Copyright
©The Author(s) 2025.
World J Stem Cells. Apr 26, 2025; 17(4): 103314
Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.103314
Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.103314
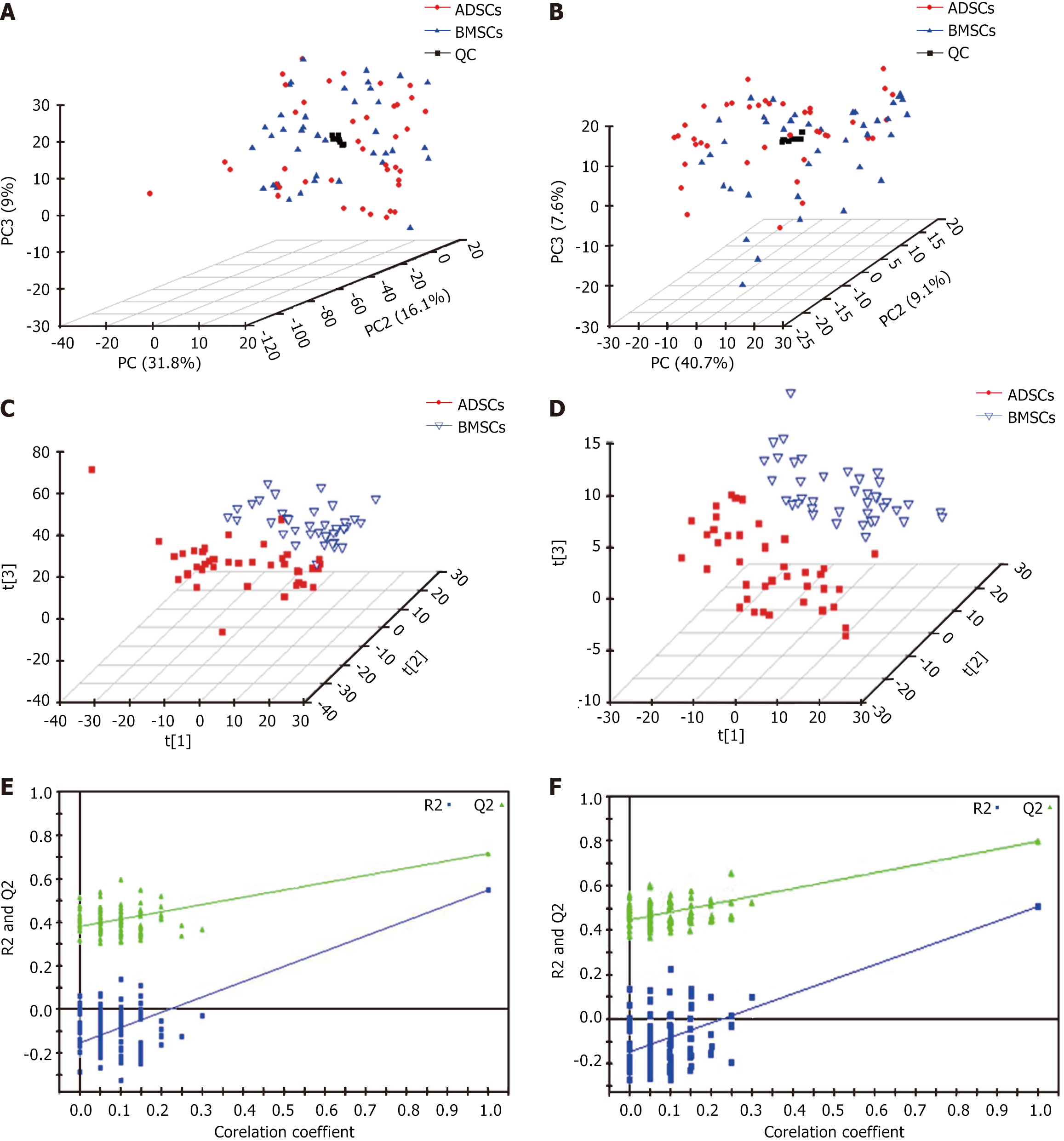
Figure 1 Principal component analysis and partial least squares discriminant analysis score three-dimensional score plots and validation plots for discriminating bone marrow-derived mesenchymal stem cells and adipose tissue-derived mesenchymal stem cells in electron spray ionization in the positive and negative modes.
A: Principal component analysis plot in electron spray ionization (ESI)+ mode; B: Principal component analysis plot in ESI- mode; C: Partial least squares discriminant analysis score plot in ESI+ mode; D: Partial least squares discriminant analysis score plot in ESI- mode; E: Validation plot in ESI+ mode; F: Validation plot in ESI- mode. QC: Quality control; ADSCs: Adipose tissue-derived mesenchymal stem cells; BMSCs: Bone marrow-derived mesenchymal stem cells.
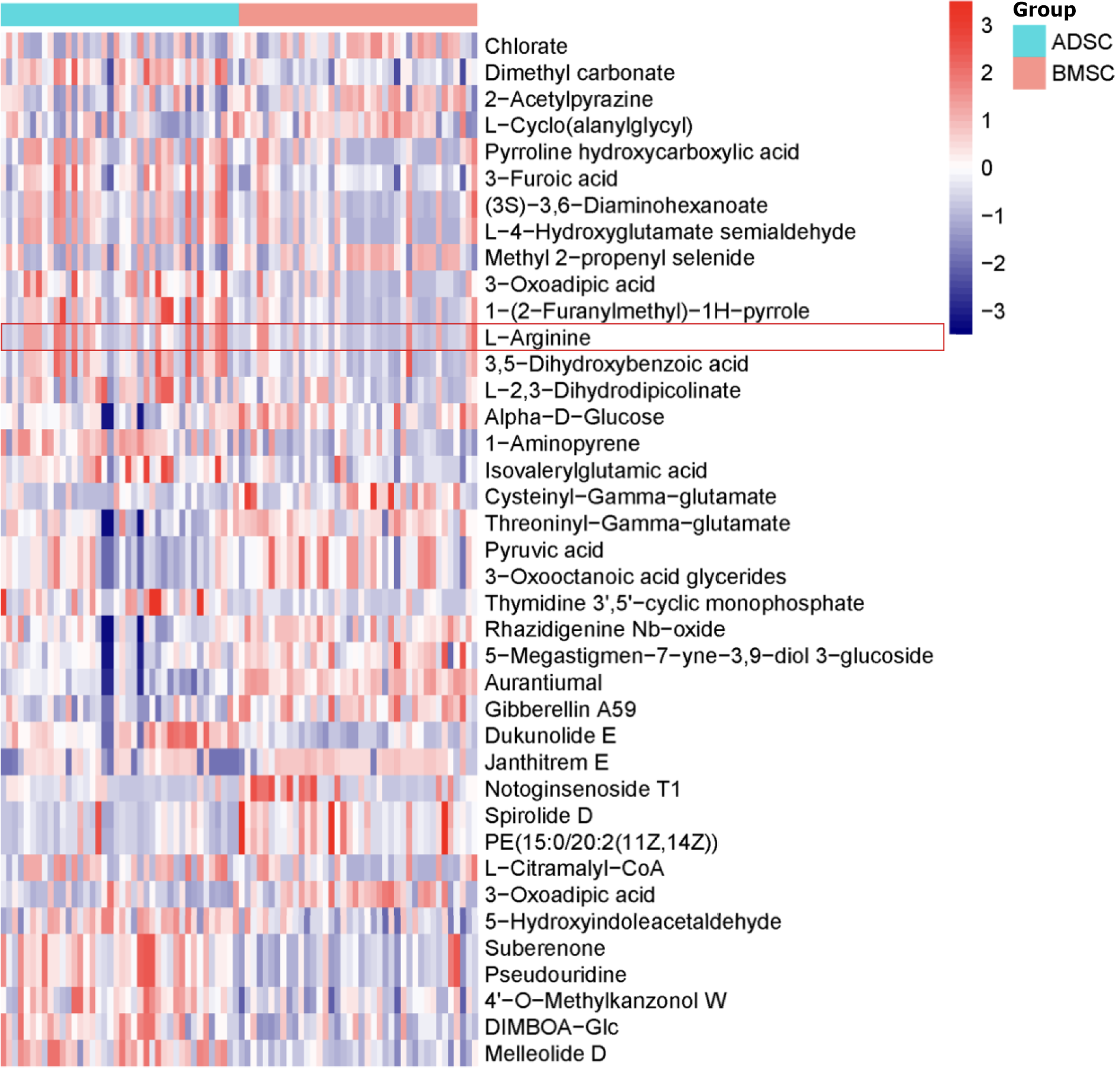
Figure 2 Heat map demonstrated 39 coronary heart disease-related metabolites in adipose tissue-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells.
Metabolites detected in positive/negative electron spray ionization mode according to Table 2. ADSCs: Adipose tissue-derived mesenchymal stem cells; BMSCs: Bone marrow-derived mesenchymal stem cells.
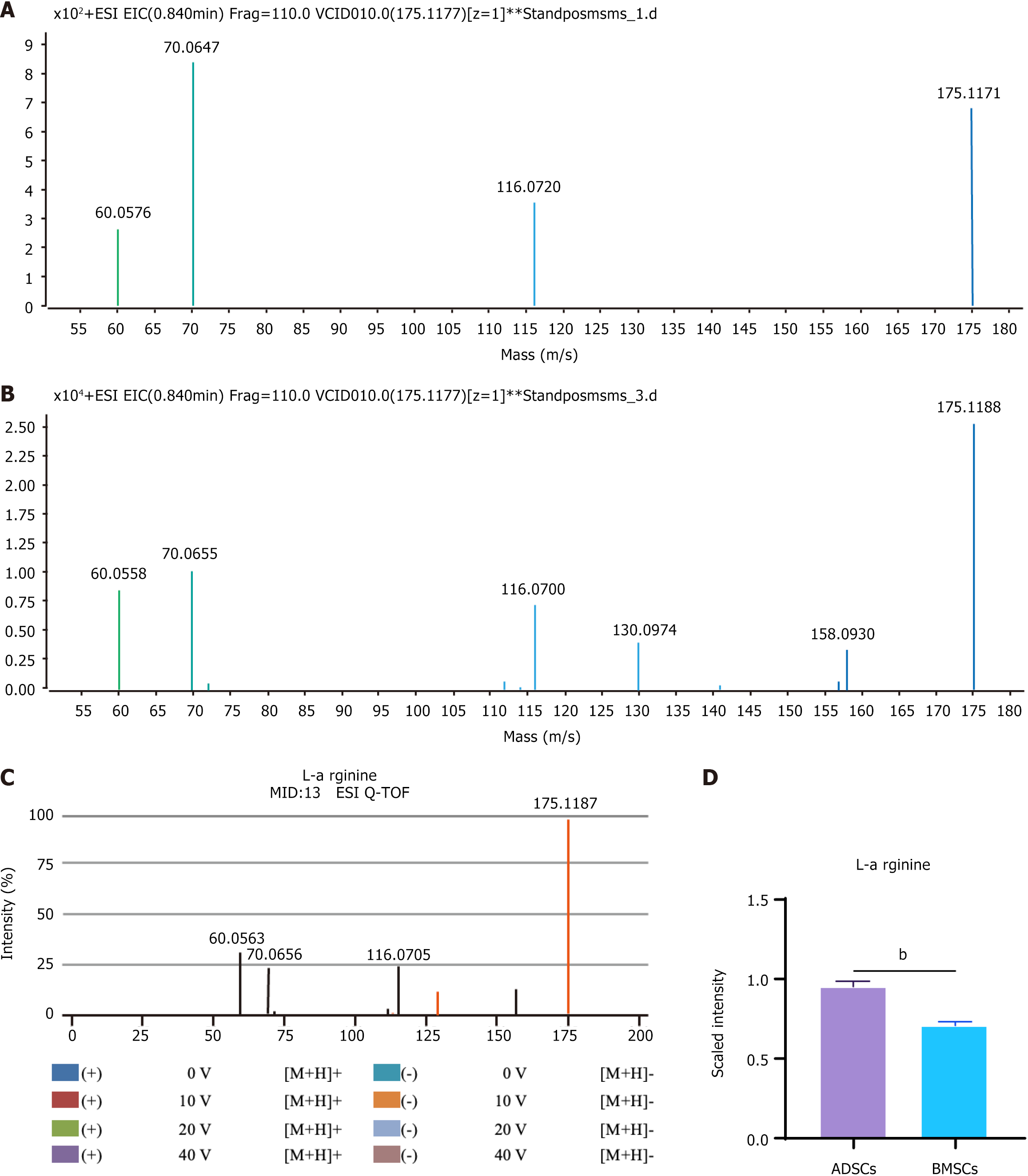
Figure 3 Identification procedure for the potential biomarker L-arginine.
A: Metabolite profiles for L-arginine differences between adipose tissue-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells in electron spray ionization in the positive mode; B: Mass spectrum (MS) at retention time of 54.32 min; C: MS/MS spectrum of L-arginine ion from online METLIN databases; D: MS/MS spectrum of L-arginine ion at 54.32 min in plasma sample. bP < 0.01. ESI: Electron spray ionization; ADSCs: Adipose tissue-derived mesenchymal stem cells; BMSCs: Bone marrow-derived mesenchymal stem cells.
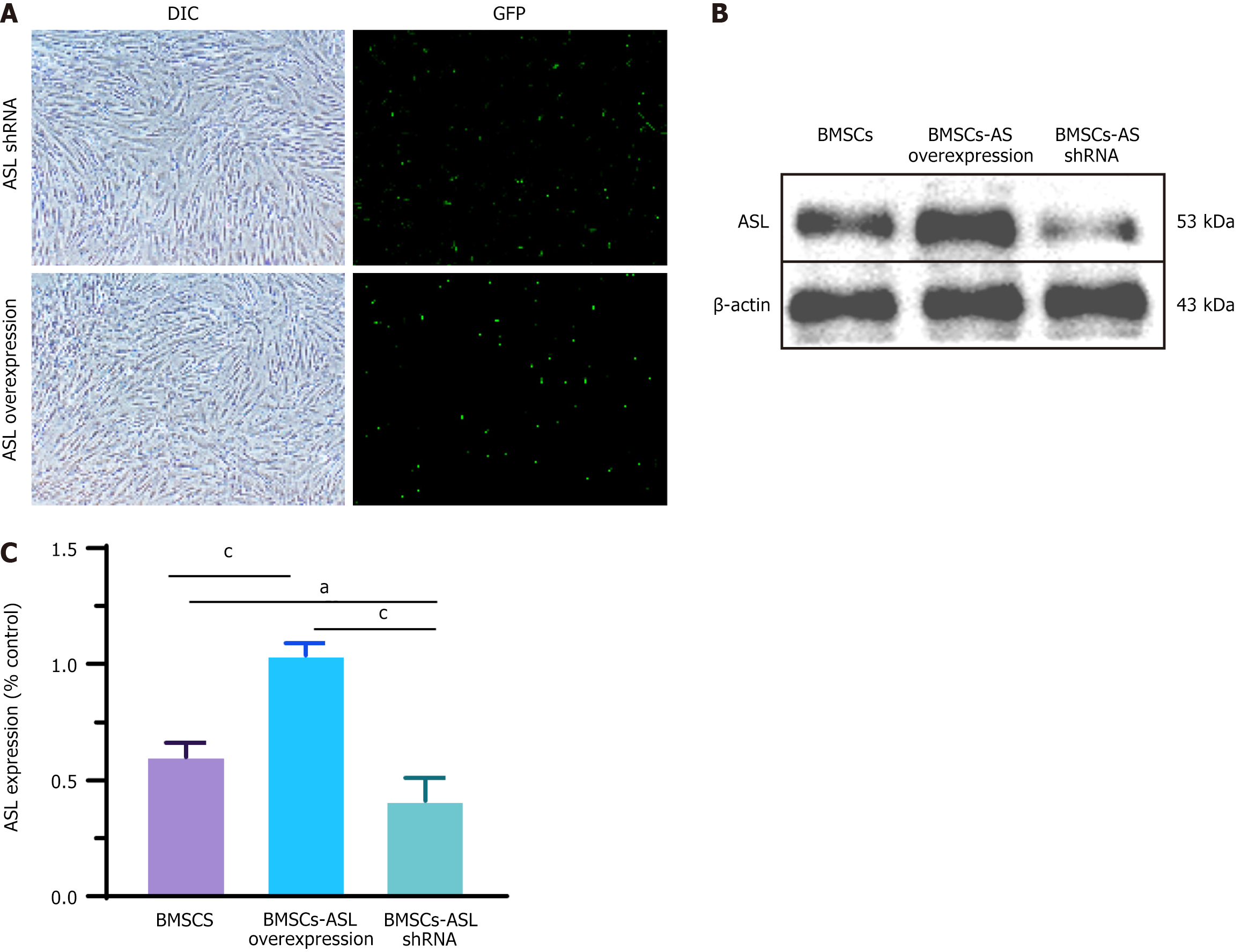
Figure 4 Verification of arginine succinate lyase overexpression and knockdown in bone marrow-derived mesenchymal stem cells.
A: Bone marrow-derived mesenchymal stem cells after plasmid transfection and green fluorescent protein were observed under normal and fluorescence microscopes. Scale bars = 200 μm; B: Arginine succinate lyase (ASL) protein levels were significantly upregulated or significantly downregulated relative to the negative control group using ASL-overexpressing or ASL-short hairpin RNA plasmids; C: Relative quantitative comparison of western blot analyses (n = 3/group). aP < 0.05,cP < 0.001. ASL: Arginine succinate lyase; DIC: Plasmid transfection; GFP: Green fluorescent protein; shRNA: Short hairpin RNA; BMSCs: Bone marrow-derived mesenchymal stem cells.
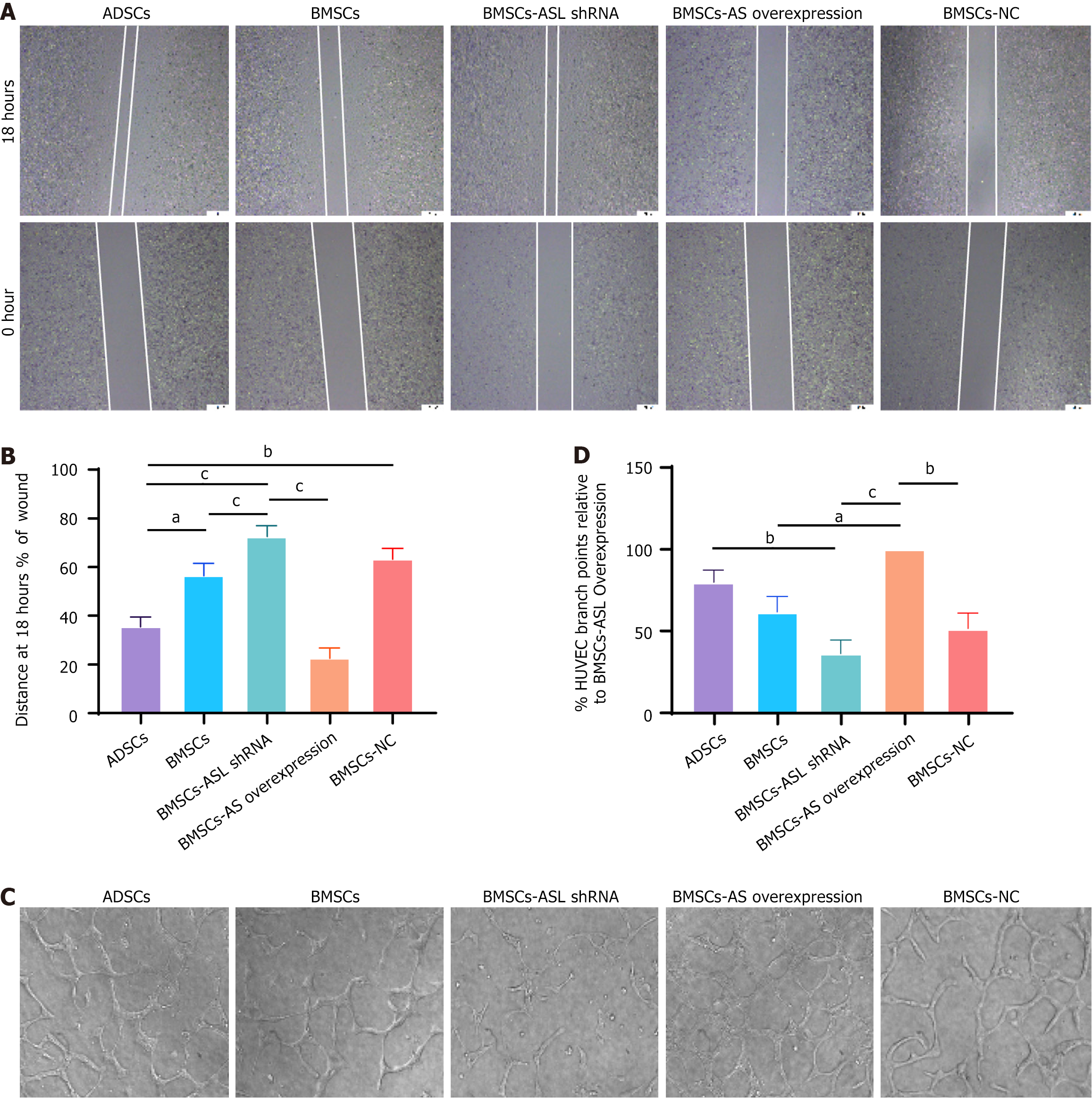
Figure 5 Wound healing and tube formation capacity in adipose tissue-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells from identical older patients cultured in serum-free and hypoxic conditions.
A: Wound healing capacity of human umbilical vein endothelial cells (HUVECs) co-cultured with different mesenchymal stem cells (MSCs); B: Relative quantitative comparison of healing speed analyses (n = 6); C: Representative photomicrographs showing tube formation in HUVECs co-cultured with different MSCs; D: Quantification of endothelial tube junction numbers demonstrated that co-culture with aged patient-derived MSCs promoted HUVECs to form capillary-like structures (n = 6). aP < 0.05, bP < 0.01, cP < 0.001. ASL: Arginine succinate lyase; shRNA: Short hairpin RNA; ADSCs: Adipose tissue-derived mesenchymal stem cells; BMSCs: Bone marrow-derived mesenchymal stem cells; HUVECs: Human umbilical vein endothelial cells; NC: Negative control.
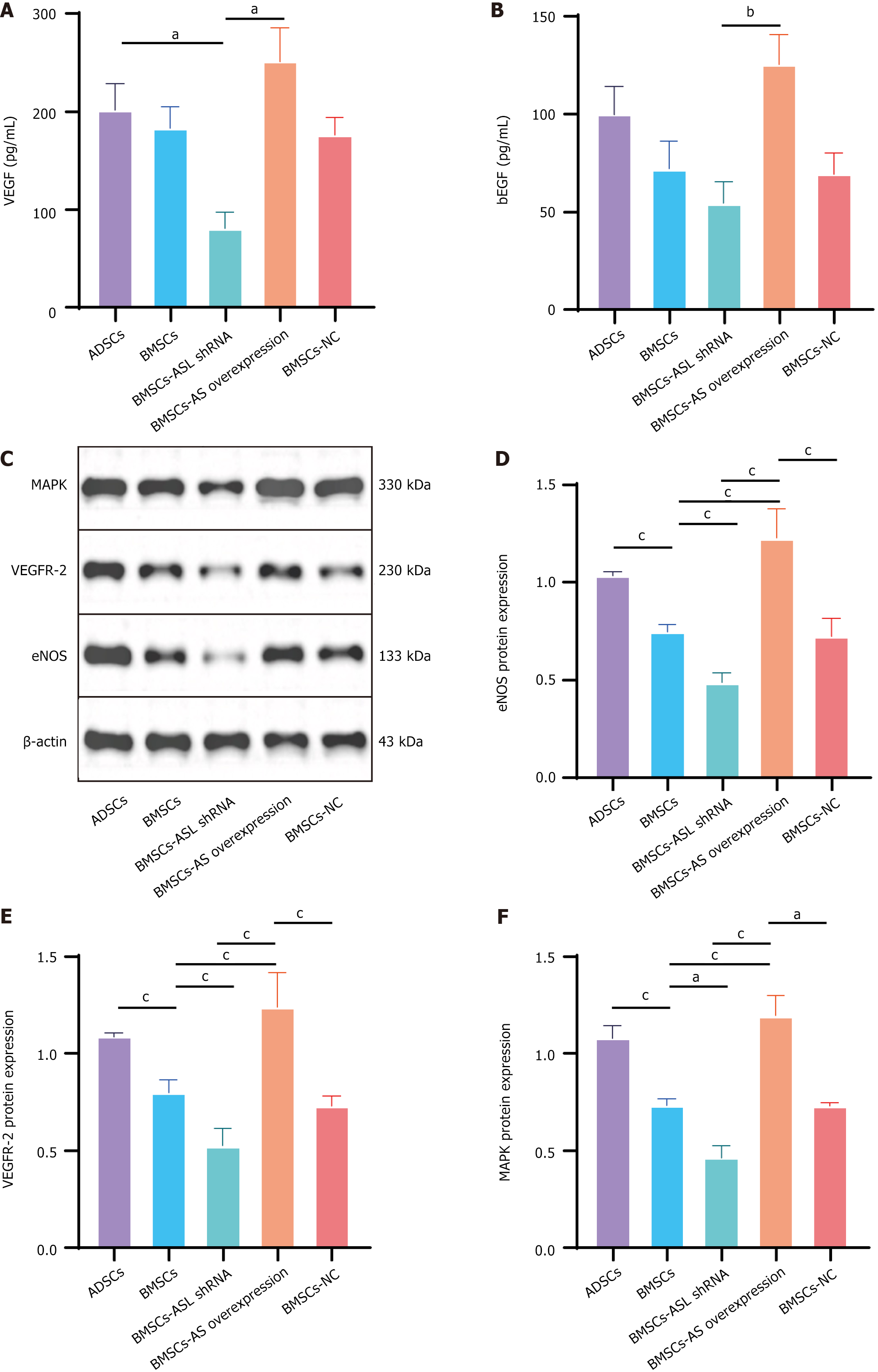
Figure 6 Vascular endothelial growth factor and basic fibroblast growth factor secretion and expression of mitogen active protein kinase, vascular endothelial growth factor receptor 2, and endothelial nitric oxide synthase in different mesenchymal stem cells from older donors cultured in serum-free and hypoxic conditions.
A and B: Vascular endothelial growth factor and basic fibroblast growth factor secretion in different aged patient-derived mesenchymal stem cells (MSCs) cultured in serum-free and hypoxic conditions. n = 3; C: Western blotting results for mitogen activated protein kinase, vascular endothelial growth factor receptor 2, and endothelial nitric oxide synthase expression in different MSCs cultured in serum-free and hypoxic conditions; D-F: Relative quantitative protein expression comparison. n = 3. aP < 0.05, bP < 0.01, cP < 0.001. ASL: Arginine succinate lyase; shRNA: Short hairpin RNA; BMSCs: Bone marrow-derived mesenchymal stem cells; ADSCs: Adipose tissue-derived mesenchymal stem cell; NC: Negative control; VEGF: Vascular endothelial growth factor; bFGF: Basic fibroblast growth factor; eNOS: Endothelial nitric oxide synthase; VEGFR2: Vascular endothelial growth factor receptor 2; MAPK: Mitogen active protein kinase.
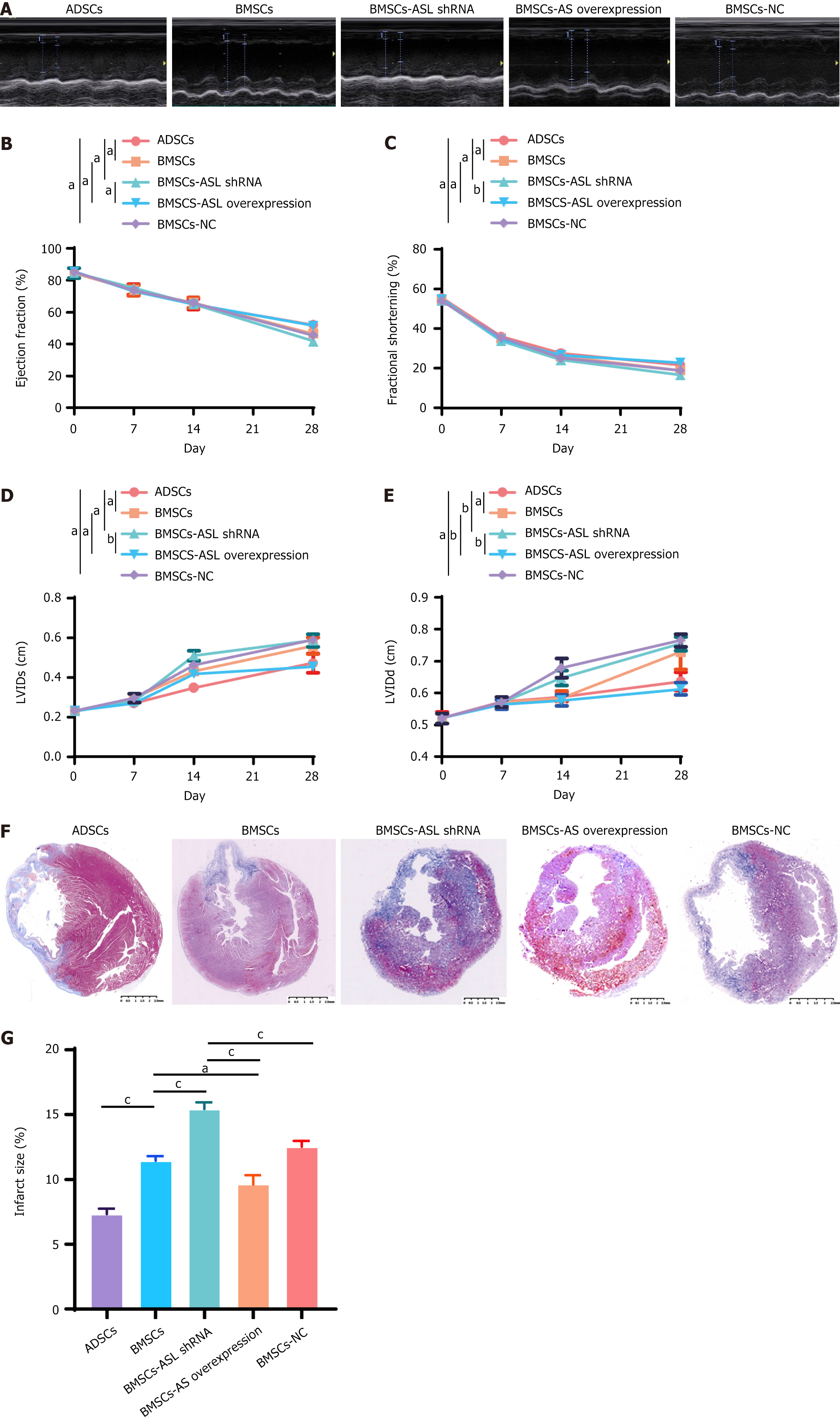
Figure 7 Enhanced cardiac function and decreased infarct size after cell transplantation with mesenchymal stem cells.
A: Representative M-mode echocardiography 28 days after cell transplantation with aged patient-derived mesenchymal stem cells (MSCs); B and C: Ejection fraction and fractional shortening of bone marrow-derived MSC-arginine succinate lyase (BMSC-ASL) overexpressed and adipose tissue-derived mesenchymal stem cell (ADSC) groups after myocardial infarction were significantly higher than those in other groups four weeks after cell transplantation (n = 5); D and E: Left ventricular internal systolic dimension and left ventricular internal diastolic dimension of BMSC-ASL overexpressed and ADSC groups were significantly lower than those in BMSC and BMSC-ASL short hairpin RNA groups 4 weeks after myocardial infarction (n = 5); F: Masson’s trichrome staining of infarct size 4 weeks after cell transplantation in all groups; G: Computerized morphometric analysis indicated that infarct size was significantly smaller in the BMSC-ASL overexpressed and ADSC groups than that in BMSCs and other groups. Infarct size in the ADSC group was smaller than that in both BMSC and BMSC-ASL short hairpin RNA groups (n = 3). aP < 0.05, bP < 0.01, cP < 0.001. ASL: Arginine succinate lyase; shRNA: Short hairpin RNA; BMSCs: Bone marrow-derived mesenchymal stem cells; ADSCs: Adipose tissue-derived mesenchymal stem cell; NC: Negative control; LVID: Left ventricular internal systolic dimension; LVIDd: Left ventricular internal diastolic dimension.
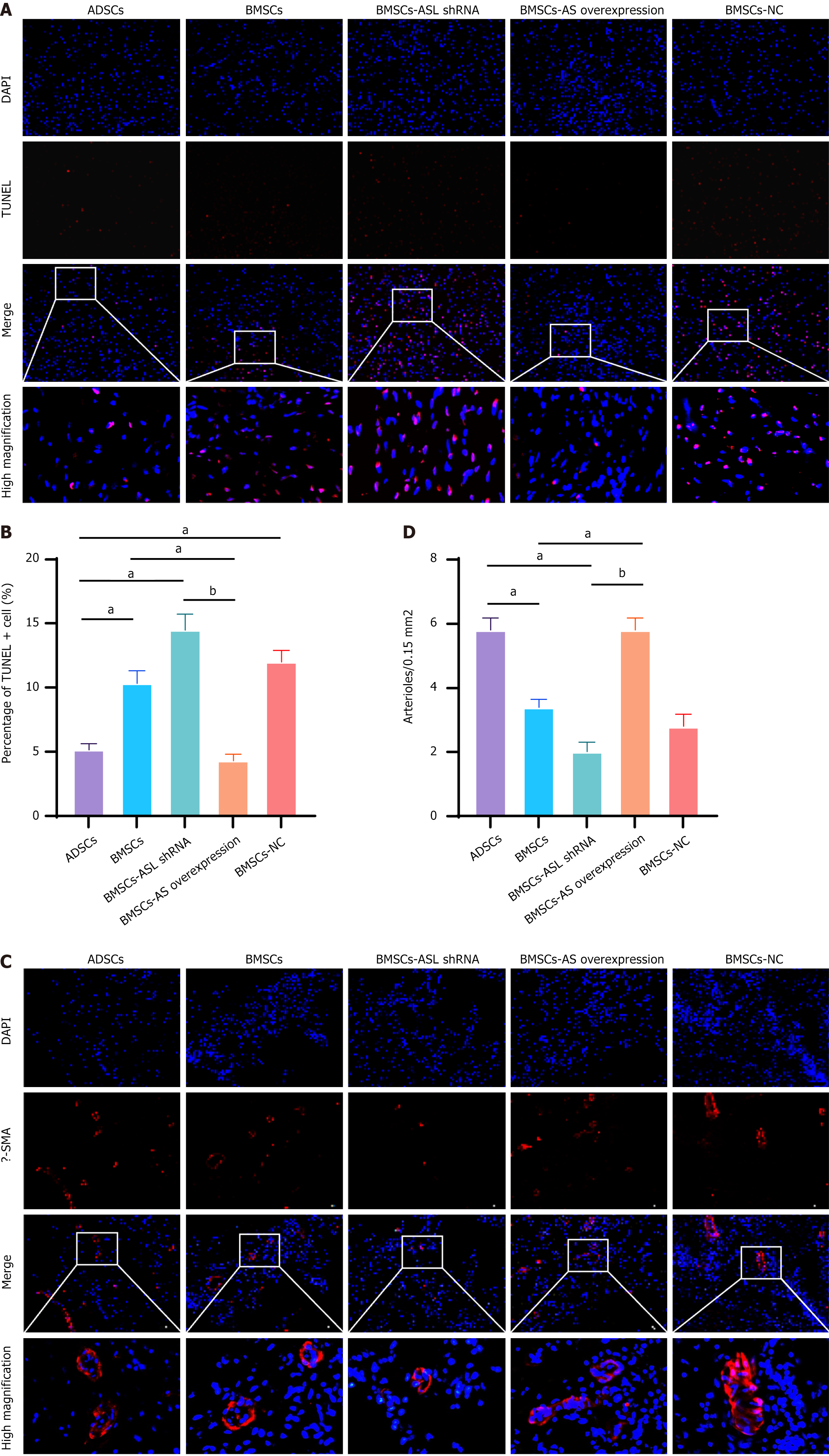
Figure 8 Cell apoptosis and vascular density.
A: Terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) staining of apoptotic cells in all groups; B: Apoptotic cell number (TUNEL+) was significantly lower in border region in bone marrow-derived mesenchymal stem cell-arginine succinate lyase (BMSC-ASL) overexpressed and adipose tissue-derived mesenchymal stem cell (ADSC) groups compared to both the BMSC and BMSC-ASL short hairpin RNA groups after MSC transplantation (n = 6); C: Representative images for anti-α-smooth muscle actin (red); D: Vascular density was significantly higher in the BMSC-ASL overexpressed and ADSC groups than in the BMSCs and BMSC-ASL short hairpin RNA groups. Vascular density in the BMSCs group was higher than in the BMSC-ASL short hairpin RNA group (n = 6). aP < 0.05, bP < 0.01. ASL: Arginine succinate lyase; shRNA: Short hairpin RNA; BMSCs: Bone marrow-derived mesenchymal stem cells; ADSCs: Adipose tissue-derived mesenchymal stem cell; NC: Negative control; TUNEL: Terminal-deoxynucleotidyl transferase mediated nick end labeling; DAPI: 4,6-diamidino-2-phenylindole; SMA: Smooth muscle actin.
- Citation: Li JZ, Zhan X, Sun HB, Chi C, Zhang GF, Liu DH, Zhang WX, Sun LH, Kang K. L-arginine from elder human mesenchymal stem cells induces angiogenesis and enhances therapeutic effects on ischemic heart diseases. World J Stem Cells 2025; 17(4): 103314
- URL: https://www.wjgnet.com/1948-0210/full/v17/i4/103314.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i4.103314