Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1640
Peer-review started: July 16, 2020
First decision: August 9, 2020
Revised: October 9, 2020
Accepted: November 5, 2020
Article in press: November 5, 2020
Published online: December 26, 2020
Processing time: 163 Days and 8.5 Hours
Human adipose-derived stromal/stem cells (hASCs) are one of the most useful types of mesenchymal stromal/stem cells, which are adult multipotent cells with great therapeutic potential for the treatment of several diseases. However, for successful clinical application, it is critical that high-quality cells can be obtained. Diverse factors seem to be able to influence cell quality and performance, especially factors related to donors’ intrinsic characteristics, such as age. Nevertheless, there is no consensus regarding this characteristic, and there is conflicting information in the literature.
To investigate the growth kinetics and differentiation potential of adipose-derived stem cells isolated from the lipoaspirates of elderly and young donors.
hASCs were harvested from liposuctioned adipose tissue obtained from female donors (aged 20-70 years). Cells were distributed into two groups according to age range: old hASCs (oASCs, ≥ 55 years, n = 9) and young hASCs (yASCs, ≤ 35 years, n = 9). For each group, immunophenotypic characterization was performed by flow cytometry. Population doubling time was assessed over seven days. For adipogenic potential evaluation, lipid deposits were assessed after 7 d, 14 d and 21 d of adipogenic induction. Osteogenic potential was verified by analyzing cell mineralization after 14 d, 21 d and 28 d of osteogenic induction. mRNA expression of PPARγ2, CEBPA and Runx2 were detected by quantitative reverse transcription polymerase chain reaction.
hASCs were successfully obtained, cultured, and grouped according to their age: yASCs (26.33 ± 4.66 years old) and oASCs (64.78 ± 4.58 years old). After maintenance of the cells in culture, there were no differences in morphology between cells from the young and old donors. Additionally, both groups showed classical immunophenotypic characteristics of mesenchymal stem/stromal cells. The average doubling time indicated that yASCs (4.09 ± 0.94 d) did not significantly differ from oASCs (4.19 ± 1.29 d). Concerning differentiation potential, after adipogenic and osteogenic induction, yASCs and oASCs were able to differentiate to greater levels than the noninduced control cells. However, no differences were found in the differentiation efficiency of yASCs and oASCs in adipogenesis or osteogenesis. Additionally, the mRNA expression of PPARγ2, CEBPA and Runx2 were similar in yASCs and oASCs.
Our findings suggest that age does not seem to significantly affect the cell division or adipogenic or osteogenic differentiation ability of adipose-derived stem cells isolated from lipoaspirates.
Core Tip: Adipose-derived stem cells are adult multipotent stem cells with great therapeutic potential for a variety of diseases. The donor’s intrinsic characteristics, such as sex, health conditions and age, have been claimed to be the main factors responsible for affecting the quality and the performance of these cells. Regarding donor age, the literature shows many conflicting observations. Here, we evaluated the proliferation and differentiation potential of adipose-derived stem cells isolated from lipoaspirates obtained from elderly and young female donors. Our results demonstrated that age does not seem to significantly affect the cell division or adipogenic or osteogenic potential of these cells.
- Citation: Horinouchi CD, Barisón MJ, Robert AW, Kuligovski C, Aguiar AM, Dallagiovanna B. Influence of donor age on the differentiation and division capacity of human adipose-derived stem cells. World J Stem Cells 2020; 12(12): 1640-1651
- URL: https://www.wjgnet.com/1948-0210/full/v12/i12/1640.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i12.1640
Mesenchymal stromal/stem cells (MSCs) are adult multipotent cells that have the capacity to differentiate into many specialized cell types in addition to having self-renewal potential and immunomodulatory properties[1]. Due to these attractive characteristics and the ease with which they can be obtained, grown in culture and processed, MSCs hold great therapeutic potential and have been successfully applied in clinical trials for several disorders over the past years[2,3]. Originally isolated and characterized from bone marrow, MSCs can be obtained from various tissues, such as adipose tissue, dental tissues, and menstrual blood[4,5].
Human adipose-derived stromal/stem cells (hASCs) have increasingly been shown to be one of the most useful types of MSCs for application in stem cell-based therapy, tissue engineering and other regenerative medicine approaches[6]. Adipose tissue is usually abundant in the body and can be easily accessed using minimally invasive techniques, and it can even be obtained from medical waste following liposuction or dermolipectomy. Additionally, studies have shown that isolation from adipose tissue yields a greater number of cells than isolation from other tissue sources[6,7].
It seems to be clear that the quality of hASCs is crucial for obtaining good results in preclinical and clinical applications. Regardless of the harvesting system, donor characteristics or processing approaches, these cells must maintain standard features concerning viability, proliferation and multilineage differentiation abilities[8].
Donor age is regularly discussed as one of the factors capable of changing stem cell performance[9]. It seems to be well established that donor age can result in microenvironmental changes, such as metabolic alterations, hormonal disturbances, and immunological disorders which can promote differences in the behavior of MSCs[10]. However, whether there are intrinsic differences in MSCs isolated from elderly or young donors is a less clear question. Regarding this issue, there are conflicting observations in the literature, as reviewed by Varghese et al[11] (2017) and Prieto Gonzalez[12] (2019).
In this study, we investigated the growth kinetics and differentiation potential of hASCs isolated from the lipoaspirates of elderly and young donors.
hASCs were isolated from adipose tissue obtained from female donors who underwent liposuction surgery, as previously described[13]. Informed consent was provided by each donor. This study was performed in accordance with the guidelines for research involving human subjects and had the approval of the Ethics Committee of Fundação Oswaldo Cruz, Brazil (CAAE: 48374715.8.0000.5248).
Isolated cells were separated into two groups based on donor age: ≥ 55 years were named old hASCs (oASCs), while those from donors ≤ 35 years were named young hASCs (yASCs). Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mmol/L L-glutamine in a humidified incubator at 37°C with 5% CO2. The culture medium was changed twice a week until the hASC cultures were 80%-90% confluent, at which point the cells were trypsinized. All experiments were performed with hASCs that were between passages 4 and 7.
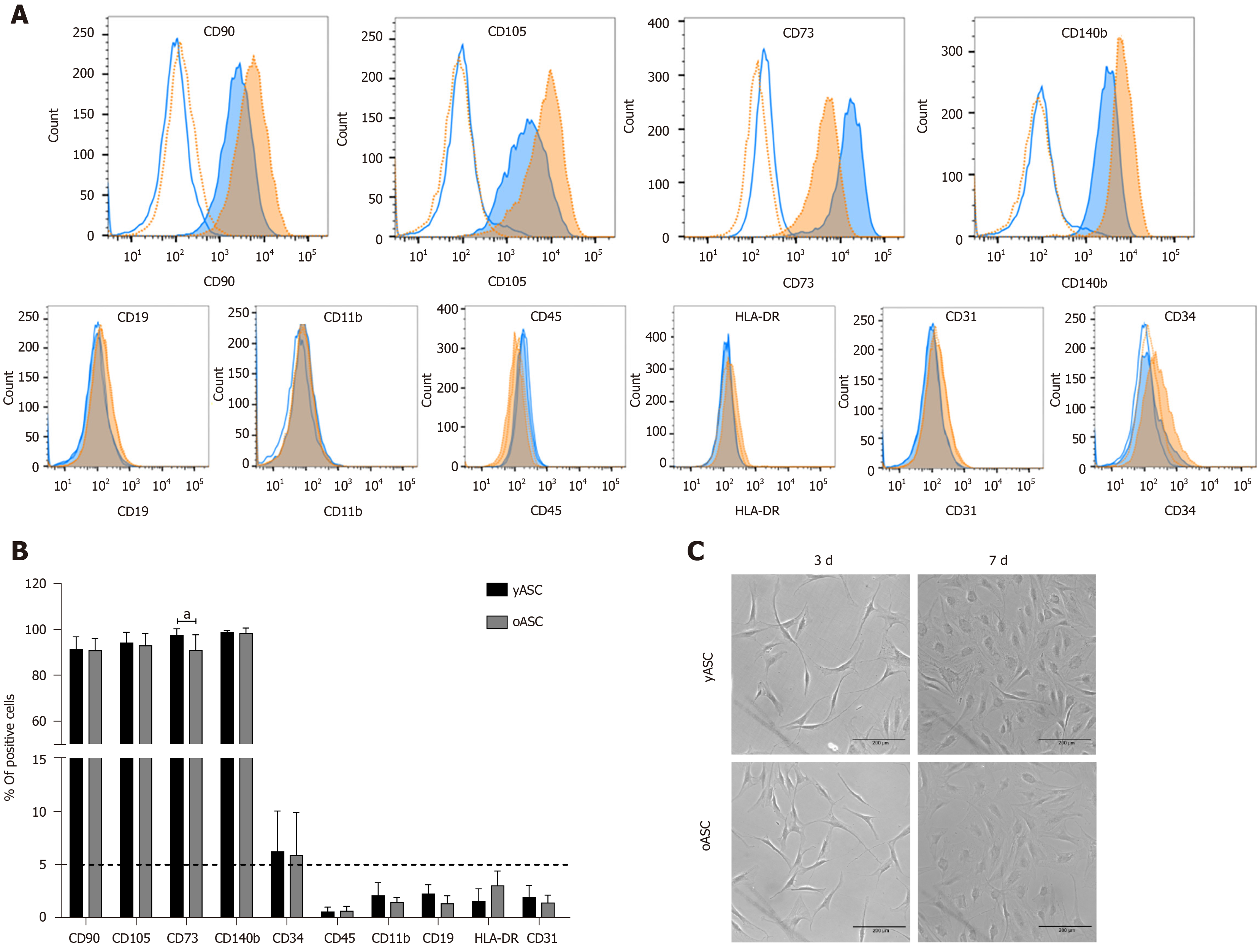
Immunophenotypic characterization was based on the parameters established for defining mesenchymal stem cells, as indicated by the International Society for Cellular Therapy[14]. The protocol used followed that previously established in our laboratory[13]. CD90, CD105, CD73 and CD140b were considered positive markers, while the negative markers were CD19, CD11b, HLA-DR, CD45, CD34 and CD31.
For doubling time determination, hASCs were plated on 96-well plates at a density of 2 × 103 cells/cm2 (time = 0). Every 24 h, for seven days, cells were fixed with 4% paraformaldehyde and stained with 1 µg/µL DAPI for nuclear labeling. Quantification of the number of cells was performed using the Operetta® high-content imaging system and Harmony® high-content analysis software 4.8 (Perkin Elmer). Doubling time for each group was calculated using the online software Doubling Time Computing Version 3.1.0[15].
For adipogenic differentiation, cells were plated at a density of 10 × 103 cells/cm2 in 96- or 6-well plates, and after 24 h (90%-100% confluency), hASCs were induced using hMSC Adipogenic Differentiation Medium (hMSC Adipogenic BulletKit, Lonza) following the manufacturer’s instructions. The medium was changed every 3-4 d, intercalating induction medium with maintenance medium for a total of 21 d. The efficiency of adipogenic differentiation was verified after 7 d, 14 d and 21 d of induction using the lipophilic fluorescent dye Nile red (Sigma-Aldrich®, Saint Louis, Missouri, United States). Briefly, cells were fixed with 4% paraformaldehyde and stained using 1 µg/mL Nile red and 1 µg/µL DAPI for nucleus quantification. The intensity of Nile red fluorescence was measured at excitation of 485 nm and emission of 555 nm on a Synergy H1 Multi-Mode Microplate Reader (Biotek, Winooski, Vermont, United States). We also quantified the number of cells (DAPI+) and the intracellular stained area with Nile red using an Operetta® high-content imaging system and Harmony® high-content analysis software 4.8 (Perkin Elmer).
For osteogenic differentiation, cells were plated at a density of 6 × 103 cells/cm2 in 96- or 6-well plates, and after 24 h (70%-80% confluency), hASCs were induced with hMSC Osteogenic Differentiation Medium (hMSC Osteogenic BulletKit, Lonza) for 28 d. Noninduced hASCs were cultured with maintenance medium composed of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mmol/L L-glutamine. The medium was replaced every 3-4 d, and hASCs were stained after 14 d, 21 d and 28 d of osteogenic induction. Mineralization deposition was quantified using an OsteoImage™ Mineralization Assay (Lonza) following the manufacturer’s instructions. Cells were also stained with 1 µg/µL DAPI for nuclear quantification. Osteoimage™ fluorescence intensity was measured at excitation of 492 nm and emission of 520 nm on a Synergy H1 Multi-Mode Microplate Reader (Biotek). The number of cells (DAPI+) and the mineralized area were also quantified by an Operetta® high-content imaging system and Harmony® high-content analysis software 4.8 (Perkin Elmer).
For microplate reader analysis, cell differentiation efficiency is expressed as the ratio between the mean fluorescence intensity of induced and control cells (cell differentiation = mean fluorescence intensity of induced cells/mean fluorescence intensity of control cells). For the high-content image system, first, the parameters of analysis were defined as the number of nuclei and the area of lipid inclusions or hydroxyapatite deposition (defined by the number of positive pixels per field in the green channel). Cell differentiation was first normalized by the total number of nuclei in the well, and then they were calculated as the adipogenic or osteogenic positive area/number of nuclei. Finally, the results were expressed as the difference between the cell differentiation of induced and control cells (cell differentiation = mean cell differentiation of induced cells minus mean cell differentiation of control cells).
After 21 d of adipogenesis and 14 d of osteogenesis, total RNA was extracted from induced and control hASCs using an RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. cDNA synthesis was performed using an ImProm-II Reverse Transcription System (Promega) and 1 µg of total RNA. The relative expression of adipogenic and osteogenic markers was quantified by quantitative PCR (qPCR) using GoTaq Polymerase Mix (Promega) and a LightCycler 96 Instrument (Roche). PPARγ2 (peroxisome proliferator-activator receptor gamma 2; forward primer 5’-ATTACAGCAAACCCCTATTCC-3’ and reverse primer 5’-GGCATCTCTGTGTCAACCAT-3’) and CEBPA (CCAAT/enhancer-binding protein α, primers acquired from Qiagen, United States) were the markers used to characterize adipogenic differentiation. Runx2 (runt-related transcription factor 2, forward primer 5’-ACTGGCGCTGCAACAAGAC-3’ and reverse primer 5’-CCCGCCATGA-CAGTAACCA-3’) was used to assess osteogenic differentiation. RNAPII (RNA polymerase II, forward primer 5’-TACCACGTCATCT-CCTTTGATGGCT-3’ and reverse primer 5’-GTGCGG-CTGCTTCCATAA-3’) was used as a housekeeping gene. Each reaction was performed in three technical replicates, and the expression ratio to RNAPII expression (Fold to RNAPII) calculated using LightCycler 96 software (Roche).
Statistical analysis was performed with GraphPad Prism version 7.0. Experimental differences between young and old hASC donors were determined by the Student’s t-test for population doubling time and mRNA expression analysis. Experimental data on differentiation were evaluated by two-way repeated measures analysis of variance. The data indicate the mean ± SD, and P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Hellen Geremias Santos from Fundação Oswaldo Cruz.
hASCs were isolated from female donors and grouped according to their age: young donors (yASCs; 26.33 ± 4.66 years old; n = 9) and old donors (oASCs; 64.78 ± 4.58 years old; n = 9). Both groups showed similar average weights (yASC: 64.23 ± 9.38 kg; oASC: 71.69 ± 5.61 kg). More information on group distribution, such as body mass index (yASC: 23.53 ± 2.28 kg/m2; oASC: 27.11 ± 1.76 kg/m2), is shown in Table 1 and Supplementary Figure 1.
| Young hASC | Old hASC | ||||||||
| ID | Age | Weight (kg) | Height (m) | BMI | ID | Age | Weigh (kg) | Height (m) | BMI |
| yASC_01 | 27 | 53 | 1.60 | 20.7 | oASC_01 | 70 | n.i | n.i | - |
| yASC_02 | 20 | 75 | 1.74 | 24.77 | oASC_02 | 64 | 64 | 1.62 | 24.39 |
| yASC_03 | 28 | 58 | 1.63 | 21.83 | oASC_03 | 70 | 73 | 1.62 | 27.82 |
| yASC_04 | 20 | 60 | 1.59 | 23.73 | oASC_04 | 64 | 66 | 1.61 | 25.46 |
| yASC_05 | 27 | 70.9 | 1.62 | 27.02 | oASC_05 | 61 | 80 | 1.73 | 26.73 |
| yASC_06 | 32 | 55 | 1.63 | 20.7 | oASC_06 | 58 | 70 | 1.62 | 26.67 |
| yASC_07 | 22 | 60 | 1.60 | 23.44 | oASC_07 | 66 | 76 | 1.65 | 27.92 |
| yASC_08 | 32 | 80.2 | 1.74 | 26.49 | oASC_08 | 60 | 68 | 1.57 | 27.59 |
| yASC_09 | 29 | 66 | 1.69 | 23.11 | oASC_09 | 70 | 76.5 | 1.59 | 30.26 |
With regard to cell morphology, yASC and oASC showed similar characteristics, exhibiting the classical fibroblast-like morphology of MSCs (Figure 1C). After immunophenotypic analysis, both groups exhibited the classical pattern of MSCs marker expression. Despite a difference in CD73, no significant differences in the frequency of positive cells for CD90, CD105 and CD140b were observed between the groups. We also confirmed the absence (less than 5%) of negative MSC markers, CD19, CD45, CD11b, HLA-DR, and CD31 (Figure 1A and B). Interestingly, a large variation was observed in the percentage of CD34+ cells, both for yASC (1.88%-14.9%) and oASC (1.43%-12.8%). Complete information on the percentage of positive hASCs for each marker and each donor can be found in Supplementary Table 1. This initial characterization showed that there were no morphological or immunophenotypic differences among hASCs from donors of different ages.
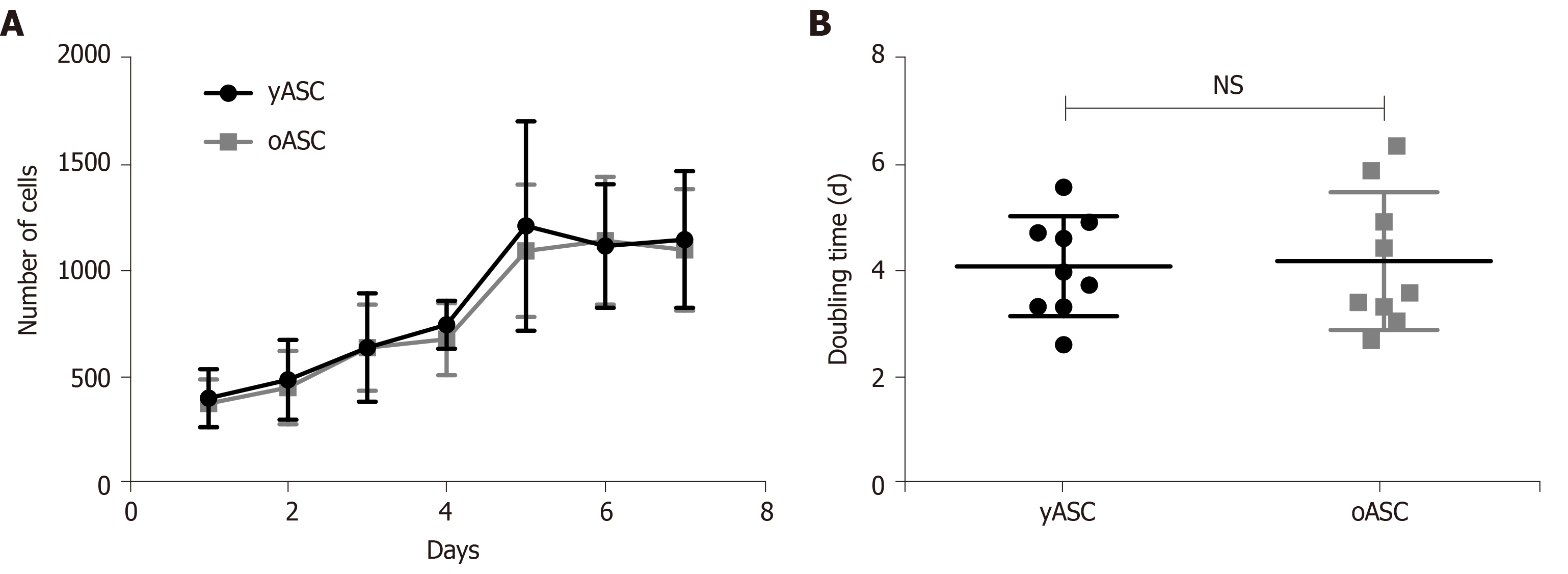
To evaluate the potential of division from hASCs derived from young and old donors, cells in a comparable range of population doubling level (PDL) (Supplementary Figure 2) were allowed to grow over seven days, and growth curves were evaluated. No significant difference was observed between growth curves from yASCs and oASCs (Figure 2A). Based on these data, the population doubling time was calculated. The yASC population doubling time ranged from 2.5 to 5.6 d, while the oASC range was 2.6 to 6.3. As visualized in Figure 2B, the average doubling time for yASCs (4.09 ± 0.94 d) was not different from the average for oASCs (4.19 ± 1.29 d), suggesting that hASC growth potential does not significantly change according to donor age.
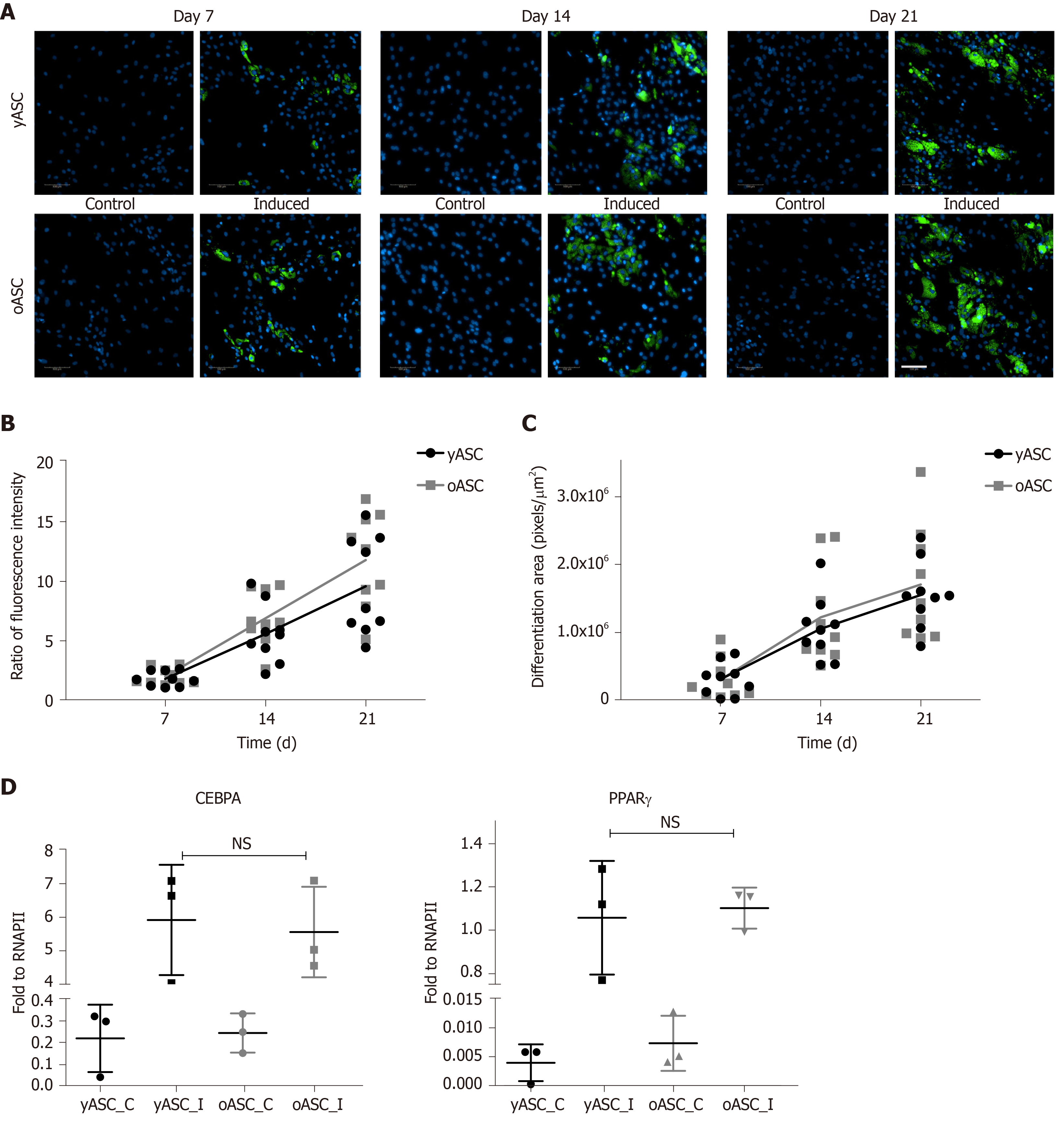
Adipogenic differentiation was assessed by staining the cytoplasmic accumulation of triglycerides using the lipid dye Nile red after 7 d, 14 d and 21 d of differentiation. As observed in Figure 3A, after 7 d of induction, it was already possible to find lipid-rich droplets in the cytoplasm of induced cells from both groups but not in the control cells. Lipid accumulation increased over the duration of the experiment, and at day 21, the morphology of induced cells was consistent with that of adipocytes, showing large intracellular Nile red-positive lipid vacuoles (Figure 3A). Quantitative analyses were performed with two different systems: a fluorescence microplate reader (Figure 3B) and a high-content image system (Figure 3C). Both yASCs and oASCs were able to differentiate better than the noninduced control cells, with a gradual increase in the differentiation rate over the duration of the experiment (Figure 3A). After quantifying the fluorescence intensity (Figure 3B) and calculating the stained area (Figure 3C), there was no significant difference when comparing oASCs and yASCs (P = 0.2406, fluorescence intensity; P = 0.6488, stained area). Another parameter used to compare the differentiation efficiency of both yASCs and oASCs was the quantification of specific adipogenic marker expression. The results from qPCR revealed that adipogenesis-induced hASCs exhibited increased expression levels of PPARγ2 and CEBPA in relation to noninduced cells in both groups. However, this change was not different between induced yASCs and oASCs (Figure 3D).
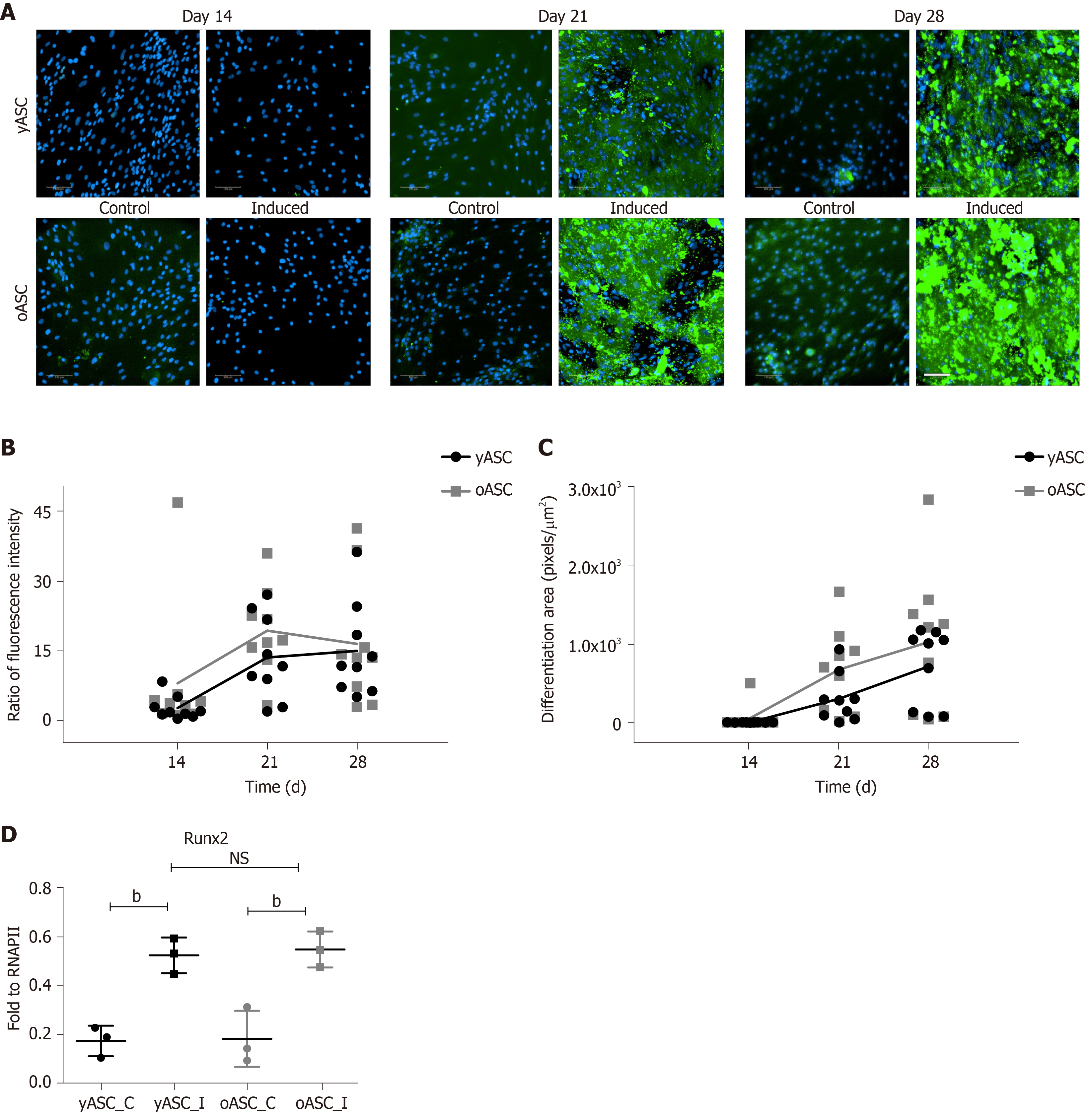
To verify osteogenic differentiation, cell mineralization was assessed after 14 d, 21 d and 28 d of differentiation induction using fluorescent staining of the hydroxyapatite deposited by cells. As observed in Figure 4A, no significant mineralization was noted on day 14. However, after 21 d of osteogenic induction, cells showed substantial mineral deposition, which was not seen in noninduced cells (control) (Figure 4A). Quantitative analyses of this mineralization, both by fluorescence microplate reader (Figure 4B) and by high-content image system (Figure 4C), indicated that yASCs and oASCs were able to differentiate better than the noninduced cells (Figure 4B and C). In addition, comparing the osteogenic potential between hASCs derived from young and elderly donors, no significant difference was observed between groups (P = 0.3129, fluorescence intensity; P = 0.1796, stained area). As an osteogenic marker, we used Runx2. This gene usually shows higher expression levels on the 14th day of osteogenesis[16]. For this reason, this differentiation time was chosen for Runx2 expression quantification. It was verified that osteogenesis-induced cells had higher marker expression when compared to noninduced cells. Nonetheless, no differences were found between induced yASCs and oASCs (Figure 4D).
The effect of donor age on the growth kinetics and differentiation potential of MSCs is still very controversial. In this work, the results showed no differences regarding population doubling time, adipogenic and osteogenic potential when comparing hASCs from lipoaspirates of young and elderly donors.
We verified that the immunophenotypic profile and cell morphology of hASCs derived from donors of different ages were similar, which corroborates data from other studies[17-19]. However, using only these parameters to distinguish young from elderly populations might not be ideal. Block and colleagues have shown that bone marrow MSCs from elderly donors that did not show differences in classical markers when compared with cells from young donors could be separated into subpopulations based on cell size and stage-specific embryonic antigen-4 (SSEA-4) marker expression. From the resultant subpopulations, the smaller and SSEA-4-positive cells were found to represent a “younger” phenotype[20].
Previous studies have demonstrated that young donors exhibited a higher proliferation rate and/or a shorter population doubling time when comparing to old hASCs[17,19,21-23]. Zhang and colleagues (2018), for instance, have shown that after 7 d of cultivation, hASCs from older donors (> 55 years old) had a lower proliferative rate when compared to cells from younger donors[23]. Similar results were found by Choudhery et al[19], who showed that doubling time was significantly different when comparing donors < 30 years old and those > 40 years old. In another study, the authors suggested that there was reduced proliferative activity in hASCs isolated from the orbital adipose tissue of older donors; however, they also stated that these cells stopped proliferating at passage 6, showing senescent behavior[22]. In our work, although we did not evaluate senescence markers, we did not observe morphological or behavioral changes in the cells through eight passages (data not shown). We also did not identify any influence of the donor’s age on the growth potential of the cells, as previously reported by other authors[16,24,25].
The age of the hASC donors could also influence their adipogenic or osteogenic potential. As in our study, Choudhery et al[19] evaluated the influence of donor age on hASCs from lipoaspirates. Even though they did not find impairment of the adipogenic potential of aged cells, they observed that as the age of the donors increased, the abilities of the cells to proliferate and to differentiate into osteoblasts decreased significantly[19]. Zhu et al[25] also showed osteogenesis impairment of hASCs isolated from liposuction tissue of a mid-aged (40s) group of donors. However, they did not find an influence of the donor’s age on proliferation or adipogenic potential. Nevertheless, a more recent work showed that age influenced the adipogenesis efficiency of hASCs, while no differences in proliferation, osteogenesis or chondrogenesis were observed with increasing age[24]. Another study evaluating hASCs isolated from liposuction material demonstrated that the age of the donors did not have an effect on the differentiation potential of the cells; however, cells from younger donors showed a slightly better clonogenic ability[26]. Interestingly, our results showed no significant difference in adipogenic or osteogenic differentiation efficiency between young and elderly hASC donors, although oASCs seem to show an increased tendency to differentiation. Therefore, although it could be expected that cells from the same type of origin tissue would behave similarly, studies have shown that even cells from the same source (liposuction fat) can show huge variability in their performance.
One factor that could introduce the variability observed in the literature is the different age groups of hASC donors. There is no standard grouping among different studies. Interestingly, some authors reported that middle-aged donors (approximately 40 years) show differences in the ability to differentiate when compared to younger (approximately 20 years) but also to older (> 50 years) donors. A study evaluating the effect of aging on the adipogenic capacity of hASCs has shown that cells isolated from female donors with ages ranging from 40-45 years had an increased ability to accumulate lipids and increased expression of PPARγ2 when compared to cells from donors older than 55 and younger than 30 years[27]. Similar conditions were found in the study by Zhu et al[25], mentioned above. They showed that cells from 40-49-year-old donors had less extracellular matrix calcification than cells from donors younger than 39 years and older than 50 years. These authors suggested that age has an influence on differentiation and that a decline in estrogen levels due to the menopause could be the explanation for this transient effect[25]. In our study, we did not include this age range, and we could not find differences when comparing the groups < 35 and > 55 years, corroborating the findings of these authors.
The high variability reported in the results from different studies comparing hASCs from young and elderly donors could be due to several factors, including technical issues and intrinsic characteristics of donors and cell physiology. Adipose tissue exhibits a complex nature, with specific characteristics depending on body location and cell composition[12] . Specifically, subcutaneous adipose tissue, used in this study and by others in the literature, is composed mainly of white adipose tissue. It has been reported that depending on the extraction site (superficial or deep adipose tissue), hASCs may present phenotypic differences[28].
Furthermore, one of the major sources of variability in hASCs proliferation and differentiation potential is related to donor parameters. In addition to age, other aspects, such as general health status, medical and disease history, body mass index, or epigenetic patterns related to environment or donor habits, can influence hASCs performance, as reviewed by Prieto González[12]. Our data exhibited high intragroup variability, possibly reflecting the intrinsic heterogeneity of the patients. Kawagishi-Hotta et al[24] have shown that these individual differences are more evident as the age of patients increases and is dependent on donor sex, as the differences are more evident in cells from female donors.
We have demonstrated that the behavior of hASCs isolated from young or elderly female patients was not different in terms of cell growth and adipogenic and osteogenic differentiation ability. Taken together, our results suggest that donor age does not seem to be an issue in guaranteeing the quality of hASCs isolated from lipoaspirates.
Mesenchymal stromal/stem cells (MSCs) are adult multipotent cells successfully used in clinical trials for several health conditions over the last decades. Human adipose-derived stromal/stem cells (hASCs) are MSCs easily and abundantly obtained from fat tissue. hASCs have been shown to be one of the most advantageous type of MSCs for clinical trials. One of the keys for successful application of these cells is the guarantee of the material quality. Many factors such as the harvesting system, donor characteristics and processing methods are responsible for the good viability, proliferation and multilineage differentiation abilities of these cells.
The aging process is well known to modify the microenvironment and consequently cells performance in the organism. However, it is not understood if donor age promotes intrinsic alterations in cell functions regardless of environmental stimuli. Contradictory evidence is described in the literature regarding the influence of donor age on hASCs functionating after isolation.
The main objective of the present study was to evaluate the growth and differentiation abilities of hASCs isolated from the lipoaspirates of elderly and young donors.
hASCs were isolated from liposuctioned adipose tissue obtained from female donors and distributed into two groups according to age range: old hASCs (oASCs) (≥ 55 years) and young hASCs (yASCs) (≤ 35 years). For hASCs characterization, immunophenotypic markers were assessed by flow cytometry. Growth kinetics were assessed over seven days. Adipogenic and osteogenic differentiation was evaluated. For adipogenic potential evaluation, lipid deposits were assessed after 7 d, 14 d and 21 d of adipogenic induction. Osteogenic potential was verified by analyzing cell mineralization after 14 d, 21 d and 28 d of osteogenic induction. mRNA expression of PPARγ2, CEBPA and Runx2 were detected by quantitative reverse transcription polymerase chain reaction.
No differences were observed in morphology or performance between cells from young (26.33 ± 4.66 years old) and old donors (64.78 ± 4.58 years old) during cultivation and maintenance of these cells. Both groups showed classical immunophenotypic characteristics of mesenchymal stem/stromal cells. The potential for cell division did not significantly differ between yASCs and oASCs. Regarding differentiation potential, yASCs and oASCs were able to efficiently differentiate after adipogenic and osteogenic induction. No differences were observed in the adipogenesis or osteogenesis effectiveness between yASCs and oASCs.
hASCs isolated from lipoaspirate material obtained from young or elderly female patients were not different in terms of in vitro performance considering cell growth and adipogenic and osteogenic differentiation potentiality.
Donor age does not seem to interfere with the intrinsic characteristics of hASCs isolated from lipoaspirates. Therefore, donor age should not interfere in guaranteeing the quality of these cells.
The authors would like to acknowledge Hellen Geremias Santos for reviewing the statistical methods applied to this study and the Program for Technological Development in Tools for Health-PDTIS FIOCRUZ for use of its facilities. BD and MJB thank CNPq and CDSH thanks Fiocruz for fellowship support.
Manuscript source: Invited manuscript
Specialty type: Cell biology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pham PV, Yong KW S-Editor: Zhang H L-Editor: Webster JR P-Editor: Xing YX
| 1. | Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 2. | Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018;2018:8031718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 3. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1256] [Article Influence: 209.3] [Reference Citation Analysis (0)] |
| 4. | Álvarez-Viejo M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J Stem Cells. 2020;12:100-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Gomez-Salazar M, Gonzalez-Galofre ZN, Casamitjana J, Crisan M, James AW, Péault B. Five Decades Later, Are Mesenchymal Stem Cells Still Relevant? Front Bioeng Biotechnol. 2020;8:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 6. | Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 386] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 7. | Dubey NK, Mishra VK, Dubey R, Deng YH, Tsai FC, Deng WP. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Papadopulos NA, Wigand S, Kuntz N, Piringer M, Machens HG, Klüter H, Bieback K, Karagianni M. The Impact of Harvesting Systems and Donor Characteristics on Viability of Nucleated Cells in Adipose Tissue: A First Step Towards a Manufacturing Process. J Craniofac Surg. 2019;30:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Fafián-Labora JA, Morente-López M, Arufe MC. Effect of aging on behaviour of mesenchymal stem cells. World J Stem Cells. 2019;11:337-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Sui BD, Hu CH, Zheng CX, Jin Y. Microenvironmental Views on Mesenchymal Stem Cell Differentiation in Aging. J Dent Res. 2016;95:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Varghese J, Griffin M, Mosahebi A, Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther. 2017;8:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 12. | Prieto González EA. Heterogeneity in Adipose Stem Cells. Adv Exp Med Biol. 2019;1123:119-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Marcon BH, Spangenberg L, Bonilauri B, Robert AW, Angulski ABB, Cabo GC, Cofré AR, Bettes PSL, Dallagiovanna B, Shigunov P. Data describing the experimental design and quality control of RNA-Seq of human adipose-derived stem cells undergoing early adipogenesis and osteogenesis. Data Brief. 2020;28:105053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 15. | Roth V. Doubling time computing. 2006. In: Doubling Time [Internet]. Available from: http://www.doubling-time.com/compute.php. |
| 16. | Liu M, Lei H, Dong P, Fu X, Yang Z, Yang Y, Ma J, Liu X, Cao Y, Xiao R. Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties. Cell Transplant. 2017;26:1505-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:2152435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 20. | Block TJ, Marinkovic M, Tran ON, Gonzalez AO, Marshall A, Dean DD, Chen XD. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res Ther. 2017;8:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Kornicka K, Marycz K, Tomaszewski KA, Marędziak M, Śmieszek A. The Effect of Age on Osteogenic and Adipogenic Differentiation Potential of Human Adipose Derived Stromal Stem Cells (hASCs) and the Impact of Stress Factors in the Course of the Differentiation Process. Oxid Med Cell Longev. 2015;2015:309169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Ye X, Liao C, Liu G, Xu Y, Tan J, Song Z. Age-Related Changes in the Regenerative Potential of Adipose-Derived Stem Cells Isolated from the Prominent Fat Pads in Human Lower Eyelids. PLoS One. 2016;11:e0166590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Zhang M, Wang Z, Zhao Y, Zhang L, Xu L, Cao L, He W. The Effect of Age on the Regenerative Potential of Human Eyelid Adipose-Derived Stem Cells. Stem Cells Int. 2018;2018:5654917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Kawagishi-Hotta M, Hasegawa S, Igarashi T, Yamada T, Takahashi M, Numata S, Kobayashi T, Iwata Y, Arima M, Yamamoto N, Yagami A, Nakata S, Uzawa T, Matsunaga K, Sugiura K, Akamatsu H. Enhancement of individual differences in proliferation and differentiation potentials of aged human adipose-derived stem cells. Regen Ther. 2017;6:29-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | de Girolamo L, Lopa S, Arrigoni E, Sartori MF, Baruffaldi Preis FW, Brini AT. Human adipose-derived stem cells isolated from young and elderly women: their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy. 2009;11:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |