Copyright
©The Author(s) 2015.
World J Stem Cells. Sep 26, 2015; 7(8): 1090-1108
Published online Sep 26, 2015. doi: 10.4252/wjsc.v7.i8.1090
Published online Sep 26, 2015. doi: 10.4252/wjsc.v7.i8.1090
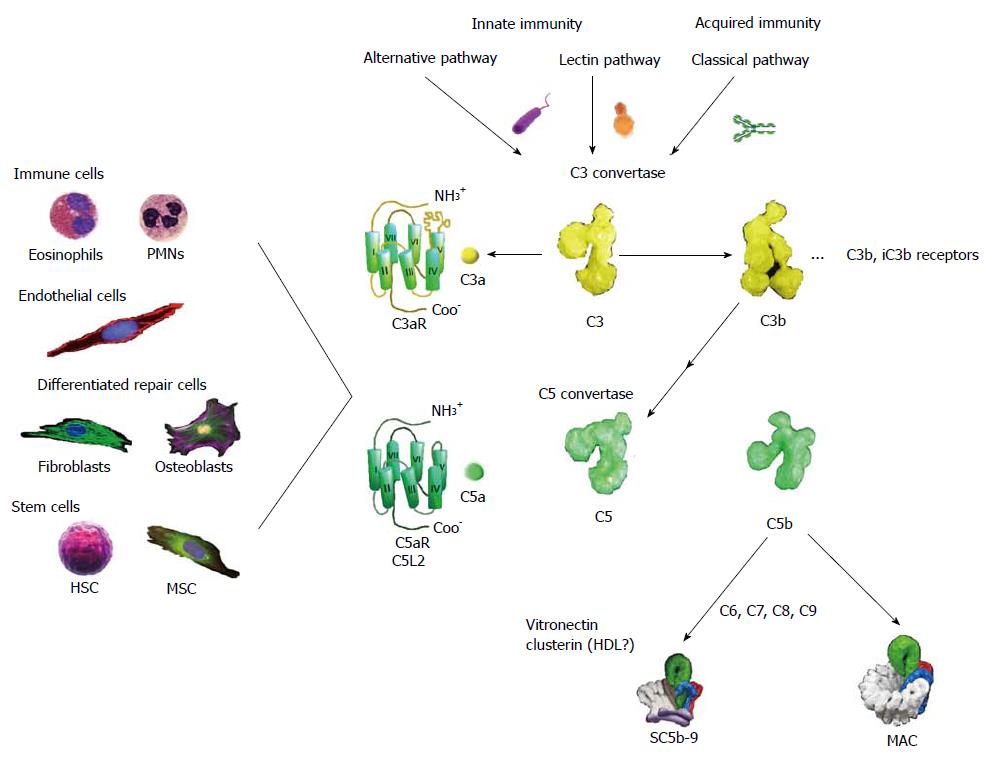
Figure 1 Schematic presentation of the complement pathways with emphasis on outcomes relevant to tissue repair and regeneration.
The complement system can be activated by three pathways, two of which are part of innate immunity, the alternative and lectin pathways, whereas the classical pathway is normally initiated by immunoglobulins. All routes converge on the cleavage by complex enzymes referred to as C3 convertases of component C3 (Mr: about 195000) to C3a (Mr: about 8500) and C3b (Mr: about 185000). As a consequence of C3b deposition on C3 convertase, C5 convertase is created that acts similarly producing from component C5 (Mr: about 196000) the activation peptide C5a (Mr: about 11000) and C5b (Mr: about 185000). The anaphylatoxins, C3a and C5a, are important for elaboration of mechanisms of wound healing and regeneration. These small mediators are recognized by their cognate receptors: C3aR and C5aR/C5L2 that are GPCRs found on a diversity of cells inclusive of immune cells, endothelial cells, differentiated repair cells, and stem cells. In addition C3b and its split product iC3b, C3d are recognized by receptors inclusive of CR1-4 that assist clearance of microorganisms, cellular debris, immune complexes, and apoptotic cells. The ultimate outcome of complement activation is the formation of the MAC that is a transmembrane pore (100 Ǻ) assembly that embeds in target cell membranes. In the absence of proximal phospholipid membranes, the terminal components of complement associate into complexes referred to as SC5b-9. These are probable heterogeneous and contain multiple copies of vitronectin and clusterin (apolipoprotein J). Because vitronectin in an oligomeric state can present the canonical tripeptide, Arg-Gly-Asp, to integrins on a variety of restorative cells, such as fibroblasts and keratinocytes, SC5b-9 may have a wound healing function. MAC: Membrane attack complex; MSC: Mesenchymal stem cells; HDL: High density lipo-protein; HSC: Hematopoietic stem cells; PMNs: Polymorphonuclear cells.
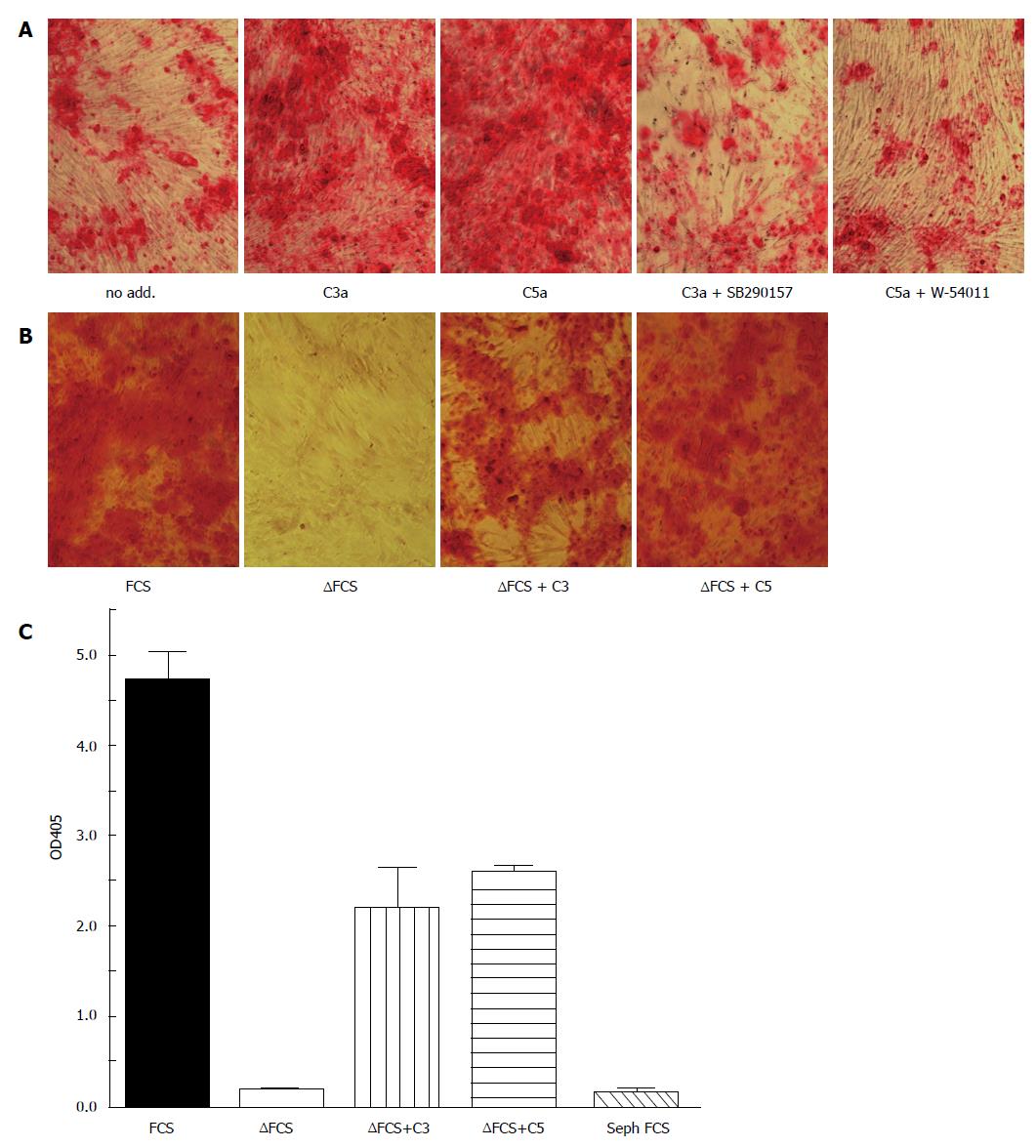
Figure 2 Complement activation accelerates osteogenic differentiation of bone marrow mesenchymal stem cells.
MSC were cultured in osteogenic media (α-MEM containing 16.5% FCS, not heat inactivated, 10 nmol/L dexamethasone, 20 mmol/L β-glycerolphosphate, and 50 μmol/L ascorbic acid 2-phosphate) for the indicated time. Osteogenesis was detected by alizarin red S staining. A: All cells were cultured in the presence of the carboxypeptidase inhibitor 2-mercaptomethyl-3-guanidinoethylthioproprionic acid (80 nmol/L) to maintain C3a and C5a activity. C3a (100 nmol/L) or C5a (10 nmol/L) were added during the first 3 d of cultures in the presence or absence of the C3aR inhibitor SB290157 (1 nmol/L) or the C5aR inhibitor W-54001 (1 nmol/L). Both C3a and C5a accelerated calcification in a C3aR and C5aR specific fashion as detected by Alizarin red staining on day 14; B: Osteogenesis was considerably delayed in heat-inactivated FCS (FCS), in which complement components are inactivated. Addition of either C3 or C5 partially reconstituted the effect of FCS. Alizarin staining on day 21; C: Quantitation of the alizarin staining of figure 2B following solubilizing in acid SDS solution. FCS: Fetal calf serum; MSC: Mesenchymal stem cells.
- Citation: Schraufstatter IU, Khaldoyanidi SK, DiScipio RG. Complement activation in the context of stem cells and tissue repair. World J Stem Cells 2015; 7(8): 1090-1108
- URL: https://www.wjgnet.com/1948-0210/full/v7/i8/1090.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i8.1090