Published online Jun 26, 2020. doi: 10.4252/wjsc.v12.i6.448
Peer-review started: February 24, 2020
First decision: April 25, 2020
Revised: May 9, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 26, 2020
Processing time: 121 Days and 21.1 Hours
Normal cells mainly rely on oxidative phosphorylation as an effective energy source in the presence of oxygen. In contrast, most cancer cells use less efficient glycolysis to produce ATP and essential biomolecules. Cancer cells gain the characteristics of metabolic adaptation by reprogramming their metabolic mechanisms to meet the needs of rapid tumor growth. A subset of cancer cells with stem characteristics and the ability to regenerate exist throughout the tumor and are therefore called cancer stem cells (CSCs). New evidence indicates that CSCs have different metabolic phenotypes compared with differentiated cancer cells. CSCs can dynamically transform their metabolic state to favor glycolysis or oxidative metabolism. The mechanism of the metabolic plasticity of CSCs has not been fully elucidated, and existing evidence indicates that the metabolic phenotype of cancer cells is closely related to the tumor microenvironment. Targeting CSC metabolism may provide new and effective methods for the treatment of tumors. In this review, we summarize the metabolic characteristics of cancer cells and CSCs and the mechanisms of the metabolic interplay between the tumor microenvironment and CSCs, and discuss the clinical implications of targeting CSC metabolism.
Core tip: Accumulating evidence indicates that the inadequacy of many treatments is due to their failure to target cancer stem cells (CSCs). Therefore, CSCs are a promising target for cancer treatment. Recently, it has been reported that CSCs exhibit a unique metabolic phenotype compared to normal cancer cells (non-CSCs), and CSCs can dynamically transform their metabolic state to favor glycolysis or oxidative metabolism. However, the mechanism of the metabolic plasticity of CSCs has not been fully elucidated, and existing evidence indicates that the metabolic phenotype of cancer cells is closely related to the tumor microenvironment (TME). In this article, we summarize the metabolic characteristics of non-CSCs and CSCs, highlight the mechanisms by which CSCs alter their energy metabolism via interactions with the surrounding TME, and discuss the potential therapeutic strategies to target energy metabolism in CSCs.
- Citation: Zhu X, Chen HH, Gao CY, Zhang XX, Jiang JX, Zhang Y, Fang J, Zhao F, Chen ZG. Energy metabolism in cancer stem cells. World J Stem Cells 2020; 12(6): 448-461
- URL: https://www.wjgnet.com/1948-0210/full/v12/i6/448.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i6.448
According to the World Health Organization statistics, cancer is still the most common cause of death, although multiple therapy strategies have significantly improved the overall survival rate of cancer patients. Accumulating evidence indicates that the inadequacy of many treatments is due to their failure to target cancer stem cells (CSCs). CSCs widely exist in different types of tumors and possess the ability to form tumors. Therefore, CSCs are a promising target for cancer treatment. Unfortunately, there are currently few therapeutic options for CSCs because CSCs are resistant to conventional therapies[1].
Recently, it has been reported that CSCs exhibit a unique metabolic phenotype compared to normal cancer cells (non-CSCs). Non-CSCs metabolize glucose to produce lactate through glycolysis even in the presence of sufficient oxygen[2], which is now known as the Warburg effect. Unlike non-CSCs, CSCs may be highly glycolytic or oxidative phosphorylation (OXPHOS)-dependent depending on the niches where the CSCs are located. Targeting the metabolism of CSCs would be a new strategy for CSC treatment.
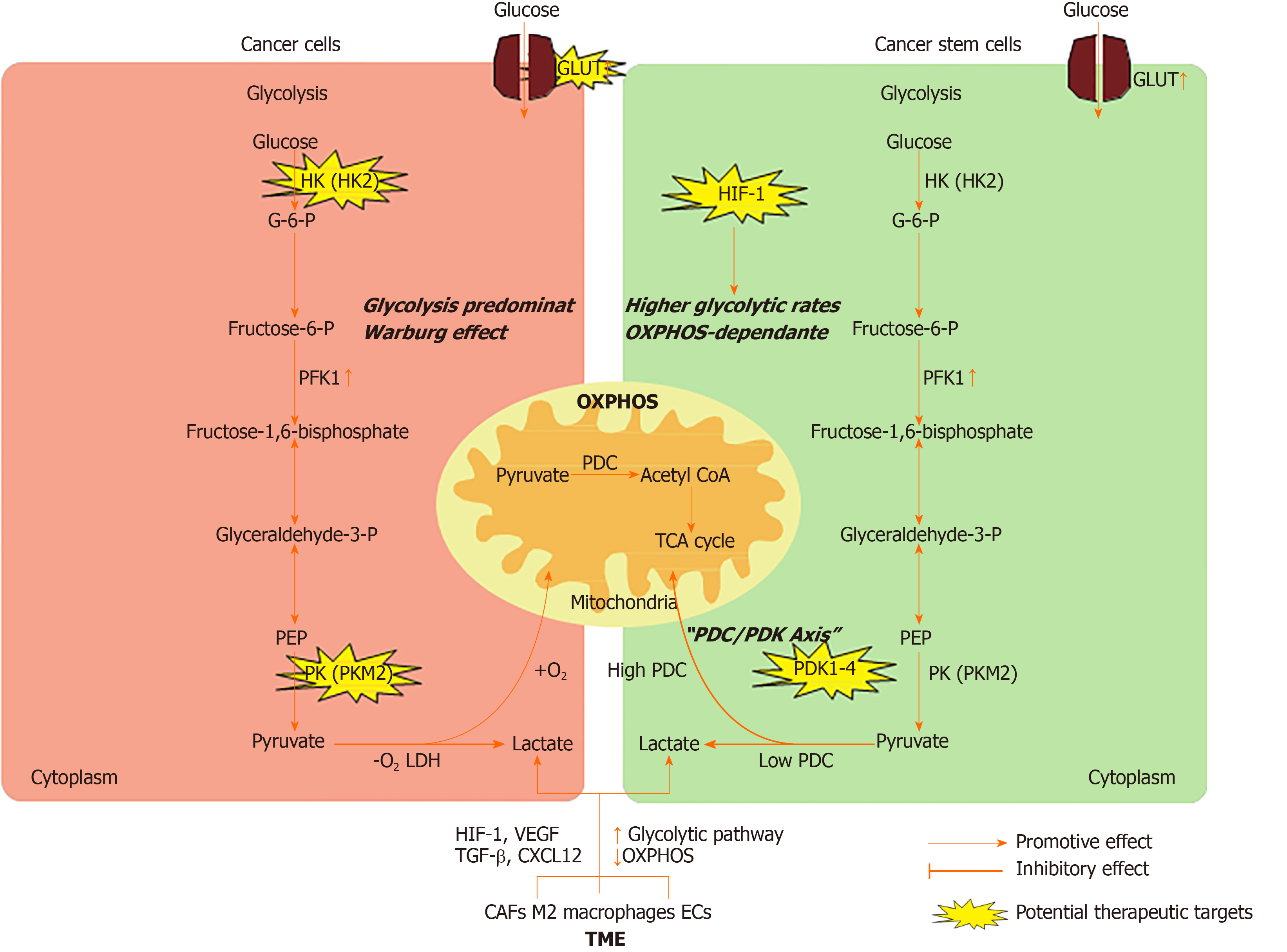
In this review, we summarize the metabolic characteristics of non-CSCs and CSCs, highlight the mechanisms by which CSCs alter their energy metabolism via interactions with the surrounding tumor microenvironment (TME), and discuss the potential therapeutic strategies to target energy metabolism in CSCs (Figure 1).
Somatic cells obtain energy or ATP mainly through the tricarboxylic acid (TCA) cycle and OXPHOS in a normoxic environment. Unlike normal cells, non-CSCs are highly proliferating and produce ATP mainly by glycolysis even in the presence of sufficient oxygen supply, which is called the Warburg effect or aerobic glycolysis[3,4]. The reason is that ATP produced by glycolysis is 100 times faster than that produced by OXPHOS[5]. Meanwhile, cancer cells upregulate the expression of glucose transporters (GLUTs) to gain more glucose. Increasing studies have demonstrated that various GLUTs are upregulated in different types of tumors[6]. This abnormal energy metabolism is a hallmark of cancer cells and is believed to be the root of tumor formation and growth[7]. Several common mechanisms have been reported to be involved in the regulation of glycolysis in cancer cells.
The typical glycolytic pathway includes several reversible enzyme reactions and three irreversible reactions, which are known as the committed steps. The first committed step is the phosphorylation of glucose to glucose-6-phosphate, a process catalyzed by hexokinase (HK). There are four known HK isoforms in mammalian cells, and HK2 is expressed at high levels in cancer cells[8]. The high expression and activity of HK2 in cancer cells have been exploited to detect and image tumors by a method known as [18F]-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET). HK2 ablation reverses tumor formation in vitro and in vivo in non-small-cell lung cancer and breast cancer cells[9]. Recently, HK2 was shown to be overexpressed in human colorectal cancer tissues and cell lines, and knockout of HK2 inhibited cell proliferation, colony formation, and xenograft tumor growth[10]. The second committed step of glycolysis is catalyzed by 6-phosphofructo-1-kinase (PFK1), whereby fructose-6-phosphate (F6P) is converted to fructose-1,6-bisphosphate. Researchers have demonstrated that fructose-2,6-bisphosphate (F2,6BP) controls the rate of glycolysis by allosterically activating PFK1. F2,6BP is generated from F6P by the bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK2/F2,6BPase or PFKB) family, which contains four isoforms. Generally, the kinase activity of the PFKFB3 isoform is increased in cancer, thereby increasing the intracellular concentration of F2,6BP and the allosteric activation capacity for PFK1[11]. PFK1 is also elevated in cancer cells and controls the most important steps of glycolysis[12]. The last committed step of glycolysis is catalyzed by pyruvate kinases (PKs), which transform phosphoenolpyruvate into pyruvate. There are four PK isoforms, and PKM2 is expressed in rapidly proliferating cells, including cancer cells[13]. It has been reported that PKM2 was overexpressed in various malignancies, including lung, breast, prostate, blood, cervical, kidney, bladder, and colon cancers[14,15]. During the last step of glycolysis, PK mediates the production of pyruvate. At this point, the gatekeeper enzyme pyruvate dehydrogenase complex (PDC) determines whether glucose metabolism ends in glycolysis or progresses to the TCA cycle and OXPHOS in the mitochondria. When energy production ends in glycolysis, lactate dehydrogenases (LDHs) convert pyruvate to lactate. Because LDHs are reversible, cells secrete lactate through monocarboxylic acid transporters (MCTs) to promote the reaction and prevent a highly acidic environment. Currently, the expression of LDHs and MCTs has been observed in many tumors, and the effects of LDH and MCT inhibition in cancer cells are under investigation[16]. When pyruvate enters mitochondria for OXPHOS, it is irreversibly converted to acetyl-CoA by PDC decarboxylation. Phosphorylation mediated by pyruvate dehydrogenase kinase enzymes inhibits PDC activity and is involved in the pathophysiology of many metabolically integrated diseases, including cancer[17]. Several studies have shown that both the decrease in PDK activity and the enhancement of PDC activity through drug suppression or reduced expression are associated with reduced tumor growth in vivo[18]. In gastric cancer, compared with that in normal tissue, the expression of PDC is lower in tumor tissue, which predicts a poor prognosis[19].
Hypoxia and activation of oncogenes have been reported to be involved in the upregulation of glycolytic flux[20]. Irregular perfusion and subsequent tissue hypoxia are common features of solid tumors. Under hypoxic conditions, overexpression of hypoxia-inducible factor-1 (HIF-1) upregulates and activates glycolytic proteins, such as GLUTs and glycolytic enzymes, to increase glucose absorption and phosphorylation[21]. Meanwhile, a number of oncogenes, including RAS, SRC, and Myc, and activated AKT have been known to promote glycolysis by increasing the expression of GLUTs and glycolytic enzymes[22-24]. In addition, mutations in tumor suppressors (e.g., PTEN, p53, and VHL) are related to the acceleration of glycolytic flux in cancer cells[25]. In prostate cancer, mutation of PTEN increases the translation of HK2 mRNA by activating the AKT-mTORC1-4EBP1 axis, and deletion of P53 enhances the stability of HK2 mRNA by inhibiting miR143 biogenesis[26].
The metabolic characteristics of CSCs have been the focus of attention. Numerous studies have shown that the metabolic characteristics of CSCs are highly heterogeneous. Unlike non-CSCs, which mainly utilize glycolysis, CSCs exhibit a glycolytic or OXPHOS-dependent metabolic phenotype[27,28].
Normal stem cells mainly use glycolysis to generate energy[29]. Therefore, CSCs were hypothesized to mainly rely on glycolysis, similar to normal stem cells[30]. Indeed, a series of studies in osteosarcoma, glioblastoma, breast cancer, lung cancer, ovarian cancer, and colon cancer have proven that CSCs are more glycolytic than non-CSCs, both in vitro and in vivo[31-33]. Moreover, it has been observed that the glucose uptake, glycolytic enzyme expression, lactate production, and ATP content of CSCs are significantly increased compared with those of non-CSCs[34]. Many genes, including PFKFB4, PDK1, and PKM2, are upregulated in brain CSCs[35]. Inhibition of glycolysis or glucose deprivation can lead to the death of CSCs[36,37].
The glycolytic switch in CSCs plays a key role in stemness rather than being a consequence of achieving pluripotency[38]. Studies have demonstrated that inducing the metabolic transition from OXPHOS to glycolysis can increase CSC-like property of CD44+CD24lowEPCAM+ cells in basal-like breast cancer[39]. Interestingly, HIF-1 has been identified as a central driver of the cascade of events that initiates CSC metabolic reprogramming from OXPHOS to glycolysis[40]. Furthermore, the role of HIF-1 in tumors is related to stem cell characteristics, including self-renewal, pluripotency, tumorigenicity, and therapy resistance, as demonstrated in breast, hematologic, prostate, bladder, and central nervous system malignancies[36,41,42]. In CSCs, HIF-1 alters glucose uptake and metabolism through upregulating GLUT expression, HK2 and PK activity during glycolysis, and LDHA levels at the end of glycolysis and downregulating pyruvate dehydrogenase (PDH) levels[40]. Moreover, HIF-1 reduces mitochondrial reactive oxygen species (ROS) production by increasing the glycolytic pathway and decreasing the TCA cycle. Dynamic maintenance of ROS homeostasis is necessary to induce breast cancer stem cell phenotypes in response to hypoxia or cytotoxic chemotherapy[43].
On the other hand, some studies have shown that CSCs from multiple tumor types (e.g., acute myeloid leukemia, glioblastoma, melanoma, and pancreatic cancer) rely on OXPHOS and have low glycolytic reserves[44-47]. According to these studies, CSCs consume less glucose, produce less lactate, maintain higher ATP levels, and are more inclined to mitochondrial OXPHOS than their differentiated offspring. It is not ideal to study the metabolism of CSCs in an experimental environment lacking a relevant microenvironment. In the absence of better models that preserve the physiological state of CSCs, to keep the metabolic characteristics of CSCs intact, the best experimental strategy is to isolate them directly from patients and analyze them immediately or in the first step of in vitro culture. In glioblastoma specimens, low-passage, patient-derived CSCs are more dependent on OXPHOS than their differentiated offspring[48].
Although OXPHOS produces energy at a much lower rate than glycolysis, it is a much more efficient energy source. Moreover, CSCs also increase the utilization of extracellular metabolites, such as pyruvate, lactate, glutamine, glutamic acid, alanine, and ketone bodies, to adapt to OXPHOS metabolism [49-51]. Similarly, OXPHOS-dependent CSCs may gain selective advantages in specific tumor microenvironments[4]. In addition, studies have shown that elevated OXPHOS levels in CSCs can promote chemotherapeutic resistance. It has been demonstrated that Myc and MCL1 synergistically promote chemotherapy-resistant CSCs by increasing mitochondrial OXPHOS[52]. Additionally, compared with non-CSCs, CSCs have higher mitochondrial mass and mitochondrial membrane potential (Δψm), which reflects that CSCs are more prone to mitochondrial metabolism[53-55]. Although the specific settings leading to the OXPHOS phenotype in all tumor types mentioned above have not been fully characterized, studies have shown that the mitochondrial biogenesis regulator and transcription coactivator peroxisome proliferator-activator 1 alpha (PGC1α) may play an important role in maintaining stemness characteristics[56]. In breast cancer, the inhibition of PGC1α prevents mammosphere formation and CSC marker expression[57]. In addition, in pancreatic CD133+ CSCs, PGC1α is essential for OXPHOS function, self-renewal ability, and tumorigenesis[54]. Growing evidence indicates that mitochondrial function is the basis for maintaining CSCs and can be a target for cancer treatment.
Tumor cells are located in the niche and constantly interact with the surrounding microenvironment. The TME is composed of extracellular matrix (ECM), cancer-associated fibroblasts (CAFs), macrophages, endothelial cells (ECs), immune cells, and a complex network of signaling molecules. It is well acknowledged that the metabolic phenotype of CSCs is regulated by the changing TME, and CSCs also remodel the metabolism of cells in the TME[58].
Recently, it was reported that CAFs could significantly promote the formation and growth of tumor spheres, which indicates an increased tumor formation potential[59]. In addition, in pancreatic cancers, CAFs have been shown to induce epigenetic and metabolic changes in cancer cells and CSCs, thereby promoting tumor progression[60]. CAFs also have metabolic adaptations, and these metabolic adaptations are considered to support glycolysis and play a key role in the use of nutrition by cancer cells and CSCs. Drivers of metabolic changes in CAF activation may include transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), hypoxia, HIF-1α, and ROS-mediated caveolin 1 inhibition[61], which induce CAFs to switch from OXPHOS to aerobic glycolysis. After metabolic reprogramming, CAFs show enhanced catabolism and produce metabolites (lactate, glutamine, and ketones), which are used by cancer cells and CSCs to promote the production of oxidative energy and are associated with their potential for tumorigenicity and resistance to treatment[62]. The metabolic interaction between cancer cells and CAFs in the TME is called the reverse Warburg effect[63]. In the TME, cancer cells, CSCs, and CAFs express different types of lactate MCTs. Previous studies have suggested that epithelial cancer cells with a stem-like phenotype express MCT-1, while CAFs and other differentiated cancer cells express MCT-4[64]. MCT-4-expressing CAFs and differentiated cancer cells secrete lactate through glycolysis, which is absorbed by epithelial CSCs that express MCT-1 and subsequently serves as a substrate for the TCA cycle[63].
Moreover, the autocrine and paracrine effects of CAFs on cancer cells have now been fully studied[65]. Compared with normal fibroblasts, CAFs have the ability to enhance the production of ECM components and secrete unique cytokines, including stromal cell-derived factor-1 (SDF-1)/C-X-C-motif chemokine 12 (CXCL12), vascular endothelial growth factor, PDGF, and hepatocyte growth factor[66,67]. In breast cancer, CAFs promote the growth of breast cancer cells by secreting SDF-1[67]. In addition, CAFs can secrete TGF-β and induce epithelial-mesenchymal transition (EMT) and eventually develop drug resistance[66]. The induction of EMT is involved in the acquisition of stemness, leading to reduced mitochondrial metabolism and increased glycolytic flux[68]. Many EMT regulators, such as TGF-β, Wnt, Snail, and distal-less homeobox-2 (Dlx-2), are involved in the metabolic reprogramming of cancer cells[69].
Tissue inflammation is an important part of the TME, and inflammatory cells and soluble mediators of inflammation, such as cytokines and chemokines, are abundant in the TME. Evidence suggests that inflammation has a dual role in tumor development[70].
Macrophages are one of the most abundant components of the TME and play an active role in tumorigenesis. CSCs can attract macrophages into tumors by producing proinflammatory cytokines and chemokines[71]. Once entering the tumor, macrophages are activated by factors such as IL-4 and transformed into tumor-associated macrophages (TAMs), which need to be metabolically adapted to survive the harsh tumor environment. The M1 polarization of macrophages is induced by endotoxin, interferon-γ, and interleukin (IL)-1α, with a pro-inflammatory role while the M2 phenotype, induced by IL-4, IL-10, IL-13, TGF-β, and glucocorticoids, has anti-inflammatory effects and is involved in the resolution of inflammation[72]. In the TME, TAMs are forced to undergo metabolic reprogramming to compete with cancer cells for nutrition. In solid tumors, hypoxia is one of the important factors determining the vascular structure of tumors. It was found that under hypoxic conditions, TAMs strongly upregulated the expression of the negative regulator of mTOR-DNA damage response 1 (REDD1)[73]. REDD1-mediated mTOR inhibition can block glycolysis in TAMs and reduce their excessive angiogenic response, thereby forming abnormal blood vessels. Moreover, lactate secreted by tumor cells can stabilize HIF-1α and induce the expression of vascular endothelial growth factor (VEGF) and M2 polarization of TAMs[74]. Importantly, the metabolic effects of cancer cells on TAMs are not unidirectional. TAMs exposed to hypoxia or lactate secrete a variety of metabolic cytokines, including IL-6, tumor necrosis factor-α (TNFα), and CC-motif chemokine ligand 5[75-77]. Polarized M2 TAMs secrete IL-6 to enhance 3-phosphoinositide-dependent protein kinase 1-mediated phosphoglycerate kinase 1 threonine (T) 243 phosphorylation, which promotes protein kinase 1-mediated phosphoglycerate kinase 1-catalyzed glycolysis[77]. TAMs secrete TNFα to promote tumor cell glycolysis, with increased GLUT-1 and HK-2 protein expression[75]. M2 TAMs stimulate CC-motif chemokine ligand 5 secretion, which increases cell migration, induces EMT in cancer cells, and promotes aerobic glycolysis in breast cancer cells via AMPK signaling[76]. CSCs induce the M2 phenotype in TAMs, which secrete IL-6, IL-10, TGF-β, and EGF and drive CSC self-renewal by activating the STAT3/NF-κB signaling pathway[78].
Tumor cell growth and culture require multiple strategies to meet oxygen and metabolic needs, which involve the formation of new blood vessels. The angiogenesis process during tumor progression requires the recruitment of endothelial progenitor cells in the TME, during which endothelial progenitor cells differentiate into blood vessels[79]. Angiogenesis is considered a key process for tumor progression and metastasis, and the TME and CSCs are considered to be important promoters of this phenomenon. Elevated lactate concentrations in the TME were found to affect EC signaling by enhancing IL-8/CXCL8 signaling, thereby promoting angiogenesis[80]. In addition, CSCs can induce angiogenesis by secreting HIF-1, VEGFA, CXCL12, and other factors[81]. Moreover, in recent studies, CSCs have been shown to differentiate into ECs in a process known as "vascular mimicry" and generate their own vascular system via a VEGF-dependent pathway[81]. Recently, in a study of gliomas, researchers found that the injection of CSCs into the right frontal lobe of nude mice induced stronger angiogenesis and more hemorrhagic tumors than injection of non-CSC control cells. Meanwhile, the angiogenic advantage of the CSC counterpart may be supported by the 10-20-fold increase in VEGF secretion[82]. Importantly, VEGF supports the angiogenic switch mainly by stimulating the glycolytic pathway. Studies have shown that when the glycolytic pathway is inhibited by the rate-limiting enzyme PFKFB3 in endothelial cells, the efficiency of angiogenesis is reduced[83]. Other studies have found that under different environmental states such as hypoxia or altered glucose metabolism, CSCs can differentiate into functional ECs[84].
The formation of new blood vessels and tumor angiogenesis are necessary conditions for tumor progression. Tumor blood vessels provide nutrition and oxygen for tumors and provide a pathway for tumor metastasis. Recent studies have shown that "vascular endothelial factors" released by ECs promote tumor progression[85]. In brain tumors, endothelial cells interact directly with tumor cells and secrete factors that maintain these cells in a stem cell-like state[86]. In head and neck squamous cell carcinoma, it is reported that 80% of CSCs are located near blood vessels. In addition, ECs secrete a variety of growth factors, including EGF, induce EMT through the PI3K/Akt signaling pathway, and promote the maintenance of CSC characteristics in head and neck squamous cell carcinoma[87]. In breast cancer, ECs secrete TNFα, activate the NF-κB signaling pathway in CSCs, and eventually develop chemical resistance to doxorubicin and cyclophosphamide[88]. In colorectal cancer cells, ECs activate the NANOGP8 pathway associated with CSCs in a paracrine manner[89].
Since CSCs have distinct metabolic phenotypes, which are a response to tumor progression and recurrence. On the other hand, the metabolic phenotype of CSCs is highly flexible between OXPHOS and glycolytic phenotype due to regulation of the TME. Traditional anticancer treatments aim to suppress rapidly proliferating cancer cells and fail to eradicate CSCs. Therefore, it is necessary to develop strategies to target metabolism of CSCs based on finding of the mechanisms involved in maintaining metabolic phenotype of CSCs. Recently, a large number of studies have been designed to selectively target the metabolism of CSCs.
Most CSCs satisfy their energy demands through glycolysis, which is subject to complex regulation. Therefore, various glycolytic enzymes or transporters can be targeted, such as GLUT1-4, HK, PDK1, and PKM2. A direct antiglycolytic strategy was proposed to block the glucose uptake via GLUTs, resulting in a complete disruption of energy metabolism. Previous studies have reported that several agents, such as phloretin, fasentin, and WZB117, have excellent anticancer effects through glucose uptake inhibition and energy deprivation in preclinical models[90-93]. Phloretin is an antagonist of GLUT2[90,91], which suppresses colorectal cancer cell growth by inducing cell cycle arrest and apoptosis via p53-mediated signaling[90]. In addition, phloretin significantly inhibits the migration of cancer cells through paxillin/FAK, Src, alpha smooth muscle actin (α-sMA), and E-cadherin signaling[91]. Fasentin is a GLUT1 inhibitor, which causes glucose deprivation and G0-G1 cell cycle arrest[92]. As a selective GLUT1 inhibitor, WZB117 effectively inhibits glucose uptake, reduces the amount of intracellular ATP, and causes cell cycle arrest[93]. Since GLUTs are widely expressed in normal cells, specific inhibition of glucose uptake in CSCs is challenging.
HK enzymes are responsible for glucose phosphorylation. Several HK inhibitors including 2-deoxy-D-glucose (2-DG), lonidamine (LN), genistein-27 (GEN-27), and benserazide, have been exploited for cancer treatment[94-97]. 2-DG is a well-studied antiglycolytic agent, which competitively inhibits glucose transport[98]. Several clinical trials of 2-DG activity have been designed in cancer patients[96,99,100]. However, the anticancer efficacy and safety of 2-DG are still inconclusive. Currently, 2-DG is widely used in combination with other agents like cisplatin or docetaxel[100,101]. Another HK inhibitor LN has completed preclinical studies and several clinical trials have explored its effects for cancer treatment[102]. Unfortunately, no significant survival benefit of LN has been studied in several cancer types, such as lung, breast, and ovarian cancers[97,103,104].
Pyruvate in the cytosol is converted into mitochondrial acetyl-CoA, which can enter the Krebs cycle via PDH enzymes. PDH is negatively regulated by the PDK enzyme, leading to a shift from OXPHOS to glycolytic metabolism. Thus, targeting PDK may be another attractive approach to inhibit cellular proliferation and cancer growth by inducing cancer cell or CSC metabolic reprogramming. Dichloroacetate (DCA) can activate mitochondrial PDH by inhibiting its regulator, PDK, and then enhance reprogramming of metabolism from glycolysis towards mitochondrial OXPHOS[105]. Several clinical trials are ongoing for testing DCA efficacy as an anticancer agent[106,107]. DCA is known to be relatively well tolerated with few significant side effects, and tumor response assessed by FDG-PET revealed stable disease in eight patients but no response in others[107]. Overall, the current results of DCA for cancer therapy are preliminary, supporting a favorable toxicity profile but limited anticancer efficacy.
PK converts phosphoenolpyruvate into pyruvate by dephosphorylation to generate ATP. Kefas et al[108] demonstrated that PKM2 was expressed in glioma stem cells. Furthermore, PKM2 knockdown in glioma stem cells led to decreased cell proliferation and impaired metabolism accompanied by a reduced ATP level, which indicated that inhibition of PKM2 was a potential target in glioma stem cells[108].
Moreover, CD44 was previously reported to interact with PKM2 and thereby enhance the glycolytic phenotype, suggesting that it could also become a preferential target. Tamada et al[109] reported that ablation of CD44 led to inhibition of glycolysis, with an increase in ROS, and enhanced the chemotherapeutic drug effect in glioma, colorectal cancer, or lung cancer cells. As a surface marker of CSCs, it can also be utilized for effective cytotoxic drug delivery[110].
On the other hand, CSCs can rapidly transition their metabolic phenotype under heterogeneous environmental conditions (such as hypoxia and glucose deprivation); thus, targeting adaptive mechanisms is also an optional strategy. As mentioned above, HIF-1α is one of the principle factors that reprograms cells to undergo glycolysis instead of OXPHOS[21,111] and is also involved in angiogenesis, metastasis, and cell survival[112]. Hence, targeting HIF-1α is a potential therapy for cancer treatment as well.
Previously discussed evidence for OXPHOS in CSCs indicated mitochondrial metabolism to be a potential therapeutic target for eliminating CSCs. Inhibition of the OXPHOS pathway inhibits sphere formation and tumorigenesis, manifesting the vulnerability of CSCs to mitochondria-targeted drugs[47,113]. Several pharmacological agents targeting OXPHOS are currently being explored in preclinical studies and clinical trials for cancer treatment.
The anti-diabetic agent metformin has emerged as a promising candidate for targeting oxidative metabolism in pancreatic CSCs[54]. Several clinical results suggested that cancer patients with diabetes treated with metformin had a better prognosis than those treated with other antidiabetic regimens, which aroused researchers’ interest in the mechanisms[114,115]. Wheaton’s study on metformin found that its antitumor activity involved the impairment of OXPHOS through direct inhibition of mitochondrial electron transport chain complex I[116]. Nevertheless, the use of metformin for cancer therapy remains controversial. The first clinical trial testing the effect of metformin in pancreatic ductal adenocarcinoma patients did not report a positive outcome[117]. The MYME trial failed to provide evidence in support of the efficacy of metformin in treating metastatic breast cancer patients[118], while another study on non-small-cell lung cancer showed a significant change in outcome with the addition of metformin to standard chemotherapy[119]. Phenformin, another biguanide formerly used in diabetes, can be delivered to mitochondria more efficiently than metformin[4]. This agent also induces cancer cell death by inhibiting complex I and promotes apoptosis, offering promising preclinical results in certain cancer types[120], but its clinical application remains to be studied.
Previous studies that were developed to screen compounds that selectively eliminate CSCs eventually discovered several drugs that inhibit mitochondrial activity. For example, the antibiotic salinomycin was identified and found to inhibit OXPHOS[121]. The study showed that salinomycin treatment could result in reduced expression of breast CSC genes and thus inhibit mammary tumor growth in vivo. Another antibiotic, tigecycline, was also identified in a screen using OXPHOS-dependent leukemia cells[122]. This agent was found to suppress OXPHOS by inhibiting mitochondrial translation associated with mitochondrial ribosomes. However, the clinical utility of these two drugs has not been fully elucidated.
As mentioned before, CSCs relying on OXPHOS show an elevated Δψm, which can also be exploited for selectively increasing drug delivery to the mitochondria. Triphenylphosphonium, as a delocalized lipophilic cation, accumulates in the mitochondrial matrix and can be conjugated to small compounds for selective drug delivery to mitochondria[123]. A recent study showed that conjugation of triphenylphosphonium to doxorubicin, a DNA topoisomerase II inhibitor, directed its activity towards mitochondrial DNA, promoting drug selectivity for cancer cells with reduced mitochondrial DNA integrity and preventing the acquisition of resistance by drug efflux[124].
Due to the metabolic plasticity of CSCs, dual inhibition of the glycolytic and OXPHOS energy pathways may be the best approach against tumor growth. One study using such a strategy elegantly demonstrated that sarcoma cells are more sensitive than normal cells to synergistic effects of inhibiting glycolysis with 2-DG and OXPHOS with oligomycin or metformin[125]. These results suggest that the dual inhibition of glycolytic and mitochondrial respiration may represent a better approach to eradicating CSCs and cancer treatment[126,127]. Despite limited clinical evidence, targeting CSCs by blocking their metabolic components still holds great potential in improving cancer treatments. In practice, combinational treatments involving both a standard cytotoxic therapy and a CSC-targeted therapy will probably enhance the antitumor effect.
CSCs are thought to be the source of cancer cell production, resistant to treatment, and responsible for metastasis, and eliminating them could lead to a permanent cure for patients. Increasing evidence indicates that CSCs have distinct metabolic phenotypes compared to most differentiated tumor cells. Therefore, the metabolic phenotype of CSCs has attracted great interest. CSCs have a unique metabolic phenotype and exhibit metabolic plasticity between high levels of glycolysis and OXPHOS, which may be related to the TME. However, the mechanisms of CSC metabolic plasticity still need to be clarified. Targeting CSC metabolism is considered a new treatment to eliminate cancer recurrence or metastasis. Combining CSC-targeted drugs with traditional anticancer treatments may be a more effective strategy for treating cancer. It is hypothesized that tumor stem cells may be derived from genetic changes in normal stem cells. It is necessary to accurately distinguish these two similar cell types to eliminate the damage that targeting CSCs may cause to normal stem cells.
A large number of studies have provided evidence that metabolic plasticity of CSCs exists in different malignancy, but the mechanisms involved in this process still need to be explored in the future. The role of metabolic reprogramming in CSCs in tumorigenesis, metastasis, drug resistance, and tumor relapse needs more evidence. Targeting the metabolism of CSCs is a promising approach in cancer treatment. However, it is necessary to identify specific targets to selectively inhibit the metabolism of CSCs without causing damage to normal stem cells. We expect that more preclinical and clinical studies will be carried out to find effective new anticancer agents.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Majumdar APN S-Editor: Wang J L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 2. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9924] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 3. | Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010;70:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 4. | Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 418] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 5. | Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 993] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 6. | Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism. 2016;65:124-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 7. | Garber K. Energy deregulation: licensing tumors to grow. Science. 2006;312:1158-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683-4696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, Jha AK, Smolen GA, Clasquin MF, Robey B, Hay N. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 691] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 10. | Liu W, Li W, Liu H, Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int J Biol Sci. 2019;15:2497-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Chesney J. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis. Curr Opin Clin Nutr Metab Care. 2006;9:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 806] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 13. | Li YH, Li XF, Liu JT, Wang H, Fan LL, Li J, Sun GP. PKM2, a potential target for regulating cancer. Gene. 2018;668:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 616] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 15. | Bluemlein K, Grüning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011;2:393-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 16. | Ždralević M, Marchiq I, de Padua MMC, Parks SK, Pouysségur J. Metabolic Plasiticy in Cancers-Distinct Role of Glycolytic Enzymes GPI, LDHs or Membrane Transporters MCTs. Front Oncol. 2017;7:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Stacpoole PW. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 18. | Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer. 2016;138:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 19. | Song L, Liu D, Zhang X, Zhu X, Lu X, Huang J, Yang L, Wu Y. Low expression of PDHA1 predicts poor prognosis in gastric cancer. Pathol Res Pract. 2019;215:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Chae YC, Kim JH. Cancer stem cell metabolism: target for cancer therapy. BMB Rep. 2018;51:319-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 124] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 903] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 22. | Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 583] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 23. | Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 725] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 24. | Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797-21800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 25. | Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 578] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 26. | Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, Koochekpour S, Saleem M, Huang H, Lu J, Deng Y. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014;8:1461-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 27. | Emmink BL, Verheem A, Van Houdt WJ, Steller EJ, Govaert KM, Pham TV, Piersma SR, Borel Rinkes IH, Jimenez CR, Kranenburg O. The secretome of colon cancer stem cells contains drug-metabolizing enzymes. J Proteomics. 2013;91:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Hammoudi N, Ahmed KB, Garcia-Prieto C, Huang P. Metabolic alterations in cancer cells and therapeutic implications. Chin J Cancer. 2011;30:508-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Jang H, Yang J, Lee E, Cheong JH. Metabolism in embryonic and cancer stemness. Arch Pharm Res. 2015;38:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 523] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 31. | Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D'Agostino D, Forlì F, D'Aguanno S, Todaro M, Stassi G, Di Ilio C, De Laurenzi V, Urbani A. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 220] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 33. | Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, Keating MJ, Kondo S, Huang P. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843-32853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, Hu Y, Wang P, Ju HQ, Xu RH, Huang P. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ. 2014;21:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 35. | Goidts V, Bageritz J, Puccio L, Nakata S, Zapatka M, Barbus S, Toedt G, Campos B, Korshunov A, Momma S, Van Schaftingen E, Reifenberger G, Herold-Mende C, Lichter P, Radlwimmer B. RNAi screening in glioma stem-like cells identifies PFKFB4 as a key molecule important for cancer cell survival. Oncogene. 2012;31:3235-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | De Francesco EM, Sotgia F, Lisanti MP. Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem J. 2018;475:1611-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 37. | Palorini R, Votta G, Balestrieri C, Monestiroli A, Olivieri S, Vento R, Chiaradonna F. Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J Cell Biochem. 2014;115:368-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 39. | Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 650] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 40. | Yuen CA, Asuthkar S, Guda MR, Tsung AJ, Velpula KK. Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: a new target gleaned from an old concept. CNS Oncol. 2016;5:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Schito L, Semenza GL. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer. 2016;2:758-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 683] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 42. | Tong WW, Tong GH, Liu Y. Cancer stem cells and hypoxia-inducible factors (Review). Int J Oncol. 2018;53:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 44. | Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008;68:4018-4021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Janiszewska M, Suvà ML, Riggi N, Houtkooper RH, Auwerx J, Clément-Schatlo V, Radovanovic I, Rheinbay E, Provero P, Stamenkovic I. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26:1926-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 46. | Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 1030] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 47. | Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA, Philipp SE, Krause E, Pätzold S, Villanueva J, Krepler C, Fukunaga-Kalabis M, Hoth M, Bastian BC, Vogt T, Herlyn M. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 543] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 48. | Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, Reue K, Christofk H, Mischel PS, Pajonk F. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci USA. 2011;108:16062-16067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 425] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 49. | Cuyàs E, Corominas-Faja B, Menendez JA. The nutritional phenome of EMT-induced cancer stem-like cells. Oncotarget. 2014;5:3970-3982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | LaBarge MA. The difficulty of targeting cancer stem cell niches. Clin Cancer Res. 2010;16:3121-3129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1060] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 52. | Lee KM, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL, Hutchinson KE, Nixon MJ, Estrada MV, Sánchez V, Sanders ME, Lee T, Gómez H, Lluch A, Pérez-Fidalgo JA, Wolf MM, Andrejeva G, Rathmell JC, Fesik SW, Arteaga CL. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017;26:633-647.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 468] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 53. | Lamb R, Bonuccelli G, Ozsvári B, Peiris-Pagès M, Fiorillo M, Smith DL, Bevilacqua G, Mazzanti CM, McDonnell LA, Naccarato AG, Chiu M, Wynne L, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Mitochondrial mass, a new metabolic biomarker for stem-like cancer cells: Understanding WNT/FGF-driven anabolic signaling. Oncotarget. 2015;6:30453-30471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 54. | Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Graña O, Viera CR, Yuneva M, Sainz B Jr, Heeschen C. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015;22:590-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 568] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 55. | Vlashi E, Lagadec C, Vergnes L, Reue K, Frohnen P, Chan M, Alhiyari Y, Dratver MB, Pajonk F. Metabolic differences in breast cancer stem cells and differentiated progeny. Breast Cancer Res Treat. 2014;146:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Lamb R, Ozsvari B, Bonuccelli G, Smith DL, Pestell RG, Martinez-Outschoorn UE, Clarke RB, Sotgia F, Lisanti MP. Dissecting tumor metabolic heterogeneity: Telomerase and large cell size metabolically define a sub-population of stem-like, mitochondrial-rich, cancer cells. Oncotarget. 2015;6:21892-21905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | De Luca A, Fiorillo M, Peiris-Pagès M, Ozsvari B, Smith DL, Sanchez-Alvarez R, Martinez-Outschoorn UE, Cappello AR, Pezzi V, Lisanti MP, Sotgia F. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget. 2015;6:14777-14795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 58. | Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 559] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 59. | Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3347] [Cited by in RCA: 3528] [Article Influence: 185.7] [Reference Citation Analysis (1)] |
| 60. | Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 61. | Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2209] [Cited by in RCA: 2941] [Article Influence: 326.8] [Reference Citation Analysis (0)] |
| 62. | Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and "fibroblast addiction" are new therapeutic targets for drug discovery. Cell Cycle. 2013;12:2723-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984-4001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1083] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 64. | Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P, Chiarugi P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130-5140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 450] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 65. | Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 66. | Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1048] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 67. | Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2625] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 68. | Lee SY, Jeon HM, Ju MK, Jeong EK, Kim CH, Yoo MA, Park HG, Han SI, Kang HS. Dlx-2 is implicated in TGF-β- and Wnt-induced epithelial-mesenchymal, glycolytic switch, and mitochondrial repression by Snail activation. Int J Oncol. 2015;46:1768-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 399] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 70. | Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, Kreutz M. Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment. Front Immunol. 2017;8:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 71. | Mehla K, Singh PK. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer. 2019;5:822-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 407] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 72. | Krstic J, Trivanovic D, Jaukovic A, Santibanez JF, Bugarski D. Metabolic Plasticity of Stem Cells and Macrophages in Cancer. Front Immunol. 2017;8:939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J, Prenen H, Ghesquière B, Carmeliet P, Mazzone M. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016;24:701-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 74. | Shrivastava R, Singh V, Asif M, Negi MPS, Bhadauria S. Oncostatin M upregulates HIF-1α in breast tumor associated macrophages independent of intracellular oxygen concentration. Life Sci. 2018;194:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE, Bok S, Oh JM, Gwak SH, Yoo MY, Lee MS, Chung SJ, Defrêne J, Tessier P, Pelletier M, Jeon H, Roh TY, Kim B, Kim KH, Ju JH, Kim S, Lee YJ, Kim DW, Kim IH, Kim HJ, Park JW, Lee YS, Lee JS, Cheon GJ, Weissman IL, Chung DH, Jeon YK, Ahn GO. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019;79:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 76. | Lin S, Sun L, Lyu X, Ai X, Du D, Su N, Li H, Zhang L, Yu J, Yuan S. Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5-CCR5 axis: a positive metabolic feedback loop. Oncotarget. 2017;8:110426-110443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 77. | Zhang Y, Yu G, Chu H, Wang X, Xiong L, Cai G, Liu R, Gao H, Tao B, Li W, Li G, Liang J, Yang W. Macrophage-Associated PGK1 Phosphorylation Promotes Aerobic Glycolysis and Tumorigenesis. Mol Cell. 2018;71:201-215.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 78. | Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 775] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 79. | De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 80. | Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 81. | Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1019] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 82. | Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843-7848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 967] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 83. | De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1139] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 84. | Zhao Y, Dong J, Huang Q, Lou M, Wang A, Lan Q. Endothelial cell transdifferentiation of human glioma stem progenitor cells in vitro. Brain Res Bull. 2010;82:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Maishi N, Hida K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017;108:1921-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 227] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 86. | Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1640] [Cited by in RCA: 1574] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 87. | Zhang Z, Dong Z, Lauxen IS, Filho MS, Nör JE. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014;74:2869-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 88. | Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massagué J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 884] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 89. | Wang R, Bhattacharya R, Ye X, Fan F, Boulbes DR, Xia L, Ellis LM. Endothelial cells activate the cancer stem cell-associated NANOGP8 pathway in colorectal cancer cells in a paracrine fashion. Mol Oncol. 2017;11:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Lin ST, Tu SH, Yang PS, Hsu SP, Lee WH, Ho CT, Wu CH, Lai YH, Chen MY, Chen LC. Apple Polyphenol Phloretin Inhibits Colorectal Cancer Cell Growth via Inhibition of the Type 2 Glucose Transporter and Activation of p53-Mediated Signaling. J Agric Food Chem. 2016;64:6826-6837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Wu KH, Ho CT, Chen ZF, Chen LC, Whang-Peng J, Lin TN, Ho YS. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J Food Drug Anal. 2018;26:221-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 92. | Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, Klip A, Batey RA, Schimmer AD. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7:3546-3555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 93. | Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 434] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 94. | Tao L, Wei L, Liu Y, Ding Y, Liu X, Zhang X, Wang X, Yao Y, Lu J, Wang Q, Hu R. Gen-27, a newly synthesized flavonoid, inhibits glycolysis and induces cell apoptosis via suppression of hexokinase II in human breast cancer cells. Biochem Pharmacol. 2017;125:12-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 95. | Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, Min Q, Sun W, Chen L, Xiang G, Li H. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J Exp Clin Cancer Res. 2017;36:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 96. | Coleman MC, Asbury CR, Daniels D, Du J, Aykin-Burns N, Smith BJ, Li L, Spitz DR, Cullen JJ. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radic Biol Med. 2008;44:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 97. | Berruti A, Bitossi R, Gorzegno G, Bottini A, Alquati P, De Matteis A, Nuzzo F, Giardina G, Danese S, De Lena M, Lorusso V, Farris A, Sarobba MG, DeFabiani E, Bonazzi G, Castiglione F, Bumma C, Moro G, Bruzzi P, Dogliotti L; Epirubicin-Lonidamine Group, Orbassano, Torino, Italy. Time to progression in metastatic breast cancer patients treated with epirubicin is not improved by the addition of either cisplatin or lonidamine: final results of a phase III study with a factorial design. J Clin Oncol. 2002;20:4150-4159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Dwarakanath B, Jain V. Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol. 2009;5:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 99. | Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, Eddy S, Goodin S, White E, Dipaola RS. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate. 2010;70:1388-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 100. | Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 101. | Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 102. | Di Cosimo S, Ferretti G, Papaldo P, Carlini P, Fabi A, Cognetti F. Lonidamine: efficacy and safety in clinical trials for the treatment of solid tumors. Drugs Today (Barc). 2003;39:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 103. | De Marinis F, Rinaldi M, Ardizzoni A, Bruzzi P, Pennucci MC, Portalone L, D'Aprile M, Ripanti P, Romano F, Belli M, Altavilla G, Migliorino MR, Rosso R, Salvati F. The role of vindesine and lonidamine in the treatment of elderly patients with advanced non-small cell lung cancer: a phase III randomized FONICAP trial. Italian Lung Cancer Task Force. Tumori. 1999;85:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 104. | De Lena M, Lorusso V, Bottalico C, Brandi M, De Mitrio A, Catino A, Guida M, Latorre A, Leone B, Vallejo C, Gargano G. Revertant and potentiating activity of lonidamine in patients with ovarian cancer previously treated with platinum. J Clin Oncol. 1997;15:3208-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 106. | Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs. 2014;32:452-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 107. | Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs. 2015;33:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 108. | Kefas B, Comeau L, Erdle N, Montgomery E, Amos S, Purow B. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro Oncol. 2010;12:1102-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 109. | Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, Sugihara E, Onishi N, Yamamoto T, Yanagawa H, Suematsu M, Saya H. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 110. | Snyder V, Reed-Newman TC, Arnold L, Thomas SM, Anant S. Cancer Stem Cell Metabolism and Potential Therapeutic Targets. Front Oncol. 2018;8:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 111. | Peng F, Wang JH, Fan WJ, Meng YT, Li MM, Li TT, Cui B, Wang HF, Zhao Y, An F, Guo T, Liu XF, Zhang L, Lv L, Lv DK, Xu LZ, Xie JJ, Lin WX, Lam EW, Xu J, Liu Q. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene. 2018;37:1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 112. | Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1170] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 113. | Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget. 2015;6:4569-4584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 377] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 114. | Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53:1631-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 115. | Kheirandish M, Mahboobi H, Yazdanparast M, Kamal W, Kamal MA. Anti-cancer Effects of Metformin: Recent Evidences for its Role in Prevention and Treatment of Cancer. Curr Drug Metab. 2018;19:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 116. | Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, Chandel NS. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 864] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 117. | Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 118. | Nanni O, Amadori D, De Censi A, Rocca A, Freschi A, Bologna A, Gianni L, Rosetti F, Amaducci L, Cavanna L, Foca F, Sarti S, Serra P, Valmorri L, Bruzzi P, Corradengo D, Gennari A; MYME investigators. Metformin plus chemotherapy versus chemotherapy alone in the first-line treatment of HER2-negative metastatic breast cancer. The MYME randomized, phase 2 clinical trial. Breast Cancer Res Treat. 2019;174:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 119. | Marrone KA, Zhou X, Forde PM, Purtell M, Brahmer JR, Hann CL, Kelly RJ, Coleman B, Gabrielson E, Rosner GL, Ettinger DS. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naïve Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist. 2018;23:859-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 120. | Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, Shaw RJ. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 481] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 121. | Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 1909] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 122. | Skrtić M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, Hurren R, Jitkova Y, Gronda M, Maclean N, Lai CK, Eberhard Y, Bartoszko J, Spagnuolo P, Rutledge AC, Datti A, Ketela T, Moffat J, Robinson BH, Cameron JH, Wrana J, Eaves CJ, Minden MD, Wang JC, Dick JE, Humphries K, Nislow C, Giaever G, Schimmer AD. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2011;20:674-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 123. | Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777:1028-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 419] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 124. | Chamberlain GR, Tulumello DV, Kelley SO. Targeted delivery of doxorubicin to mitochondria. ACS Chem Biol. 2013;8:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 125. | Issaq SH, Teicher BA, Monks A. Bioenergetic properties of human sarcoma cells help define sensitivity to metabolic inhibitors. Cell Cycle. 2014;13:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, Wa Cheng K, Hall H, Zhang D, Lu Y, Ravoori M, Kundra V, Ajani J, Lee JS, Ki Hong W, Mills GB. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011;10:2350-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 127. | Peiris-Pagès M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP. Cancer stem cell metabolism. Breast Cancer Res. 2016;18:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 364] [Article Influence: 6.4] [Reference Citation Analysis (0)] |