Published online Jun 26, 2020. doi: 10.4252/wjsc.v12.i6.481
Peer-review started: January 15, 2020
First decision: March 16, 2020
Revised: April 7, 2020
Accepted: May 5, 2020
Article in press: May 5, 2020
Published online: June 26, 2020
Processing time: 161 Days and 23.5 Hours
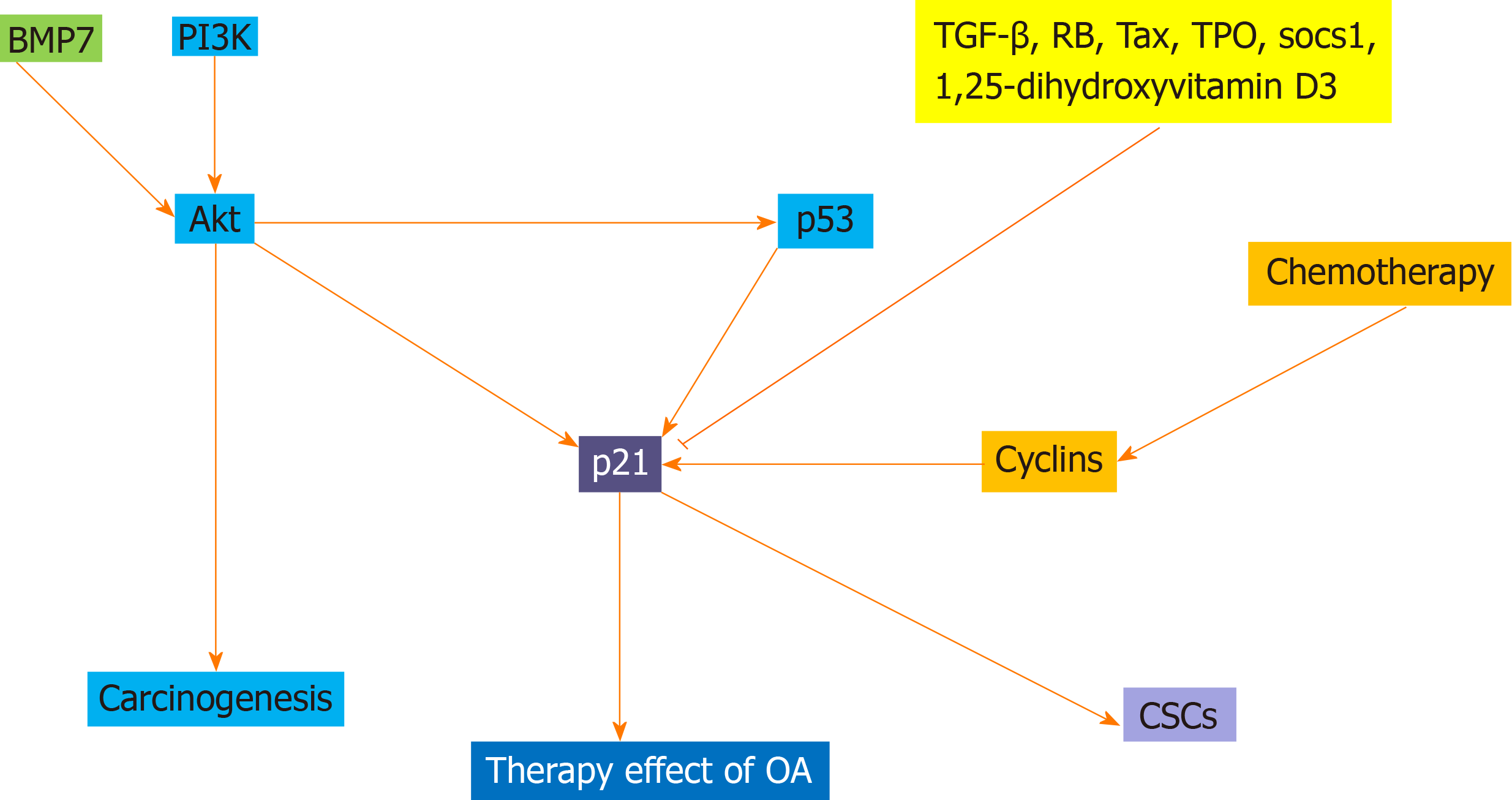
Cancer cells possess metabolic properties that are different from those of benign cells. p21, encoded by CDKN1A gene, also named p21Cip1/WAF1, was first identified as a cyclin-dependent kinase regulator that suppresses cell cycle G1/S phase and retinoblastoma protein phosphorylation. CDKN1A (p21) acts as the downstream target gene of TP53 (p53), and its expression is induced by wild-type p53 and it is not associated with mutant p53. p21 has been characterized as a vital regulator that involves multiple cell functions, including G1/S cell cycle progression, cell growth, DNA damage, and cell stemness. In 1994, p21 was found as a tumor suppressor in brain, lung and colon cancer by targeting p53 and was associated with tumorigenesis and metastasis. Notably, p21 plays a significant role in tumor development through p53-dependent and p53-independent pathways. In addition, expression of p21 is closely related to the resting state or terminal differentiation of cells. p21 is also associated with cancer stem cells and acts as a biomarker for such cells. In cancer therapy, given the importance of p21 in regulating the G1/S and G2 check points, it is not surprising that p21 is implicated in response to many cancer treatments and p21 promotes the effect of oncolytic virotherapy.
Core tip: p21, as a cyclin-dependent kinase regulator, suppresses cell cycle G1/S phase and retinoblastoma protein phosphorylation. As the downstream target gene of TP53, p21 expression is induced by wild-type p53. p21 was found as a tumor suppressor in several cancers by targeting p53 and was associated with tumorigenesis and metastasis. Notably, p21 is also associated with cancer stem cells. Moreover, p21 is closely related to cancer therapy, and it can promote antitumor effect of oncolytic virotherapy. These findings implicated multifaceted roles of p21 in cancer treatment.
- Citation: Xiao BD, Zhao YJ, Jia XY, Wu J, Wang YG, Huang F. Multifaceted p21 in carcinogenesis, stemness of tumor and tumor therapy. World J Stem Cells 2020; 12(6): 481-487
- URL: https://www.wjgnet.com/1948-0210/full/v12/i6/481.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i6.481
The cell cycle is strictly regulated by cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors to determine whether the cell is divided, quiescent or in the process of cell death in response to external stimuli and/or cellular microenvironment. Each type of cell has its own features during the cell cycle, and embryonic stem cells, germ cells and cancer cells rapidly divide. These cells also undergo cell cycle arrest and cell quiescence under adverse conditions. To harmonize the events, the cells have developed a sophisticated regulatory system. Malfunction of this mechanism results in serious disorders such as autoimmune disease and carcinogenesis.
p21 is encoded by CDKN1A gene, and was first identified as a CDK regulator that suppresses cell cycle G1/S phase and retinoblastoma protein (RB) phosphorylation. p21 is a major inhibitor of CDK2 and is therefore also known as CDKN1A (p21) or CDK-interaction protein (CIP)1[1,2]. To be noted, p21 is a non-specific but commonly used name, and it has many aliases such as p21CIP1/WAF1due to its multiple functions. In this context, p21 acts as the downstream target gene of TP53 (p53), and its expression is induced by wild-type p53 and it is not associated with mutant p53. Hence, p21 is called wild-type p53 activating fragment 1 (waf1)[3]. Since p21 was found as a potent inhibitor of G1 cyclin-dependent kinases in 1993[4], it has been characterized as a vital regulator that involves multiple cell functions, including G1/S cell cycle progression, cell growth, DNA damage, and cell stemness. Early research revealed that G1/S cell cycle progression is negatively regulated by p21 binding to CDK and obstructing CDK interaction with its substrates[5-7]. p21 inhibits tumor growth by targeting p53[3]. Interaction between p21 and proliferating cell nuclear antigen maintains G2/M arrest after DNA damage[8,9]. Importantly, the regulatory mechanism of p21 still attracts much attention in many fields.
Tumorigenesis is associated with imbalance between cell proliferation and cell death. p21, a CDK inhibitor, is related to cell cycle progression[4]. In 1994, p21 was first found as a tumor suppressor in brain, lung, and colon cancer by targeting p53[3]. Early research also revealed that the absence of p21 alters keratinocyte growth and differentiation and promotes ras-tumor progression [10]. p21 is also associated with tumor migration and invasion. For example, cyclin D1 cooperates with p21 to regulate transforming growth factor (TGF)-β-mediated breast cancer cell migration and local tumor invasion[11]. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration and invasion, and contributes to poor prognosis in patients[12]. p21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via extracellular signal-regulated- and AKT-dependent pathways[13]. In addition, environmental and microenvironmental factors also influence molecular mechanisms of carcinogenesis[14], which may be related with variable p21 expression. These factors include diet, nutrition, lifestyle such as smoking or not, the living environment such as microbiome, and will influence the genome, epigenome, transcriptome, proteome, and metabolome of tumor and normal cells[15]. Controversial aspects of p21 are decided by p21 location and p53 protein condition[16]. p21 expression is induced by p53 under conditions of DNA damage or oxidative stress. For example, gambogic acid triggers DNA damage signaling that induces p53/p21Waf1/CIP1 activation through the ATR–checkpoint kinase 1 pathway[17]. Notably, p21 plays a significant role in tumor development through p53-dependent and p53-independent pathways. For example, the cytoprotective aminothiol WR1065 activates p21 (waf-1) and downregulates cell cycle progression through a p53-dependent pathway[18]. p53-independent induction of the p21 (waf1) pathway is preserved during tumor progression[19].
p21 is regulated by the tumor suppressor gene p53 and plays a regulatory role in inhibiting tumorigenesis; therefore, it is naturally assumed that p21 is also an antioncogene. A lot of experimental evidence confirms this conjecture. In vitro, expression of p21 negatively affects the malignancy of many cancer cell lines (TETs, ATLL and skin tumor) by inhibiting growth and inducing apoptosis[20-23]. In vivo, experiments have found that upregulation of p21 leads to the arrest and invasion of breast cancer cells[24]. The proportion of leukemia stem cell precursors in vivo is decreased in mice lacking p21 expression[25]. In renal cell carcinoma, a decrease in p21 expression is seen as an important factor in the poor survival of clinical outcomes[26]. In addition, p21 as CDKN1A can regulate T cell activation and attract innate immune cells to activate immune regulation[27]. In some p53-deleted cell lines, TGF-β, RB, Tax, thrombopoietin, suppressor of cytokine signaling 1, 1,25-dihydroxyvitamin D3 and other factors can regulate p21 expression to achieve inhibitory effects[28-33].
Circular RNAs (circRNAs) are newly-identified noncoding RNAs that covalently link 3’ and 5’ ends to form a closed loop and possess high stability[34]. circRNAs can regulate tumor progression through regulating p21 expression. For example, circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21 and phosphatase and tensin homolog expression[35]. circRNA affects cell cycle progression by forming complexes with p21. For example, forkhead box O3 circRNA retards cell cycle progression by forming ternary complexes with p21 and CDK2[36]. However, there is no report on whether circRNA is expressed in p21 gene. Thus, whether p21 expresses circRNA to regulate tumorigenesis and migration and invasion is of importance.
Several studies have shown that expression of p21 is closely related to the resting state or terminal differentiation of tumor cells. Various studies have shown that p21 is a key factor for the maintenance of stem/progenitor cells[27,37,38]. Upregulation of p21 mRNA can inhibit proliferation of progenitor cells[29]. Under normal steady-state conditions, abundant p21 expression is detected in both stationary hematopoietic stem cells and terminally differentiated mature blood cells. Knockdown of p21 results in proliferation of hematopoietic stem cells[39,40]. Therefore, keeping the stem/progenitor cells at rest is crucial to prevent their premature depletion. In the bone marrow, p21 expression produces different results. In colony-formation experiments, p21 can promote the colony formation, proliferation and differentiation of murine bone marrow progenitor cells[41]. Transient overexpression of p21 can lead to the development and differentiation of mononuclear/macrophages[27,30]. The expression of p21 mRNA is increased over time in granulocytes, macrophages, megakaryocytes, and erythroblasts[42]. The accumulation of its protein directly leads to final differentiation of cells[43].
It is also reported that p21 is associated with cancer stem cells (CSCs). p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and CSC-like gene expression in vivo[44]. Bone morphogenetic protein7 regulates dormancy and recurrence of prostate CSC in bone via the p38/NDRG1/p21 signaling axis[45]. Novel function of p21-activated kinase 3 in regulating Akt phosphorylation and pancreatic CSC phenotypes[46]. p21 itself acts as a biomarker for CSCs. Gallagher et al[47] reported that cancer stemness is suppressed by p21-regulating mRNA and miRNA signatures in recurrent ovarian cancer patient samples, and presented a p53–p21 cancer stemness signature model for ovarian cancer. In addition, some researches have pointed out that miRNAs affect the stemness of CSCs by regulating p21. For example, miR-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing the KLF4/PI3K/Akt/p21 pathway[48]. miR-146b-5p overexpression attenuates stemness and radioresistance of glioma stem cells by targeting the HuR/lincRNA-p21/β-catenin pathway[49]. Thus, these studies support that p21 may play important roles in tumor stemness.
Chemotherapy is one of the main approaches for treating tumors. Given the importance of p21 in regulating the G1/S and G2 check points, it is not surprising that p21 is implicated in response to many cancer treatments. An early study from Zhao et al[50] showed that p21 is required for non-small cell lung cancer sensitivity to gefitinib treatment. In addition, it was shown that p21protects cells from cisplatin cytotoxicity[51]. For example, the p21 CDK inhibitors enhances the cytotoxic effect of cisplatin in human ovarian carcinoma cells[52]. However, exogenous expression of p21 exerts cell growth inhibition and enhances sensitivity to cisplatin in hepatoma cells[53]. Thus, p21 has different effects on cisplatin sensitivity for various tumors. Simultaneously, p21-mediated cyclins can regulate the resistance of some chemotherapeutic drugs. For example, the mechanism by which elemene reverses drug resistance of lung cancer cells is regulated and controlled by CDK8/p21 pathways[54].
Oncolytic virotherapy has become one of the most promising therapeutic strategies for solid malignancies. p21 promotes the effect of oncolytic virotherapy. For example, Flak et al[55] found that p21 promotes oncolytic adenoviral activity in ovarian cancer and is a potential biomarker. Conversely, RNA-interference-mediated knockdown of p21 enhances antitumor cell activity of oncolytic adenoviruses[56]. Recently, we found that knockdown of p21 mediated by lentivirus inhibited the antitumor effect of oncolytic vaccinia virus in breast cancer cells (unpublished data). These studies revealed that p21 has different effects in oncolytic virotherapy for various tumors. However, the effect of interaction between p21 and Newcastle disease virus, herpes simplex virus1, and reovirus is unclear in tumor therapy.
Recent molecular pathological epidemiology (MPE) of cancer has increasingly becomes as a promising transdisciplinary and interdisciplinary field[57]. According to MPE of cancer, p21 is associated with an increased risk of breast cancer in Chinese women[58]. Thus, MPE can better study the pathogenesis, especially complex multifactorial diseases, and carry out personalized prevention and treatment. Notably, with the development of MPE and artificial intelligence, MPE by the analyzing of artificial intelligence has guiding significance for how to choose chemotherapy drugs to therapy tumors[14,59].
Research on p21 has grown rapidly over the past 24 years. p21 is associated with carcinogenesis, CSCs, chemotherapy and therapeutic effect of oncolytic adenovirus (Figure 1). p21 acts as a tumor suppressor and has oncogenic potential. It was demonstrated that p21 sustained expression and its cytoplasmic localization are related to its carcinogenicity and tumor heterogeneity, and reflects its dual function depending on cellular and environmental conditions. High expression of p21 in stem cells plays a key role in this characteristic. High expression of p21 in normal stem cells is a manifestation of cellular health, from which we can speculate that p21 can serve as a marker of stem cells to respond to the state of cells. p21 is also associated with CSCs. p21 attenuates Ras- and c-Myc-dependent tumor epithelial mesenchymal transition and CSC-like gene expression. p21 itself acts as a biomarker for CSCs. p21 plays an important role in CSCs. In addition, further studies should be focused on MPE and investigate cancer risk factors, microbiome, immunity, and molecular tissue biomarkers, which was related with molecular pathologies in normal or diseased tissue. In tumor therapy, given the importance of p21 in regulating the G1/S and G2 check points, it is not surprising that p21 is implicated in response to many cancer treatments. p21 plays an important role in chemotherapeutic drug resistance, and some drugs can affect resistance by regulating p21-mediated cyclins. p21 also plays an important role in treatment with oncolytic adenoviruses. In the study of p21, a network of related genes will be established to fully understand its mechanism and make more targeted treatments.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ogino S S-Editor: Yu XQ L-Editor: MedE-Ma JY E-Editor: Liu MY
| 1. | Cheng T, Scadden DT. Cell cycle entry of hematopoietic stem and progenitor cells controlled by distinct cyclin-dependent kinase inhibitors. Int J Hematol. 2002;75:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980-3985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 651] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 3. | el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6088] [Cited by in RCA: 6307] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 4. | Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4008] [Cited by in RCA: 4202] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 5. | Saha P, Eichbaum Q, Silberman ED, Mayer BJ, Dutta A. p21CIP1 and Cdc25A: competition between an inhibitor and an activator of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4338-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Shiyanov P, Bagchi S, Adami G, Kokontis J, Hay N, Arroyo M, Morozov A, Raychaudhuri P. p21 Disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996;16:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Zhu L, Harlow E, Dynlacht BD. p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev. 1995;9:1740-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Ando T, Kawabe T, Ohara H, Ducommun B, Itoh M, Okamoto T. Involvement of the interaction between p21 and proliferating cell nuclear antigen for the maintenance of G2/M arrest after DNA damage. J Biol Chem. 2001;276:42971-42977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Gulbis JM, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 641] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 261] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Dai M, Al-Odaini AA, Fils-Aimé N, Villatoro MA, Guo J, Arakelian A, Rabbani SA, Ali S, Lebrun JJ. Cyclin D1 cooperates with p21 to regulate TGFβ-mediated breast cancer cell migration and tumor local invasion. Breast Cancer Res. 2013;15:R49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Siu MK, Chan HY, Kong DS, Wong ES, Wong OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, Tsao SW, Strömblad S, Cheung AN. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA. 2010;107:18622-18627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Huynh N, Liu KH, Baldwin GS, He H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim Biophys Acta. 2010;1803:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 14. | Ogino S, Nowak JA, Hamada T, Milner DA, Jr. Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol. 2019;14:83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 15. | Hamada T, Nowak JA, Milner DA jr. Song M, Ogino S. Integration of microbiology, molecular pathology, and epidemiology: a new paradigm to explore the pathogenesis of microbiome-driven neoplasms. J Pathol. 2019;247:615-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Georgakilas AG, Martin OA, Bonner WM. p21: A Two-Faced Genome Guardian. Trends Mol Med. 2017;23:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 374] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 17. | Rong JJ, Hu R, Song XM, Ha J, Lu N, Qi Q, Tao L, You QD, Guo QL. Gambogic acid triggers DNA damage signaling that induces p53/p21(Waf1/CIP1) activation through the ATR-Chk1 pathway. Cancer Lett. 2010;296:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | North S, El-Ghissassi F, Pluquet O, Verhaegh G, Hainaut P. The cytoprotective aminothiol WR1065 activates p21waf-1 and down regulates cell cycle progression through a p53-dependent pathway. Oncogene. 2000;19:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Chen Y, Cipriano S, Sarkar F, Ware J, Arenkiel J. P53-independent induction of p21(waf1) pathway is preserved during tumor progression. Int J Oncol. 1995;7:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Klopfleisch R, Gruber AD. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res Vet Sci. 2009;87:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, Ørntoft TF, Andersen CL, Dobbelstein M. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68:10094-10104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 22. | Qiu L, Wu J, Pan C, Tan X, Lin J, Liu R, Chen S, Geng R, Huang W. Downregulation of CDC27 inhibits the proliferation of colorectal cancer cells via the accumulation of p21Cip1/Waf1. Cell Death Dis. 2016;7:e2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Leisibach P, Schneiter D, Soltermann A, Yamada Y, Weder W, Jungraithmayr W. Prognostic value of immunohistochemical markers in malignant thymic epithelial tumors. J Thorac Dis. 2016;8:2580-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Qian X, Hulit J, Suyama K, Eugenin EA, Belbin TJ, Loudig O, Smirnova T, Zhou ZN, Segall J, Locker J, Phillips GR, Norton L, Hazan RB. p21CIP1 mediates reciprocal switching between proliferation and invasion during metastasis. Oncogene. 2013;32:2292-2303, e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Cedric t, Jesslynsaw D. Cdkn1A (p21) is required for quiescence, therapeutic resistance and clonal evolution of pre-leukemic stem cells. Exp Hematol. 2014;42:S17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Wang C, Tang K, Li Z, Chen Z, Xu H, Ye Z. Targeted p21(WAF1/CIP1) activation by miR-1236 inhibits cell proliferation and correlates with favorable survival in renal cell carcinoma. Urol Oncol. 2016;34:59.e23-59.e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Seleznik GM, Reding T, Peter L, Gupta A, Steiner SG, Sonda S, Verbeke CS, Dejardin E, Khatkov I, Segerer S, Heikenwalder M, Graf R. Development of autoimmune pancreatitis is independent of CDKN1A/p21-mediated pancreatic inflammation. Gut. 2018;67:1663-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Matsumura I, Ishikawa J, Nakajima K, Oritani K, Tomiyama Y, Miyagawa J, Kato T, Miyazaki H, Matsuzawa Y, Kanakura Y. Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21(WAF1/Cip1) by STAT5. Mol Cell Biol. 1997;17:2933-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Ducos K, Panterne B, Fortunel N, Hatzfeld A, Monier MN, Hatzfeld J. p21(cip1) mRNA is controlled by endogenous transforming growth factor-beta1 in quiescent human hematopoietic stem/progenitor cells. J Cell Physiol. 2000;184:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 636] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 31. | Decesse JT, Medjkane S, Datto MB, Crémisi CE. RB regulates transcription of the p21/WAF1/CIP1 gene. Oncogene. 2001;20:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Martindale JL, Gorospe M, Holbrook NJ. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 1996;56:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Yeganeh M, Gui Y, Kandhi R, Bobbala D, Tobelaim WS, Saucier C, Yoshimura A, Ferbeyre G, Ramanathan S, Ilangumaran S. Suppressor of cytokine signaling 1-dependent regulation of the expression and oncogenic functions of p21(CIP1/WAF1) in the liver. Oncogene. 2016;35:4200-4211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Hentze MW, Preiss T. Circular RNAs: splicing's enigma variations. EMBO J. 2013;32:923-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 35. | Yang C, Yuan W, Yang X, Li P, Wang J, Han J, Tao J, Li P, Yang H, Lv Q, Zhang W. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 36. | Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846-2858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 955] [Cited by in RCA: 1303] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 37. | de Nooij JC, Hariharan IK. Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science. 1995;270:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Mantel C, Luo Z, Canfield J, Braun S, Deng C, Broxmeyer HE. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710-3719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1011] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 40. | Cheng T, Shen H, Rodrigues N, Stier S, Scadden DT. Transforming growth factor beta 1 mediates cell-cycle arrest of primitive hematopoietic cells independent of p21(Cip1/Waf1) or p27(Kip1). Blood. 2001;98:3643-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Braun SE, Mantel C, Rosenthal M, Cooper S, Liu L, Robertson KA, Hromas R, Broxmeyer HE. A positive effect of p21cip1/waf1 in the colony formation from murine myeloid progenitor cells as assessed by retroviral-mediated gene transfer. Blood Cells Mol Dis. 1998;24:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Taniguchi T, Endo H, Chikatsu N, Uchimaru K, Asano S, Fujita T, Nakahata T, Motokura T. Expression of p21(Cip1/Waf1/Sdi1) and p27(Kip1) cyclin-dependent kinase inhibitors during human hematopoiesis. Blood. 1999;93:4167-4178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Topley GI, Okuyama R, Gonzales JG, Conti C, Dotto GP. p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proc Natl Acad Sci USA. 1999;96:9089-9094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S, Ju X, Li Z, Yu Z, Zhou J, Johnson M, Fortina P, Hyslop T, Windle JJ, Pestell RG. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci USA. 2009;106:19035-19039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Xing F, Kobayashi A, Okuda H, al e. Abstract 1416: BMP 7 regulates dormancy and recurrence of prostate cancer stem cell in bone via P38/NDRG1/P21 signaling axis. Cancer Res. 2013;73:1416-1416. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Wu HY, Yang MC, Chu PC, Kulp SK, Chen CS. Abstract 1360: Novel function of p21-activated kinase 3 (PAK3) in regulating Akt phosphorylation and pancreatic cancer stem cell phenotypes. Cancer Res. 2017;77:1360-1360. [DOI] [Full Text] |
| 47. | Gallagher MF, Heffron CC, Laios A, O'Toole SA, Ffrench B, Smyth PC, Flavin RJ, Elbaruni SA, Spillane CD, Martin CM, Sheils OM, O'Leary JJ. Suppression of cancer stemness p21-regulating mRNA and microRNA signatures in recurrent ovarian cancer patient samples. J Ovarian Res. 2012;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Chang YL, Zhou PJ, Wei L, Li W, Ji Z, Fang YX, Gao WQ. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget. 2015;6:24017-24031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Yang W, Yu H, Shen Y, Liu Y, Yang Z, Sun T. MiR-146b-5p overexpression attenuates stemness and radioresistance of glioma stem cells by targeting HuR/lincRNA-p21/β-catenin pathway. Oncotarget. 2016;7:41505-41526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 50. | Zhao YF, Wang CR, Wu YM, Ma SL, Ji Y, Lu YJ. P21 (waf1/cip1) is required for non-small cell lung cancer sensitive to Gefitinib treatment. Biomed Pharmacother. 2011;65:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2005;289:F514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Lincet H, Poulain L, Remy JS, Deslandes E, Duigou F, Gauduchon P, Staedel C. The p21(cip1/waf1) cyclin-dependent kinase inhibitor enhances the cytotoxic effect of cisplatin in human ovarian carcinoma cells. Cancer Lett. 2000;161:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Qin LF, Ng IO. Exogenous expression of p21(WAF1/CIP1) exerts cell growth inhibition and enhances sensitivity to cisplatin in hepatoma cells. Cancer Lett. 2001;172:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Menges CW, Sementino E, Talarchek J, Xu J, Chernoff J, Peterson JR, Testa JR. Group I p21-activated kinases (PAKs) promote tumor cell proliferation and survival through the AKT1 and Raf-MAPK pathways. Mol Cancer Res. 2012;10:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Flak MB, Connell CM, Chelala C, Archibald K, Salako MA, Pirlo KJ, Lockley M, Wheatley SP, Balkwill FR, McNeish IA. p21 Promotes oncolytic adenoviral activity in ovarian cancer and is a potential biomarker. Mol Cancer. 2010;9:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Shiina M, Lacher MD, Christian C, Korn WM. RNA interference-mediated knockdown of p21(WAF1) enhances anti-tumor cell activity of oncolytic adenoviruses. Cancer Gene Ther. 2009;16:810-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 58. | Ma H, Jin G, Hu Z, Zhai X, Chen W, Wang S, Wang X, Qin J, Gao J, Liu J, Wang X, Wei Q, Shen H. Variant genotypes of CDKN1A and CDKN1B are associated with an increased risk of breast cancer in Chinese women. Int J Cancer. 2006;119:2173-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Hamada T, Keum N, Nishihara R, Ogino S. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol. 2017;52:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |