Published online Mar 26, 2020. doi: 10.4252/wjsc.v12.i3.188
Peer-review started: November 4, 2019
First decision: December 6, 2019
Revised: December 12, 2019
Accepted: February 17, 2020
Article in press: February 17, 2020
Published online: March 26, 2020
Processing time: 142 Days and 18.6 Hours
In steady state, the intestinal epithelium forms an important part of the gut barrier to defend against luminal bacterial attack. However, the intestinal epithelium is compromised by ionizing irradiation due to its inherent self-renewing capacity. In this process, small intestinal bacterial overgrowth is a critical event that reciprocally alters the immune milieu. In other words, intestinal bacterial dysbiosis induces inflammation in response to intestinal injuries, thus influencing the repair process of irradiated lesions. In fact, it is accepted that commensal bacteria can generally enhance the host radiation sensitivity. To address the determination of radiation sensitivity, we hypothesize that Paneth cells press a critical “button” because these cells are central to intestinal health and disease by using their peptides, which are responsible for controlling stem cell development in the small intestine and luminal bacterial diversity. Herein, the most important question is whether Paneth cells alter their secretion profiles in the situation of ionizing irradiation. On this basis, the tolerance of Paneth cells to ionizing radiation and related mechanisms by which radiation affects Paneth cell survival and death will be discussed in this review. We hope that the relevant results will be helpful in developing new approaches against radiation enteropathy.
Core tip: In healthy individuals, Paneth cells restrict the overgrowth of commensal bacteria in the gut while killing luminal pathogenic bacteria by secreting antimicrobial peptides. Such a property protects crypt intestinal stem cells against bacterial infection, thus ensuring epithelial homeostasis in steady state. Among the active pool of intestinal stem cells, apoptosis commonly occurs as a result of ionizing irradiation. Nevertheless, the intestinal epithelium will recover its integrity after sublethal irradiation. On this basis, the mechanism by which Paneth cells provide growth signals for intestinal stem cells to facilitate epithelial regeneration is easy to understand, whereas the automatic recovery of irradiated intestine from sublethal irradiation is perplexing. Being challenged with luminal bacteria, the degranulation of Paneth cells can be stimulated in a cholinergic- or inflammatory-substance-dependent manner. Then, Paneth cells can perform an antibacterial function that influences the inflammatory milieu in irradiated intestine. Therefore, radiation-induced intestinal bacterial dysbiosis can be managed.
- Citation: Gao YL, Shao LH, Dong LH, Chang PY. Gut commensal bacteria, Paneth cells and their relations to radiation enteropathy. World J Stem Cells 2020; 12(3): 188-202
- URL: https://www.wjgnet.com/1948-0210/full/v12/i3/188.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i3.188
Ionizing irradiation is indeed a useful tool for treating malignant tumors. In current guidelines, radiation therapy is highly recommended for local rectal cancers with indications after preoperative or postoperative evaluations[1]. However, the standard target volume includes the iliac lymph drainage area, thus enabling a portion of the small intestine and colon to be irradiated unavoidably[2]. Clinically, the gut is regarded as an early responding organ to ionizing irradiation, and acute enteritis commonly occurs during treatment[3]. Although acute injuries can be self-limited, more severe lesions, such as intestinal obstruction, bleeding or perforation, potentially increase their morbidities in the postirradiation period among some patients, thus poorly affecting their quality of life[3].
It has been well accepted that radiation-induced intestinal injury is an independent disease, which is termed as radiation enteropathy (RE). The pathogenesis of RE is indeed complicated, and several factors are involved in this process[3]. First, radiation-induced DNA damage occurs at the initial stage of RE. As is known, the intestinal epithelium represents one tissue with fast self-renewing capability in humans, thus enabling the epithelium to be compromised by ionizing irradiation[4]. However, the cells that form intestinal tissues differ in their radiation sensitivities. For example, smooth muscle cells are more resistant to ionizing irradiation than lymphocytes or endothelial cells partially due to their inactive cell cycle[5]. Moreover, the large bowel is well tolerant to ionizing irradiation compared to the small bowel[6,7]. Apart from the potential differences between the small and large bowels in their histological structures, several other factors also account for radiation sensitivity determination. Herein, commensal bacteria have emerged as critical candidates because they function in shaping host immunity along with strengthening intestinal epithelial homeostasis[8,9]. In clinical practice, most colorectal cancer (CRC) patients undergoing radiation therapy bear tumor burdens. Critically, intestinal bacterial dysbiosis has been proven in gut carcinogenesis, as has its promotion of tumor progression[10,11]. Moreover, radiation itself is able to potentiate intestinal bacterial dysbiosis as well[8]. In this regard, although radiation therapy induces in-field tumor shrinkage, it should be argued whether radiation-induced intestinal bacterial dysbiosis further aggravates the immunological milieu, which potentially increases the risk of local or distant CRC relapse. If so, radiation-induced intestinal bacterial dysbiosis will enable radiation therapy to be contraindicated in CRC patients. In fact, it is well known that several types of cells in the gut can produce antimicrobial substances, such as secretory IgA (sIgA) by B cells or plasma cells and antimicrobial peptides by epithelial cells. Herein, Paneth cells are specialized epithelial cells of the small intestine, which provide a wider range of secretions than other epithelial cells in this process. In this regard, we hypothesize that Paneth cells are critical in regulating microbial ecology postirradiation.
Experts in radiation-associated fields have long understood the importance of commensal bacteria in the pathogenesis of RE. To elaborate on this issue, some landmark studies should be mentioned here. Several decades ago, McLaughlin et al[12] reported that germfree mice were more resistant to whole-body irradiation (WBI) than conventional mice, thus confirming specific roles of commensal bacteria in determining host radiation sensitivity. Afterwards, Potten[13] identified that the numbers of crypt apoptotic cells did not differ within six hours postirradiation when using doses from 1 Gy to 10 Gy. Likewise, Beck et al[14] found that either 6 Gy or 14 Gy could induce a significant reduction in the number of goblet cells at the third day postirradiation, suggesting no discrimination between these doses in damaging goblet cells. Nevertheless, it is widely observed that mice can recover from sublethal irradiation even though they lack foreign interventions. To support this view, basic research revealed that intestinal injuries could be repaired automatically if irradiated using doses from 6 Gy to 12 Gy, whereas greater than 15 Gy led to an irreversible breakdown of the epithelium[15]. Moreover, although 0.01 Gy is enough to induce apoptosis in a portion of Lgr5-positive intestinal stem cells (ISCs)[16], doses less than 6 Gy barely impair epithelial structures[15]. In this case, what is the force in discriminating the biological effects between lethal and sublethal irradiation? In fact, a previous study reported that SCID mice could survive no more than two weeks if they were irradiated using doses larger than 5 Gy[17], suggesting the participation of adaptive immunity in controlling the tolerance of hosts to radiation. In general, it has been determined that ionizing irradiation can affect host immunity. After extensive exploration, it is gradually deduced that radiation therapy affects host damage and repair processes by regulating the balance between effector T (Teff) cells and regulatory T (Treg) cells or by altering the numbers of other lineage-derived promoters or suppressors infiltrating into lesioned sites[18]. According to this concept, several strategies should become potential candidates for RE treatment, such as regenerative medicine by using mesenchymal stem cells, which exhibit capacities for activating host repair responses[19]. In contrast to immunomodulatory effects, the beneficial implications of stem cell therapy in intestinal bacterial dysbiosis are rarely reported. To resolve this imbalance, bacteria-supportive care (BSC) can be used for RE because several lines of evidence from clinical trials have indicated the therapeutic efficacies of probiotics, prebiotics and symbiotics[8]. Therefore, commensal bacteria play critical roles in determining intestinal radiation sensitivity[20].
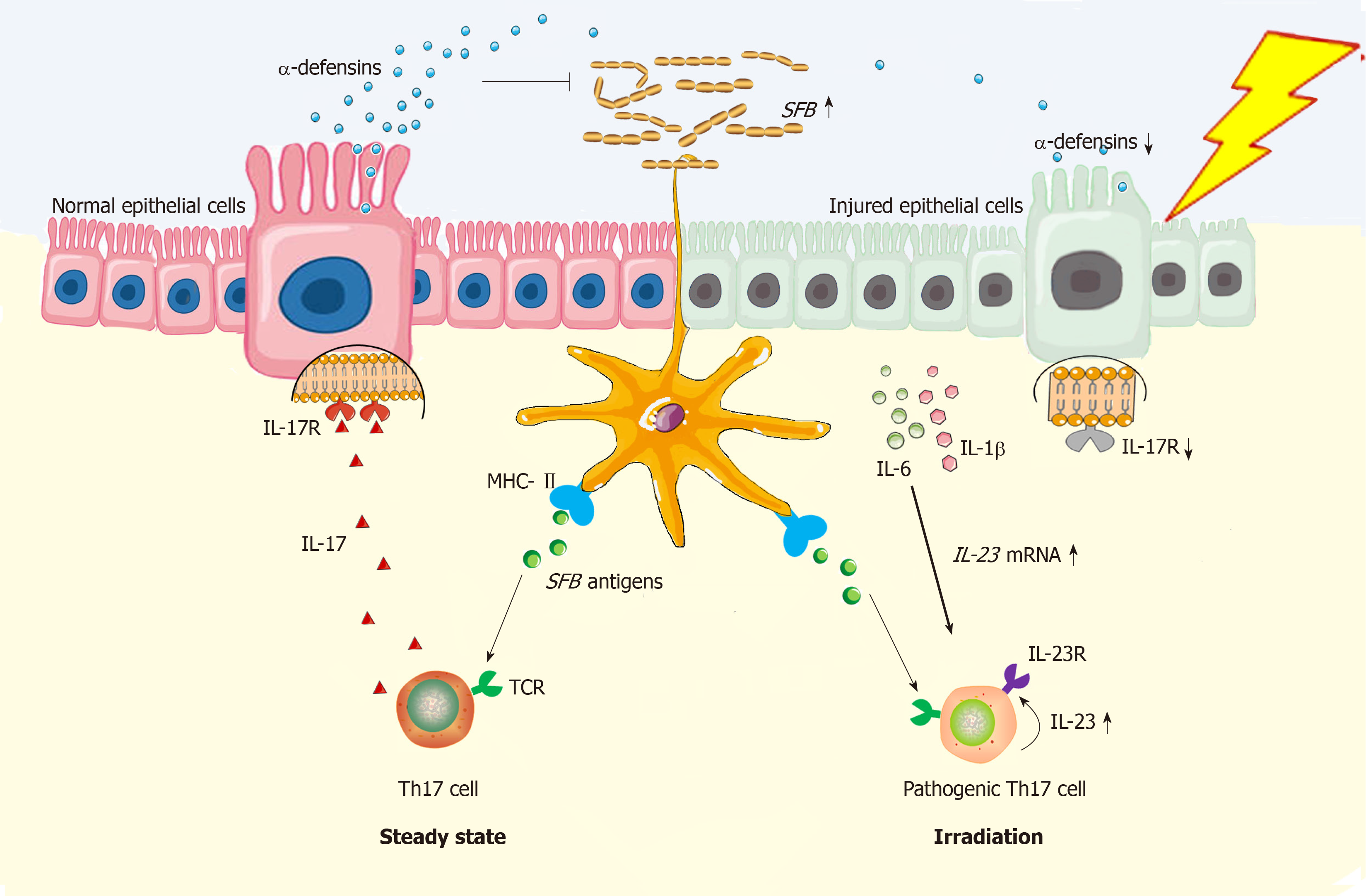
As mentioned above, intestinal commensal bacteria shape the host immunity. Herein, small intestinal bacterial overgrowth occurs as a result of radiation[21], thus enabling the immune milieu within irradiated sites to be altered reciprocally. In this process, Treg cells and their counterparts, Th17 cells, should be highlighted here because the mutual restriction between Treg and Th17 cells certainly impacts the prognosis in various diseases, especially in autoimmune diseases[22]. In the gut, Treg and Th17 cells can be induced from CD4+ naïve T cells by luminal commensal bacteria. In steady state, the human colon contains higher frequencies of commensal bacteria than the small intestine[23]. Herein, polysaccharide (PSA)–producing Bacteroides fragilis (B. fragilis) are mainly distributed in the colon, and these bacteria primarily exert the function of inducing Treg cell generation in colonic laminar propria (LP)[24]. By contrast, Th17 cells show peak numbers in the LP of the small intestine both in humans and mice[25]. Then, these cells are redistributed into other sites to defend against bacterial infection[26]. In mice, the terminal ileum was reported to contain the highest numbers of segmented filamentous bacteria (SFB) and cytophaga-flavobacterium-bacteroidetes (CFB), which specifically induced Th17 cell generation[27,28]. In contrast to mice, although commensal bacteria accounting for Th17 induction in the human gut are still unclear so far, species including enterotoxigenic B. fragilis and Bifidobacterium adolescentis are able to induce Th17 cell generation from the gut of germfree mice[25,29], while colonizing mice with feces from inflammatory bowel disease (IBD) patients also induces colonic accumulation of Th17 cells[30]. Likewise, fecal microbiota transplantation from irradiated conventional mice into germfree mice predisposes the recipients to colitis, demonstrating that such fecal bacteria are critical agents in increasing intestinal sensitivity to radiation[31]. Nevertheless, an important question should be raised here, proposing whether intestinal bacterial dysbiosis occurrence relies on a threshold dose? To this end, it is known that intestinal bacterial dysbiosis occurs secondary to epithelial injuries because the intestinal epithelium exerts selection pressures on the gut composition of commensal bacteria by secreting antibacterial substances[32]. As previously reported, genetic depletion of the IL-17 receptor (IL-17R) resulted in a dramatic loss of α-defensins, which specifically led to the overgrowth of SFB[33]. Normally, IL-17R is widely expressed by intestinal epithelial cells[34]. However, radiation-induced incomplete epithelium enables IL-17R protein levels to be reduced. On this basis, intestinal tissue will be attacked by excessive SFB, while the infiltrated Th17 cells will become pathogenic due to high levels of Th17-polarized cytokines, such as IL-1β, IL-6 and IL-23 in lesioned sites[31,35]. However, such cytokine milieus antagonize the generation and immunosuppressive function of Treg cells[35]. Moreover, in vitro studies showed that irradiation using 6 Gy potentiated TRAF6 reductions in pancreatic cancer cells[36]. Originally, the expression of TRAF6 by intestinal dendritic cells (DCs) is critical for gut immune tolerance induction because intestinal DCs induce Treg cell generation by producing IL-2[37]. Conversely, 10 Gy was reported to be able to induce a significant accumulation of Treg cells in irradiated intestine, whereas these cells were impotent in immunosuppression[38]. In that way, the above results indicate that ionizing irradiation seems to establish a paradigm that favors Th17 cells rather than Treg cells. However, a previous study showed that high dose rate irradiation differed in its effect on TRAF6 expression by tumor cells compared to low dose rate irradiation[39]. At least two approaches may have different impacts on Treg cell generation in the gut. In fact, several issues remain unknown in this process. For example, which kind of cell is mostly responsible for intestinal bacterial dysbiosis formation during RE pathogenesis? In this situation, will sublethal and lethal irradiation give rise to intestinal bacterial dysbiosis with similar characteristics or exert similar radioimmune responses alternatively? Last, how does a lethal dose cause irreversible injuries or even death among irradiated hosts? These questions should be explored in future work. Nevertheless, it is hopeful that the epithelium will become a therapeutic target[40].
In steady state, DCs are potent in Th17 induction in gut of mice because the T-cell receptor (TCR) recognizes the SFB antigen presenting by DCs[28]; Meanwhile, MHC class II molecule on DCs can provide all essential signals for Th17 polarization[41]. Functionally, Th17 cells can stimulate synthesis of α-defensins by epithelial cells depending on IL-17/IL-17R interaction, thus protecting against SFB overgrowth in gut lumen[33]. However, under the irradiated condition, epithelial injuries will augment the local concentrations of IL-1β and IL-6[31,35], which functionally upregulate expression of gene encoding IL-23[35,42]. By binding with IL-23 receptor (IL-23R) on Th17 cells, IL-23 is able to stimulate Th17 cell expansion[35]. Herein, both IL-23R/IL-22 loop and IL-23/IL-17 loop are able to increase Th17 cell-mediated immune response[26,43], thus enabling the inflammation in irradiated gut to persist. In this regard, the Th17 cells are pathogenic (Figure 1). Besides, due to epithelial loss, low production of α-defensins will somewhat facilitate SFB overgrowth in gut lumen, thus facilitating Th17 induction as well. Collectively, Th17 cell induction will be robust in irradiated gut.
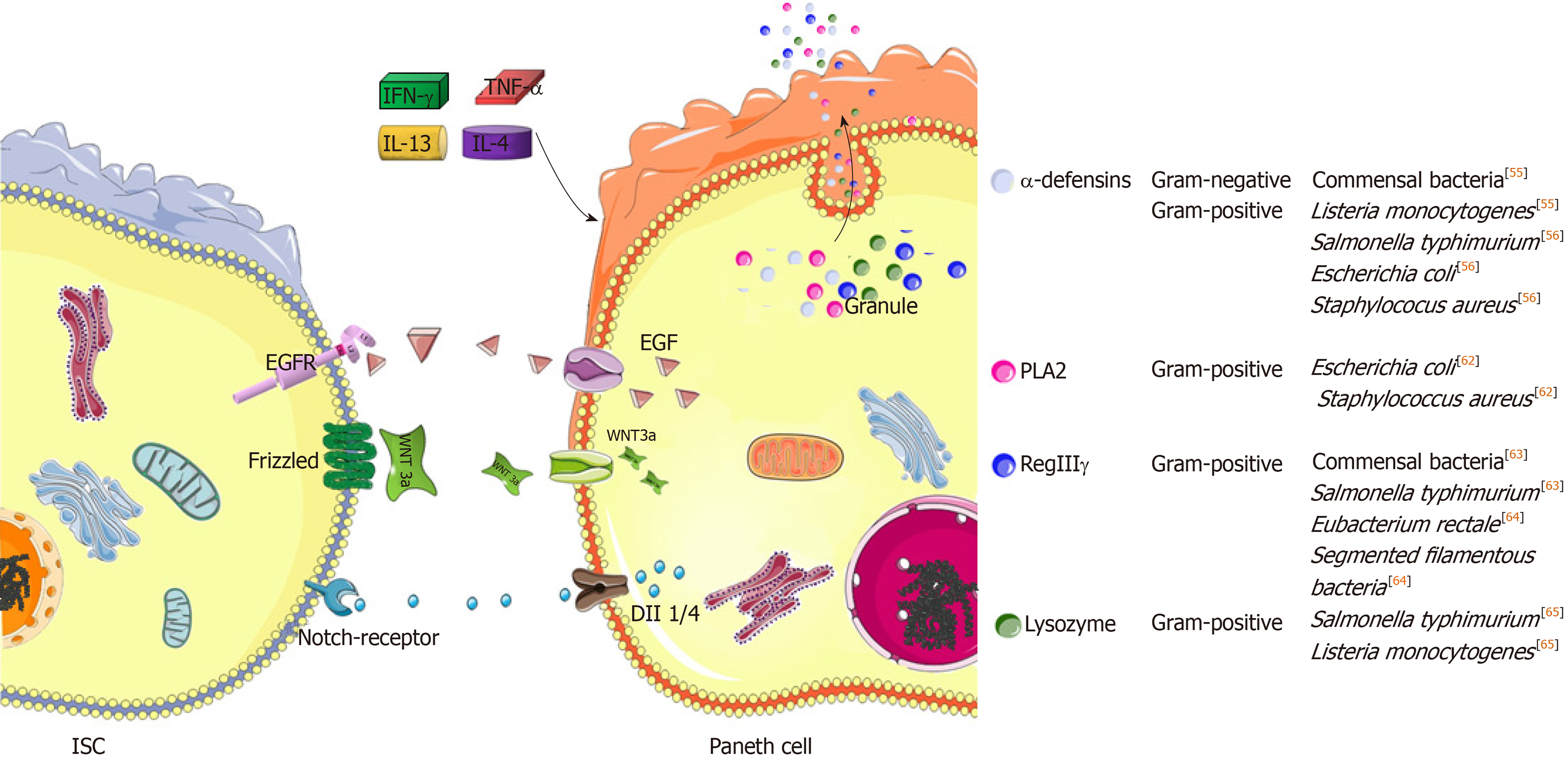
The gut possesses defensive functions in addition to nutrient absorption. Regarding the composition of the intestinal barrier, the epithelium is a critical portion[40]. In healthy adults, the intestinal epithelium is rapidly renewed, and one turnover takes about 4 to 5 d[44]. Such a capacity not only strengthens epithelial integrity but also establishes an optimal paradigm to avoid the accumulation of genetic mutations, thus protecting the gut against malformation[45]. Herein, ISCs accounts for epithelial homeostasis[44], while in their niches, Paneth cells are specialized feeders due to high secretions of epithermal growth factor (EGF), Wnt3 and Dll1/4 (Notch ligands) to neighboring ISCs[46]. Moreover, Paneth cells are derived from ISCs, and they are distributed in the basement of the crypts of Lieberkühn, tiny invaginations that line the mucosal surface all along the small intestine. The commitment of ISCs into functional Paneth cells is regulated by different signaling pathways, such as the Wnt/Sox9 and Notch/Krüppel-like factor 4 (Klf4) pathways[47]. Herein, the former promotes Paneth cell development, which can be enhanced by high-mobility group A1 (HMGA1) chromatin remodeling proteins[48]. In contrast, the retinoic acid receptor α (RARα)/Klf4 pathway antagonizes this process, implying that retinoic acids or their precursor vitamin A serve as inhibitors during Paneth cell development[49]. In fact, several other genes downstream of Wnt and Notch jointly control the equilibrium number of Paneth cells, such as the agonists of Math1 and Gfi1, along with the antagonists of Hes1 and Elf3[47]. Through their actions, the number of Paneth cells in each crypt will be constantly maintained, thus profiting epithelial integrity and disease prevention.
Paneth cells feature several characteristics. Unlike absorptive cells or other secretory lineage cells, Paneth cells are not swiftly replaced through epithelial turnover. In mice, the life span of Paneth cells is estimated as two months[46]. Such a long-lived potential ensures the stability of the number of ISCs in each crypt, which relies on Paneth cell peptides in regulating ISC development as well as in defending against luminal microbial attack. Several important peptides with anti-infective functions are derived from Paneth cells, such as α-defensins, β-defensins, regenerating islet-derived protein IIIγ (RegIIIγ), lysozyme, phospholipase A2 (PLA2) and matrix metalloproteinase 7 (MMP7)[50] (Figure 2). These peptides form a defensive network together with other lineages of cells, such as M cells in Peyer’s patches (PPs), goblet cells, absorptive cells, and LP innate or adaptive immune cells. For example, goblet cells enable Paneth-cell-derived antimicrobial peptides to be well preserved in the mucus layer[51]. Moreover, α-defensins will acquire antibacterial function if processed by MMP7[52]. In this regard, Paneth cells serve as gatekeepers in the gut.
In fact, the peptides mentioned above enable Paneth cells to possess a wide antimicrobial spectrum. Herein, the degranulation of Paneth cells is one of the most critical events in defending against luminal microbiota. In addition, degranulation can be stimulated by other factors, such as inflammatory cytokines and cholinergic substances[53]. Afterwards, antimicrobial peptides achieve high concentrations on the surface of the epithelium. In general, defensins exert lethal effects on bacteria, fungi and viruses because most defensins can bind to microbes to perforate their membranes, thus leading to microbial death[54] (Figure 2). To this end, Paneth cells mainly rely on α-defensins[50]. In steady state, α-defensins potently restrict the overgrowth of commensal bacteria[55]. In addition, pathogenic bacteria, including Salmonella typhimurium (S. typhimurium), Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), are sensitive to α-defensins[56]. Likewise, their antigens, including lipid A, lipopolysaccharide (LPS) and lipoteichoic acid (LTA), are able to induce the secretion of α-defensins by Paneth cells reciprocally[56]. Herein, the secretion of α-defensins is stimulated by LPS in a concentration-dependent manner[56]. Moreover, lipid A and LTA are the most common components of gram-negative and gram-positive bacteria, respectively[57], indicating the wide antibacterial spectrum of Paneth cells by using α-defensins. In fact, among six isoforms, α-defensins 5 and 6 are the most important peptides. For example, human α-defensin 5 was tested to preferentially and powerfully defend against S. typhimurium infection in mice[55]. In contrast to α-defensin 5, α-defensin 6 seldom exerts bactericidal function in a straightforward manner. Herein, a previous study found that nanonets of α-defensin 6 bound luminal S. typhimurium to prevent infection[58]. However, unlike α-defensins, the antibacterial spectra of other peptides are relatively narrow; in particular, RegIIIγ, lysozyme and PLA2 particularly antagonize gram-positive bacteria[50]. Nevertheless, in vivo depletions of the α-defensins and RegIIIγ could predispose the mice to spontaneous enteritis and colitis, respectively[59,60]. In this regard, intestinal inflammation is largely attributed to intestinal bacterial dysbiosis occurring as a result of the loss of the bacterial selection pressures by these peptides[55,56,61-65].
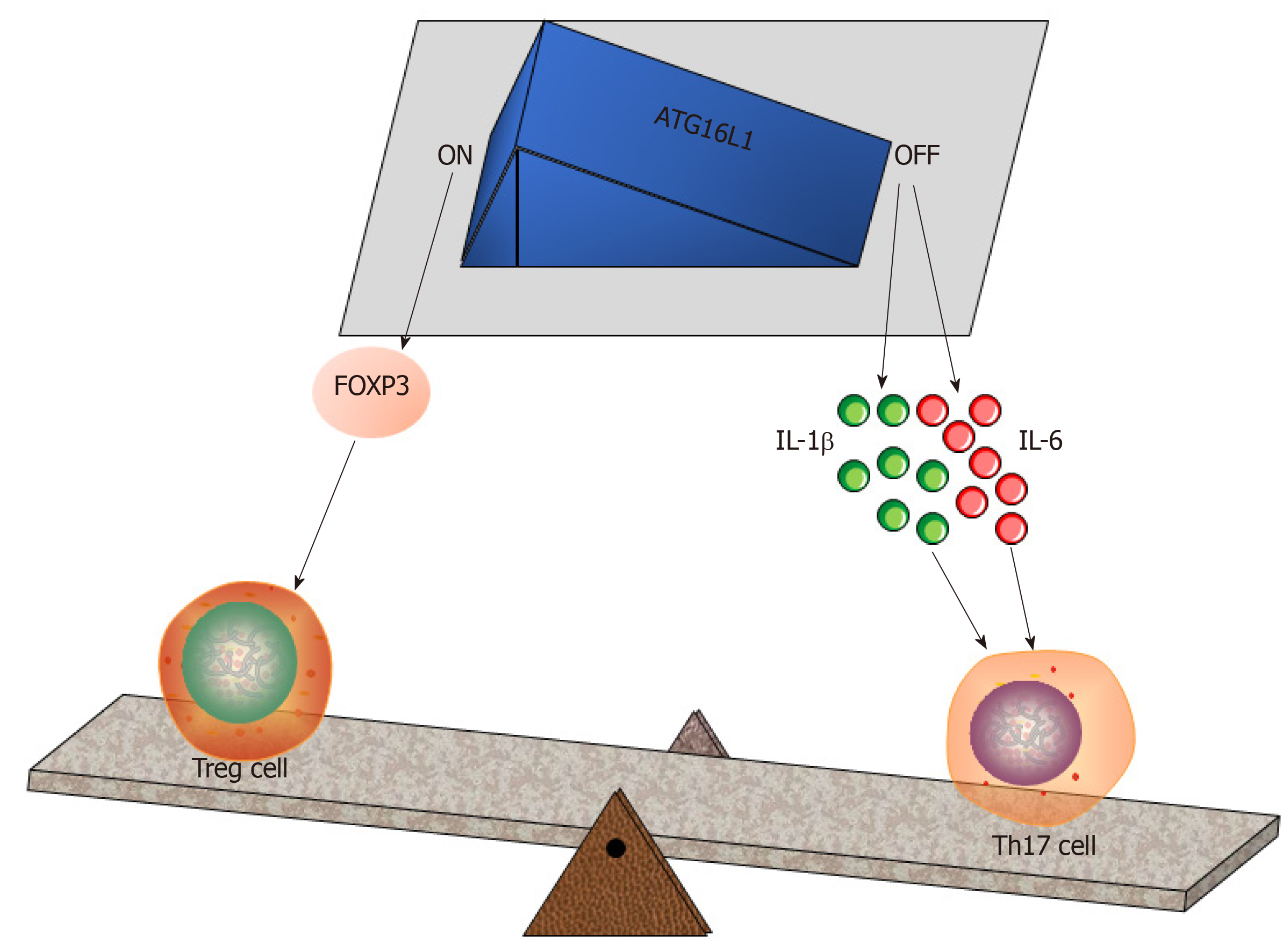
Although epithelium-derived, Paneth cells serve as critical originators of intestinal inflammation[66]. TNF-α, a prevalent cytokine regulating innate immune responses, exists in the granules of Paneth cells[67]. Herein, the specific roles of TNF-α in intestinal inflammation have been well documented in several aspects (reviewed in[68]). For example, TNF-α is a pathogenic cytokine that facilitates the pathogenesis of Crohn’s disease[69]. In this process, the endoplasmic reticulum (ER) stress of Paneth cells occurs as a result of defects in the recognition of the autophagy-related 16-like1 (ATG16L1) gene, thus impairing cell autophagy[70]. Normally, autophagy in Paneth cells is a central event against S. typhimurium infection[71], which potentially increases the intestinal number of Paneth cells as well[72]. In addition, ATG16L1 defects enable the granules of Paneth cells to be abnormal and hamper degranulation, proposing that ATG16L1 is essential for Paneth cell differentiation[70,71]. In fact, ATG16L1 is required for Treg cell induction in the gut[24]. Conversely, in response to S. typhimurium infection, the absence of ATG16L1 will increase the levels of IL-1β and IL-6 in the terminal ileum and cecum[71], the sites of which are inhabited by Th17 cells. In this regard, Paneth cells regulate Treg/Th17 balance by relying on ATG16L1 (Figure 3).
In addition to immunological participation, the antimicrobial peptides of Paneth cells also predispose the host to immune tolerance[57]. Herein, a previous study confirmed that enteric α-defensins 5 and 6 could be detected in the medullary epithelial cells of the human thymus[73]. In this situation, α-defensins 5 and 6 acted as self-reactive antigens, which could be specifically recognized by autoreactive CD4+ or CD8+ subpopulations[73]. Normally, through the action of negative selection in the thymus, the leakage of such cells into the periphery can be radically prevented. However, defects in AIRE, a key autoimmune regulator that normally controls the thymic expression of a set of genes encoding tissue-specific antigens, including α-defensins 5 and 6[74], will result in Th17 cell generation and spontaneous enteritis due to autoaggression targeting Paneth cells[73]. In contrast, mice overexpressing genes encoding human α-defensin 5 significantly reduced their gut frequencies of SFB and the numbers of Th17 cells[55]. These results further confirm the role of Paneth cells in restricting Th17 cell induction; moreover, the presence of AIRE is certainly required for Paneth cell survival. In fact, AIRE also exerts a negative impact on Paneth cell survival. For example, AIRE is required for the development of invariant natural killer T (iNKT) cells, which potentiate the degranulation of Paneth cells in an IFN-γ-dependent manner[75,76]. Herein, Paneth cells will rapidly and completely lose their granules in response to IFN-γ, which impairs the survival of Paneth cells as well[76]. In this regard, either the excessive activation or absence of AIRE seems to potentially reduce the number of Paneth cells.
Here, it is essential to mention Paneth cell degranulation in response to cytokines (Table 1). In line with IFN-γ, TNF-α, IL-13 and IL-4 cytokines induce Paneth cell degranulation as well[77,78]. In contrast to agonists of toll-like receptor (TLR) 3 & 9, oral administrations of TLR4 & 5 ligands were tested to induce Paneth cell degranulation in a TNF-α-dependent manner, thus confirming the specific role of TNF-α in this process[78]. Additionally, IL-13 receptor α1 (IL-13Rα1) is profoundly expressed by Paneth cells. The IL-13/IL-13Rα1 interaction is able to activate STAT6 and PI3K/Akt, thus upregulating the expression of lysozymes and MMP7[77]. Moreover, IL-4 is a member of the iNKT-secreted cytokines[79], further enhancing the effect of iNKT cells on inducing Paneth cell degranulation. As is known, iNKT cells are positive for CD1d, an MHC class-I-like molecule responsible for foreign antigen presentation. In addition to this function, Paneth cell degranulation is CD1d-dependent. Herein, a previous study confirmed that both cholinergic stimulation by using pilocarpine and E. coli infection were not able to reduce the crypt lysozyme intensities under the CD1d-absent condition[80]. Likewise, CD1d depletion also rendered the granules of Paneth cells abnormal in several aspects, mainly alterations in size, morphology and oligosaccharide content[80]. Furthermore, SFB overgrowth occurred if CD1d was depleted[80]. This result indicates that CD1d is required for the biosynthesis of functional α-defensins by Paneth cells because commensal SFB are sensitive to these peptides[33]. Similarly, when being colonized with E. coli or S. aureus, CD1d-deficient mice exhibited increased gut frequencies of these bacteria along with their translocation into the periphery compared to wild-type mice[80], further confirming the role of CD1d in mediating the protection against bacterial infections. In this process, CD1d is not a unique factor, and some other immune cells are able to assist Paneth cell degranulation or antimicrobial peptide secretion in addition to iNKT cells. Commonly, Th1 and group 1 innate lymphoid cells (ILC1s) are potent in producing IFN-γ, while IL-4 and IL-13 are typical cytokines produced by Th2 or ILC2[81,82]. Moreover, IL-4 and IL-13 potentiate the secretion of retinoic acids by intestinal DCs[83], thus potentially resulting in Paneth cell reduction by antagonizing the development process[47]. In addition to this function, retinoic acids preferentially induce the commitment of naïve T cells into Treg cells rather than Th17 cells[83]. Hereby, retinoic acids will synergize with α-defensin 5 in preventing the excessive generation of intestinal Th17 cells. Alternatively, in response to IL-23, intestinal TCRVγ7+γδ intraepithelial lymphocytes (IELs) can produce IL-22, which is able to induce angiogenin 4 secretion by Paneth cells to clear S. typhimurium infection[84]. Herein, IL-23 and IL-22 are also classified as Th17-type cytokines[85]. In this regard, Th17 cells potentially improve the anti-infective function of Paneth cells.
| Sort | Object | Pathway | Effect |
| Cytokines | IFN-γ | IFN-γ-dependent manner[75] | Impairment of the survival of Paneth cells[76] |
| TNF-α | TNF-α-dependent manner | Paneth cell degranulation[78] | |
| IL-13 | STAT6 and PI3K/Akt | Upregulation of the expressions of lysozyme and MMP7[77] | |
| IL-4 | Antagonizing the development process[47] | Enhancing the effect of iNKT cells[79] | |
| TLR | TLR3 / 9 | TLR9 and TLR3 dependent manner | Paneth cell degranulation[78] |
| TLR4 / 5 | TNF-α-dependent manner | Paneth cell degranulation[78] | |
| CD1d | iNKT cells | CD1d-dependent | Reducing the crypt lysozyme[80] |
| Mediating the protection against bacterial infections[80] | |||
| Cholinergic | Pilocarpine and E. coli | CD1d-dependent | Crypt lysozyme intensities[80] |
Although Paneth cells ensure the security of ISCs in steady state, the antimicrobial dysfunction of Paneth cells potentially enables ISCs to be attacked by luminal invaders. To address the importance of the gut microbiota in this process, it is documented that germfree mice with double depletions of genes encoding Rag2 and TGF-β exhibit no sporadic intestinal tumors, in contrast to conventional mice with the same phenotype[86]. This finding suggests that intestinal commensal bacteria independently induce gut carcinogenesis even though they lack adaptive immunity. Recently, several studies revealed that carcinogenesis in the human gut occurred as a result of intestinal bacterial dysbiosis[10,11]. In this situation, the feces could be used for human CRC screening[87,88]. Actually, it is well accepted that infection-associated chronic inflammation will drive the genomic instability of cells[61,89]. Herein, ISCs serve as major sources orchestrating gut malformation. In the process of phenotype conversion from ISCs to CRC stem cells, mutations or epigenetic alterations will accumulate in the genome[90]. In the gut, several commensal bacteria are capable of eliciting carcinogenesis. For example, the genotoxic island of polyketide synthase (pks) from the pathogenic strains of E. coli is required for CRC induction[91]. Instead of exerting genomic toxicity, the nonpathogenic E. coli K-12 strain potentiates the oncogenicity of colon epithelial cells by improving the activities of NF-κB and β-catenin[92]. Moreover, albeit indirectly, Enterococcus faecalis (E. faecalis) confers colon epithelial cells with oncogenicity by using their polarized macrophages, which induce cellular transformation along with gene mutation[93]. In addition to tumor induction, some other bacteria promote CRC progression. Herein, Fusobacterium nucleatum (F. nucleatum) improves the proliferative and invasive capacities of CRC cells by upregulating their miRNA-21 expression[94]. In addition, the Fab2 protein released by F. nucleatum will bind to TIGIT (T cell immunoglobulin and ITIM domain) on human T or NK cells, thus reducing their anticancer effects[95]. Similarly, enterotoxins from B. fragilis will increase the expression of c-Myc, an important oncogene driving CRC progression[96]. Moreover, enterotoxigenic B. fragilis induces Th17 cell generation[29]. However, the infiltration of massive Th17 cells in tumors predicts a poor prognosis in CRC patients[97]. To a certain extent, Th17 cells direct CRC progression by producing IL-22, which potently activates STAT3 to increase the “stemness” of tumor cells[98]. Moreover, IL-22 elicits transient ER stress in intestinal epithelial cells[99]. In concert with ATG16L1 defects, IL-22-induced epithelial necrosis will be aggravated due to robust activation of STING-dependent type I interferon (IFN-I) signaling, thus inducing excessive TNF-α production[99]. As a result, intestinal bacterial dysbiosis will be further enhanced due to the augmented defects in the epithelial barrier.
In steady state, Paneth cells are critical in protecting against intestinal bacterial dysbiosis. Here, the mission of Paneth cells postirradiation should be discussed. Foremost, autophagy will occur in Paneth cells in response to 9.25 Gy γ-irradiation[100]. Meanwhile, α-defensin 4 increases its production by Paneth cells[101]. Concerning the radiation sensitivity of Paneth cells, two previous studies confirmed that the phenotype conversion from the reserve pool of ISCs (Bmi1-positive) to the active pool of ISCs (Lgr5-positive) was a manifestation upon automatic recovery of the intestinal epithelium from radiation-induced damage, probably due to the interchange of their niche signals[102-104]. In this regard, it is reasonable to conceive that Paneth cells mediate this process, not only because they act as niche cells of ISCs but also because the numbers of Paneth cells are not significantly reduced in murine guts when doses are no more than 12 Gy[102,103]. Conversely, if doses are larger than 15 Gy, Paneth cells will dramatically lose their numbers[15]. Herein, it has been documented that ISCs are normally found in small intestine of mice albeit complete elimination of Paneth cells by genetic depletion of Math1[105]. However, Math1-mutant miniguts halt their growth in vitro [105]. This case can be translated into Wnt3-mutant miniguts as well, suggesting the essential role of Paneth cells in support of ISC expansion[105]. Moreover, conditional depletion of the gene encoding Frizzled-5, the receptor of Wnt3, will inactivate the MMP7/defensin maturation programme in Paneth cells of adult mice, suggesting the role of Wnt3 in eliciting antimicrobial function of Paneth cell[106]. Therefore, radiation-induced lethal effect on Paneth cells potentially impairs ISC regeneration due to loss of Paneth cell-derived niche signals and antimicrobials. In fact, Paneth cells are more resistant to ionizing irradiation than ISCs. The long-lived potential of Paneth cells is certainly attributed to their high genetic stability, while the survival of Paneth cells after irradiation can be controlled by their capacity to repair DNA lesions through nonhomologous end-joining[107]. A recent study found that mutation in Tyr4046 of DNA-dependent protein kinase, catalytic subunit with synchronous Trp53 depletion, significantly increased the sensitivity of mice to 8 Gy of WBI because such a genetic background hampered the survival of Paneth cells postirradiation[107]. To this end, it is proposed that Paneth cells press the button of controlling RE pathogenesis. In this process, intestinal bacterial dysbiosis occurs postirradiation, thus eliciting a pro-inflammatory milieu in lesioned gut[21]. In this context, the production of antimicrobial peptides by Paneth cells can be increased to overcome intestinal bacterial dysbiosis[101]. Hence, maintaining Paneth cell survival postirradiation appears to be critical for epithelial regeneration.
In terms of RE treatment, current clinical strategies are mainly selected according to the standard classification of intestinal toxicity reported by the Radiation Therapy Oncology Group (RTOG). Herein, the principle of treatment for Grade 1 or 2 toxicity occurring during radiation therapy mainly includes anti-inflammation; symptomatic care for nausea, vomiting or diarrhea; and nutritional support[3]. Concerning Grade 3/4 toxicities or more severe complications, multidisciplinary diagnosis and treatment are highly recommended[3], yet the relevant strategies seldom support the regeneration of lesioned intestine. In fact, it has been presented that the histological features of RE overlap with those of IBD[3,8]. Herein, MSCs have been demonstrated to be effective in patients with Crohn’s disease[108]. However, at the time of this writing, clinical trials with the purpose of managing RE using MSCs have still not been carried out. Nevertheless, clinical cases of prostate cancer with complications related to radiation-induced rectal injury could be well managed by using MSCs[109]. In this management, the efficacies of MSCs mainly include relieving pain, stanching bleeding or repairing fistula, indicating the perspective of such a stem cell therapy[109]. Additionally, TNF-α monoclonal antibody (infliximab) achieves good therapeutic effects in IBD patients. Therefore, this drug should be effective in RE, but this deserves further investigation. In parallel, some other issues should be addressed, particularly prior to RE treatment in clinical settings. For example, antibiotics are recommended for RE treatment only if infection occurs. As is known, long-lasting use of antibiotics will induce intestinal bacterial dysbiosis, but it is still unclear whether short-term use of antibiotics improves radiation-induced intestinal bacterial dysbiosis. Nevertheless, antibiotics exhibited potential in delaying CRC progression in an animal model[110]. This finding means that antibiotics serving as candidates for BSC therapy may be available in the defense against CRC-related intestinal bacterial dysbiosis. As mentioned above, BSC using prebiotics or probiotics is effective in relieving diarrhea[9]. In fact, BSC has become the hotspot for various diseases. To overcome bacterial dysbiosis, the administration of defensins or omega-3 polyunsaturated fatty acids is also promising in clinical settings[111,112]. Instead of BSC, the reduction in and/or the dysfunction of pathogenic cells will be available choices for RE treatment as well. Herein, RORγt antagonists against Th17 cell commitment were tested to be useful in the IBD model[113]. In this process, RORγt inhibition will potentially improve Treg cell generation because RORγt functionally antagonizes the transcriptional activity of Foxp3[114]. In particular, Th17 cells are pathogenic cells of RE and CRC as well, thus predicting the perspective of RORγt antagonists in these diseases. Collectively, targeting any critical event during RE pathogenesis should become a candidate option for RE treatment. For the development of novel treatment targets of RE, related mechanisms deserve further exploration in the future.
Pathogenesis of radiation enteropathy is highly associated with intestinal bacterial dysbiosis. Herein, Paneth cells probably control the process of bacterial dysbiosis by using their antimicrobial peptides.
Manuscript source: Invited Manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gassler N, Miloso M, Tanabe S S-Editor: Gong ZM L-Editor: A E-Editor: Xing YX
| 1. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1199] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 2. | Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, Fanfani F, Joye I, Kachnic L, Maingon P, Marijnen C, Ngan S, Haustermans K. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol. 2016;120:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 4. | Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed JC. Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene. 1996;12:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Garg S, Zheng J, Wang J, Authier S, Pouliot M, Hauer-Jensen M. Segmental Differences in Radiation-Induced Alterations of Tight Junction-Related Proteins in Non-Human Primate Jejunum, Ileum and Colon. Radiat Res. 2016;185:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Hua G, Wang C, Pan Y, Zeng Z, Lee SG, Martin ML, Haimovitz-Friedman A, Fuks Z, Paty PB, Kolesnick R. Distinct Levels of Radioresistance in Lgr5+ Colonic Epithelial Stem Cells versus Lgr5+Small Intestinal Stem Cells. Cancer Res. 2017;77:2124-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Ferreira MR, Muls A, Dearnaley DP, Andreyev HJ. Microbiota and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol. 2014;15:e139-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3003] [Cited by in RCA: 2990] [Article Influence: 230.0] [Reference Citation Analysis (0)] |
| 10. | Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, Zhang D, Xia H, Xu X, Jie Z, Su L, Li X, Li X, Li J, Xiao L, Huber-Schönauer U, Niederseer D, Xu X, Al-Aama JY, Yang H, Wang J, Kristiansen K, Arumugam M, Tilg H, Datz C, Wang J. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 970] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 11. | Nakatsu G, Li X, Zhou H, Sheng J, Wong SH, Wu WK, Ng SC, Tsoi H, Dong Y, Zhang N, He Y, Kang Q, Cao L, Wang K, Zhang J, Liang Q, Yu J, Sung JJ. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 512] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 12. | McLaughlin MM, Dacquisto MP, Jacobus DP, Horowitz RE. Effects of the germfree state on responses of mice to whole-body irradiation. Radiat Res. 1964;23:333-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev. 1992;11:179-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 225] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology. 2004;126:796-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 450] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 16. | Zhu Y, Huang YF, Kek C, Bulavin DV. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell. 2013;12:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Wu DD, Bosch B, Keating A. Hematopoietic cytokines enhance survival of SCID mice undergoing high-dose irradiation. Exp Hematol. 1994;22:411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Schaue D. A Century of Radiation Therapy and Adaptive Immunity. Front Immunol. 2017;8:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Chang PY, Qu YQ, Wang J, Dong LH. The potential of mesenchymal stem cells in the management of radiation enteropathy. Cell Death Dis. 2015;6:e1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci USA. 2005;102:13254-13259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol. 2007;8:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Deng Y, Chang C, Lu Q. The Inflammatory Response in Psoriasis: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;50:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 293] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 23. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2501] [Cited by in RCA: 2749] [Article Influence: 183.3] [Reference Citation Analysis (1)] |
| 24. | Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, Targan SR, Xavier RJ, Ernst PB, Green DR, McGovern DP, Virgin HW, Mazmanian SK. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 476] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 25. | Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, Pasman L, Ortiz-Lopez A, Jupp R, Wu HJ, Kasper DL, Benoist C, Mathis D. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci USA. 2016;113:E8141-E8150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 26. | Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, Bonneau R, Littman DR. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell. 2015;163:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 423] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 27. | Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1480] [Cited by in RCA: 1400] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 28. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3525] [Article Influence: 220.3] [Reference Citation Analysis (0)] |
| 29. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1309] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 30. | Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov II, Sugiyama T, Nuñez G, Camp JG, Hattori M, Umesaki Y, Honda K. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163:367-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 815] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 31. | Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O, Dheer R, Abreu MT, Koren O, Kashi Y, Chowers Y. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut. 2018;67:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 32. | Bunker JJ, Erickson SA, Flynn TM, Henry C, Koval JC, Meisel M, Jabri B, Antonopoulos DA, Wilson PC, Bendelac A. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017;358:pii: eaan6619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 343] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 33. | Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, Good M, Schoenborn AA, Bibby K, Montelaro RC, Metzger DW, Gulati AS, Kolls JK. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 2016;44:659-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 34. | Tang C, Kakuta S, Shimizu K, Kadoki M, Kamiya T, Shimazu T, Kubo S, Saijo S, Ishigame H, Nakae S, Iwakura Y. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 35. | Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1001] [Cited by in RCA: 984] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 36. | Chiu HW, Lin SW, Lin LC, Hsu YH, Lin YF, Ho SY, Wu YH, Wang YJ. Synergistic antitumor effects of radiation and proteasome inhibitor treatment in pancreatic cancer through the induction of autophagy and the downregulation of TRAF6. Cancer Lett. 2015;365:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Han D, Walsh MC, Cejas PJ, Dang NN, Kim YF, Kim J, Charrier-Hisamuddin L, Chau L, Zhang Q, Bittinger K, Bushman FD, Turka LA, Shen H, Reizis B, Defranco AL, Wu GD, Choi Y. Dendritic cell expression of the signaling molecule TRAF6 is critical for gut microbiota-dependent immune tolerance. Immunity. 2013;38:1211-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Billiard F, Buard V, Benderitter M, Linard C. Abdominal γ-radiation induces an accumulation of function-impaired regulatory T cells in the small intestine. Int J Radiat Oncol Biol Phys. 2011;80:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Shin SC, Lee KM, Kang YM, Kim K, Lim SA, Yang KH, Kim JY, Nam SY, Kim HS. Differential expression of immune-associated cancer regulatory genes in low- versus high-dose-rate irradiated AKR/J mice. Genomics. 2011;97:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 865] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 41. | Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 42. | Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1566] [Cited by in RCA: 1702] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 43. | Cătană CS, Berindan Neagoe I, Cozma V, Magdaş C, Tăbăran F, Dumitraşcu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823-5830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | Cui S, Chang PY. Current understanding concerning intestinal stem cells. World J Gastroenterol. 2016;22:7099-7110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 1933] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 47. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1327] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 48. | Xian L, Georgess D, Huso T, Cope L, Belton A, Chang YT, Kuang W, Gu Q, Zhang X, Senger S, Fasano A, Huso DL, Ewald AJ, Resar LMS. HMGA1 amplifies Wnt signalling and expands the intestinal stem cell compartment and Paneth cell niche. Nat Commun. 2017;8:15008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Jijon HB, Suarez-Lopez L, Diaz OE, Das S, De Calisto J, Parada-kusz M, Yaffe MB, Pittet MJ, Mora JR, Belkaid Y, Xavier RJ, Villablanca EJ. Intestinal epithelial cell-specific RARα depletion results in aberrant epithelial cell homeostasis and underdeveloped immune system. Mucosal Immunol. 2018;11:703-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Kurashima Y, Kiyono H. Mucosal Ecological Network of Epithelium and Immune Cells for Gut Homeostasis and Tissue Healing. Annu Rev Immunol. 2017;35:119-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 51. | Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Pütsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 52. | Mastroianni JR, Costales JK, Zaksheske J, Selsted ME, Salzman NH, Ouellette AJ. Alternative luminal activation mechanisms for paneth cell α-defensins. J Biol Chem. 2012;287:11205-11212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65:3019-3027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 373] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 55. | Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 911] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 56. | Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 782] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 57. | Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2123] [Cited by in RCA: 2125] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 58. | Chu H, Pazgier M, Jung G, Nuccio SP, Castillo PA, de Jong MF, Winter MG, Winter SE, Wehkamp J, Shen B, Salzman NH, Underwood MA, Tsolis RM, Young GM, Lu W, Lehrer RI, Bäumler AJ, Bevins CL. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science. 2012;337:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 59. | Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129-18134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 775] [Cited by in RCA: 732] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 60. | Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1159] [Cited by in RCA: 1075] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 61. | Park CH, Eun CS, Han DS. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest Res. 2018;16:338-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Laine VJ, Grass DS, Nevalainen TJ. Resistance of transgenic mice expressing human group II phospholipase A2 to Escherichia coli infection. Infect Immun. 2000;68:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858-20863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 767] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 64. | Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1078] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 65. | Elphick DA, Mahida YR. Paneth cells: their role in innate immunity and inflammatory disease. Gut. 2005;54:1802-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, Flak MB, Cusick JL, Kohno K, Iwawaki T, Billmann-Born S, Raine T, Bharti R, Lucius R, Kweon MN, Marciniak SJ, Choi A, Hagen SJ, Schreiber S, Rosenstiel P, Kaser A, Blumberg RS. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 584] [Cited by in RCA: 576] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 67. | Ouellette AJ. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology. 1997;113:1779-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Bamias G, Cominelli F. Cytokines and intestinal inflammation. Curr Opin Gastroenterol. 2016;32:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 764] [Cited by in RCA: 716] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 70. | Deuring JJ, Fuhler GM, Konstantinov SR, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn's disease. Gut. 2014;63:1081-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Conway KL, Kuballa P, Song JH, Patel KK, Castoreno AB, Yilmaz OH, Jijon HB, Zhang M, Aldrich LN, Villablanca EJ, Peloquin JM, Goel G, Lee IA, Mizoguchi E, Shi HN, Bhan AK, Shaw SY, Schreiber SL, Virgin HW, Shamji AF, Stappenbeck TS, Reinecker HC, Xavier RJ. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology. 2013;145:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 72. | Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1429] [Cited by in RCA: 1188] [Article Influence: 148.5] [Reference Citation Analysis (1)] |
| 73. | Dobeš J, Neuwirth A, Dobešová M, Vobořil M, Balounová J, Ballek O, Lebl J, Meloni A, Krohn K, Kluger N, Ranki A, Filipp D. Gastrointestinal Autoimmunity Associated With Loss of Central Tolerance to Enteric α-Defensins. Gastroenterology. 2015;149:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1836] [Cited by in RCA: 1751] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 75. | Lindh E, Rosmaraki E, Berg L, Brauner H, Karlsson MC, Peltonen L, Höglund P, Winqvist O. AIRE deficiency leads to impaired iNKT cell development. J Autoimmun. 2010;34:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Farin HF, Karthaus WR, Kujala P, Rakhshandehroo M, Schwank G, Vries RG, Kalkhoven E, Nieuwenhuis EE, Clevers H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-γ. J Exp Med. 2014;211:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 77. | Stockinger S, Albers T, Duerr CU, Ménard S, Pütsep K, Andersson M, Hornef MW. Interleukin-13-mediated paneth cell degranulation and antimicrobial peptide release. J Innate Immun. 2014;6:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Rumio C, Sommariva M, Sfondrini L, Palazzo M, Morelli D, Viganò L, De Cecco L, Tagliabue E, Balsari A. Induction of Paneth cell degranulation by orally administered Toll-like receptor ligands. J Cell Physiol. 2012;227:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 79. | Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 494] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 80. | Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, Brugman S, Yamaguchi K, Ishikawa H, Aiba Y, Koga Y, Samsom JN, Oshima K, Kikuchi M, Escher JC, Hattori M, Onderdonk AB, Blumberg RS. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 81. | Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014;841:15-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 82. | Ishizuka IE, Constantinides MG, Gudjonson H, Bendelac A. The Innate Lymphoid Cell Precursor. Annu Rev Immunol. 2016;34:299-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Iwata M, Yokota A. Retinoic acid production by intestinal dendritic cells. Vitam Horm. 2011;86:127-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Walker CR, Hautefort I, Dalton JE, Overweg K, Egan CE, Bongaerts RJ, Newton DJ, Cruickshank SM, Andrew EM, Carding SR. Intestinal intraepithelial lymphocyte-enterocyte crosstalk regulates production of bactericidal angiogenin 4 by Paneth cells upon microbial challenge. PLoS One. 2013;8:e84553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, Monteleone G, Stolfi C. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 459] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 86. | Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 87. | Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, Wang X, Xu X, Chen N, Wu WK, Al-Aama J, Nielsen HJ, Kiilerich P, Jensen BA, Yau TO, Lan Z, Jia H, Li J, Xiao L, Lam TY, Ng SC, Cheng AS, Wong VW, Chan FK, Xu X, Yang H, Madsen L, Datz C, Tilg H, Wang J, Brünner N, Kristiansen K, Arumugam M, Sung JJ, Wang J. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 88. | Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Costea PI, Amiot A, Böhm J, Brunetti F, Habermann N, Hercog R, Koch M, Luciani A, Mende DR, Schneider MA, Schrotz-King P, Tournigand C, Tran Van Nhieu J, Yamada T, Zimmermann J, Benes V, Kloor M, Ulrich CM, von Knebel Doeberitz M, Sobhani I, Bork P. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol. 2014;10:766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 858] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 89. | DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 90. | Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell. 2014;15:692-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 91. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1667] [Article Influence: 128.2] [Reference Citation Analysis (1)] |
| 92. | Sahu U, Choudhury A, Parvez S, Biswas S, Kar S. Induction of intestinal stemness and tumorigenicity by aberrant internalization of commensal non-pathogenic E. coli. Cell Death Dis. 2017;8:e2667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Wang X, Yang Y, Huycke MM. Commensal bacteria drive endogenous transformation and tumour stem cell marker expression through a bystander effect. Gut. 2015;64:459-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 94. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 716] [Article Influence: 89.5] [Reference Citation Analysis (1)] |
| 95. | Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklić K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 977] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 96. | Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 97. | Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 898] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 98. | Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, Vatan L, Szeliga W, Dou Y, Owens S, Zgodzinski W, Majewski M, Wallner G, Fang J, Huang E, Zou W. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2014;40:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 99. | Aden K, Tran F, Ito G, Sheibani-Tezerji R, Lipinski S, Kuiper JW, Tschurtschenthaler M, Saveljeva S, Bhattacharyya J, Häsler R, Bartsch K, Luzius A, Jentzsch M, Falk-Paulsen M, Stengel ST, Welz L, Schwarzer R, Rabe B, Barchet W, Krautwald S, Hartmann G, Pasparakis M, Blumberg RS, Schreiber S, Kaser A, Rosenstiel P. ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med. 2018;215:2868-2886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 100. | Gorbunov NV, Kiang JG. Up-regulation of autophagy in small intestine Paneth cells in response to total-body gamma-irradiation. J Pathol. 2009;219:242-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Gorbunov NV, Garrison BR, Kiang JG. Response of crypt paneth cells in the small intestine following total-body gamma-irradiation. Int J Immunopathol Pharmacol. 2010;23:1111-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 656] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 103. | Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 935] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 104. | Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 954] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 105. | Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 490] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 106. | van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 515] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 107. | Gurley KE, Ashley AK, Moser RD, Kemp CJ. Synergy between Prkdc and Trp53 regulates stem cell proliferation and GI-ARS after irradiation. Cell Death Differ. 2017;24:1853-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn's Disease: A Randomized Controlled Clinical Trial. Gut Liver. 2018;12:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 109. | Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, Fouillard L, Chapel A. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | Bhattacharya N, Yuan R, Prestwood TR, Penny HL, DiMaio MA, Reticker-Flynn NE, Krois CR, Kenkel JA, Pham TD, Carmi Y, Tolentino L, Choi O, Hulett R, Wang J, Winer DA, Napoli JL, Engleman EG. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8(+) T Cell-Mediated Immunity in Colorectal Cancer. Immunity. 2016;45:641-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 111. | Pachón-Ibáñez ME, Smani Y, Pachón J, Sánchez-Céspedes J. Perspectives for clinical use of engineered human host defense antimicrobial peptides. FEMS Microbiol Rev. 2017;41:323-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 112. | Zhang Y, Zhang B, Dong L, Chang P. Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis. Adv Nutr. 2019;10:133-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 113. | Xiao S, Yosef N, Yang J, Wang Y, Zhou L, Zhu C, Wu C, Baloglu E, Schmidt D, Ramesh R, Lobera M, Sundrud MS, Tsai PY, Xiang Z, Wang J, Xu Y, Lin X, Kretschmer K, Rahl PB, Young RA, Zhong Z, Hafler DA, Regev A, Ghosh S, Marson A, Kuchroo VK. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity. 2014;40:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 114. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1541] [Article Influence: 90.6] [Reference Citation Analysis (0)] |