Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.55
Peer-review started: March 8, 2019
First decision: April 16, 2019
Revised: May 13, 2019
Accepted: November 29, 2019
Article in press: November 29, 2019
Published online: January 26, 2020
Processing time: 298 Days and 14 Hours
Critically sized bone defects represent a significant challenge to orthopaedic surgeons worldwide. These defects generally result from severe trauma or resection of a whole large tumour. Autologous bone grafts are the current gold standard for the reconstruction of such defects. However, due to increased patient morbidity and the need for a second operative site, other lines of treatment should be introduced. To find alternative unconventional therapies to manage such defects, bone tissue engineering using a combination of suitable bioactive factors, cells, and biocompatible scaffolds offers a promising new approach for bone regeneration.
To evaluate the healing capacity of platelet-rich fibrin (PRF) membranes seeded with allogeneic mesenchymal bone marrow-derived stem cells (BMSCs) on critically sized mandibular defects in a rat model.
Sixty-three Sprague Dawley rats were subjected to bilateral bone defects of critical size in the mandibles created by a 5-mm diameter trephine bur. Rats were allocated to three equal groups of 21 rats each. Group I bone defects were irrigated with normal saline and designed as negative controls. Defects of group II were grafted with PRF membranes and served as positive controls, while defects of group III were grafted with PRF membranes seeded with allogeneic BMSCs. Seven rats from each group were killed at 1, 2 and 4 wk. The mandibles were dissected and prepared for routine haematoxylin and eosin (HE) staining, Masson's trichrome staining and CD68 immunohistochemical staining.
Four weeks postoperatively, the percentage area of newly formed bone was significantly higher in group III (0.88 ± 0.02) than in groups I (0.02 ± 0.00) and II (0.60 ± 0.02). The amount of granulation tissue formation was lower in group III (0.12 ± 0.02) than in groups I (0.20 ± 0.02) and II (0.40 ± 0.02). The number of inflammatory cells was lower in group III (0.29 ± 0.03) than in groups I (4.82 ± 0.08) and II (3.09 ± 0.07).
Bone regenerative quality of critically sized mandibular bone defects in rats was better promoted by PRF membranes seeded with BMSCs than with PRF membranes alone.
Core tip: Our findings are derived from a rat model for treating critical-sized mandibular bone defects. Defects were grafted with platelet-rich fibrin (PRF) membranes seeded with allogeneic bone marrow-derived stem cells (BMSCs). Our findings confirm the in vivo anti-inflammatory effects of allogenic BMSCs. In addition, BMSCs seeded on the PRF membranes exhibited beneficial syngeneic effects in promoting and accelerating the healing of critically sized mandibular defects. Routine and specific histological and immunohistochemical staining demonstrated for the first time that experimentally treated critically sized mandibular defects with PRF membrane and BMSC combined therapy increased the amount and the rate of the newly formed bone and decreased the amount of granulation tissue with a reduction in the number of inflammatory cell infiltrates.
- Citation: Awadeen MA, Al-Belasy FA, Ameen LE, Helal ME, Grawish ME. Early therapeutic effect of platelet-rich fibrin combined with allogeneic bone marrow-derived stem cells on rats' critical-sized mandibular defects. World J Stem Cells 2020; 12(1): 55-69
- URL: https://www.wjgnet.com/1948-0210/full/v12/i1/55.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i1.55
Animal models are often considered appropriate analogues to clinical conditions. Such models have been appropriately used to check the reliability of a particular hypothesis to recognize the pathogenesis of new toxicity before clinical settings[1]. Rodents share many features with humans, and they are suitable for answering many research questions. They have biological, genetic, and behavioural characteristics closely resembling those of humans, and many symptoms of human disorders can be replicated in rats[2]. One of the major surgical challenges in regenerative medicine is the reconstruction of critically sized bone defects in the craniofacial complex. The treatment of such defects remains debated, particularly defects that are of a critical size caused by removal of a tumour, trauma or congenital malformations and abnormalities. Current treatments depend on the use of autologous bone grafts as a gold standard and consequently have many disadvantages, such as an insufficient amount of bone for prosthetic rehabilitation and donor site morbidity[3].
The advent of a new era of tissue engineering-based strategies has led to promising techniques for the reconstruction of cranio-maxillofacial defects that are of critical size[4]. Significant progress has been made in craniofacial surgery with the usage of tissue engineering-based therapies that employ biomaterial scaffolds covered with adult osteogenic cells and/or osteoinductive factors[5]. Adult mesenchymal stem cells and biomaterials/scaffold-based bone substitutes are a favourable alternative to natural bone grafts[6]. Tissue-engineered bone alternates are fundamentally intended to reproduce bone autograft performance with the least injury and morbidity to the patient while achieving the mechanical properties that are mandatory for bone regeneration and reconstruction. Several techniques have been developed that integrate combinations of osteoinductive signals, osteogenic cells and osteoconductive scaffolds or matrices[7].
Platelet concentrates are used to enhance osseous tissue healing in oral and craniofacial surgery[8]. They can stimulate bone regeneration with minimum inflammatory response and unwanted complications. The usage of these concentrates was derived from the high content of growth factors that can be liberated from platelets at the time of tissue damage; these growth factors are essential for hard and soft tissue repair mechanisms. Among the advantages of platelet concentrates, their safety as an autologous source helps enhance early stability of grafts[9]. In recent years, platelet-rich fibrin (PRF) has gained wide attention for its utilization as a biocompatible regenerative material not only in the dental field but also in medical fields[10].
Mesenchymal stem cells (MSCs) are multipotent, can be isolated from multiple distinctive tissues, and have the ability to differentiate into several cell types of cells, such as osteoblasts and pre-osteogenic chondroblasts[11]. The use of MSCs in tissue engineering is highly recommended because they have a high osteogenic differentiation capacity[12]. The integration of MSCs into bone tissue-engineered biomaterials is a widely studied technique for enhancing bone osteointegration and formation in the repair of bony defects. These cells can migrate to sites of injury, they are capable of suppressing the local immune response, and they are available in large quantities from the patients themselves[13]. Cumulative evidence has proven that bone marrow-derived stem cells (BMSCs) play an efficient role in bone regeneration in a variety of orthopaedic diseases; however, some restrictions still hinder their use in clinical settings. A major obstacle lies in their very low yield, and accordingly, an adequate number of MSCs for successful bone regeneration may be transplanted into defect sites[14].
Previous studies have reported the use of combined therapy of MSCs with PRF concentrate for the treatment of articular cartilage defects[15-18], mandibular reconstruction and regeneration[19,20], alveolar bone defects and clefts[21,22], tibial bone defects[23] and bone remodelling[24]. However, none of these studies performed experiments on a critically sized defect model. We hypothesized that combination therapy of PRF membranes and BMSCs may enable the reconstruction of critically sized mandibular defects in rats. The main purpose of the present study was to assess the possible regenerative capacity of PRF membranes with/without allogeneic BMSCs on critically sized defects in rat mandibles. Our null hypothesis was that PRF membranes, in combination with BMSCs, have no effect on the regenerative capacity of critically sized mandibular defects in rats. The ARRIVE Checklist (https://www.nc3rs.org.uk/arrive-guidelines) and the guidelines of the Animal Research: Reporting In Vivo Experiments were followed in performing this study.
G* Power 3.1.9.2 software was used to statistically compute the sample size of this animal study. An a priori analysis was performed to compute the required sample size, and ANOVA was then performed to test for fixed effects, special effects, main effects, and interactions. The input parameters were an α error probability of 0.05, an effect size f of 0.40, a power of 0.95 and 3 degrees of freedom, as the predictor variables included 3 examination time points and 3 groups. The estimated sample size was 121. Five additional rats were included to allow division of the total number by 3 without a remainder, and consequently, the sample size was 126 (42 rats/group). The mandibular surgical defects were performed bilaterally (21 rats/group); thus, the sample size was 63 rats.
Sixty-three adult, male, pathogen-free Sprague Dawley rats were selected, housed and cared for in standard cages in accordance with the guiding principles of the Faculty of Medicine, Medical Research Centre, Mansoura University, Egypt. The rats were housed at a temperature under 22 °C and at 65%-70% relative humidity. All rats were maintained in a 12-h light and 12-h dark cycle and were fed a regular diet with water. The Research Randomizer software package (https://www.randomizer.org/) was used to randomly assign the rats into 3 equal groups (I, II, and III) of 21 animals each. All experimental steps and protocols were approved by the Ethical Committee of Research Center at Faculty of Dentistry, Mansoura University, Egypt.
Blood samples were collected from the orbital sinus of ten rats under anaesthesia (xylazine + ketamine) through a punctured tube devoid of anticoagulant into 10-mL test tubes, which were rapidly centrifuged (Centrifuge Z 206 A HERMLE Labortechnik GmbH, Germany) for 10 min at 3000 rpm. After centrifugation, three layers formed in the test tube: red blood cells collected at the bottom, a PRF clot formed in the middle and cellular plasma collected at the surface. The PRF clot was easily separated from the tube and then squeezed between two hard objects to transform it into a thin PRF membrane (Figure 1A).
Second passage rat allogeneic BMSCs were purchased and obtained through a cryopreserved sub-cultured primary cell line of 106 cell density from Nile Center for Experimental Researches, Mansoura, Egypt (Figure 1B). After six months of cryopreservation, cells were thawed under a proper aseptic technique, and work was performed in a laminar flow hood. The lower half of the cryovial containing the frozen cells was rapidly thawed for 60 s in a 37 °C water bath. The cryovial was decontaminated by spraying and wiping the exterior of the vial with 70% ethanol. In a biosafety hood, cells were gently resuspended and transferred to a sterile 15-mL conical tube containing 5 mL DMEM with 10% foetal calf serum (FCS), pre-warmed at 37 °C, using a sterile transfer pipette. The BMSCs were centrifuged for 3 min at 200× g, and the supernatant was aspirated without disturbing the cell pellet. The cell pellet was resuspended in fresh, pre-warmed DMEM and transferred to a T25 flask. The flask was gently rocked and incubated in a humidified incubator specified for tissue culture at 37 °C with 5% CO2.
The cell viability was determined by adding 10 μL trypan blue to 10 μL cell suspensions and mixing. Finally, 10 μL of the mixture was placed in a haemocytometer chamber (Cambridge Instruments, Buffalo, NY, United States), and the cell number was determined.
Four million BMSCs were trypsinized and harvested. They were washed and then resuspended in phosphate-buffered saline (PBS) enriched with 3% foetal bovine serum that contained a saturating concentration (1:100) of the six subsequent fluorescein isothiocyanate-conjugated monoclonal antibodies anti-CD14, anti-CD19, anti-CD44, anti-CD45, anti-CD105 and anti-CD90 and one phycoerythrin-conjugated monoclonal antibody, anti-CD34. The cells were incubated against isotype controls in the dark for 30 min at room temperature. Normal rat IgG peridinin chlorophyll protein complex was used as an isotype control to differentiate nonspecific background signals from specific antibody signals. Then, the cells were washed using 2 mL PBS and centrifuged for 5 min at 1500 rpm, and the resulting supernatant was discarded. The cells were suspended in 0.2 mL of 0.5% paraformaldehyde in PBS. Fluorescein activated cell sorting [(FACS) Canto, BD, United States)] was used for acquisition and analysis of CD34 and CD45, and the data were analysed with BD CellQuestTM Pro version 6.0 software (dot plot). A BD Accuri C6 flow cytometer was used for the analysis of CD14, CD19, CD44, CD105 and CD90, and the data were analysed with BD Accuri C6 program software (histogram plot). All these steps were carried out at the Genetic Department, Children’s Hospital, Mansoura University.
The in vitro differentiation of BMSCs toward an osteogenic lineage was induced using an alizarin red assay with an ELISA reader on the 14th day. The osteogenic differentiation potential of BMSCs was assessed using a protocol described by Saeed et al[25].
Using 48-well plates, BMSCs of passage 3 were seeded on PRF pieces at a density of 5 × 103 cells/well. The cells were cultured on the PRF membrane pieces in 200 μL of DMEM with 5% CO2 at 37 °C in a humidified atmosphere for 3 d. The cultures were microscopically observed at this stage.
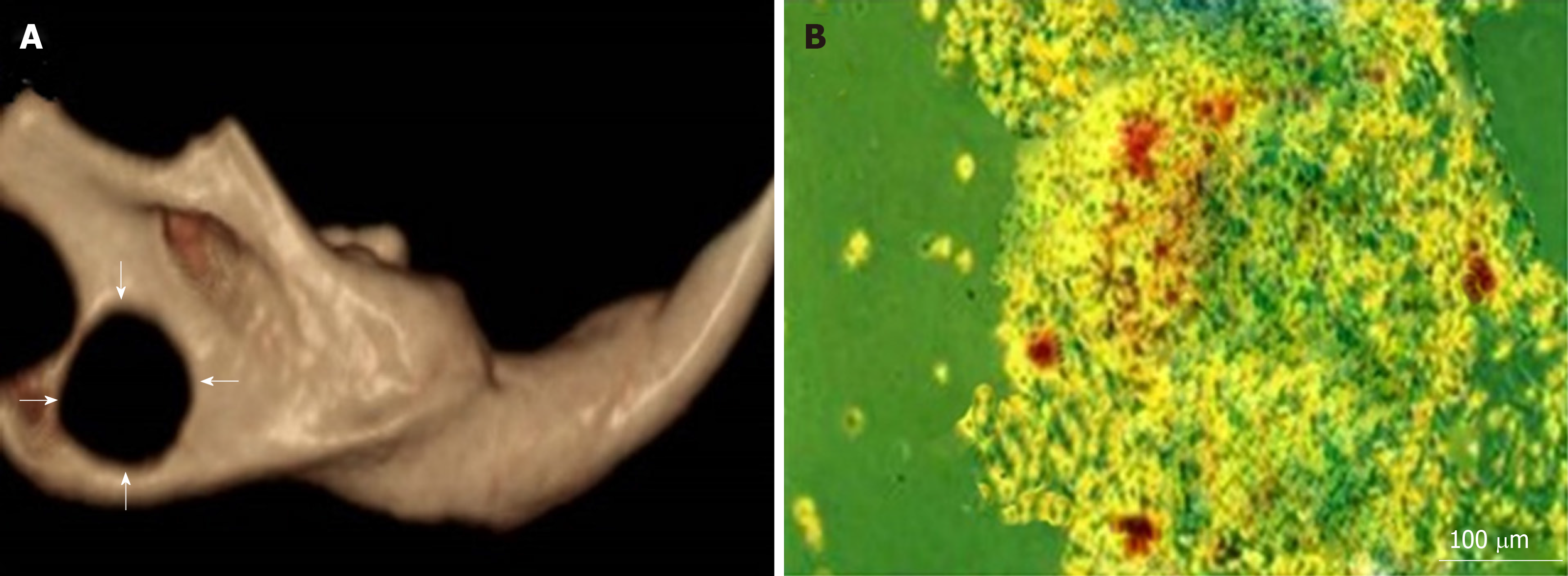
Critical-sized bone defects were created in accordance with the method reported by Zhang et al[26]. Briefly, all rats were anaesthetized by an intraperitoneal injection of 75 mg/kg body weight ketamine and 25 mg/kg body weight xylazine. The operative areas of all animals were shaved, and the skin covering these regions was scrubbed thoroughly with povidone-iodine (Betadine). A 1-cm incision above and parallel with the mandible was made using Bard-Parker No. 15 blade. The lower boundary of the mandible was exposed by blunt dissection. A bone defect of critical size with a depth of 2 mm was produced using a 5 mm diameter trephine bur, contiguous with the inferior border of the mandible and posterior to the incisor (Figure 2A).
In group I, defects were irrigated with saline solution and left empty to serve as controls. Group II defects were grafted with PRF membrane by which the incubated PRF membrane was sliced into 5 mm pieces and then carefully placed into the defects using sterilized scissors. Group III defects were grafted with PRF membrane seeded with BMSCs. The previously isolated and cultured BMSCs were seeded on PRF membrane that was sliced into 5 mm segments and placed into the defects. The surgical area was washed with normal saline, and the two edges of the skin were sutured using 3/0 silk mounted on a 3/8 half-circle needle. Seven rats from each group were euthanized at time points of 1, 2 and 4 wk.
The mandibles were removed, fixed in buffered formalin for 4 h, decalcified in ethylenediaminetetraacetic acid solution and embedded in paraffin. Serial sections were cut at 4 µm thickness. Sections of specimens were processed for routine haematoxylin and eosin (HE) staining, Masson’s trichrome staining (for revealing collagen fibres and newly formed bone), and CD68 immunohistochemical staining to detect the number of inflammatory cell infiltrates.
Slides stained with Masson’s trichrome were investigated using an Olympus microscope with a 1/2 photo adaptor. Digital images were captured by a ToupView digital camera with an objective lens for a magnification of ×4. Seven images with 300 dpi resolution from each group at each time point were digitally analysed with Fiji Image processing software (https://fiji.sc/). The parameters assessed were the total tissue area, including unmineralized bone or osteoid area (OA), and granulation tissue (GT)[27]. To count the number of inflammatory cell infiltrates, VideoTest Morphology® software (Russia) on an Intel® Core I3® based computer was used for staining quantification and area measurements of the resultant immunostained images.
Statistical Package for the Social Sciences, version 21.0 (SPSS, IBM Corp., Armonk, NY, United States) was used for statistical evaluation of the tabulated raw data. Normality of the distribution was evaluated using the Shapiro–Wilk statistical test, and homogeneity of variance was tested using Levene’s test. OA, GT, and the number of inflammatory cell infiltrates were calculated as descriptive values. Two-way ANOVA was used to determine significant differences between the different groups, followed by Tukey's post hoc statistical test. Student’s t-test was used to determine significant differences between the two groups. Mean differences were considered statistically significant at P < 0.05.
Rats generally recovered quickly within 2 d after surgery and returned to their routine activities, such as grooming, eating and drinking. The rats showed normal chewing efficiency without any weight loss or postoperative complications at the three experimental time points.
Mineralized nodules were formed and stained with alizarin red within all wells (Figure 2B).
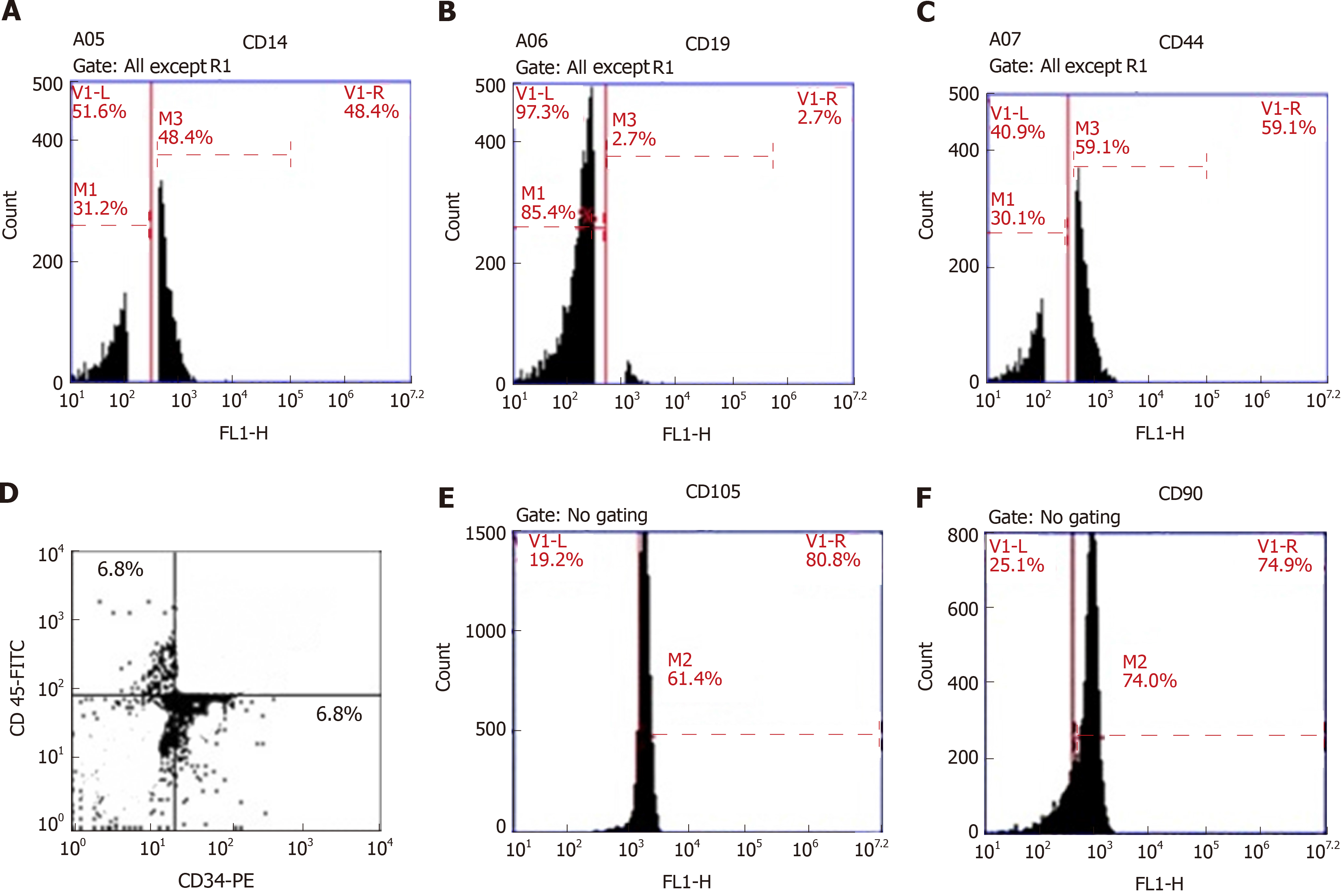
The results of flow cytometric analysis revealed that surface markers of the BMSCs were strongly negative for CD34 (6.8%), CD45 (6.8%) and CD19 (2.7%) and moderately negative for CD14 (48.4%), while their surface markers were positive for CD44 (59.1%), CD105 (61.4%) and CD90 (74%) (Figure 3), confirming the immunophenotypic profile of the BMSCs and the adequate collection and isolation of these cells from bone marrow samples.
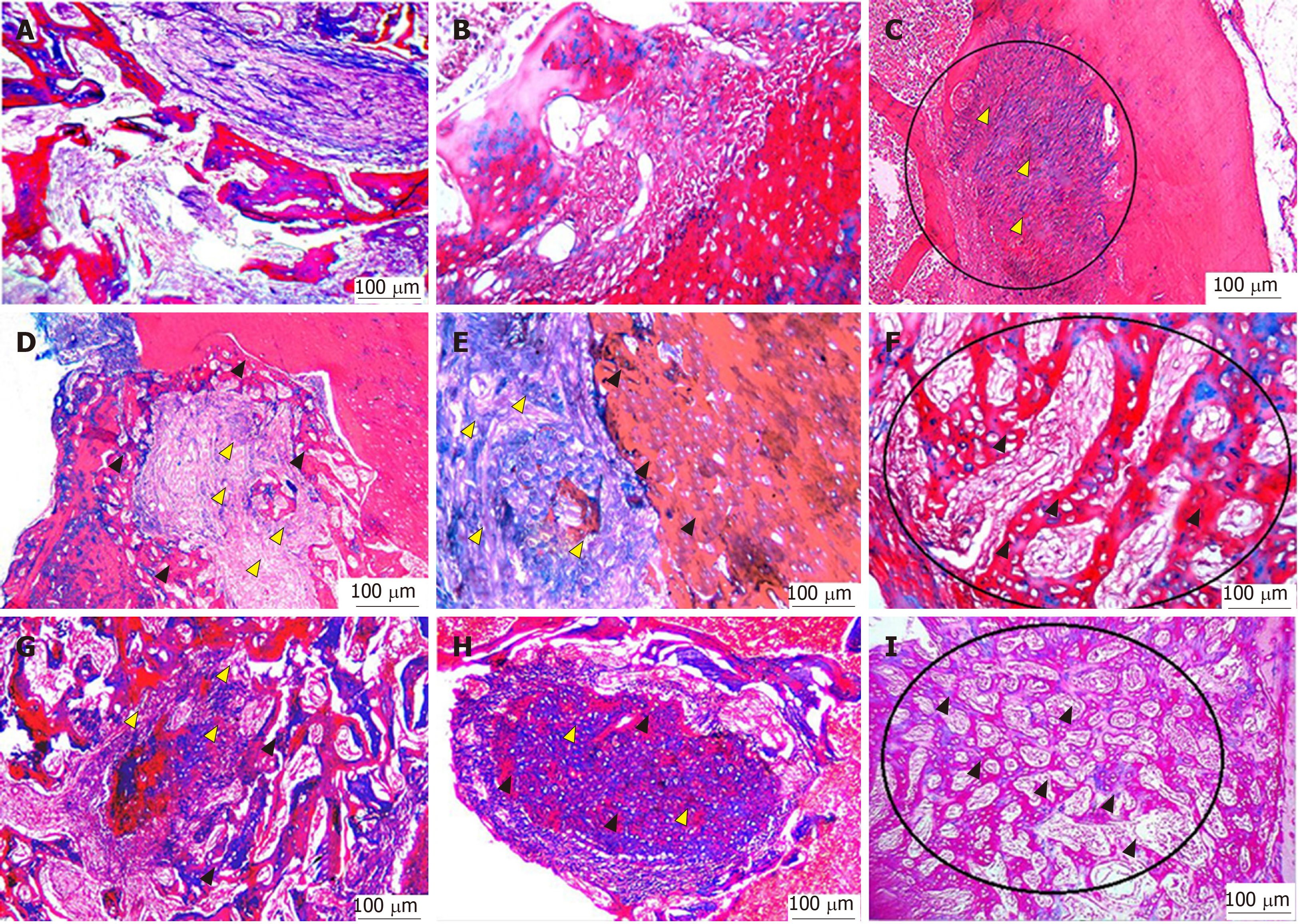
One week postoperatively, group I empty bone defects that were irrigated only with normal saline showed loose connective tissue containing many inflammatory cells and debris of bone spicules. At 2 wk postoperatively, fibroblast-like cells were increased in number, and the connective tissue became more organized concomitant with a decrease in the number of inflammatory cells. Four weeks postoperatively, the majority of the area of the bone defects remained free of bone and was filled only with dense connective tissue that contained fewer cells and more collagen fibres with a very minute amount of newly formed bone limited to the borders of the defects. After the first postoperative week, the bone defects of group II were filled with connective tissue of high vascularity consisting of proliferating fibroblasts, newly formed capillaries and a residual of inflammatory cells. In addition, thin layers of osteoid were formed at the edges of the defects. At the second postoperative week, newly formed thin projections of interconnected trabecular bone were formed and extended from the lateral walls to the central regions of the defects. The GT at the edges of the defects was markedly decreased. At the fourth postoperative week, the defects showed thin osteoid bone trabeculae oriented perpendicular to the old bone and radiated to the centre of the defects with wide bone marrow spaces. In group III, the bone defects after the first postoperative week showed newly formed thin bone trabeculae lined by osteoblasts at the borders of the defects and osteoid tissue formation intermingled with GT with inflammatory cell infiltration in the central area. At 2 wk postoperatively, there was an increased number of more organized bone trabeculae with narrow bone marrow spaces. At 4 wk postoperatively, the borders of the bone defects became indistinct and were difficult to differentiate from the original surrounding bone (Figure 4).
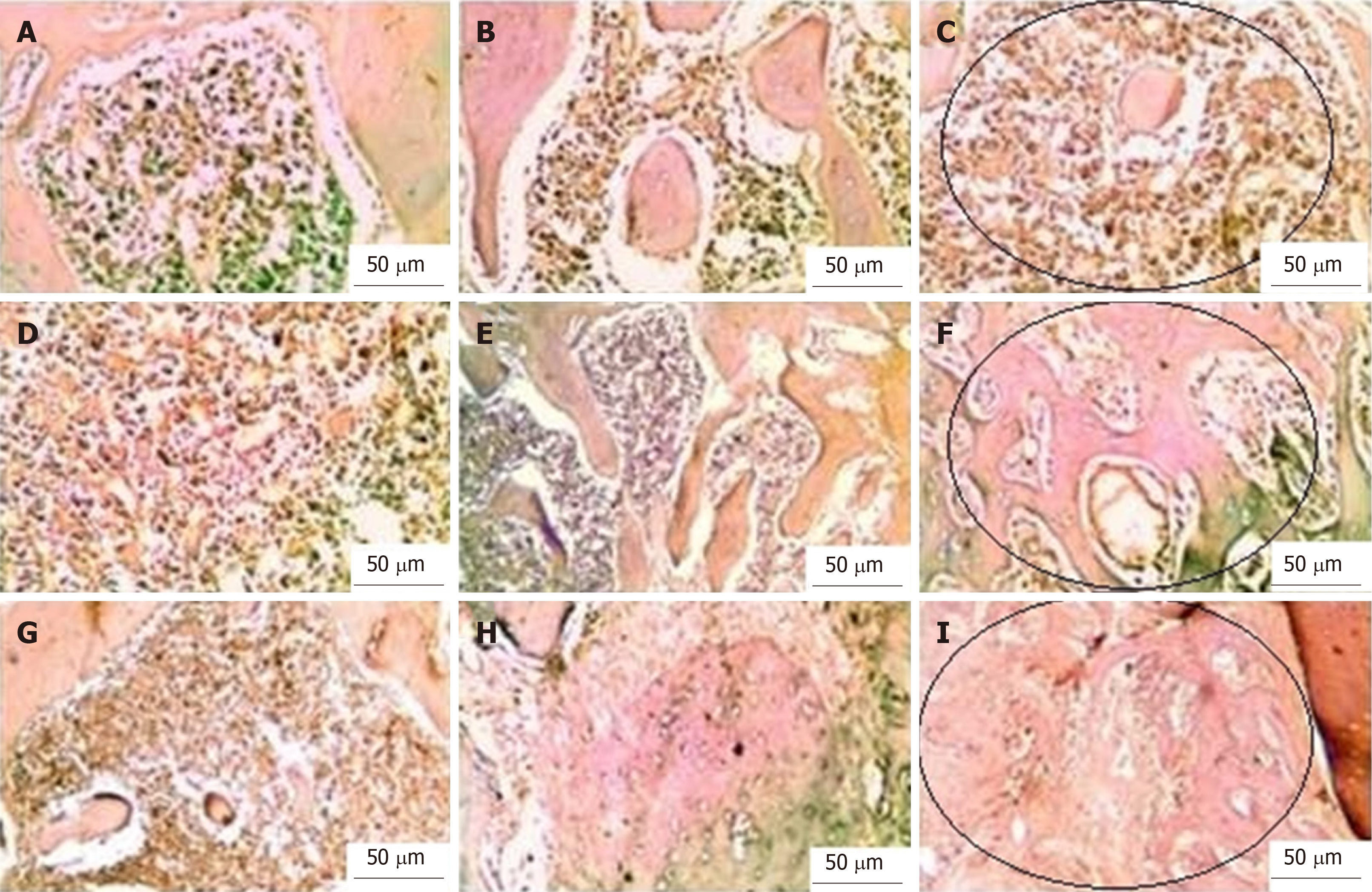
With Masson’s trichrome staining, the cytoplasm and osteoids are coloured red, collagen and mineralized bone are coloured blue, and nuclei are stained black to dark brown. Four weeks postoperatively, the group I bone defects showed a small amount of loose irregular connective tissue without new bone formation throughout the whole defect (Figure 5A-C). Engrafting the defects with PRF membranes in the defects of group II encouraged the formation of bony projections that enlarged to form thin bone tissue strips in the central area of the bony defect. These centrally located bony islands were enclosed by dense connective tissue of blue colour. The bone defects revealed intimate integration of newly formed osteoid tissue with the old mature lamellar bone, characterized by more red-stained newly formed osteoid tissue and deep blue-stained mineralized areas (Figure 5D-F). A marked increase in the amount of new bone formation was observed after the fourth postoperative week in group III, which was treated with both PRF membrane and BMSCs. The development of high-quality lamellar mature bone was clearly observed at 4 wk postoperatively, and the defect was ultimately occupied with bone (Figure 5G-I).
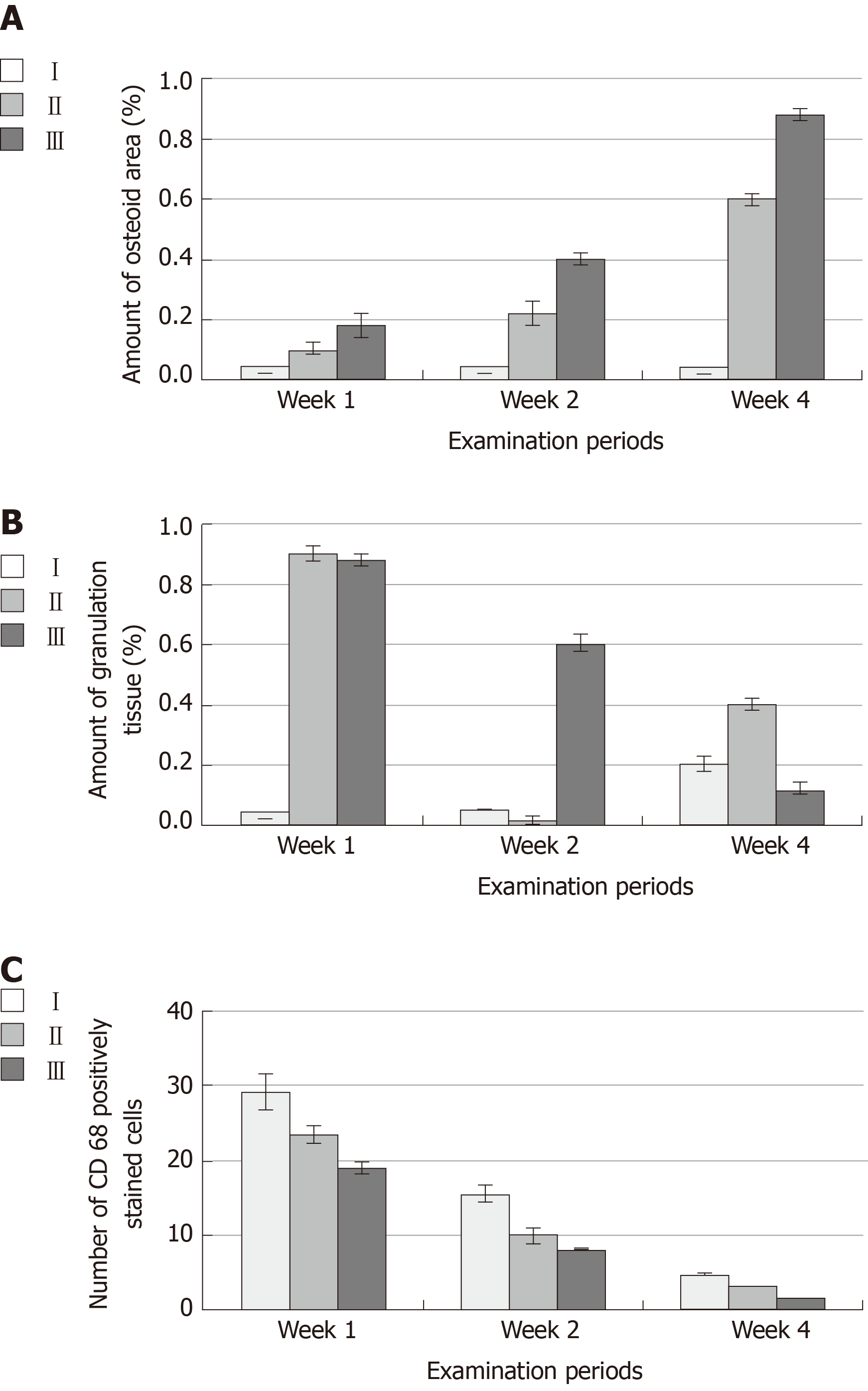
Two-way ANOVA of the mean values of OA (Figure 6) and the amount of GT (Figure 6) revealed significant differences between different time points (P < 0.05) and different groups (P < 0.05) and a significant interaction of time and group (P < 0.05). After weeks 1, 2, and 4, group III exhibited the highest mean values of OA (0.18 ± 0.04, 0.40 ± 0.02 and 0.88 ± 0.02, respectively), whereas group I exhibited the lowest mean values at the same time points (0.01 ± 0.00, 0.02 ± 0.00 and 0.02 ± 0.00, respectively). The mean values of OA in group II after weeks 1, 2, and 4 were 0.10 ± 0.02, 0.22 ± 0.04 and 0.60 ± 0.02, respectively (Table 1). After weeks 1, 2 and 4, the mean values of the amount of GT in group III were 0.88 ± 0.02, 0.60 ± 0.03 and 0.12 ± 0.02, respectively, whereas mean values of GT in group I after the same time points were 0.03 ± 0.00, 0.05 ± 0.00 and 0.20 ± 0.02, respectively. The mean values of GT after weeks 1, 2 and 4 in group II were 0.90 ± 0.03 and 0. 88 ± 0.03 and 0.40 ± 0.02, respectively (Table 2). Tukey's post hoc test revealed significant differences in OA between groups I and II, groups I and III and groups II and III at each examination time point. The statistical test revealed significant differences in the amount of GT between groups I and II, groups I and III and groups II and III at 2 and 4 wk. At the 1-wk examination, no significant difference was found between groups II and III, whereas significant differences were found between groups I and II and groups I and III. Student’s t-test revealed significant differences in the mean OA between 1 and 2 wk, 1 and 4 wk and 2 and 4 wk for group II and group III but no significant differences between these time points for group I. Significant differences were found in the amount of GT between 1 and 4 wk and 2 and 4 wk for groups I, II and III. No significant differences were found between 1 and 2 wk for groups I and II, while a significant difference was found for group III (Tables 1 and 2).
| Groups | mean ± SD | Student t-test (P value) | Two-way ANOVA (P value) | ||||
| Week 1 | Week 2 | Week 4 | 1 × 2 | 1 × 4 | 2 × 4 | ||
| I | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.05 |
| II | 0.10 ± 0.02 | 0.22 ± 0.04 | 0.60 ± 0.02 | P < 0.05 | P < 0.05 | P < 0.05 | |
| III | 0.18 ± 0.04 | 0.40 ± 0.02 | 0.88 ± 0.02 | P < 0.05 | P < 0.05 | P < 0.05 | |
| Tukey's test (P value) | |||||||
| I × II | P < 0.05 | P < 0.05 | P < 0.05 | ||||
| I × III | P < 0.05 | P < 0.05 | P < 0.05 | ||||
| II ×III | P < 0.05 | P < 0.05 | P < 0.05 | ||||
| Groups | mean ± SD | Student t-test (P value) | Two-way ANOVA (P value) | ||||
| Week 1 | Week 2 | Week 4 | 1 × 2 | 1 × 4 | 2 × 4 | ||
| I | 0.03 ± 0.00 | 0.05 ± 0.00 | 0.20 ± 0.02 | P > 0.05 | P < 0.05 | P < 0.05 | P < 0.05 |
| II | 0.90 ± 0.03 | 0. 88 ± 0.03 | 0.40 ± 0.02 | P > 0.05 | P < 0.05 | P < 0.05 | |
| III | 0.88 ± 0.02 | 0.60 ± 0.03 | 0.12 ± 0.02 | P < 0.05 | P < 0.05 | P < 0.05 | |
| Tukey's test (P value) | |||||||
| I × II | P < 0.05 | P < 0.05 | P < 0.05 | ||||
| I × III | P < 0.05 | P < 0.05 | P < 0.05 | ||||
| II x× III | P > 0.05 | P < 0.05 | P < 0.05 | ||||
According to CD68 immunostaining, the bone defects of group I at the 4th week showed intense positive immune reactivity to CD68. The GT of group II at the 4th week showed moderate immune reactivity to the CD68 antibody. The bone defects of group III at the 4th week showed negativity for the immune reaction after the formation of large bone trabeculae. Two-way ANOVA of the number of inflammatory cell infiltrates (Figure 6) revealed a significant difference between different time points (P < 0.05) and groups (P < 0.05) and a significant interaction of time and group (P < 0.05). The highest mean values of CD68-immunostained inflammatory cells were found in group I, which corresponded to 29.22 ± 2.53, 15.62 ± 1.09 and 4.82 ± 0.08 at weeks 1, 2, and 4, respectively. The lowest mean values of CD68-immunostained inflammatory cells were found in group III, which were 19.04 ± 0.95, 8.13 ± 0.13, and 0.29 ±0.03 at weeks 1, 2, and 4, respectively. The mean values in group II were 23.57 ± 1.08, 9.92 ± 1.07 and 3.09 ± 0.07 at weeks 1, 2, and 4, respectively (Table 3, Figure 7). Tukey's post hoc test revealed significant differences in CD68-immunostained inflammatory cells between groups I and II, groups I and III and groups II and III at each examination time point. Student’s t-test for the mean values of CD68 immunostained inflammatory cells revealed significant differences between 1 and 2 wk, 1 and 4 wk and 2 and 4 wk for each group (Table 3).
| Groups | mean ± SD | Student t-test (P value) | Two-way ANOVA (P value) | ||||
| Week 1 | Week 2 | Week 4 | 1 × 2 | 1 × 4 | 2 × 4 | ||
| I | 29.22 ± 2.53 | 15.62 ± 1.09 | 4.82 ± 0.08 | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 |
| II | 23.57 ± 1.08 | 9.92 ± 1.07 | 3.09 ± 0.07 | P < 0.05 | P < 0.05 | P < 0.05 | |
| III | 19.04 ± 0.95 | 8.13 ± 0.13 | 0.29 ±0.03 | P < 0.05 | P < 0.05 | P < 0.05 | |
| Tukey's test (P value) | |||||||
| I × II | P < 0.05 | P < 0.05 | P < 0.05 | ||||
| I × III | P < 0.05 | P < 0.05 | P < 0.05 | ||||
| II × III | P < 0.05 | P < 0.05 | P < 0.05 | ||||
Bone healing depends on the coordinated action of several cell types and a cascade of biological events. Bone healing is an extremely complex process and has been considered a major medical concern[28]. The current study evaluated the efficacy of BMSCs seeded on PRF membrane in comparison with PRF membrane alone for the treatment of critically sized mandibular defects in a rat model. The HE staining results revealed that the bone defects of group II grafted with PRF membranes exhibited faster GT formation and more newly formed osteoid tissue than the bone defects of group I that were irrigated with normal saline. These results are in accordance with those of He et al[29], who reported that PRF membranes released autologous and multiple growth factors that gradually induced a more durable and stronger effect on the differentiation and proliferation of rat osteoblasts. Usage of the PRF membrane appears to be a highly favourable approach for improving bone healing in a manageable and reasonably long-term effect.
The slow and natural polymerization that occurs during centrifugation of PRF leads to the development of a fibrin network with a consistent 3-dimensional pattern. Massive platelet activation occurs as a consequence of the absence of anticoagulant in the test tube. The structural configuration of PRF with progressive polymerization significantly increases the incorporation of circulating intrinsic cytokines into the fibrin meshes. This configuration implies an increase in the lifespan for these cytokines, as they will be released and used only at the time of initial cicatricial remodelling[30].
The present study reports that group III, which was treated with a combination of BMSCs and PRF membrane, exhibited faster healing of bone defects than group II, and thus, this combination could be used to repair alveolar bone defects without the need for exogenous scaffolds or additional growth factors. These results are in accordance with those of Chen et al[31], who reported that PRF membrane stimulates the proliferation of BMSCs and improves osteogenic capacity in vivo and in vitro more than PRF membrane alone. In addition, Gassling et al[32] evaluated the use of PRF membranes as scaffolds for periosteal tissue engineering and compared the in vitro biocompatibility and effects of both PRF membranes and collagen membranes on the proliferation of periosteal stem cells. They found that the PRF membrane is preferable to collagen as a scaffold material for human periosteal cell proliferation and is a suitable candidate for the in vitro cultivation of periosteal cells for engineering bony tissues.
The seeding of BMSCs on PRF membranes for topical engraftments in bone defects was performed in accordance with the report of Knapen et al[33], who found that the PRF membrane has no limited effect on the quantity, quality and kinetics of bone regeneration. This result could be attributed to the early engraftment of the PRF membrane at the time of surgery, when neither osteoblast precursors nor connective-vascularized tissue are yet available on site. In addition, the use of autologous PRF membranes seeded with BMSCs in alveolar bone defects is beneficial for organizing formative cells (especially osteoblasts) and promoting neovascularization with more rapid and faster apposition of bone matrix[34].
These results were supported by the increase in the amount of trabecular bone with more extended trabecular width and cortical width and the greater number of osteoblasts, osteocytes and blood vessels in the bone defects in group III than in group I and group II. These results were in accordance with Simonpieri et al[35], who explained that the PRF membrane can integrate with the fibrin network and facilitate cellular migration and angiogenesis.
The statistical analysis of the histomorphometric results, namely, the amount of GT and osteoid tissue formation, revealed that the use of PRF membranes seeded with BMSCs not only enhanced new bone formation but also decreased the amount of GT formation. These results are in accordance with those of Yuanzheng et al[22], who used a combination of PRF membranes and BMSCs to enhance osteointegration of autologous iliac bone grafts in dogs and reported that complete healing was achieved according to histologic and histomorphometric analysis of the specimens.
In the current work, the statistical results of the immunohistochemical analysis of the presence of macrophages in bony defects suggested that the bone defects of group I showed the highest degree of early macrophage presence in the first week, compared with the other two groups. The obvious presence of macrophages in group III decreased markedly by the end of the 4th week, while the other groups showed higher levels of macrophages. Our results were in accordance with those of Andrew et al[36], who reported that macrophages present in wound sites increase during the early differentiation of osteoblasts and decrease during bone formation. Macrophages are important angiogenic effector cells that produce a number of growth inhibitors, stimulators and proteolytic enzymes that have the capacity to modulate new vessel formation[37].
Compared with the results of a study performed by Alge et al[38], flow cytometric analysis of the surface markers used in the present study showed a higher profile in the negativity of CD34 and CD45 and a lower profile in the positivity of CD14, CD19, CD44, CD105, and CD90. These features may be attributed to the differences in medium composition, cell seeding density, and oxygen partial pressure, as these conditions influence cell phenotype.
Within the limitations of the current animal model and the present findings, we reject the null hypothesis and conclude that bone regenerative quality was better promoted with the use of PRF membranes seeded with BMSCs than with PRF membranes alone in critically sized bone defects in rats. However, further long-term and large-scale in vivo studies are necessary to verify our results in terms of determining the most suitable method for PRF membrane application and the adequate number of BMSCs for treating critical-sized mandibular defects. Moreover, more in-depth studies are needed to identify how the presence of BMSCs contributes to more effective bone regeneration in the presence of PRF and whether BMSCs improve growth factor release from the PRF membrane or excrete other factors that synergistically promote bone regeneration.
Regeneration of critical-sized bone defects remains a major clinical problem in the field of orthopaedic surgery, and therefore, novel treatment methods must be developed. Currently, the management of such defects mainly depends on the use of autologous bone grafts. However, complications such as donor site morbidity drive us to find other lines of treatments.
To investigate whether allogenic bone marrow-derived stem cells (BMSCs) seeded on platelet-rich fibrin (PRF) membranes have the ability to regenerate critical-sized mandibular defects in rats and, therefore, whether this combination therapy is a suitable approach for developing a new line of treatment for such bony defects.
The objectives of the present study were to create critical-sized mandibular defects, to seed BMSCs on PRF membranes, to fill these defects with the combination therapy, and finally, to assess the possible regenerative effect of PRF membranes with or without allogeneic BMSCs on such bony defects in rat models.
We induced critically sized defects and treated these defects with a combined therapy, followed by performing histological and immunohistochemical analyses. The data of the histomorphometric analysis were statistically analysed.
The percentage area of newly formed bone was significantly higher in the defects treated with the combined therapy than in the defects treated with the PRF membrane alone and untreated defects. However, the amount of granulation tissue formation and the number of inflammatory cells were lower in the defects treated with the combined therapy than in the defects treated with the PRF membrane alone.
The combined therapy of BMSCs and PRF membrane showed a regenerative effect in critically sized bone defects and may represent a potential therapeutic alternative for bone regeneration.
Based on our results, we believe that BMSCs seeded on platelet-rich plasma could be clinically applied for treating critically sized bone defects and promoting wound regeneration in the future.
Manuscript source: Invited Manuscript.
Specialty type: Cell and tissue engineering
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goebel WS, Jun VM, Miloso M, Li SC S-Editor: Ma YJ L-Editor: A E-Editor: Ma YJ
| 1. | Morgan SJ, Elangbam CS, Berens S, Janovitz E, Vitsky A, Zabka T, Conour L. Use of animal models of human disease for the nonclinical safety assessment of novel pharmaceuticals. Toxicol Pathol. 2013;41:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Vandamme TF. Use of rodents as models of human diseases. J Pharm Bioallied Sci. 2014;6:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Shah SR, Young S, Goldman JL, Jansen JA, Wong ME, Mikos AG. A composite critical-size rabbit mandibular defect for evaluation of craniofacial tissue regeneration. Nat Protoc. 2016;11:1989-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Petrovic V, Zivkovic P, Petrovic D, Stefanovic V. Craniofacial bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e1-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Khaled EG, Saleh M, Hindocha S, Griffin M, Khan WS. Tissue engineering for bone production- stem cells, gene therapy, and scaffolds. Open Orthop J. 2011;5 Suppl 2:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Wang X, Xing H, Zhang G, Wu X, Zou X, Feng L, Wang D, Li M, Zhao J, Du J, Lv Y, E L, Liu H. Restoration of a Critical Mandibular Bone Defect Using Human Alveolar Bone-Derived Stem Cells and Porous Nano-HA/Collagen/PLA Scaffold. Stem Cells Int. 2016;2016:8741641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 449] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 8. | Daif ET. Effect of autologous platelet-rich plasma on bone regeneration in mandibular fractures. Dent Traumatol. 2013;29:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Lee HR, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J Control Release. 2012;159:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Verma UP, Yadav RK, Dixit M, Gupta A. Platelet-rich Fibrin: A Paradigm in Periodontal Therapy - A Systematic Review. J Int Soc Prev Community Dent. 2017;7:227-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 11. | Oryan A, Kamali A, Moshiri A, Baghaban Eslaminejad M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs. 2017;204:59-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (1)] |
| 12. | Chen JP, Chang YS. Preparation and characterization of composite nanofibers of polycaprolactone and nanohydroxyapatite for osteogenic differentiation of mesenchymal stem cells. Colloids Surf B Biointerfaces. 2011;86:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | De Witte TM, Fratila-Apachitei LE, Zadpoor AA, Peppas NA. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater. 2018;5:197-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 14. | Jin YZ, Lee JH. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin Orthop Surg. 2018;10:271-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, El Shewy MT, Azzam A, Abdel Aziz MT. The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage. 2010;1:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: Macroscopic and histological assessments. Bone Joint Res. 2017;6:98-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Hsu YK, Sheu SY, Wang CY, Chuang MH, Chung PC, Luo YS, Huang JJ, Ohashi F, Akiyoshi H, Kuo TF. The effect of adipose-derived mesenchymal stem cells and chondrocytes with platelet-rich fibrin releasates augmentation by intra-articular injection on acute osteochondral defects in a rabbit model. Knee. 2018;25:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Xu F, Yang Y, Yang T, Dai T, Shao X, Xu H, An R, Liu Y, Liu B. The use of allogenic adipose-derived stem cells in combination with platelet-rich fibrin for the treatment of cartilage defects in rabbit ear. Am J Transl Res. 2018;10:1900-1907. [PubMed] |
| 19. | Liao HT, Chen CT, Chen CH, Chen JP, Tsai JC. Combination of guided osteogenesis with autologous platelet-rich fibrin glue and mesenchymal stem cell for mandibular reconstruction. J Trauma. 2011;70:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Maĭborodin IV, Matveeva VA, Kolesnikov IS, Drovosekov MN, Toder MS, Shevela AI. [Regeneration of the damaged mandibular bone in rat after the injection of autologous mesenchymal stem cells of bone marrow origin adsorbed on the fibrin clot]. Morfologiia. 2011;140:79-85. [PubMed] |
| 21. | Zhou C, Li S, Wenqiguli N, Yu L, Zhao L, Wu P, Nijiati T. [The expressions of the Notch and Wnt signaling pathways and their significance in the repair process of alveolar bone defects in rabbits with bone marrow stem cells compounded with platelet-rich fibrin]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2016;34:130-135. [PubMed] |
| 22. | Yuanzheng C, Yan G, Ting L, Yanjie F, Peng W, Nan B. Enhancement of the repair of dog alveolar cleft by an autologous iliac bone, bone marrow-derived mesenchymal stem cell, and platelet-rich fibrin mixture. Plast Reconstr Surg. 2015;135:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Rady D, Mubarak R, Abdel Moneim RA. Healing capacity of bone marrow mesenchymal stem cells versus platelet-rich fibrin in tibial bone defects of albino rats: an in vivo study. F1000Res. 2018;7:1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Nugraha AP, Narmada IB, Ernawati DS, Dinaryanti A, Hendrianto E, Riawan W, Rantam FA. Bone alkaline phosphatase and osteocalcin expression of rat's Gingival mesenchymal stem cells cultured in platelet-rich fibrin for bone remodeling (in vitro study). Eur J Dent. 2018;12:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Saeed MA, El-Rahman MA, Helal ME, Zaher AR, Grawish ME. Efficacy of Human Platelet Rich Fibrin Exudate vs Fetal Bovine Serum on Proliferation and Differentiation of Dental Pulp Stem Cells. Int J Stem Cells. 2017;10:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Feng Z, Wei J, Yu Y, Luo J, Zhou J, Li Y, Zheng X, Tang W, Liu L, Long J, Li X, Jing W. Repair of Critical-Sized Mandible Defects in Aged Rat Using Hypoxia Preconditioned BMSCs with Up-regulation of Hif-1α. Int J Biol Sci. 2018;14:449-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Egan KP, Brennan TA, Pignolo RJ. Bone histomorphometry using free and commonly available software. Histopathology. 2012;61:1168-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Pountos I, Georgouli T, Blokhuis TJ, Pape HC, Giannoudis PV. Pharmacological agents and impairment of fracture healing: what is the evidence? Injury. 2008;39:384-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 345] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 30. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 692] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 31. | Chen B, Sun HH, Wang HG, Kong H, Chen FM, Yu Q. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials. 2012;33:5023-5035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Gassling V, Douglas T, Warnke PH, Açil Y, Wiltfang J, Becker ST. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Knapen M, Gheldof D, Drion P, Layrolle P, Rompen E, Lambert F. Effect of leukocyte- and platelet-rich fibrin (L-PRF) on bone regeneration: a study in rabbits. Clin Implant Dent Relat Res. 2015;17 Suppl 1:e143-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Stübinger S, Dard M. The rabbit as experimental model for research in implant dentistry and related tissue regeneration. J Invest Surg. 2013;26:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Simonpieri A, Del Corso M, Sammartino G, Dohan Ehrenfest DM. The relevance of Choukroun's platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part I: a new grafting protocol. Implant Dent. 2009;18:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Andrew JG, Andrew SM, Freemont AJ, Marsh DR. Inflammatory cells in normal human fracture healing. Acta Orthop Scand. 1994;65:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Polverini PJ. Role of the macrophage in angiogenesis-dependent diseases. EXS. 1997;79:11-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, Gabriel Chu TM, Goebel WS. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |