Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1591
Peer-review started: March 21, 2020
First decision: August 22, 2020
Revised: August 24, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: December 26, 2020
Processing time: 280 Days and 11.9 Hours
Spinal cord injury (SCI) is an important cause of traumatic paralysis and is mainly due to motor vehicle accidents. However, there is no definite treatment for spinal cord damage.
To assess the outcome of rat embryonic stem cells (rESC) and autologous bone marrow-derived neurocytes (ABMDN) treatment in iatrogenic SCI created in rats, and to compare the efficacy of the two different cell types.
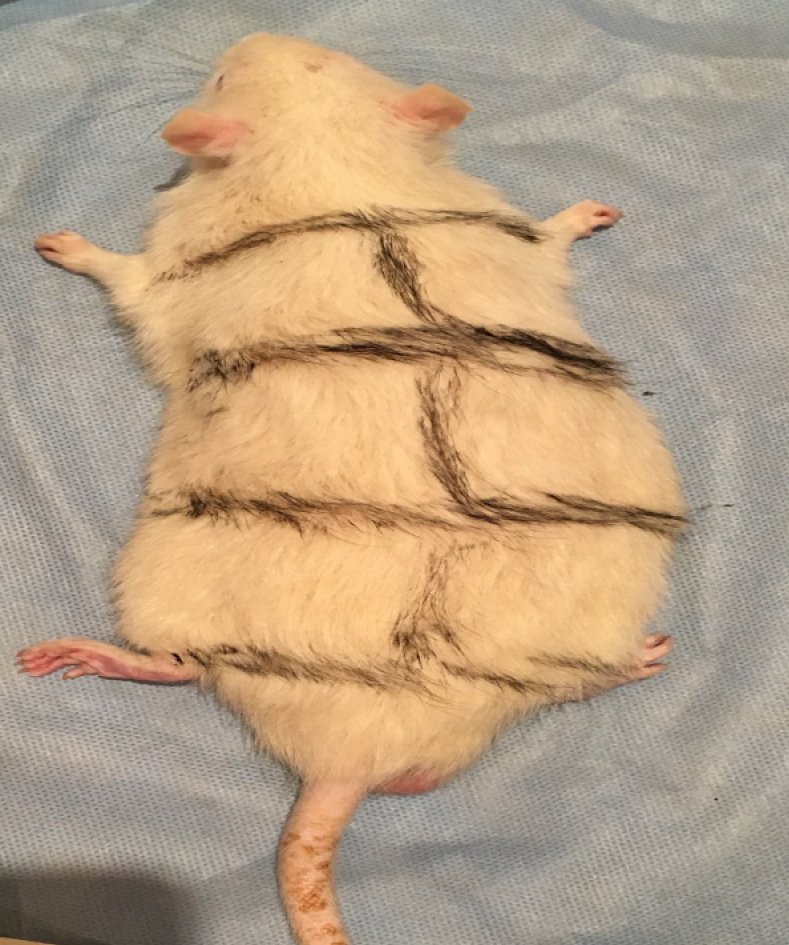
The study comprised 45 male Wistar rats weighing between 250 and 300 g, which were divided into three groups, the control, rESC and ABMDN groups. The anesthetized animals underwent exposure of the thoracic 8th to lumbar 1st vertebrae. A T10-thoracic 12th vertebrae laminectomy was performed to expose the spinal cord. A drop-weight injury using a 10 g weight from a height of 25 cm onto the exposed spinal cord was conducted. The wound was closed in layers. The urinary bladder was manually evacuated twice daily and after each evacuation Ringer lactate 5 mL/100 g was administered, twice daily after each bladder evacuation for the first 7 postoperative days. On the 10th day, the rats underwent nerve conduction studies and behavioral assessment [Basso, Beattie, Brenham (BBB)] to confirm paraplegia. Rat embryonic stem cells, ABMDN and saline were injected on the 10th day. The animals were euthanized after 8 wk and the spinal cord was isolated, removed and placed in 2% formalin for histopathological analysis to assess the healing of neural tissues at the axonal level.
All the animals tolerated the procedure well. The BBB scale scoring showed that at the end of the first week no recovery was observed in the groups. Post-injection, there was a strong and significant improvement in rats receiving rESC and ABMDN as compared to the control group based on the BBB scale, and the Train-of-four-Watch SX acceleromyography device exhibited statistically significant (P < 0.0001) regeneration of neural tissue after SCI. Histological evaluation of the spinal cord showed maximum vacuolization and least gliosis in the control group compared to the rESC and ABMDN treated animals. In the ABMDN group, limited vacuolization and more prominent gliosis were observed in all specimens as compared to the control and rESC groups.
This study provided strong evidence to support that transplantation of rESC and ABMDN can improve functional recovery after iatrogenic SCI. The transplanted cells showed a beneficial therapeutic effect when compared to the control group.
Core Tip: We performed a comparative study of rats with iatrogenic SCI treated with control, rat embryonic stem cells and autologous bone marrow-derived neurocytes. Regeneration was assessed using three classic parameters and it was found that neurocytes were superior in the regeneration process.
- Citation: Sadat-Ali M, Al-Dakheel DA, Ahmed A, Al-Turki HA, Al-Omran AS, Acharya S, Al-Bayat MI. Spinal cord injury regeneration using autologous bone marrow-derived neurocytes and rat embryonic stem cells: A comparative study in rats. World J Stem Cells 2020; 12(12): 1591-1602
- URL: https://www.wjgnet.com/1948-0210/full/v12/i12/1591.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i12.1591
Spinal cord injury (SCI) is an important cause of traumatic paralysis and is mainly due to motor vehicle accidents. It is reported that in United States over 200000 patients suffer injury to the spinal cord and over 50% of spinal cord injured individuals are considered paraplegic and about 48% quadriplegic. Approximately > 10000 new injuries occur each year of which 82% occur in males and 56% of injuries occur in young people < 30 years[1]. The total direct cost for care of spinal cord injured patients in the United States is approximately $7.736 billion[2]. The yearly incidence of traumatic SCI worldwide is about 15-40 cases per million[3]. Despite serious preventive measures to reduce the injuries causing traumatic paraplegia, the incidence of such injuries is increasing. In addition, although many treatments to reverse total paralysis have been tried, we currently do not have effective therapies to reverse this disabling condition.
Spinal cord bypass surgery using peripheral nerves[4], olfactory ensheathing cells[5,6] Schwann cells for transplantation[7] and neural stem cells (NSC)[8] have been performed. Studies in animals have shown improvements in injured spinal cord function when cells derived from the embryonic central nervous system of mice were transplanted[9]. In another report, adult stem cells from the central nervous system were also used with limited success[10-12]. Recently Syková et al[13] (2006) injected mesenchymal stem cells (MSCs) into the spinal cord in rats with iatrogenic injury and found acceptable functional recovery, which was confirmed histologically. These results were similar to those reported by Kang et al[14]. In a recent review, Han et al[15] (2019) reported the extraction of MSCs from various sources, which included bone marrow, adipose tissue, synovium, umbilical cord and human blood. In animal models, MSCs-based therapies had a beneficial effect on locomotor recovery[16,17], but the results could not be replicated or in vitro.
Expanded MSCs have a short life span and they disappear within 24 h[18], while terminal cells such as neurocytes remain alive for about 90 d.
Our objective was to study the outcome of rat embryonic stem cells (rESC) and autologous bone marrow-derived neurocytes (ABMDN) in iatrogenic SCI created in rats, and to compare the efficacy of the two different cell types.
This study was carried out following approval by the Institutional Review Board of the University of Dammam, which was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
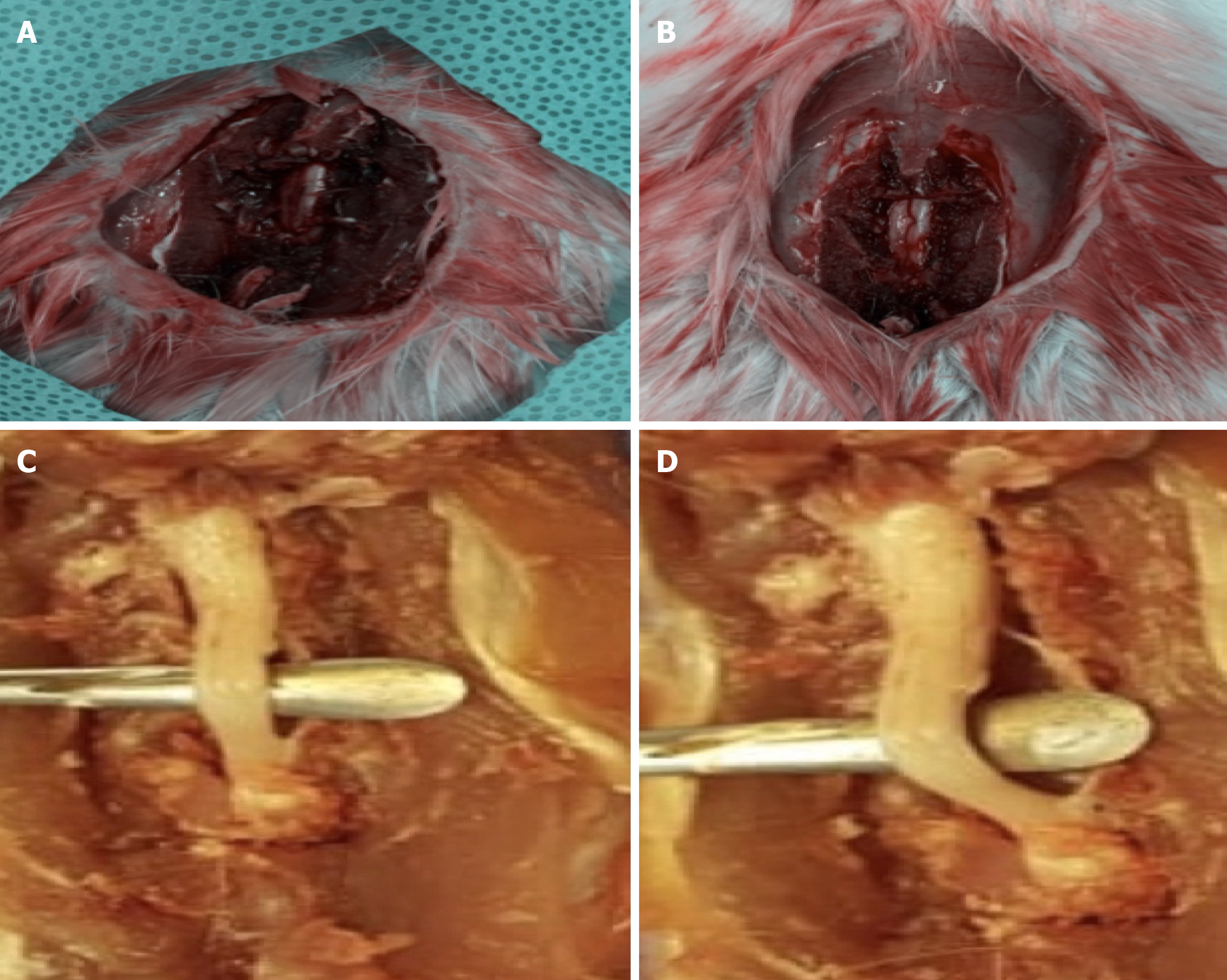
A drop weight device was standardized, which consisted of a 10 g weight dropped from a height of 25 cm. The study included 45 male Wistar rats weighing between 250 and 300 g (Figure 1). The animals were kept in the similar cages and had similar feeding habits. They were divided into the following three groups: Group I was the control group, Group II was the rESC group and Group III was the ABMDN group. rESC were obtained from Celprogen Inc., Torrance, CA, United States and ABMDN were harvested at the university’s stem cell laboratory. The animals were anesthetized with ketamine 50 mg/kg body weight and xylazine 35 mg/kg). Sterile eye drops were instilled to keep the eyes moist during surgery. The fur was clipped and the surgical area was cleaned with betadine. An incision was made over the lower thoracic area (Figure 2), muscle and connective tissues were bluntly dissected to expose the thoracic 8th to lumbar 1st vertebrae. A T10-thoracic 12th vertebrae laminectomy was performed, avoiding any injury to the spinal cord. A 10 g weight was dropped from a height of 25 cm onto the exposed spinal cord (Figure 3A and B). The wound was closed in layers. Diclofenac 1 mg/kg body weight as an analgesic and floxacillin 3 mg/kg body weight were given. Postoperatively, Ringer lactate 5 mL/100 g was given subcutaneously. Following surgery, the rats were monitored thrice a day for one week. The urinary bladder was manually evacuated twice daily and Ringer lactate 5 mL/100 g was administered for the first week after surgery.
On the 10th day after creation of the SCI all the rats underwent nerve conduction studies and behavioral assessment (Basso, Beattie, Bresnahan [BBB])[19] to confirm paraplegia. The BBB scale, which was described in 1995, is a 21-point scale which assesses trunk stability, hind limb movements, stepping with coordination and clearance of toes, and tail position. Following the drop weight SCI, injection of rESC, ABMDN or saline was administered on the 10th day. Behavioral assessment using the BBB scale was conducted prior to cell transplantation. Once paraplegia was confirmed the injured site was reopened and in the controls 1 mL of normal saline (Group I), 4 million rESC (Group II) and 4 million ABMDN (Group III) was injected. The wound was closed in layers. At the end of the 8-wk period nerve conduction tests were performed using Train-of-four (TOF)-Watch SX, which is the current "clinical gold standard", for measuring recovery of nerve blockade. In TOF nerve stimulation, the ratio of the fourth muscle contraction (twitch) to the first twitch is recorded; when this ratio is 90% (or 0.9) or greater, full recovery of muscle strength is present indicating nerve recovery.
The animals were euthanized after 8 wk and spinal cords were isolated (Figure 4A and B) and placed in 2% formalin for histopathological analysis to assess healing of neural tissues at the axonal level.
All the animals tolerated the procedure well. Eight weeks after implantation, the assessment of locomotion by BBB and electromyographic studies was carried out and animals were then euthanized and spinal cord was dissected out and sent for histopathological studies.
BBB scale scoring showed that at the end of the first week, no recovery was observed in the groups and by the 1st week there was recovery in the rESC and ABMDN groups. There was a significant improvement in rats receiving ESC and ABMDN. In the rESC group the final score was 11/21, was 13/21 in the ABMDN group and was 2/21 in the control group (Table 1). A statistically significant difference between the control group and the rESC group was observed (P < 0.0001), between the control group and the ABMDN group (P < 0.0001) and between the rESC group and the ABMDN group (P < 0.01). Overall, the transplanted cells resulted in a remarkable recovery at 8 wk.
| Week | Control | rESC | ABNDN |
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 3 |
| 3 | 0 | 3 | 3 |
| 4 | 1 | 3 | 4 |
| 5 | 1 | 5 | 6 |
| 6 | 1 | 7 | 8 |
| 7 | 2 | 7 | 10 |
| 8 | 2 | 11 | 13 |
The TOF-Watch SX acceleromyography device (Organon, Ireland) is accepted as the standard monitor for use in clinical trials, and was used to assess the improvement in neural function in the lower limbs. In the rESC group, the amplitude was statistically significant in the rESC and ABMDN groups as compared with the control group (P < 0.0001), exhibiting regeneration of neural tissue after SCI (Tables 2-4). Table 5 shows the results of a comparison between the three groups, which revealed that recovery was much better in the ABMDN group followed by the rESC group.
| Mode | Tw1 (%) | Tw2 (%) | Tw3 (%) | Tw4 (%) | TOF (%) | Temp (°C) | Stim (mA) | T (μs) | Sens. | |
| 1 | TOF | 4 | 0 | 0 | 1 | 25 | 33.6 | 50 | 200 | 157 |
| 2 | TOF | 0 | 0 | 0 | 0 | 33.1 | 50 | 200 | 157 | |
| 3 | TOF | 0 | 0 | 0 | 0 | 32.6 | 50 | 200 | 157 | |
| 4 | TOF | 0 | 0 | 0 | 0 | 33.3 | 50 | 200 | 157 | |
| 5 | TOF | 0 | 0 | 0 | 0 | 32.7 | 50 | 200 | 157 | |
| 6 | TOF | 0 | 0 | 0 | 0 | 327 | 50 | 200 | 157 | |
| 7 | TOF | 6 | 3 | 3 | 2 | 33.3 | 32.9 | 50 | 200 | 157 |
| 8 | TOF | 4 | 3 | 0 | 3 | 52 | 33.8 | 50 | 200 | 157 |
| 9 | TOF | 0 | 0 | 0 | 0 | 32.4 | 50 | 200 | 157 | |
| 10 | TOF | 0 | 0 | 0 | 0 | 32.0 | 50 | 200 | 157 | |
| 11 | TOF | 0 | 0 | 0 | 0 | 32.7 | 50 | 200 | 157 | |
| 12 | TOF | 5 | 0 | 2 | 3 | 60 | 33.1 | 50 | 200 | 157 |
| 13 | TOF | 4 | 3 | 3 | 0 | 32.9 | 50 | 200 | 157 | |
| 14 | TOF | 0 | 0 | 0 | 0 | 33.1 | 50 | 200 | 157 | |
| 15 | TOF | 7 | 3 | 4 | 3 | 42 | 33.6 | 50 | 200 | 157 |
| Mode | Tw1 (%) | Tw2 (%) | Tw3 (%) | Tw4 (%) | TOF (%) | Temp (°C) | Stim (mA) | T (μs) | Sens. | |
| 1 | TOF | 85 | 81 | 67 | 86 | 96 | 32.8 | 50 | 200 | 157 |
| 2 | TOF | 87 | 43 | 56 | 60 | 86 | 33.1 | 50 | 200 | 157 |
| 3 | TOF | 76 | 57 | 52 | 42 | 68 | 33.2 | 50 | 200 | 157 |
| 4 | TOF | 79 | 71 | 78 | 65 | 82 | 32.8 | 50 | 200 | 157 |
| 5 | TOF | 65 | 77 | 43 | 59 | 46 | 33.1 | 50 | 200 | 157 |
| 6 | TOF | 52 | 57 | 52 | 42 | 80 | 32.2 | 50 | 200 | 157 |
| 7 | TOF | 43 | 77 | 75 | 75 | 89 | 31.8 | 50 | 200 | 157 |
| 8 | TOF | 33 | 34 | 34 | 30 | 90 | 31.1 | 50 | 200 | 157 |
| 9 | TOF | 52 | 57 | 52 | 42 | 81 | 31.2 | 50 | 200 | 157 |
| 10 | TOF | 66 | 48 | 69 | 24 | 88 | 32.8 | 50 | 200 | 157 |
| 11 | TOF | 33 | 34 | 34 | 65 | 90 | 33.1 | 50 | 200 | 157 |
| 12 | TOF | 52 | 57 | 52 | 42 | 80 | 33.2 | 50 | 200 | 157 |
| 13 | TOF | 75 | 71 | 72 | 24 | 88 | 32.8 | 50 | 200 | 157 |
| 14 | TOF | 39 | 34 | 55 | 70 | 76 | 32.1 | 50 | 200 | 157 |
| 15 | TOF | 56 | 57 | 52 | 42 | 75 | 33.6 | 50 | 200 | 157 |
| Mode | Tw1 (%) | Tw2 (%) | Tw3 (%) | Tw4 (%) | TOF (%) | Temp (°C) | Stim (mA) | T (μs) | Sens. | |
| 1 | TOF | 45 | 61 | 66 | 44 | 67 | 32.1 | 50 | 200 | 157 |
| 2 | TOF | 39 | 24 | 34 | 28 | 71 | 32.5 | 50 | 200 | 157 |
| 3 | TOF | 58 | 53 | 42 | 32 | 55 | 33.2 | 50 | 200 | 157 |
| 4 | TOF | 62 | 61 | 87 | 45 | 72 | 32.8 | 50 | 200 | 157 |
| 5 | TOF | 49 | 67 | 35 | 34 | 57 | 32.9 | 50 | 200 | 157 |
| 6 | TOF | 60 | 59 | 51 | 50 | 69 | 32.7 | 50 | 200 | 157 |
| 7 | TOF | 61 | 23 | 29 | 43 | 70 | 33.2 | 50 | 200 | 157 |
| 8 | TOF | 66 | 43 | 61 | 39 | 59 | 32.6 | 50 | 200 | 157 |
| 9 | TOF | 52 | 49 | 51 | 44 | 84 | 31.9 | 50 | 200 | 157 |
| 10 | TOF | 67 | 31 | 27 | 37 | 55 | 32.3 | 50 | 200 | 157 |
| 11 | TOF | 39 | 43 | 54 | 29 | 74 | 32.8 | 50 | 200 | 157 |
| 12 | TOF | 65 | 64 | 57 | 35 | 45 | 32.7 | 50 | 200 | 157 |
| 13 | TOF | 58 | 61 | 59 | 61 | 67 | 32.9 | 50 | 200 | 157 |
| 14 | TOF | 53 | 60 | 51 | 49 | 77 | 32.1 | 50 | 200 | 157 |
| 15 | TOF | 62 | 59 | 56 | 61 | 79 | 32.6 | 50 | 200 | 157 |
| Variable | Control | rESC | ABMDN |
| Twitch1 height | 2 | 49.5 | 52.9 |
| Twitch2 height | 0.8 | 50 | 48.7 |
| Twitch3 height | 0.8 | 51 | 43.7 |
| Twitch4 height | 0.8 | 42 | 38.9 |
| TOF ratio = (Twitch 4/Twitch 1) × 100 (%) | 47.6 ± 20.3 | 69.33 ± 13.9 | 80.2 ± 11.9 |
| Stimulation current (60 mA) | 50 | 50 | 50 |
| Pulse width (200 μs) | 200 | 200 | 200 |
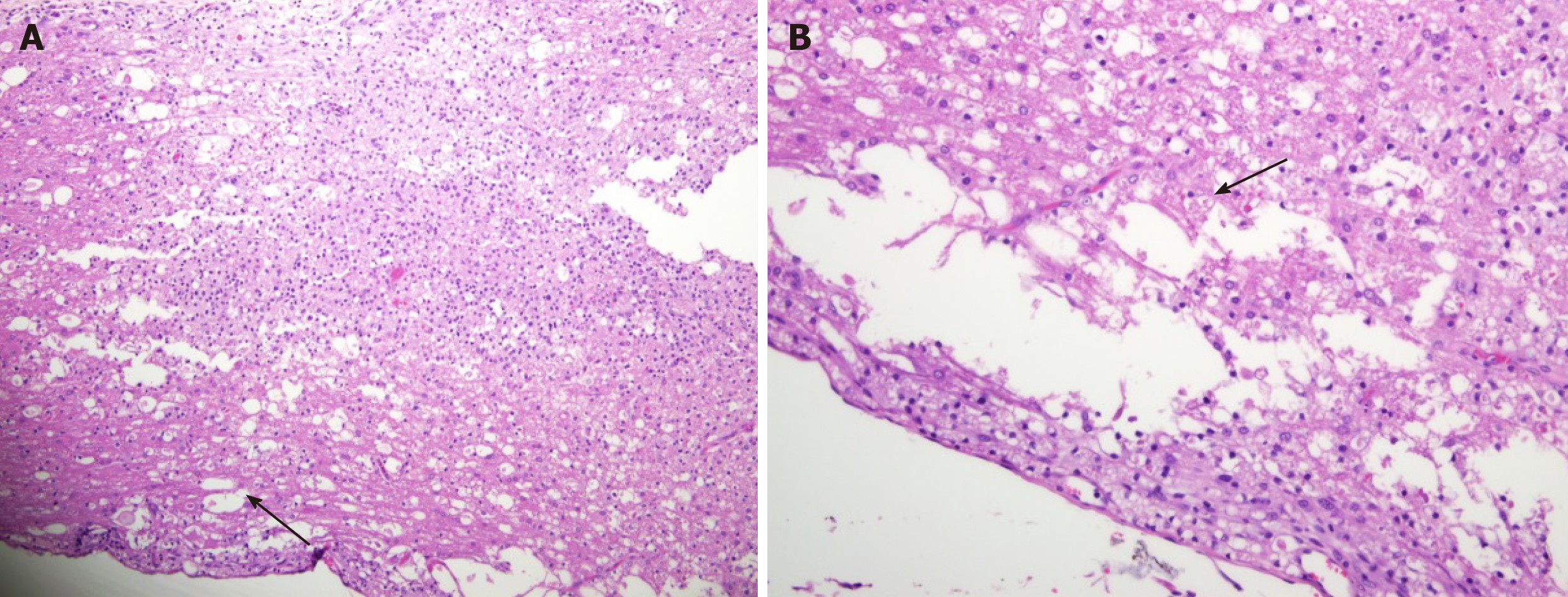
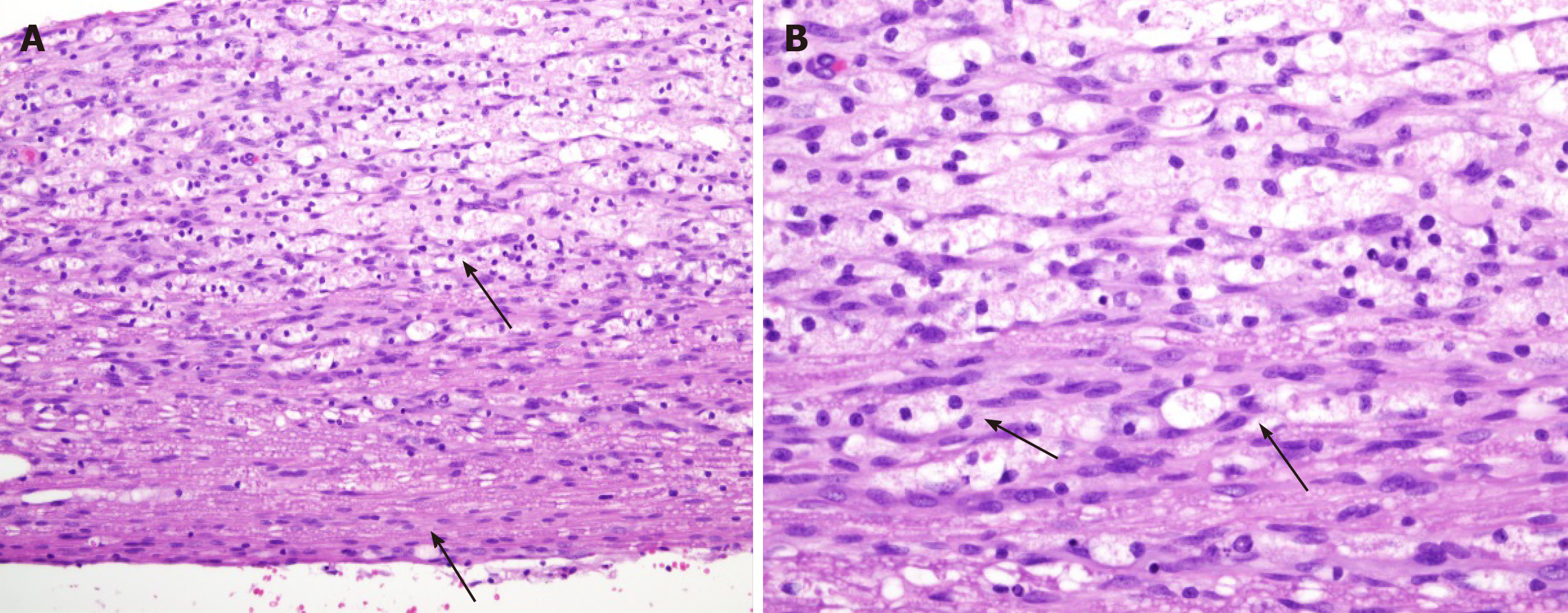
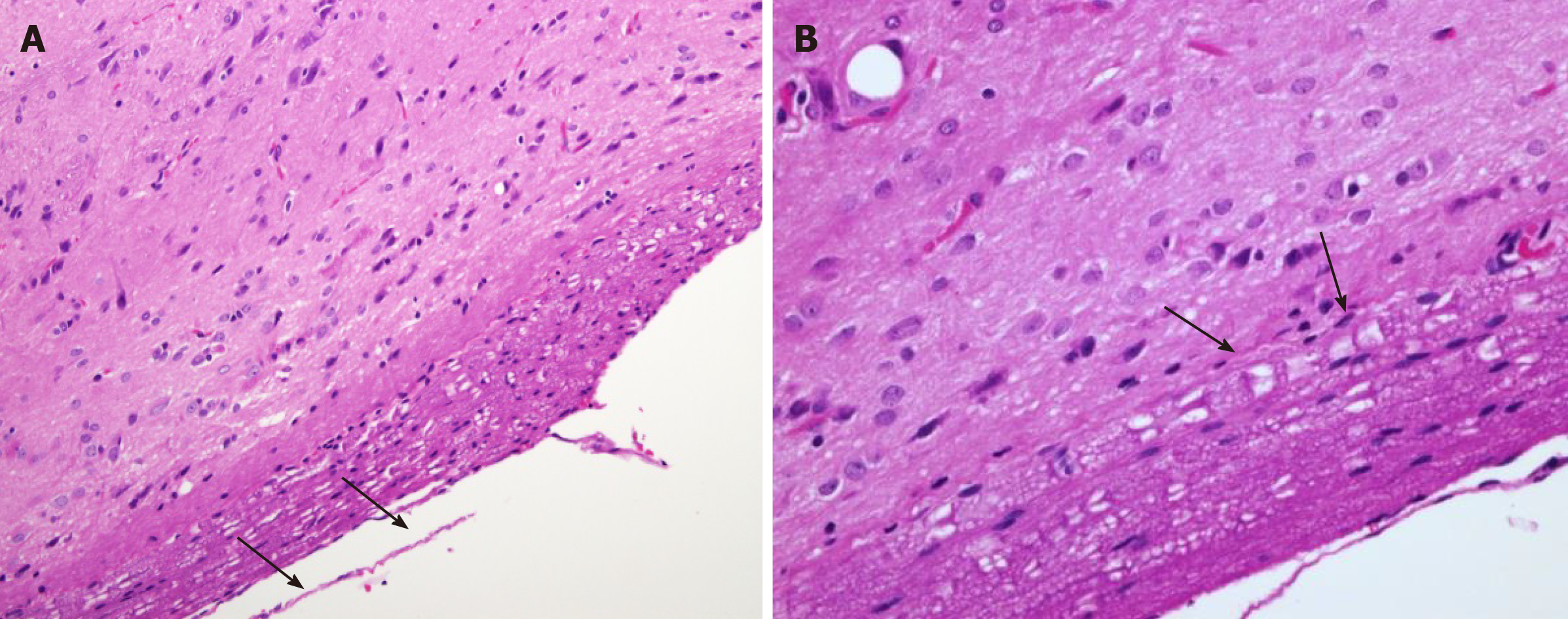
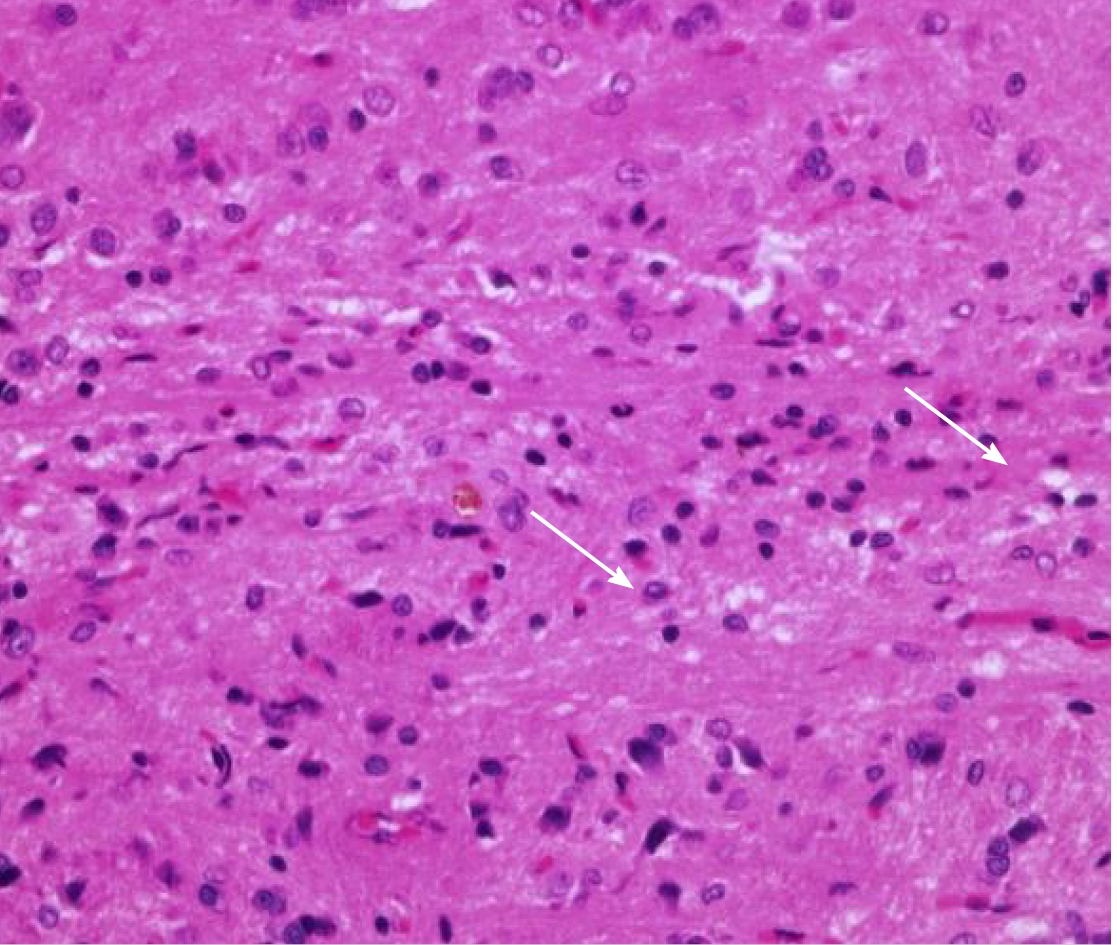
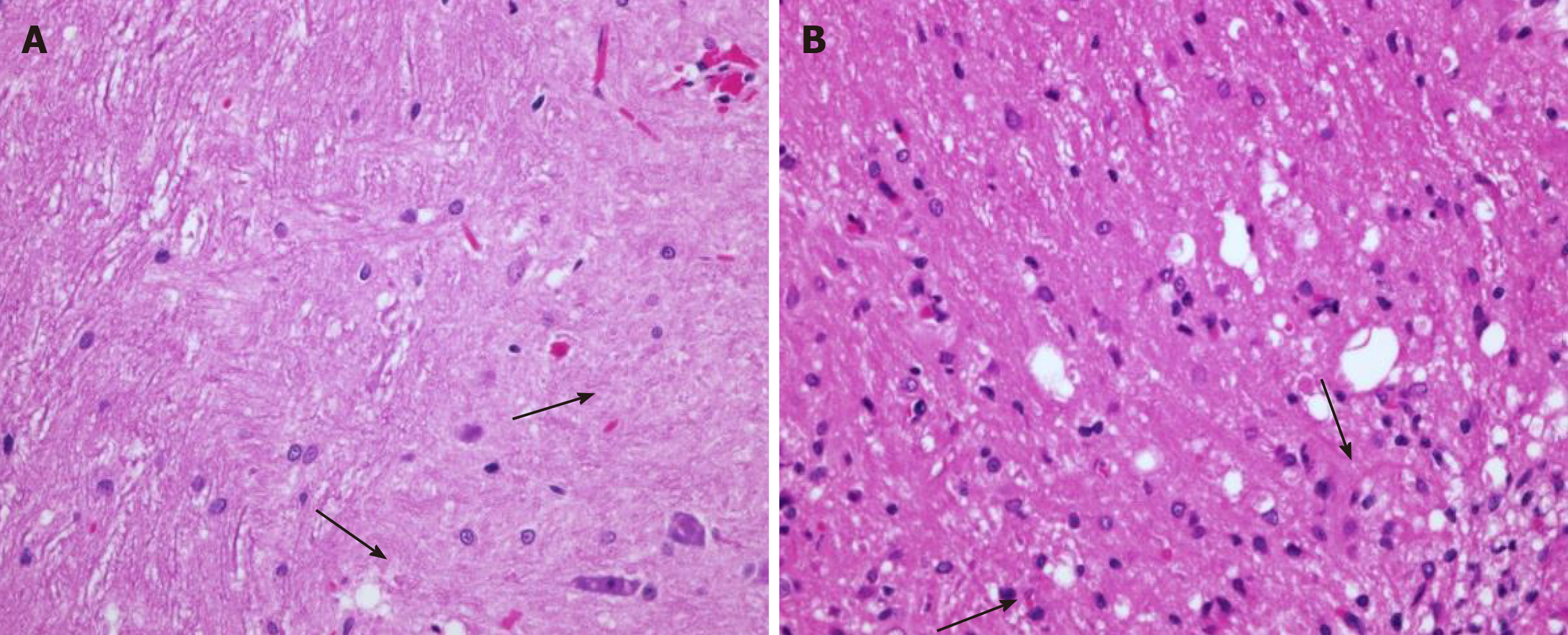
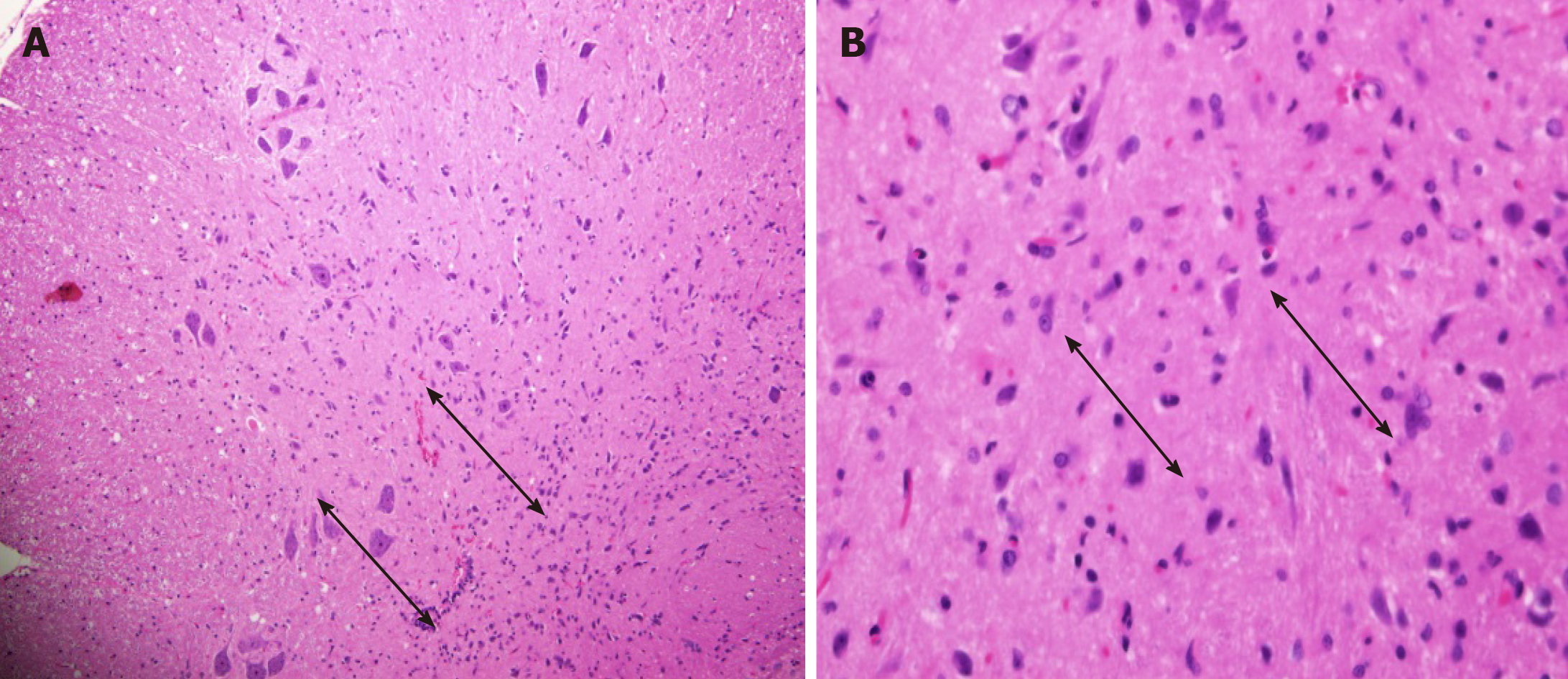
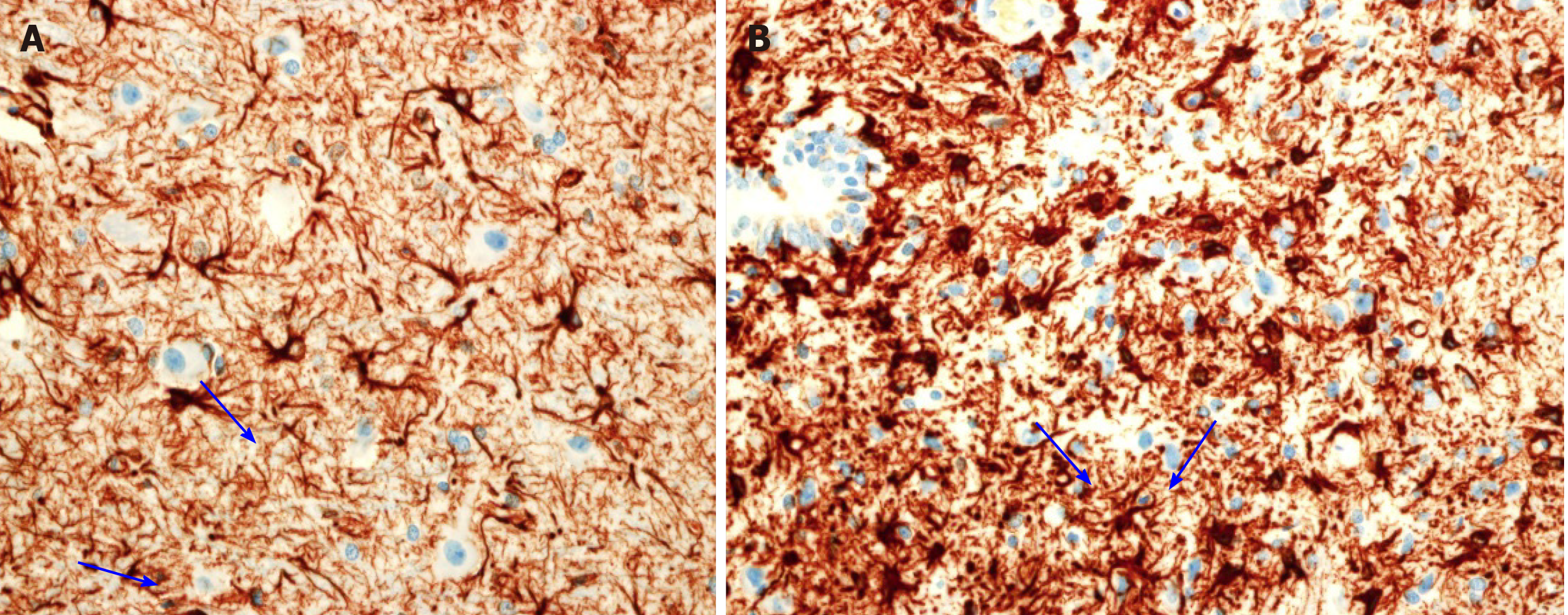
Cross-section of the spinal cord showed dissolved and degenerated cells with the formation of varying extents of vacuolization and reactive gliosis in the control group (Figures 3 and 4), whereas the spinal cords in rESC transplanted animals were smaller and further apart with more reactive gliosis and no tumor formation (Figures 5 and 6). In the ABMDN group, limited vacuolization and more prominent gliosis were observed in all specimens compared to the controls (Figures 7 and 8). Overall, the specimens exposed to ABMDN demonstrated less pronounced vacuolization and enhanced reactive gliosis as compared to specimens exposed to rESC. This was further confirmed by glial fibrillary acidic protein staining (Figure 9).
Our study found that iatrogenic SCI can functionally and neurologically recover after rESC and ABMDN transplantation compared with the control group. When the outcome in the rESC and ABMDN groups was compared, the latter showed better results on the BBB scale, electromyographic and histological analysis. Ye et al[20] (2018) conducted a trial using bone mesenchymal stem cells (BMSCs) and BMSC neural-like cells and showed that human cerebrospinal fluid-derived neural-like cells fared better when the cells were transplanted into the lateral ventricles. Our results also confirmed that ABMDN resulted in a better outcome than rESC. Muniswami et al[21] (2018) used bone marrow-derived MSCs and found that these cells improved healing of the injured spinal cord.
The question arises whether to use MSCs or rESC or the terminal neurocytes. When MSCs enter the circulation, they need to be transformed to terminal cells for reasonable function. During the course of transformation to neurocytes, transformation can be arrested at any time. Secondly, rESC when given intravenously can differentiate into any cell type with a short life span and carry the risk of teratoma formation[22]. The life span of neurocytes has been reported to be between 90-120 d with no risk of tumor formation as these are autologous derived cells.
Jung et al[23] (2009) compared allogenous, autogenous, and control stem cells, and reported that in the control group the recovery was minimal compared to either the allogenous or autogenous stem cell group. This indicates that allogenous stem cells do work but the immune reactions need to be overcome.
Different routes of administration for cell therapies in SCI have been tried. Amemori et al[24] (2015) injected NSCs either intraspinally or directly into the subarachnoid space of rats and found that both methods resulted in recovery. The report by Cheng et al[25] (2012) further supported this method of injection with acceptable recovery.
In the present study, we transplanted the cells directly at the injury site with improved results. Our results are similar to those reported by other researchers in the field. Intravenous administration of hNSCs has been reported to have good therapeutic potential, is less invasive, and avoids a second surgery. This type of therapy needs to be investigated further[26,27]. The literature on stem cell therapy has shown that it is quite promising and can be used in many diseases after appropriate controlled trials. Of these diseases, SCI has shown slow recovery following many modes of treatment. The use of stem cell therapy in SCI in recent years has shown improvement in neural function, which was once believed to be unachievable[28,29].
Recently, trials to heal SCI have used different types of cells for transplantation, including neural stem/progenitor cells (NS/PCs)[16,30], Schwann cells[31], olfactory ensheathing glia[32], MSCs[33] and oligodendrocyte progenitor cells[34] with varying success. The use of scaffolds especially biological scaffolds is emerging as a potential game changer for reducing necrosis, local inflammatory reactions, otherwise could increase necrosis at the site of SCI[35]. Recently, Koffler et al[36] (2019) convincing showed that 3D biomimetic scaffolds with the use of neural progenitor cells enhanced growth and regeneration in host cells. For the first time we used ABMDN in rats and these cells provided better results than rESCs even without a scaffold. It is probable that recovery using a scaffold could lead to further improvement.
In conclusion, the present study provides strong evidence, based on the BBB scale, electromyographic and histopathological studies, to support that transplantation of ABMDN could improve functional recovery after iatrogenic SCI. The transplanted ABMDNs showed a beneficial therapeutic effect. As recovery was ≥ 60% in 8 wk, this is sufficient to warrant large-scale, randomized controlled studies to evaluate their efficacy in larger animals and in humans.
Different methods have been used to heal damaged neural tissue and the results have not been promising.
Many young people have been paralyzed due to trauma and adequate treatment to heal neural tissue is unavailable.
To heal damaged neural tissue using stem cells.
Wistar rats were paralyzed using direct trauma and then treated with rat embryonic stem cells (rESC), autologous bone marrow-derived neurocytes (ABMDN) and saline and the results were observed using electromyography and histology.
ABMDN resulted in significantly better recovery than rESC.
ABMDN led to significantly better recovery than rESC in rats with iatrogenic SCI.
These findings provide adequate evidence for conducting trials in larger animals followed by phase I human trials.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding JX S-Editor: Fan JR L-Editor: Webster JR P-Editor: Li JH
| 1. | DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med. 2002;25:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord. 1997;35:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 192] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 473] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 4. | Oppenheim JS, Spitzer DE, Winfree CJ. Spinal cord bypass surgery using peripheral nerve transfers: review of translational studies and a case report on its use following complete spinal cord injury in a human. Experimental article. Neurosurg Focus. 2009;26:E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Kocsis JD, Lankford KL, Sasaki M, Radtke C. Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neurosci Lett. 2009;456:137-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Hu ZJ, Ma YH. [An update of repairing spinal cord injury by olfactory ensheathing cells]. Zhongguo Gu Shang. 2009;22:68-71. [PubMed] |
| 7. | Tetzlaff W, Kobayashi NR, Giehl KM, Tsui BJ, Cassar SL, Bedard AM. Response of rubrospinal and corticospinal neurons to injury and neurotrophins. Prog Brain Res. 1994;103:271-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1125] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 9. | Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system "bridge" and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405-7415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 839] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 11. | Kim GM, Xu J, Xu J, Song SK, Yan P, Ku G, Xu XM, Hsu CY. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci. 2001;21:6617-6625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA. 2000;97:6126-6131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 388] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Syková E, Jendelová P, Urdzíková L, Lesný P, Hejcl A. Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26:1113-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Kang KS, Kim SW, Oh YH, Yu JW, Kim KY, Park HK, Song CH, Han H. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005;7:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 731] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 17. | Oliveri RS, Bello S, Biering-Sørensen F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta-analyses of rat models. Neurobiol Dis. 2014;62:338-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 19. | Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3140] [Cited by in RCA: 3555] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 20. | Ye Y, Feng TT, Peng YR, Hu SQ, Xu T. The treatment of spinal cord injury in rats using bone marrow-derived neural-like cells induced by cerebrospinal fluid. Neurosci Lett. 2018;666:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Muniswami DM, Kanthakumar P, Kanakasabapathy I, Tharion G. Motor Recovery after Transplantation of Bone Marrow Mesenchymal Stem Cells in Rat Models of Spinal Cord Injury. Ann Neurosci. 2019;25:126-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2:198-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 23. | Jung DI, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009;285:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Amemori T, Ruzicka J, Romanyuk N, Jhanwar-Uniyal M, Sykova E, Jendelova P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther. 2015;6:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Cheng I, Mayle RE, Cox CA, Park DY, Smith RL, Corcoran-Schwartz I, Ponnusamy KE, Oshtory R, Smuck MW, Mitra R, Kharazi AI, Carragee EJ. Functional assessment of the acute local and distal transplantation of human neural stem cells after spinal cord injury. Spine J. 2012;12:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, Tsuji O, Fujiyoshi K, Ebise H, Toyama Y, Okano H, Nakamura M. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, Kocsis JD. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 568] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 29. | Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol Med. 2017;23:831-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, Knackert J, Poplawski G, Dulin JN, Strobl H, Takashima Y, Biane J, Conner J, Zhang SC, Tuszynski MH. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 31. | Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerström-Noga E, Wood P, Levi AD. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J Neurotrauma. 2017;34:2950-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 32. | Gomes ED, Mendes SS, Assunção-Silva RC, Teixeira FG, Pires AO, Anjo SI, Manadas B, Leite-Almeida H, Gimble JM, Sousa N, Lepore AC, Silva NA, Salgado AJ. Co-Transplantation of Adipose Tissue-Derived Stromal Cells and Olfactory Ensheathing Cells for Spinal Cord Injury Repair. Stem Cells. 2018;36:696-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | May Z, Kumar R, Fuehrmann T, Tam R, Vulic K, Forero J, Lucas Osma A, Fenrich K, Assinck P, Lee MJ, Moulson A, Shoichet MS, Tetzlaff W, Biernaskie J, Fouad K. Adult skin-derived precursor Schwann cell grafts form growths in the injured spinal cord of Fischer rats. Biomed Mater. 2018;13:034101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, Sugai K, Nishimura S, Iwai H, Isoda M, Shibata S, Kohyama J, Iwanami A, Toyama Y, Matsumoto M, Nakamura M, Okano H. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Reports. 2016;6:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | Zhang Q, Shi B, Ding J, Yan L, Thawani JP, Fu C, Chen X. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019;88:57-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 36. | Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |