Copyright
©The Author(s) 2024.
World J Stem Cells. May 26, 2024; 16(5): 575-590
Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.575
Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.575
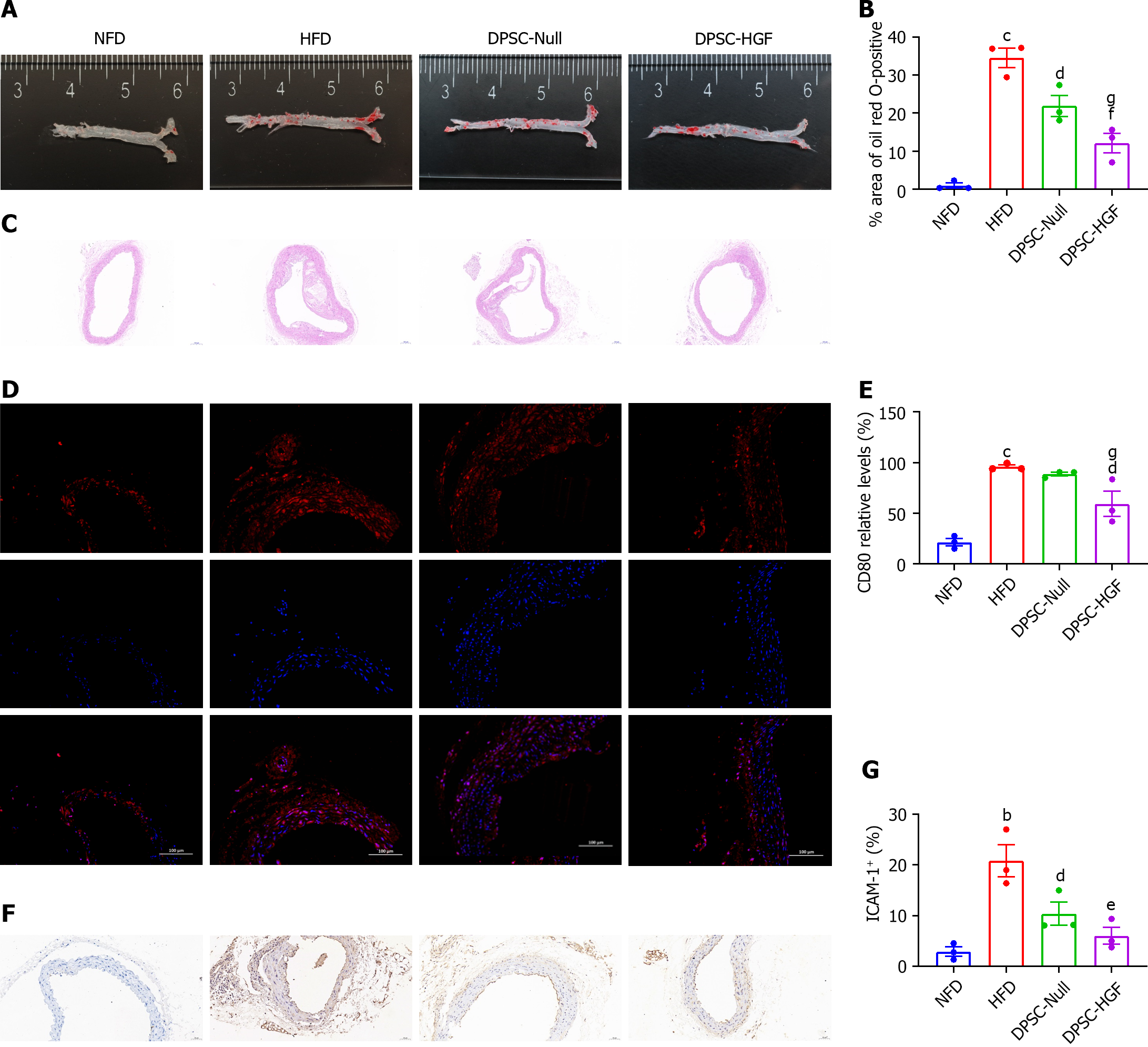
Figure 1 Ad-Null and Ad-hepatocyte growth factor modified dental pulp stem cells treatments alleviated atherosclerotic lesions in apolipoprotein E-knockout mice.
A: Representative images of oil Red O-stained aortas; B: Atherosclerotic plaque areas are expressed as the ratio of oil Red O-stained areas to total aortic areas (n = 3); C: Representative images of hematoxylin and eosin (HE) stained sections showing the plaque size in the aortic lumen (scale bar = 100 μm); D: Representative images of aortic arch sections stained with an anti-mCD80 antibody (scale bar = 100 μm); E: The CD80-positive areas in the aortic tissues are expressed as the ratios of positively stained cells to total cells (n = 3); F: Representative images of intercellular cell adhesion molecule-1 (ICAM-1) expression in atherosclerotic lesions of the aortic arch (scale bar = 50 μm); G: The ICAM-1 expression levels are shown as the ratios of positively stained cells to total endothelial cells (n = 3). All results are presented as the means ± SEMs; bP < 0.01, cP < 0.001 vs. the normal fat diet group; dP < 0.05, eP < 0.01, fP < 0.001 vs. the high-fat diet group; gP < 0.05 vs. the Ad-Null modified dental pulp stem cells group. DPSC-HGF: Ad-hepatocyte growth factor modified dental pulp stem cells; HFD: High-fat diet; NFD: Normal formula diet; DPSC: Dental pulp stem cell.
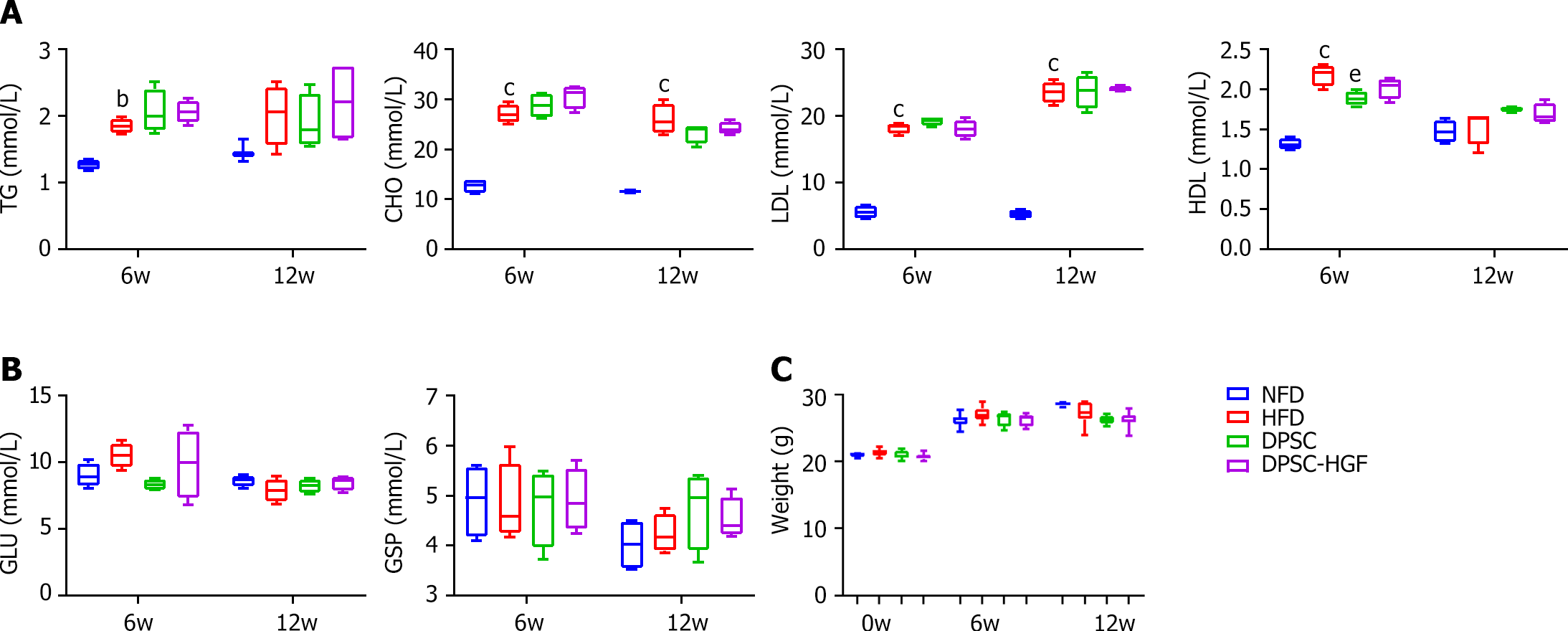
Figure 2 Neither Ad-Null modified dental pulp stem cells nor Ad-hepatocyte growth factor modified dental pulp stem cells altered the biochemical parameters of apolipoprotein E-knockout mice fed a high-fat diet.
A: Serum concentrations of triglycerides, total cholesterol, low-density lipoprotein, and high-density lipoprotein in apolipoprotein E-knockout (ApoE-/-) mice at weeks 6 and 12 (n = 4); B: Serum concentrations of glucose (GLU) and glycosylated serum protein (GSP) in ApoE-/- mice at weeks 6 and 12 (n = 4); C: Body weight (n = 9). All results are presented as the means ± SEMs; bP < 0.01, cP < 0.001 vs. the normal fat diet group. eP < 0.01 vs. high-fat diet group. DPSC-Null: Ad-Null modified dental pulp stem cells; DPSC-HGF: Ad-hepatocyte growth factor modified dental pulp stem cells; HFD: High-fat diet; NFD: Normal formula diet; TG: Triglycerides; CHO: Cholesterol; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; GLU: Glucose; GSP: Glycosylated serum protein.
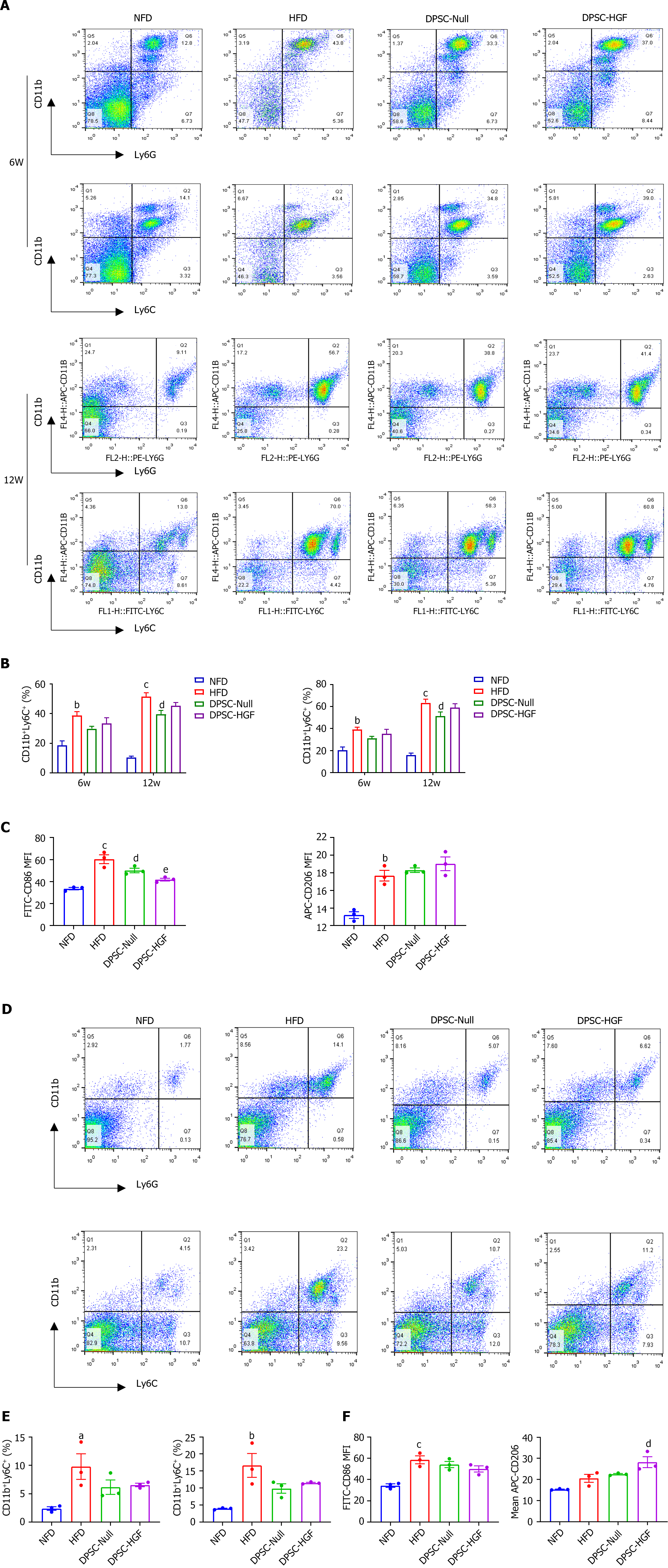
Figure 3 Ad-Null and Ad-hepatocyte growth factor modified dental pulp stem cells treatments reduced the percentages of monocytes, neutrophils and inflammatory macrophages in apolipoprotein E-knockout mice.
Peripheral blood cells were stained with anti-mouse CD11b, anti-mouse Ly6G, and anti-mouse Ly6C at weeks 6 and 12 to determine the percentages of CD11b+Ly6G+ and CD11b+Ly6C+ cells (n = 3). A: Representative flow cytometry images of peripheral blood; B: Statistical charts of peripheral blood are shown; C: At the end of the experiment, peripheral blood cells were stained with anti-mouse CD86 and anti-mouse CD206, and the mean fluorescence intensity (MFI) of CD86 and CD206 were analyzed (n = 3). Splenocytes were stained with anti-mCD11b, anti-mLy6G, and anti-mLy6C to determine the percentages of CD11b+Ly6G+ and CD11b+Ly6C+ cells (n = 3); D: Representative flow cytometry images of the spleen; E: Statistical graphs of the spleen are shown; F: Splenocytes were stained with anti-mCD86 and anti-mCD206 antibodies, and the MFI of CD86 and CD206 were analyzed (n = 3). All results are represented as the mean ± SEM; aP < 0.05, bP < 0.01, cP < 0.001 vs. normal fat diet group; dP < 0.05, eP < 0.01 vs. high-fat diet group. DPSC-Null: Ad-Null modified dental pulp stem cells; DPSC-HGF: Ad-hepatocyte growth factor modified dental pulp stem cells; HFD: High-fat diet; NFD: Normal formula diet.
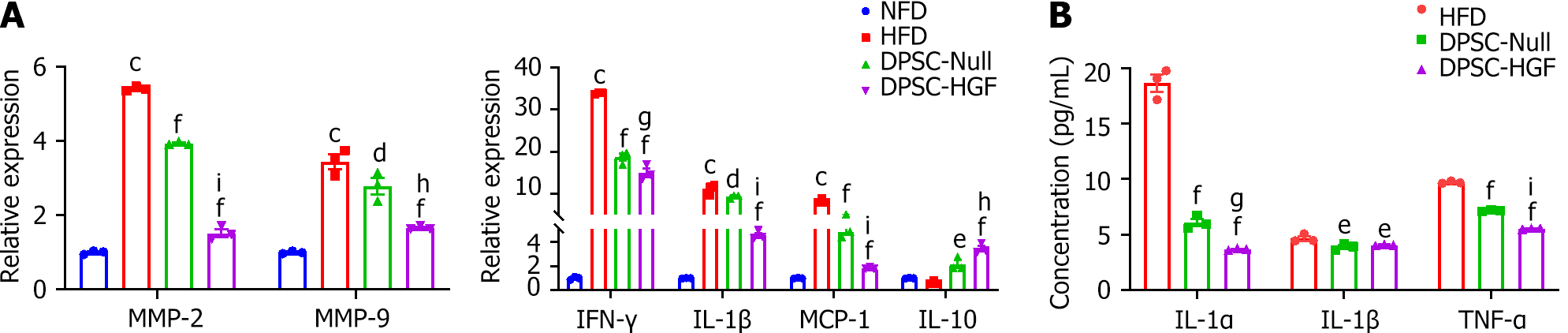
Figure 4 Ad-hepatocyte growth factor modified dental pulp stem cells treatment reduces the expression of inflammatory cytokines in aortic tissues and serum.
The expression levels of matrix metallopeptidase-2 (MMP-2), MMP-9, interferon-γ, interleukin-1β (IL-1β), monocyte chemotactic protein-1 and IL-10 in aortic tissue were detected by real-time reverse transcription polymerase chain reaction (n = 3). A: The serum inflammatory factor expression levels of interleukin-1α (IL-1α), IL-1β and tumor necrosis factor-α were detected by enzyme-linked immunosorbent assay (n = 3); B: All results are represented as the mean ± SEM; cP < 0.001 vs. normal fat diet group; dP < 0.05, eP < 0.01, fP < 0.001 vs. high fat diet group; gP < 0.05, hP < 0.01, iP < 0.001 vs. the Ad-Null modified dental pulp stem cells group. DPSC-Null: Ad-Null modified dental pulp stem cells; DPSC-HGF: Ad-hepatocyte growth factor modified dental pulp stem cells; HFD: High-fat diet; NFD: Normal formula diet; MMP: Matrix metallopeptidase; IFN: Interferon; IL: Interleukin; MCP: Monocyte chemotactic protein; TNF: Tumor necrosis factor.
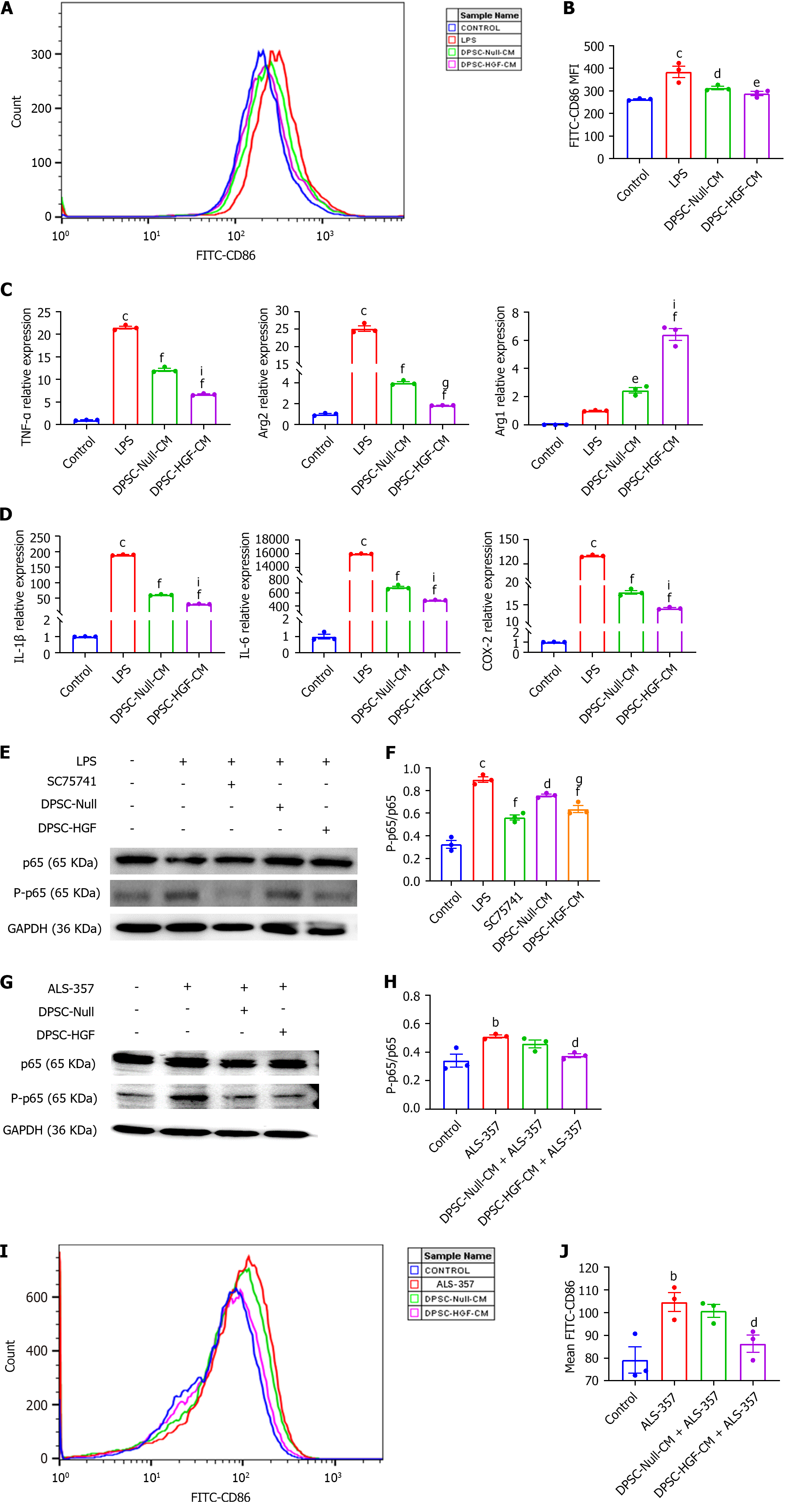
Figure 5 Effects of the supernatant of Ad-Null and Ad-hepatocyte growth factor modified dental pulp stem cells on the polarization and inflammatory cytokine expression of RAW264.
7 mouse macrophages under inflammatory stimulation. RAW264.7 cells were indirectly co-cultured with the supernatant of Ad-Null modified dental pulp stem cells (DPSCs) and Ad-hepatocyte growth factor modified DPSCs for 24 h under lipopolysaccharide (150 ng/mL) stimulation. The mean fluorescence intensity of the anti-CD86 antibody was detected by flow cytometry. A: Representative image; B: A statistical plot of the mean fluorescence intensity (MFI) of CD86 was generated (n = 3); C: The mRNA expression levels of the M1 macrophage markers tumor necrosis factor-α (TNF-α) and arginase 2 (Arg2) and the M2 macrophage marker Arg1 were detected by real-time reverse transcription polymerase chain reaction (RT-PCR) under inflammatory stimulation (n = 3); D: The mRNA expression levels of the inflammatory factors interleukin-1β (IL-1β), IL-6, and cyclooxygenase-2 were detected by RT-PCR under inflammatory stimulation (n = 3); E: The expression of phosphorylated p65 and p65 in RAW264.7 cells under inflammatory stimulation conditions was investigated using western blotting; F: The gray values were analyzed; G: The expression levels of phosphorylated p65 and p65 in RAW264.7 cells under nuclear factor-κB (NF-κB) activator treatment were detected by western blotting; H: Analyzed for gray values; I: After NF-κB activator treatment, the CD86 MFI was detected by flow cytometry; J: A statistical plot of the MFI of CD86 was generated (n = 3). All results are representative of the mean ± SEM; bP < 0.01, cP < 0.001 vs. control group; dP < 0.05, eP < 0.01, fP < 0.001 vs. lipopolysaccharide group; gP < 0.05, iP < 0.001 vs. supernatant of Ad-Null modified dental pulp stem cells group. LPS: Lipopolysaccharide; DPSC-Null-CM: Supernatant of Ad-Null modified dental pulp stem cells; DPSC-HGF-CM: Supernatant of Ad-hepatocyte growth factor modified dental pulp stem cells; IL: Interleukin; TNF: Tumor necrosis factor; Arg2: Arginase 2; COX-2: Cyclooxygenase-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
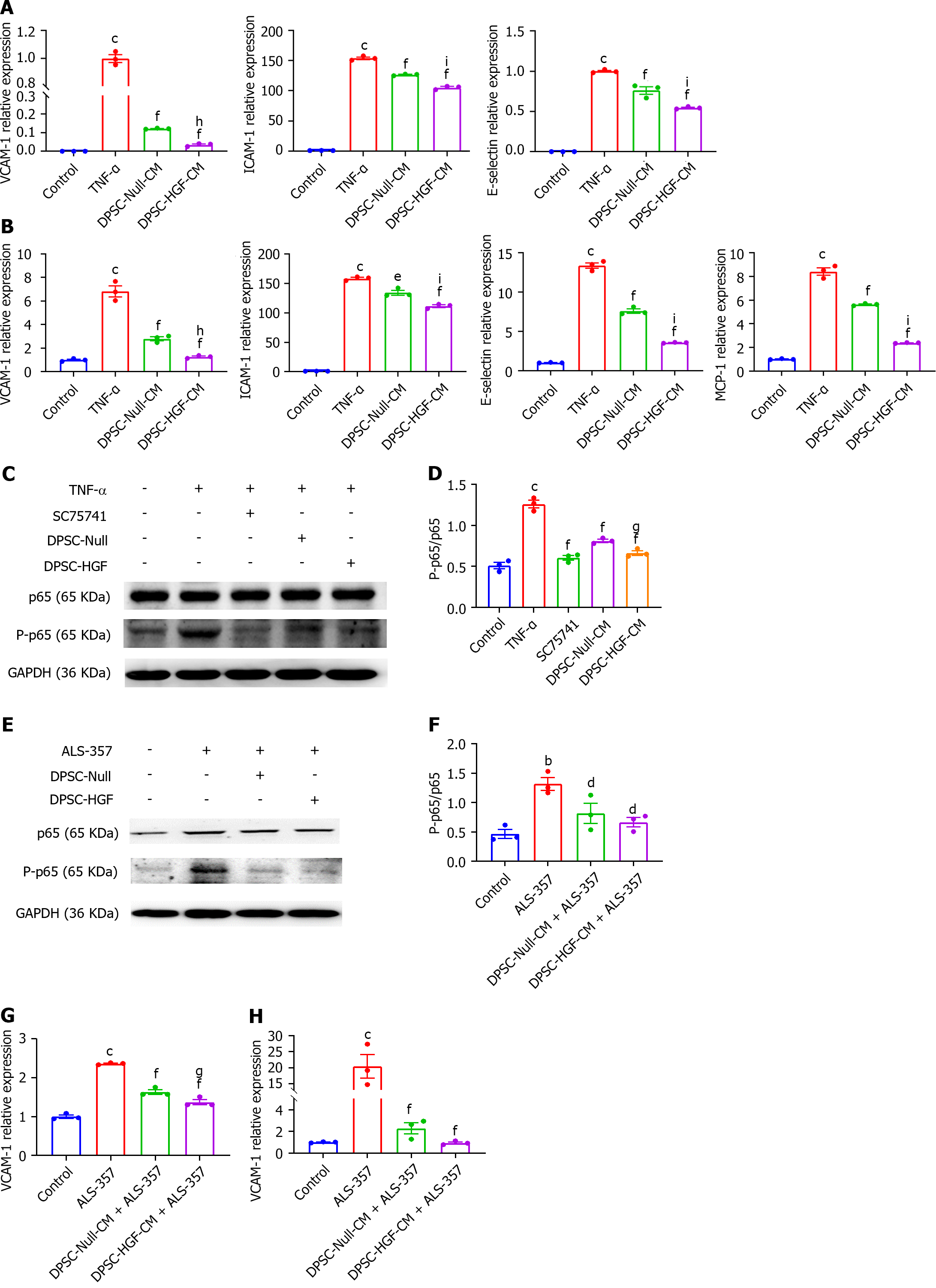
Figure 6 Effects of the supernatant of Ad-Null and Ad-hepatocyte growth factor modified dental pulp stem cells on the expression of adhesion molecules by human aortic endothelial cells under inflammatory stimulation.
A and B: Human aortic endothelial cells (HAOECs) were indirectly co-cultured with the supernatant of Ad-Null modified dental pulp stem cells (DPSC-Null-CM) or Ad-hepatocyte growth factor modified DPSCs (DPSC-HGF-CM) for 2 h or 8 h under tumor necrosis factor-α (TNF-α) (100 ng/mL) stimulation. The expression of the adhesion molecules vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1, E-selectin, and monocyte chemotactic protein-1 in HAOECs under inflammatory stimulation was detected by real-time reverse transcription polymerase chain reaction (RT-PCR) at 2 h (A) and 8 h (B) (n = 3). HAOECs were pretreated with DPSC-Null-CM or DPSC-HGF-CM for 4 h, and TNF-α (100 ng/mL) was then added for 5 min; C: The expression of phosphorylated p65 and p65 in inflammation-stimulated HAOECs was investigated using western blotting; D: Gray values analysis of Figure C; E: The expression of phosphorylated p65 and p65 in HAOECs under nuclear factor-κB (NF-κB) activator treatment was investigated using western blotting; F: Gray values analysis of Figure E; G and H: VCAM-1 was detected in HAOECs treated with the NF-κB activator by RT-PCR at 2 h (G) and 8 h (H) (n = 3). All results are represented as the mean ± SEM; bP < 0.01, cP < 0.001 vs. control group; dP < 0.05, eP < 0.01, fP < 0.001 vs. tumor necrosis factor-α group; gP < 0.05, hP < 0.01, iP < 0.001 vs. supernatant of Ad-Null modified dental pulp stem cells group. DPSC-Null-CM: Supernatant of Ad-Null modified dental pulp stem cells; DPSC-HGF-CM: Supernatant of Ad-hepatocyte growth factor modified dental pulp stem cells; TNF: Tumor necrosis factor; VCAM-1: Vascular cell adhesion molecule-1; ICAM-1: Intercellular adhesion molecule-1; MCP-1: Monocyte chemotactic protein-1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
- Citation: Duan H, Tao N, Lv L, Yan KX, You YG, Mao Z, Wang CY, Li X, Jin JY, Wu CT, Wang H. Hepatocyte growth factor enhances the ability of dental pulp stem cells to ameliorate atherosclerosis in apolipoprotein E-knockout mice. World J Stem Cells 2024; 16(5): 575-590
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/575.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.575