Copyright
©The Author(s) 2017.
World J Stem Cells. Aug 26, 2017; 9(8): 118-126
Published online Aug 26, 2017. doi: 10.4252/wjsc.v9.i8.118
Published online Aug 26, 2017. doi: 10.4252/wjsc.v9.i8.118
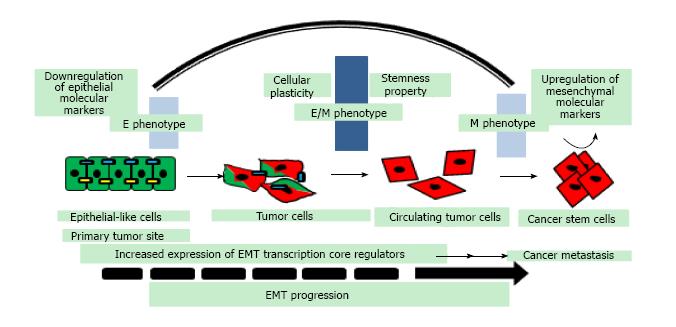
Figure 1 Epithelial-mesenchymal transition progression in epithelial cancer cells: Cancer cells with E phenotype exhibit epithelial-mesenchymal transition at primary tumor site, loose cell-cell contacts, gain migratory abilities, undergo morphological change and acquire M phenotype.
Co-expression of epithelial and mesenchymal marker proteins in cancer cells with partial E/M hybrid phenotype is associated with increased cellular plasticity and stemness. Cancer stem cells with hybrid E/M phenotype undergoing partial EMT and not complete EMT gain self-renewability, migratory and invasive traits during cancer metastasis. EMT: Epithelial-mesenchymal transition; E: Epithelial; M: Mesenchymal.
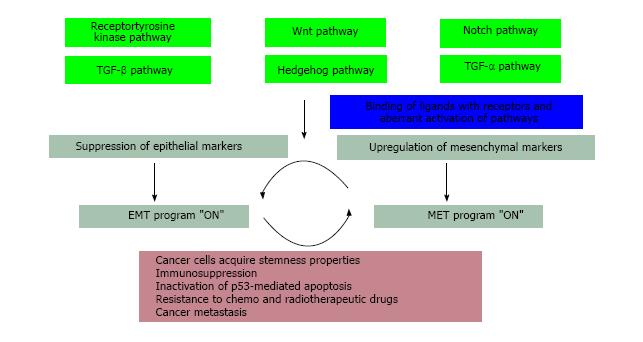
Figure 2 Signaling pathways regulating epithelial-mesenchymal transition and mesenchymal-epithelial transition: Aberrant activation of signaling pathways including Notch, Wnt, Hedgehog, receptor tyrosine kinase, Transforming growth factor-beta, tumor necrosis factor-alpha regulate the expression of epithelial-mesenchymal transition-activating transcription factors.
EMT-ATFs induce EMT by repressing and activating the expression of epithelial and mesenchymal genes respectively. Epithelial plasticity confers long term survival advantages to the disseminated cancer stem cells at distant sites, makes them resistant to conventional therapies and allows the cancer to relapse. EMT: Epithelial-mesenchymal transition; MET: Mesenchymal-epithelial transition; TGF-β: Transforming growth factor-beta; TNF-α: Tumor necrosis factor-alpha; EMT-ATFs: EMT-activating transcription factors.
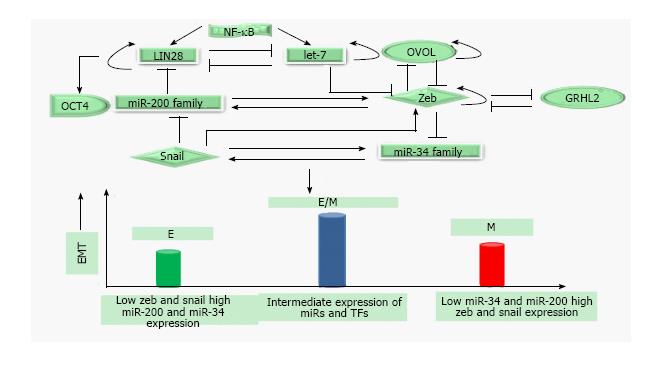
Figure 3 Epithelial-mesenchymal transition regulatory network: Mutually exclusive inhibitory loops including miR-200family/Zeb; miR-34family/Snail; LIN28/let-7 bring about bistable switch between epithelial (E) and mesenchymal (M) phenotypes, control Epithelial-mesenchymal transition/mesenchymal-epithelial transition and stemness.
Phenotypic stability factors like OVOL and GRHL2 couple to core-EMT decision making circuits and stabilize hybrid E/M phenotype. NF-κB controls LIN28/let-7 regulation and elevates the likelihood of hybrid E/M phenotype. Solid arrows represent the activation; solid lines represent the repression and circular loops represent the self-activation. Hybrid E/M: Hybrid epithelial/mesenchymal; NF-κB: Nuclear factor kappa B; miR: MicroRNA; EMT: Epithelial-mesenchymal transition.
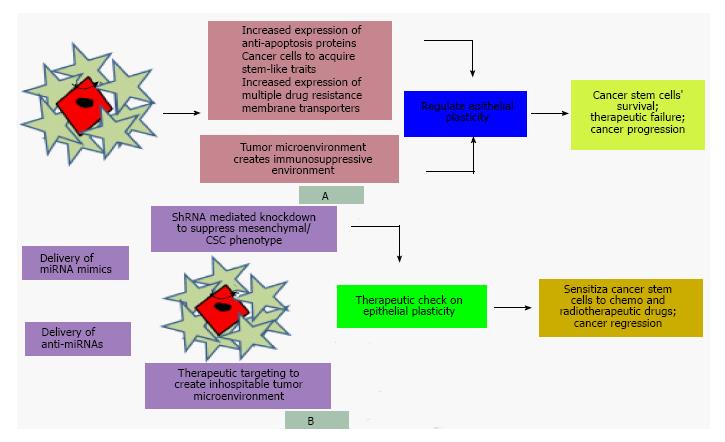
Figure 4 Cancer stem cells, epithelial plasticity and therapeutic strategies.
A: Existence of quiescent CSCs that possess the potential to self-renew, ability to proliferate and aberrantly differentiate into heterogeneous lineages of cancer cells and tumor microenvironment by creating immunosuppressive environment regulate epithelial plasticity and enable CSCs to survive, exhibit resistance to growth inhibitory drugs and cause tumor to progress; B: Therapeutic strategies including delivery of miRNA mimics to enforce the expression of tumor suppressor genes, administration of anti-miRNAs to downregulate the expression of oncogenes, shRNA mediated knockdown of oncogenic factors to revert the mesenchymal/CSC phenotype to epithelial non-CSC phenotype and creating inhospitable tumor microenvironment not only confer therapeutic check on epithelial plasticity but also sensitize cancer stem cell populations to the killing effects of therapeutic drugs. CSC: Cancer stem cell; miRNAs: MicroRNAs; ShRNA: Short hairpin RNA.
- Citation: Garg M. Epithelial plasticity and cancer stem cells: Major mechanisms of cancer pathogenesis and therapy resistance. World J Stem Cells 2017; 9(8): 118-126
- URL: https://www.wjgnet.com/1948-0210/full/v9/i8/118.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i8.118