Copyright
©The Author(s) 2025.
World J Stem Cells. Jul 26, 2025; 17(7): 108202
Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.108202
Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.108202
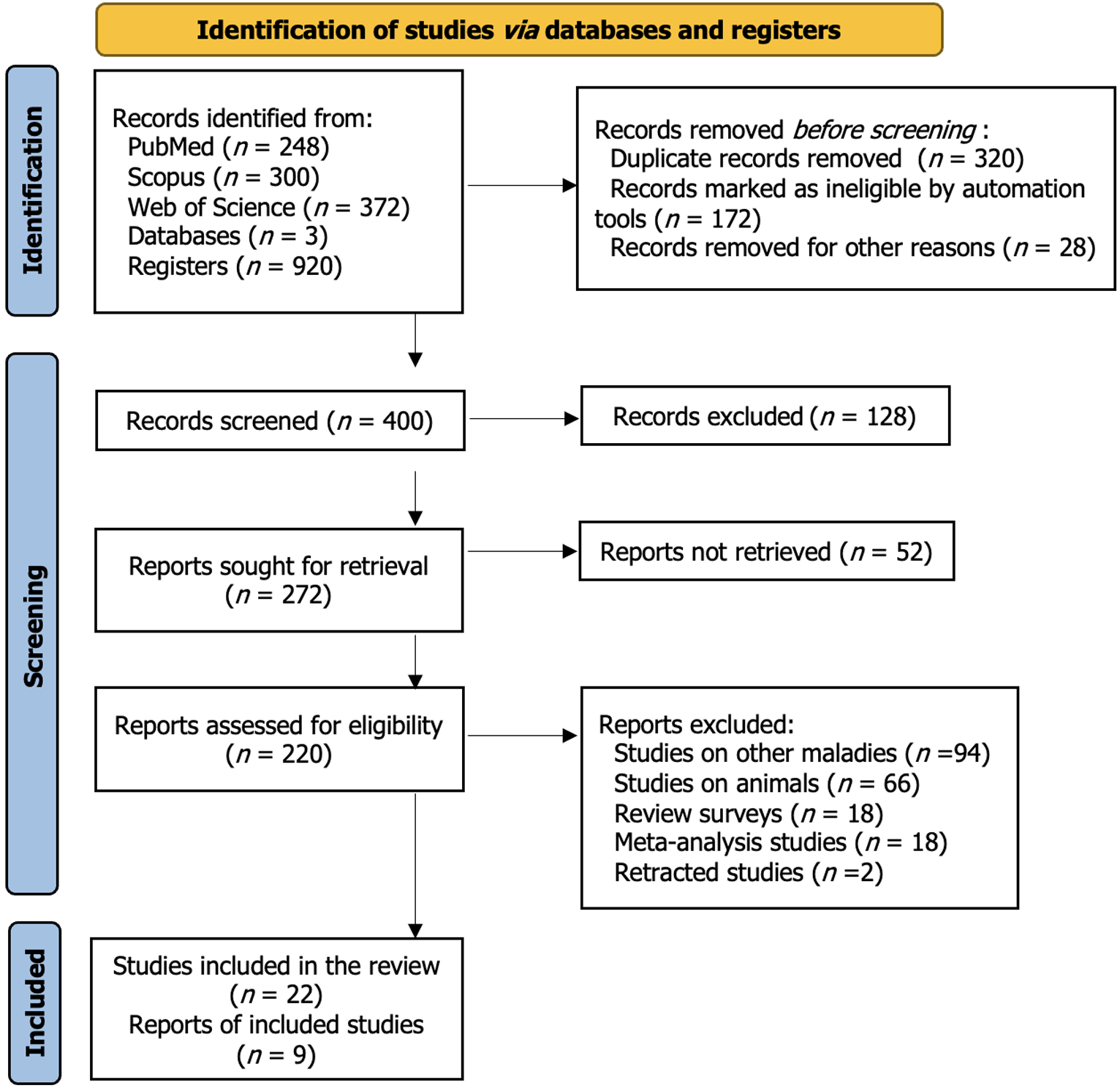
Figure 1 Preferred Reporting Items for Systematic Reviews - a flow chart of the study selection process for the effects of mesenchymal stem cell-based therapies for diabetes mellitus.
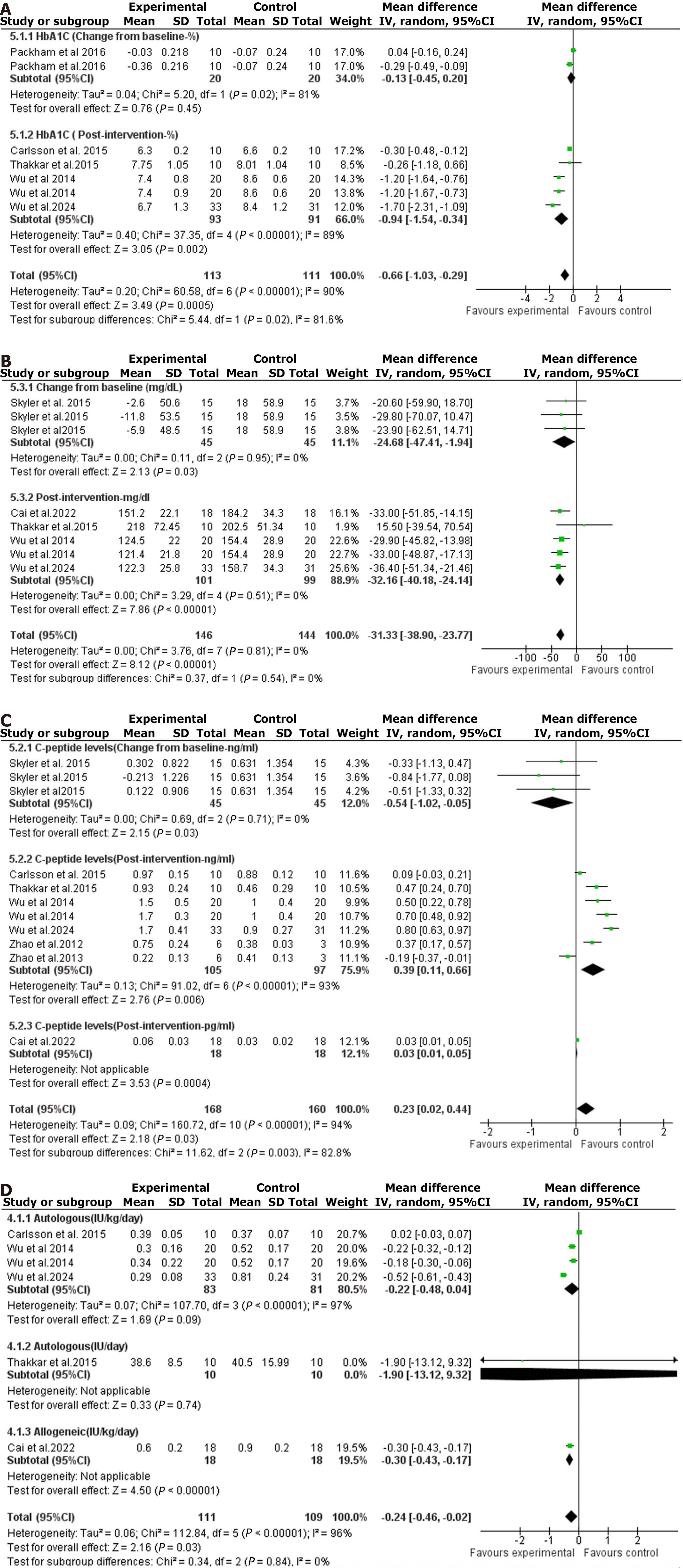
Figure 2 The forest plots resulted from the meta-analysis of the effect of bone marrow-derived mesenchymal stem cell-based therapies on various glycemic outcomes.
A: Hemoglobin A1c; B: Fasting blood glucose; C: C-peptide level; D: Insulin requirements. CI: Confidence interval.
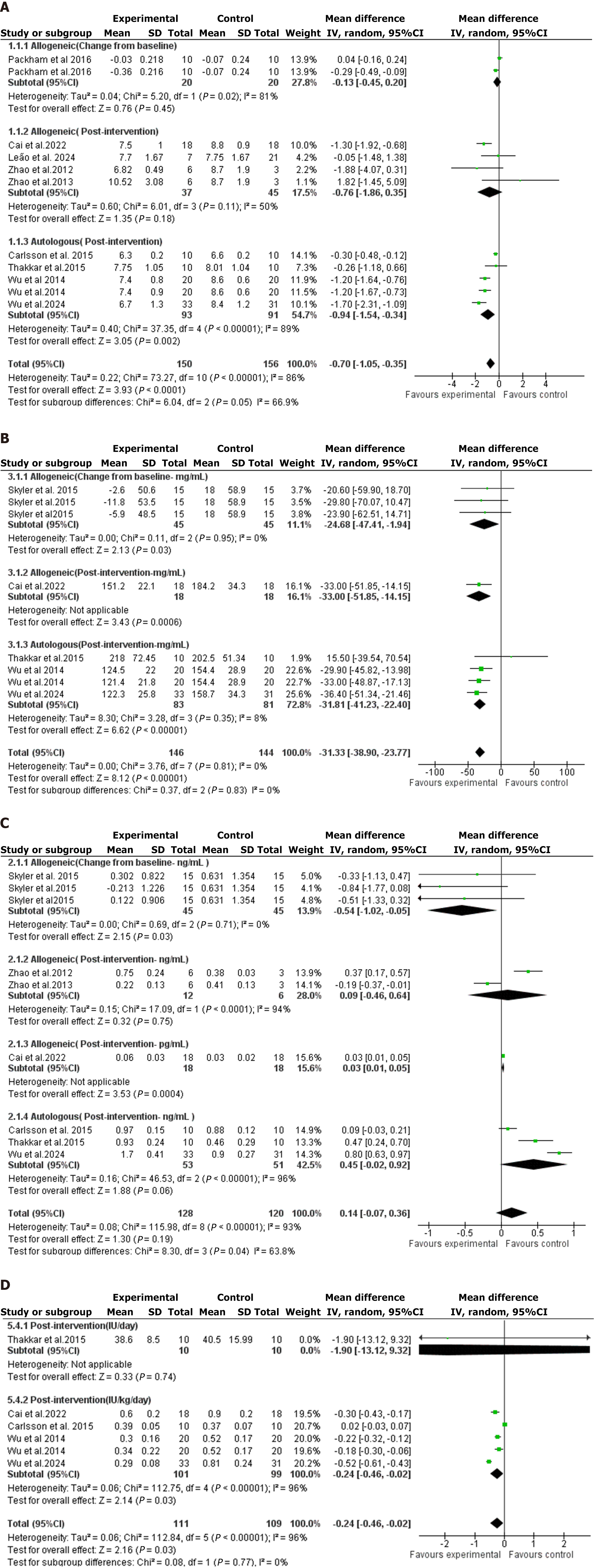
Figure 3 The forest plots resulted from the meta-analysis of the effect of allogeneic and autologous transplanted mesenchymal stem cell-based therapies on various glycemic outcomes.
A: Hemoglobin A1c; B: Fasting blood glucose; C: C-peptide level; D: Insulin requirements. CI: Confidence interval; HbA1c: Hemoglobin A1c.
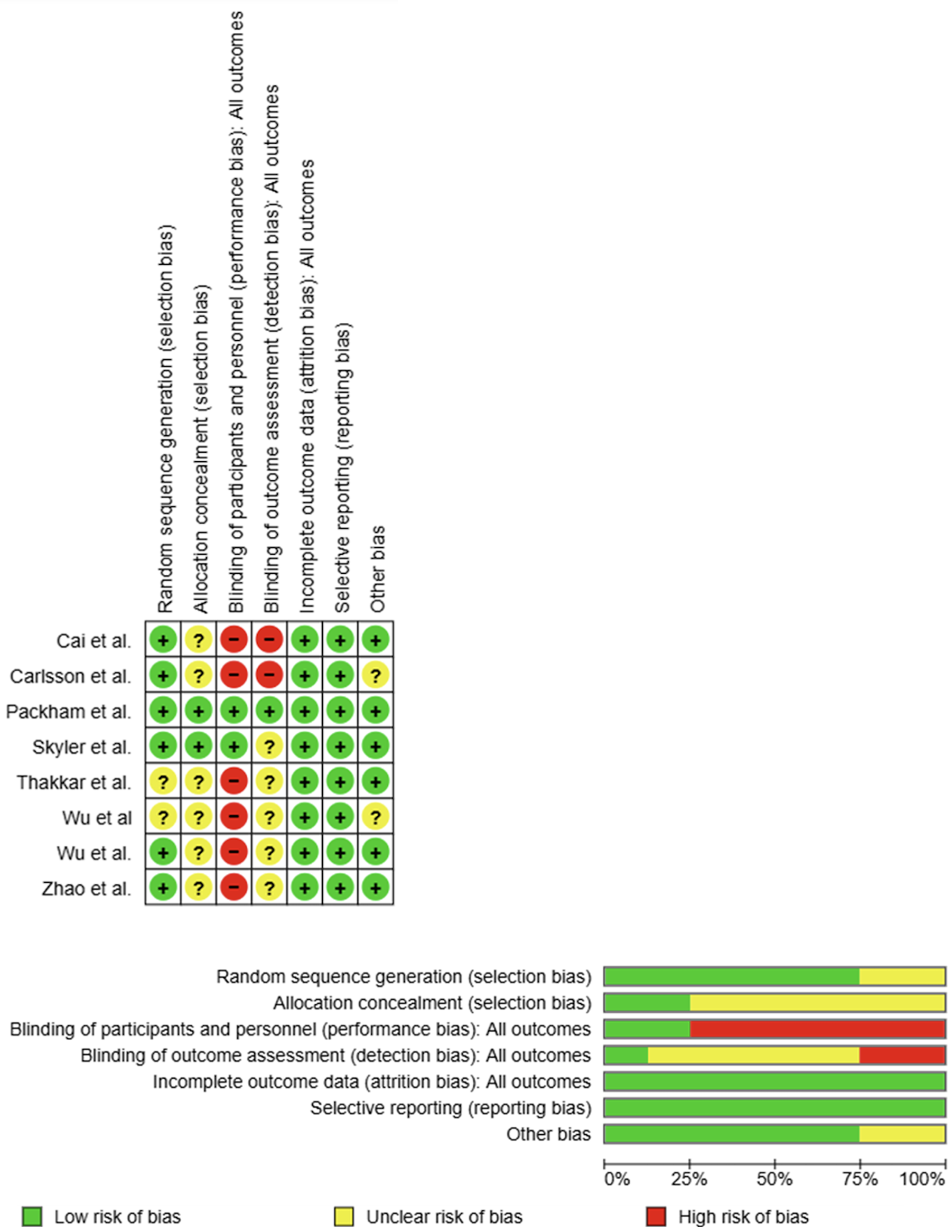
Figure 4 The results of assessment of risk of bias in the studies that underwent meta-analysis.
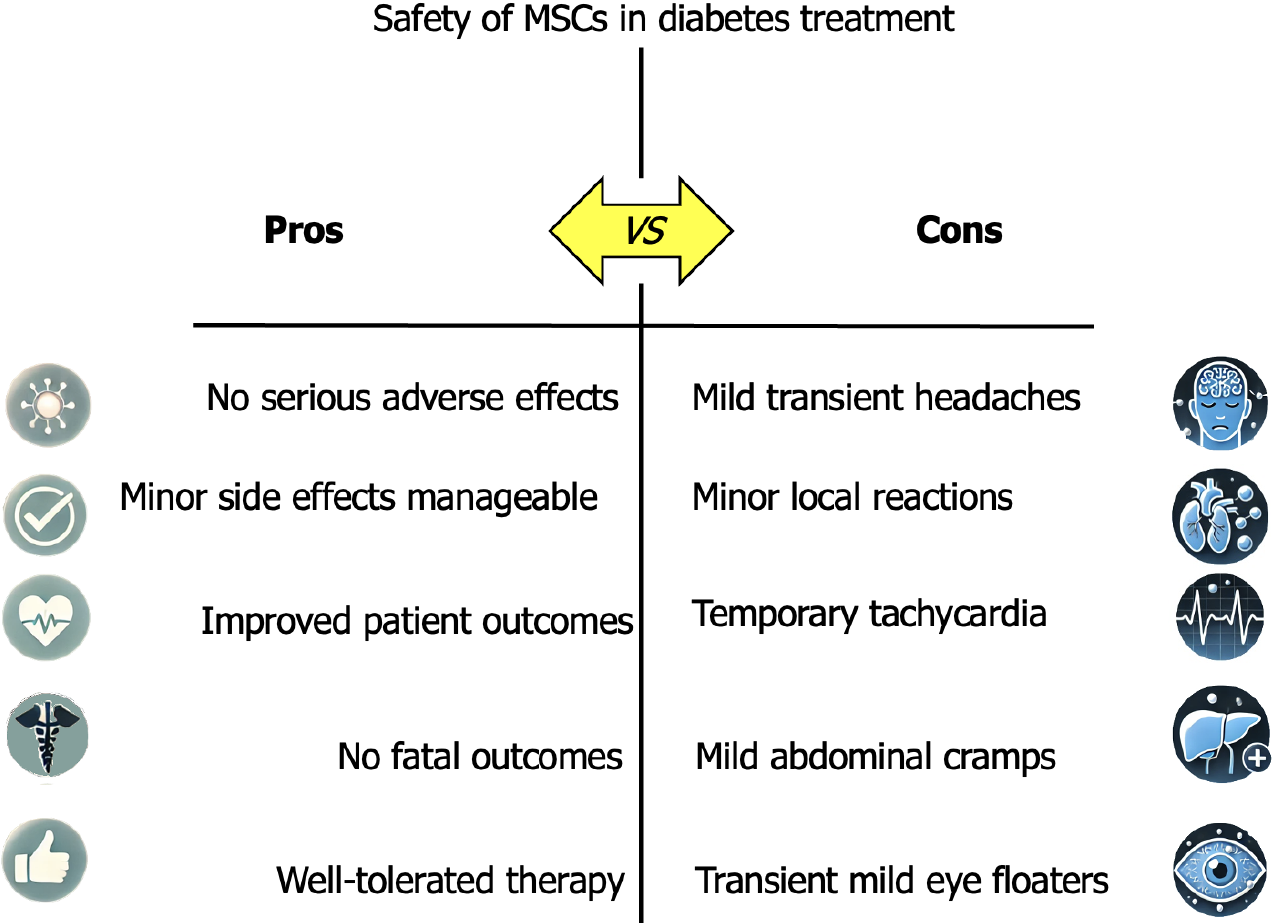
Figure 5 The safety assessment of mesenchymal stem cell-based therapies in patients suffering from diabetes mellitus.
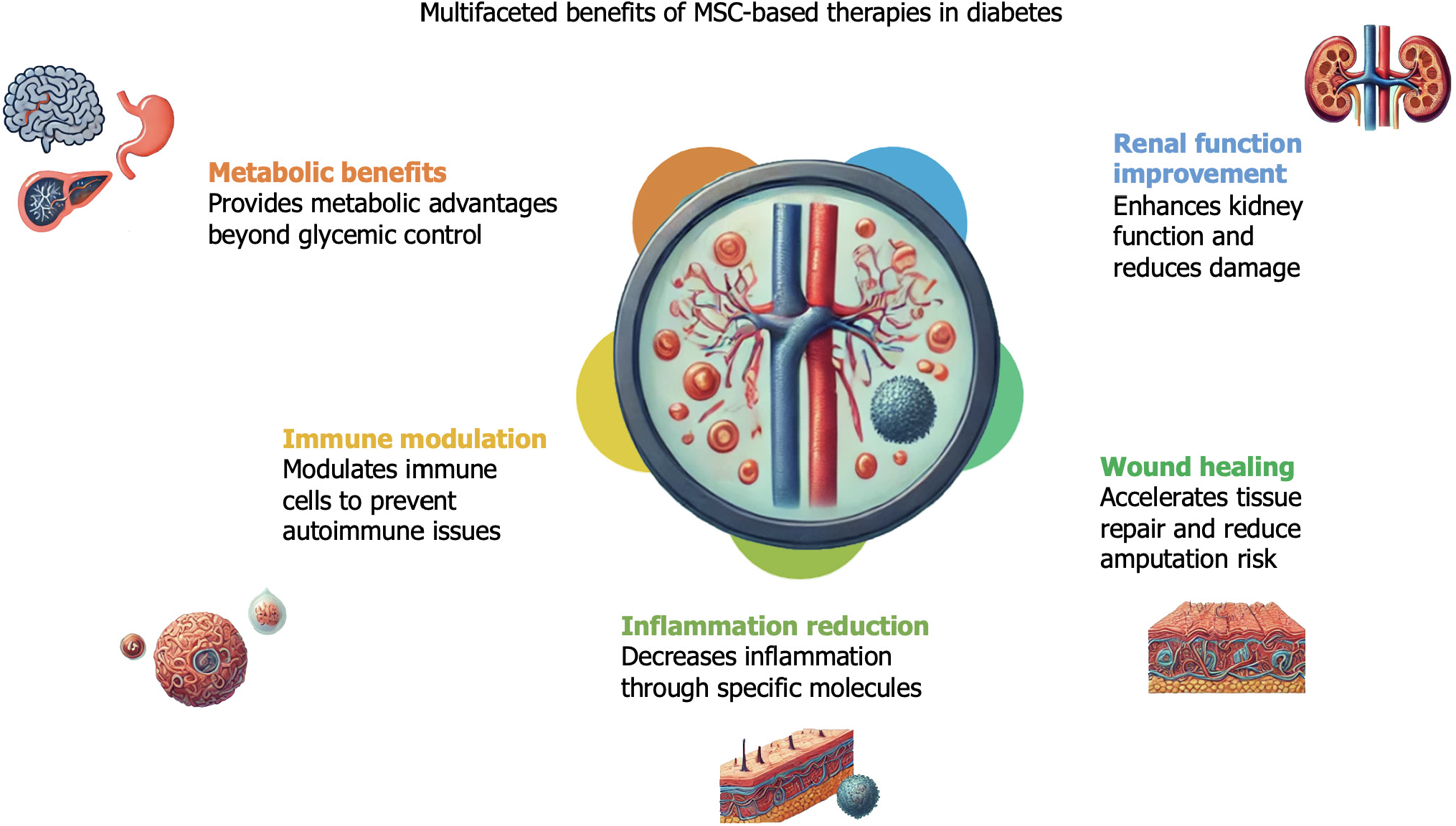
Figure 6 Various non-glycemic effects of mesenchymal stem cell-based therapies on patients with diabetes mellitus.
MSC: Mesenchymal stem cell.
- Citation: Aringazina RA, Zare A, Mousavi SM, Abenova N, Mussin NM, Tamadon A. Autologous and allogeneic mesenchymal stem cell-based therapies for diabetes mellitus: A systematic review and meta-analysis. World J Stem Cells 2025; 17(7): 108202
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/108202.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.108202