II = III |
| 2 yr | 8.4 ± 6.0 | 13.4 ± 8.2 | 17.3 ± 7.4 | < 0.001 | I > II = III |
| 3 yr | 6.5 ± 6.0 | 10.5 ± 5.6 | 12.6 ± 8.3 | < 0.001 | I > II = III |
| P value2 | < 0.001 | < 0.001 | 0.001 | ||
| P value3 | < 0.001 | 0.001 | 0.607 | ||
| P value4 | 0.003 | 0.002 | 0.018 | ||
| VAS | |||||
| Preoperative | 7.6 ± 1.4 | 7.5 ± 1.1 | 7.7 ± 0.8 | 0.817 | |
| 1 yr | 2.1 ± 1.7 | 3.0 ± 1.6 | 3.3 ± 1.2 | 0.002 | I > II = III |
| 2 yr | 1.5 ± 1.4 | 2.7 ± 1.8 | 3.1 ± 1.2 | < 0.001 | I > II = III |
| 3 yr | 1.1 ± 1.5 | 2.0 ± 1.4 | 2.6 ± 0.9 | < 0.001 | I > II = III |
| P value2 | < 0.001 | < 0.001 | 0.001 | ||
| P value3 | < 0.001 | 0.229 | 0.658 | ||
| P value4 | 0.004 | 0.019 | 0.103 | ||
Preoperative VAS scores differed significantly between the trochlear lesion group and the non-lesion group (P = 0.046); however, no significant differences in outcomes observed between these two groups at 1, 2, and 3 years postoperatively (P > 0.05 for all time points) (Table 3). Similarly, the WOMAC and IKDC scores did not differ significantly between the trochlear lesion group and the non-lesion group (P > 0.05 for all time points). The preoperative WOMAC score increased with increasing age (P < 0.001) but did not affect other outcomes (P > 0.05 for all). The lesion size of the MFC did not affect the IKDC score (P > 0.05 for all follow-up time points). The preoperative WOMAC score increased significantly with increased lesion size but did not affect the postoperative WOMAC score (P < 0.001 for preoperative WOMAC, and P > 0.05 for postoperative WOMAC). The VAS score increased significantly with increased lesion size at the preoperative stage (P < 0.001). However, there was no significant difference in postoperative outcomes (P > 0.05) (Table 4).
| Trochlear lesion (n = 73) | No trochlear lesion (n = 52) | P value1 | |
| IKDC | |||
| Preoperative | 29.3 ± 7.3 | 28.5 ± 7.6 | 0.794 |
| 1 yr | 56.7 ± 9.7 | 56.7 ± 9.7 | 0.960 |
| 2 yr | 61.6 ± 10.7 | 61.4 ± 9.5 | 0.916 |
| 3 yr | 64.7 ± 11 | 65.3 ± 11.4 | 0.493 |
| WOMAC | |||
| Preoperative | 44.8 ± 10.3 | 43.1 ± 11.1 | 0.288 |
| 1 yr | 13.8 ± 7.5 | 13.2 ± 8.2 | 0.471 |
| 2 yr | 10.7 ± 6.9 | 11.3 ± 8.5 | 0.958 |
| 3 yr | 8.4 ± 6.4 | 8.4 ± 6.8 | 0.755 |
| VAS | |||
| Preoperative | 7.8 ± 1.2 | 7.3 ± 1.3 | 0.046 |
| 1 yr | 2.6 ± 1.7 | 2.3 ± 1.6 | 0.331 |
| 2 yr | 2.1 ± 1.5 | 2.0 ± 1.7 | 0.555 |
| 3 yr | 1.6 ± 1.4 | 1.4 ± 1.6 | 0.203 |
| Age (n = 125) | Lesion size (n = 125) | |||||||
| β | t | R2 | P value1 | β | t | R2 | P value1 | |
| IKDC | ||||||||
| Pre-op | -0.128 | -1.429 | 0.016 | 0.156 | -0.148 | -1.661 | 0.022 | 0.099 |
| 1 yr | -0.095 | -1.053 | 0.009 | 0.294 | -0.025 | -0.282 | 0.001 | 0.779 |
| 2 yr | -0.053 | -0.593 | 0.003 | 0.554 | -0.120 | -1.339 | 0.014 | 0.183 |
| 3 yr | 0.049 | 0.549 | 0.002 | 0.584 | 0.024 | 0.266 | 0.001 | 0.791 |
| WOMAC | ||||||||
| Pre-op | 0.327 | 3.831 | 0.107 | < 0.001 | 0.305 | 3.550 | 0.093 | 0.001 |
| 1 yr | 0.173 | 1.945 | 0.030 | 0.054 | 0.040 | 0.440 | 0.002 | 0.661 |
| 2 yr | -0.005 | -0.055 | < 0.001 | 0.956 | 0.052 | 0.575 | 0.003 | 0.566 |
| 3 yr | -0.048 | -0.532 | 0.002 | 0.595 | -0.064 | -0.716 | 0.004 | 0.475 |
| VAS | ||||||||
| Pre-op | 0.115 | 1.286 | 0.013 | 0.201 | 0.335 | 3.943 | 0.112 | < 0.001 |
| 1 yr | 0.114 | 1.278 | 0.013 | 0.204 | 0.113 | 1.266 | 0.013 | 0.208 |
| 2 yr | 0.001 | 0.013 | <0.001 | 0.989 | 0.112 | 1.249 | 0.013 | 0.214 |
| 3 yr | -0.047 | -0.519 | 0.002 | 0.605 | 0.164 | 1.842 | 0.027 | 0.068 |
The results obtained in this study show that cartilage was regenerated to ICRS grade III or better in all cases after implantation of hUCB-MSCs with concomitant HTO. Jung et al[7] showed that cartilage was regenerated in MFC and MTP in second-look arthroscopy after medial opening-wedge HTO without any cartilage regeneration surgery. Although regenerated cartilage is mostly immature, we believe that reduced joint loading of the medial compartment after HTO provides an environment in which cartilage is regenerated. Accordingly, implantation or injection of MSCs with concomitant HTO has been used to enhance insufficient cartilage regeneration. Wong et al[23] investigated the injection of BM-derived MSCs with HA 3 weeks after MFx with HTO. They reported improved short-term outcomes, as well as magnetic resonance observation of cartilage repair tissue (MOCART) scores compared with those of the control group. Koh et al[24] compared a group treated with an injection of platelet-rich plasma (PRP) and concomitant HTO to a group treated with a dose of platelet-rich plasma (PRP), HTO, and an additional infusion of adipose-tissue-derived MSCs. Their results demonstrated that the group receiving MSC injection showed improved cartilage recovery and clinical outcomes compared with the group receiving a PRP injection only[24]. Kim et al[22] confirmed that injection of adipose tissue-derived MSCs in 50 patients of MCOA improved clinical outcomes more than did HTO alone. Although we have not included a control group, we show that regenerated cartilage affected clinical outcomes in patients with varus deformity of more than 5° and full-thickness articular cartilage lesion of ICRS grade IV with more than 4 cm2 in the medial compartment of the knee.
Regenerated cartilage in the ICRS grades I, II, and III groups improved the clinical outcomes of these patients, with the ICRS grade I group showing the best clinical outcomes among the three groups. Indeed, all scores in the ICRS grade I group improved over time compared with those of the ICRS grade II and III groups. These results indicate that cartilage regeneration via hUCB-MSCs implantation with concomitant HTO, is an effective approach to cartilage regeneration. In our present study, the regeneration status of articular cartilage in 73 (58.4%) patients, assessed via second-look arthroscopy, was judged to be ICRS grade I and accounted for the largest proportion of the patients. Although some patients presented with partially regenerated cartilage, none showed a lack of cartilage regeneration (ICRS grade IV). We did not perform a histological examination to avoid damaging the regenerated cartilage in these patients. However, in patients with large chondral lesions, the regenerated cartilage fully covered the lesions, and showed adequate thickness and elasticity as assessed via palpation with a probe during second-look arthroscopy. Similarly, Park et al[38] reported that hyaline-like cartilage was regenerated after hUCB-MSCs implantation in patients with OA, resulting in improved clinical outcomes in these patients.
Although the mechanisms involved in hUCB-MSC-mediated cartilage regeneration are only partially characterized[46-48], it is clear that hUCB-MSC-based strategies are effective in treating patients with OA. Allogeneic hUCB-MSCs are not only standardized as off-the-shelf medicinal products, but also non-invasively yield a sufficient number of stem cells that can be applied according to the size of the cartilage lesion. Jo et al[49] reported that patients with OA showed reduced pain levels and improved function after being treated with a high dose of adipose tissue-derived MSCs (1.0 × 108 cells) compared with a low (1.0 × 107 cells) or moderate dose (5.0 × 107 cells) administered intra-articularly. In addition, the cartilage defect area was regenerated into hyaline-like cartilage in the high dose group. However, the number of stem cells with respect to defect sizes has not been standardized and requires further investigation.
We also investigated whether patient age, presence of trochlear lesion, and size of lesion of MFC influenced clinical outcomes in patients with varus deformity. Several studies have shown that although OA is exacerbated by increased pressure in the patellofemoral (PF) joint after HTO, this process does not deteriorate clinical results or affect anterior knee pain[50,51]. In our present study, patients with trochlear lesions showed significantly increased preoperative VAS scores compared to those without trochlear lesions; however, there were no differences in other scores between these two patient groups. The results of our present study show that implantation of hUCB-MSCs into the trochlea exerted a positive effect on cartilage regeneration. However, further studies are required to evaluate cartilage regeneration in the trochlea after hUCB-MSCs implantation with concomitant HTO. Furthermore, the preoperative WOMAC scores were the only variable affected by advanced patient age. Autologous sources of MSCs such as adipose tissue and BM are age-dependent[52-54]; however, hUCB-MSCs maintain cell quality regardless as it is a cell therapy product. Finally, increased lesion size caused a subsequent increase in the preoperative VAS and WOMAC scores, but it did not affect other postoperative scores. These results indicate that implantation of hUCB-MSCs with concomitant HTO was applicable in patients with trochlear lesions and may even be a viable treatment option in patients with older or more extensive lesions.
Certain limitations were noted in this study. First, it was retrospective and did not include a control group. However, the presence of regenerated cartilage was confirmed via second-look arthroscopy in all the 125 patients. We also evaluated how the status of regenerated cartilage affected clinical outcomes. Thus, we can suggest the necessity of cartilage repair procedure during HTO. Second, although the presence of regenerated cartilage was confirmed visually and palpated using a probe, it was not evaluated histologically as that would have required a biopsy, which could damage the regenerated cartilage. Finally, a second-look arthroscopy was performed during hardware removal at an average time of 20.2 ± 6.5 mo post-surgery. This was a relatively short-term evaluation considering that cartilage remodeling requires an extended period of time. However, reducing the pressure in the medial compartment via HTO would preserve the regenerated cartilage and allow it to remain intact over time.
In conclusion, our results show that the implantation of hUCB-MSCs with concomitant HTO was an effective treatment option for patients with MCOA. We confirmed that regenerated cartilage improved clinical outcomes in this patient population. In addition, our results suggest that the presence of the trochlear lesions, the advanced age of the patient, or large cartilage lesions did not significantly affect clinical outcomes in patients with MCOA undergoing HTO with hUCB-MSCs implantation.
High tibial osteotomy (HTO) is widely used to treat medial compartment osteoarthritis (MCOA) of the knee with varus deformity. HTO reduces knee pain and improves knee function by decreasing the pressure in the medial compartment of the knee.
HTO alone offers excellent short- and mid-term outcomes; however, these outcomes tend to deteriorate over time. For further improvement in knee joint condition, cartilage regeneration can be combined with HTO. Autologous chondrocyte implantation (ACI), osteochondral autologous transplantation (OAT), and microfracture have been known to be effective therapies for articular cartilage regeneration, but they are not suitable in case of osteoarthritis (OA) therapy. Recently, mesenchymal stem cells (MSCs) have been identified as a new option in the field of cartilage regeneration for the treatment of OA patients. The MSCs isolated from human umbilical cord blood (hUCB-MSCs) demonstrate higher proliferation and chondrogenic capacity than other MSCs. Reports on the clinical application of hUCB-MSCs are scarce, and there are no studies examining the use of hUCB-MSCs with concomitant HTO.
This study aimed to evaluate clinical outcomes and cartilage regeneration via second-look arthroscopy after implantation of hUCB-MSCs with concomitant HTO, for treatment of osteoarthritic knee with varus deformity.
A total of 125 patients were included in this study with an average age of 58.3 ± 6.8 years (range: 43-74 years). All the patients had a varus deformity of more than 5° and a full-thickness International Cartilage Repair Society (ICRS) grade IV articular-cartilage lesion of more than 4 cm2 in the medial compartment of the knee. All patients underwent second-look arthroscopy during hardware removal. Cartilage regeneration was evaluated macroscopically using the ICRS grading system in second-look arthroscopy. We also assessed the effects of patient characteristics, such as trochlear lesions, patient age, and lesion size, using the patients’ medical records.
The results obtained in this study show that cartilage was regenerated to ICRS grade III or better in all the cases after implantation of hUCB-MSCs with concomitant HTO. Regenerated cartilage in the ICRS grades I, II, and III groups improved the clinical outcomes of these patients. The ICRS grade I group showed the best clinical outcomes among the three groups. Indeed, all the scores in the ICRS grade I group improved over time compared with those of the ICRS grade II and III groups. Although some patients presented with partially regenerated cartilage, none of the patients showed lack of cartilage regeneration (ICRS grade IV).
Our results show that implantation of hUCB-MSCs with concomitant HTO is an effective treatment option for patients with medial compartment osteoarthritis (MCOA). In addition, our results also suggest that the presence of trochlear or large cartilage lesions, or advanced age of the patient, does not significantly affect clinical outcomes in patients with MCOA undergoing HTO with hUCB-MSC implantation.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish M, Labusca L S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Shiomi T, Nishii T, Tanaka H, Yamazaki Y, Murase K, Myoui A, Yoshikawa H, Sugano N. Loading and knee alignment have significant influence on cartilage MRI T2 in porcine knee joints. Osteoarthritis Cartilage. 2010;18:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Cerejo R, Dunlop DD, Cahue S, Channin D, Song J, Sharma L. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 217] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386:376-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1989] [Article Influence: 198.9] [Reference Citation Analysis (0)] |
| 4. | Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1054] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 5. | Yoon JR. Some Considerations in High Tibial Osteotomy. Knee Surg Relat Res. 2018;30:273-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Hui C, Salmon LJ, Kok A, Williams HA, Hockers N, van der Tempel WM, Chana R, Pinczewski LA. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Jung WH, Takeuchi R, Chun CW, Lee JS, Ha JH, Kim JH, Jeong JH. Second-look arthroscopic assessment of cartilage regeneration after medial opening-wedge high tibial osteotomy. Arthroscopy. 2014;30:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Tang WC, Henderson IJ. High tibial osteotomy: long term survival analysis and patients' perspective. Knee. 2005;12:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Gstöttner M, Pedross F, Liebensteiner M, Bach C. Long-term outcome after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Amendola A, Bonasia DE. Results of high tibial osteotomy: review of the literature. Int Orthop. 2010;34:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 648] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 12. | Kumagai K, Akamatsu Y, Kobayashi H, Kusayama Y, Saito T. Mosaic Osteochondral Autograft Transplantation Versus Bone Marrow Stimulation Technique as a Concomitant Procedure With Opening-Wedge High Tibial Osteotomy for Spontaneous Osteonecrosis of the Medial Femoral Condyle. Arthroscopy. 2018;34:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Ferruzzi A, Buda R, Cavallo M, Timoncini A, Natali S, Giannini S. Cartilage repair procedures associated with high tibial osteotomy in varus knees: clinical results at 11 years' follow-up. Knee. 2014;21:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Matsunaga D, Akizuki S, Takizawa T, Yamazaki I, Kuraishi J. Repair of articular cartilage and clinical outcome after osteotomy with microfracture or abrasion arthroplasty for medial gonarthrosis. Knee. 2007;14:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Franceschi F, Longo UG, Ruzzini L, Marinozzi A, Maffulli N, Denaro V. Simultaneous arthroscopic implantation of autologous chondrocytes and high tibial osteotomy for tibial chondral defects in the varus knee. Knee. 2008;15:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Schuster P, Schulz M, Mayer P, Schlumberger M, Immendoerfer M, Richter J. Open-Wedge High Tibial Osteotomy and Combined Abrasion/Microfracture in Severe Medial Osteoarthritis and Varus Malalignment: 5-Year Results and Arthroscopic Findings After 2 Years. Arthroscopy. 2015;31:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Jung WH, Takeuchi R, Chun CW, Lee JS, Jeong JH. Comparison of results of medial opening-wedge high tibial osteotomy with and without subchondral drilling. Arthroscopy. 2015;31:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29:1579-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 698] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 20. | Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Saw KY, Anz A, Jee CS, Ng RC, Mohtarrudin N, Ragavanaidu K. High Tibial Osteotomy in Combination With Chondrogenesis After Stem Cell Therapy: A Histologic Report of 8 Cases. Arthroscopy. 2015;31:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Kim YS, Kwon OR, Choi YJ, Suh DS, Heo DB, Koh YG. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am J Sports Med. 2015;43:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. Arthroscopy. 2013;29:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 24. | Koh YG, Kwon OR, Kim YS, Choi YJ. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 819] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 26. | Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17:1425-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 27. | de Windt TS, Saris DB, Slaper-Cortenbach IC, van Rijen MH, Gawlitta D, Creemers LB, de Weger RA, Dhert WJ, Vonk LA. Direct Cell-Cell Contact with Chondrocytes Is a Key Mechanism in Multipotent Mesenchymal Stromal Cell-Mediated Chondrogenesis. Tissue Eng Part A. 2015;21:2536-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1405] [Cited by in RCA: 1347] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 29. | Shin YS, Yoon JR, Kim HS, Lee SH. Intra-Articular Injection of Bone Marrow-Derived Mesenchymal Stem Cells Leading to Better Clinical Outcomes without Difference in MRI Outcomes from Baseline in Patients with Knee Osteoarthritis. Knee Surg Relat Res. 2018;30:206-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet Disord. 2016;17:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 31. | Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12:e0175449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 32. | Subramanian A, Shu-Uin G, Kae-Siang N, Gauthaman K, Biswas A, Choolani M, Bongso A, Chui-Yee F. Human umbilical cord Wharton's jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. J Cell Biochem. 2012;113:1886-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Park YB, Song M, Lee CH, Kim JA, Ha CW. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33:1580-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Chung JY, Song M, Ha CW, Kim JA, Lee CH, Park YB. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther. 2014;5:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2347] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 36. | Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim JS, Jeon HB. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Ha CW, Park YB, Chung JY, Park YG. Cartilage Repair Using Composites of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronic Acid Hydrogel in a Minipig Model. Stem Cells Transl Med. 2015;4:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl Med. 2017;6:613-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 39. | Song JS, Hong KT, Kim NM, Jung JY, Park HS, Lee SH, Cho YJ, Kim SJ. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regen Ther. 2020;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 931] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 41. | Schiphof D, Boers M, Bierma-Zeinstra SM. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis. 2008;67:1034-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 42. | Han SB, Bae JH, Lee SJ, Jung TG, Kim KH, Kwon JH, Nha KW. Biomechanical properties of a new anatomical locking metal block plate for opening wedge high tibial osteotomy: uniplane osteotomy. Knee Surg Relat Res. 2014;26:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Lee DH, Han SB, Oh KJ, Lee JS, Kwon JH, Kim JI, Patnaik S, Shetty GM, Nha KW. The weight-bearing scanogram technique provides better coronal limb alignment than the navigation technique in open high tibial osteotomy. Knee. 2014;21:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1591] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 45. | McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 47. | Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim G, Kim JS, Yoon JR, Cho DH, Jeon HB. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 48. | Park YB, Ha CW, Kim JA, Han WJ, Rhim JH, Lee HJ, Kim KJ, Park YG, Chung JY. Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthritis Cartilage. 2017;25:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, Yoon KS. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am J Sports Med. 2017;45:2774-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 50. | Goshima K, Sawaguchi T, Shigemoto K, Iwai S, Nakanishi A, Ueoka K. Patellofemoral Osteoarthritis Progression and Alignment Changes after Open-Wedge High Tibial Osteotomy Do Not Affect Clinical Outcomes at Mid-term Follow-up. Arthroscopy. 2017;33:1832-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 51. | Kim KI, Kim DK, Song SJ, Lee SH, Bae DK. Medial Open-Wedge High Tibial Osteotomy May Adversely Affect the Patellofemoral Joint. Arthroscopy. 2017;33:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 53. | Wu LW, Wang YL, Christensen JM, Khalifian S, Schneeberger S, Raimondi G, Cooney DS, Lee WP, Brandacher G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl Immunol. 2014;30:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |