Copyright
©The Author(s) 2024.
World J Stem Cells. Oct 26, 2024; 16(10): 860-872
Published online Oct 26, 2024. doi: 10.4252/wjsc.v16.i10.860
Published online Oct 26, 2024. doi: 10.4252/wjsc.v16.i10.860
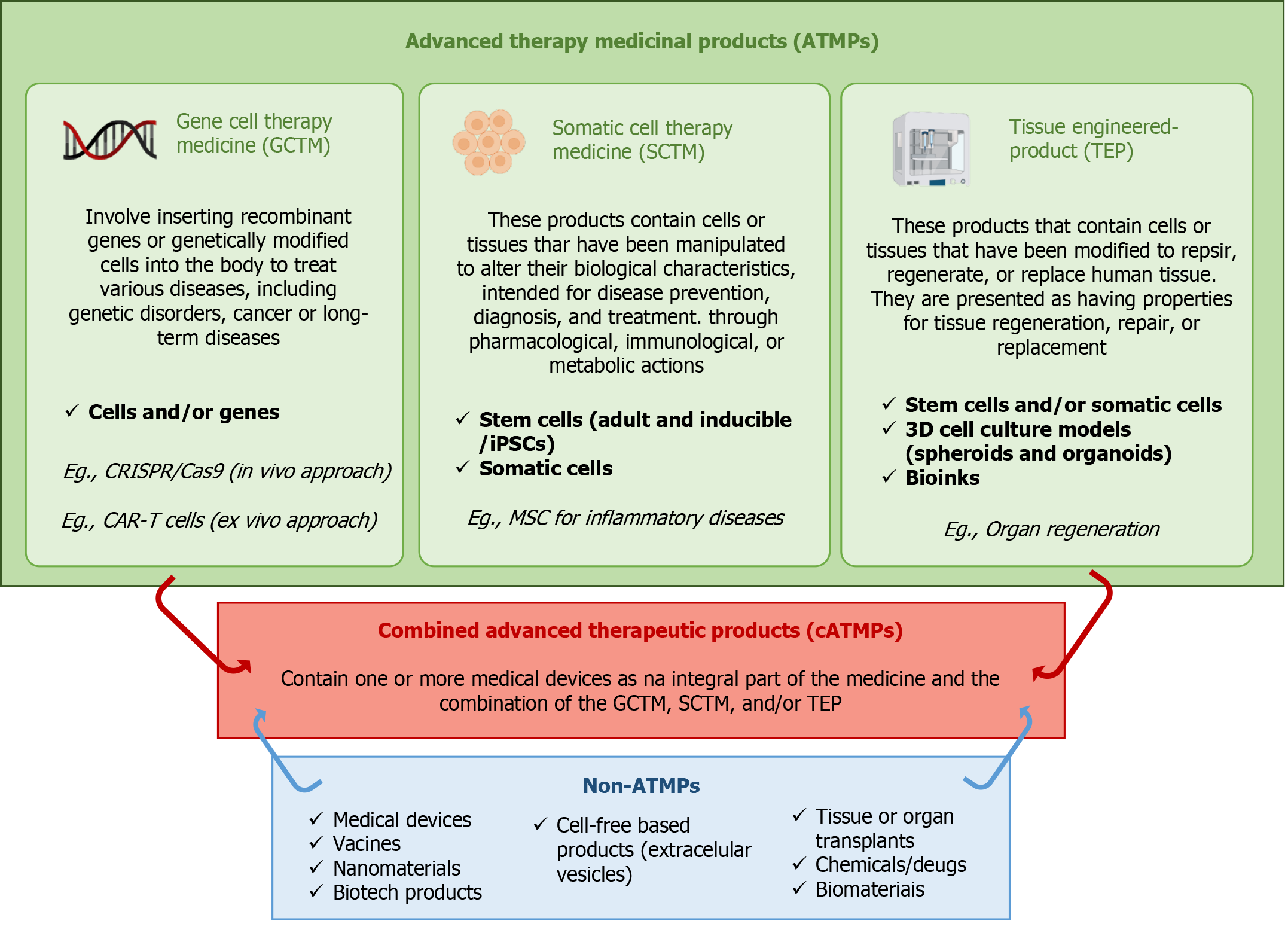
Figure 1 Three main types of advanced therapy medicinal products.
Non-advanced therapeutic products are regulated separately from advanced therapeutic products and may be used in combination with advanced therapeutic products. ATPs: Advanced therapeutic products; cATMP: Combined advanced therapy medicinal product; SCTM: Somatic cell therapy medicine; GTMP: Gene therapy medicinal product; TEP: Tissue-engineered products; iPSC: Induced pluripotent stem cell; MSC: Mesenchymal stem cell; CRISPR/Cas9: Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9; CAR-T: Chimeric antigen receptor T; 3D: Three-dimensional.
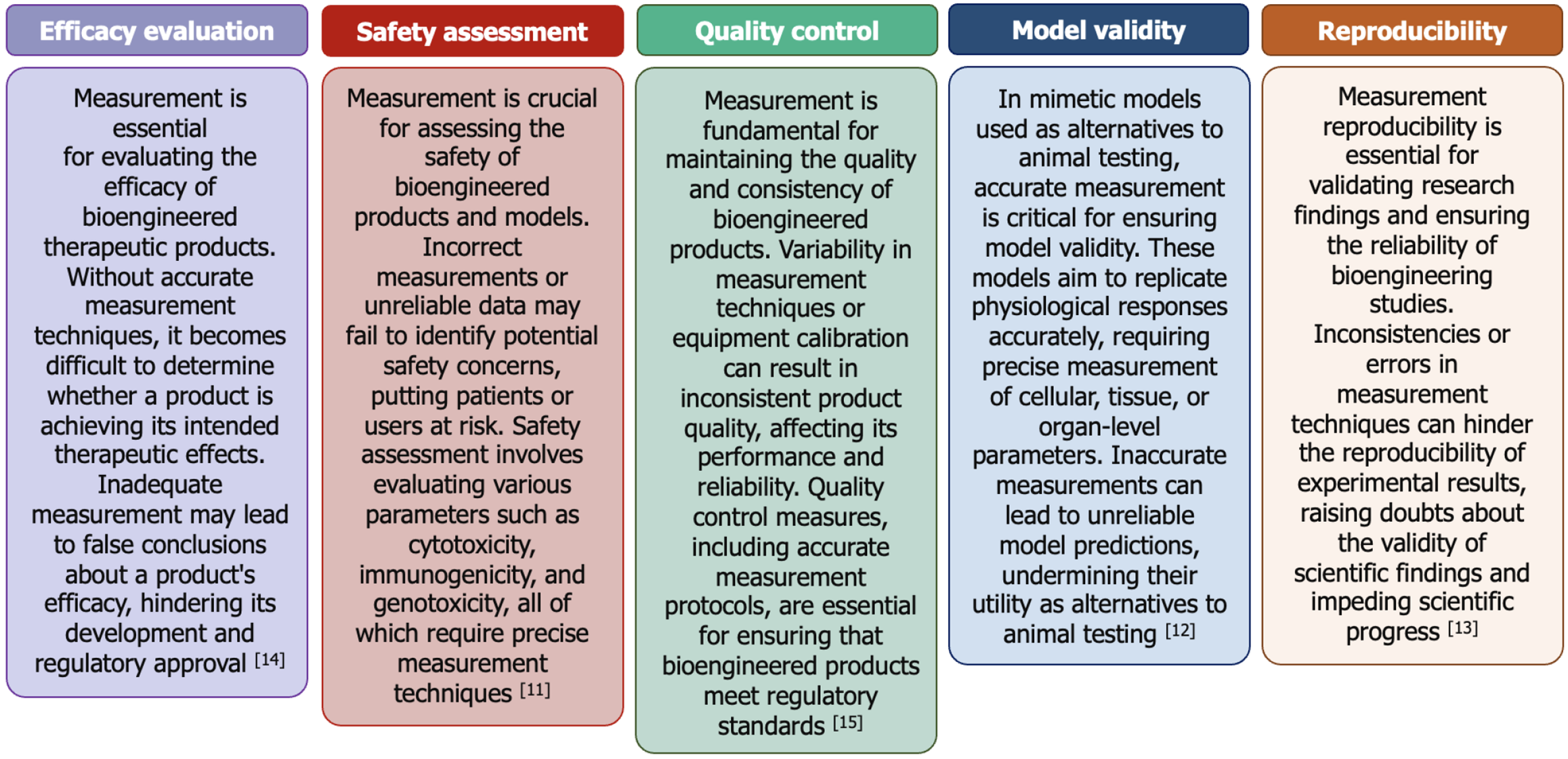
Figure 2 Measurement challenges that can hinder the progress of advanced therapeutic product development.
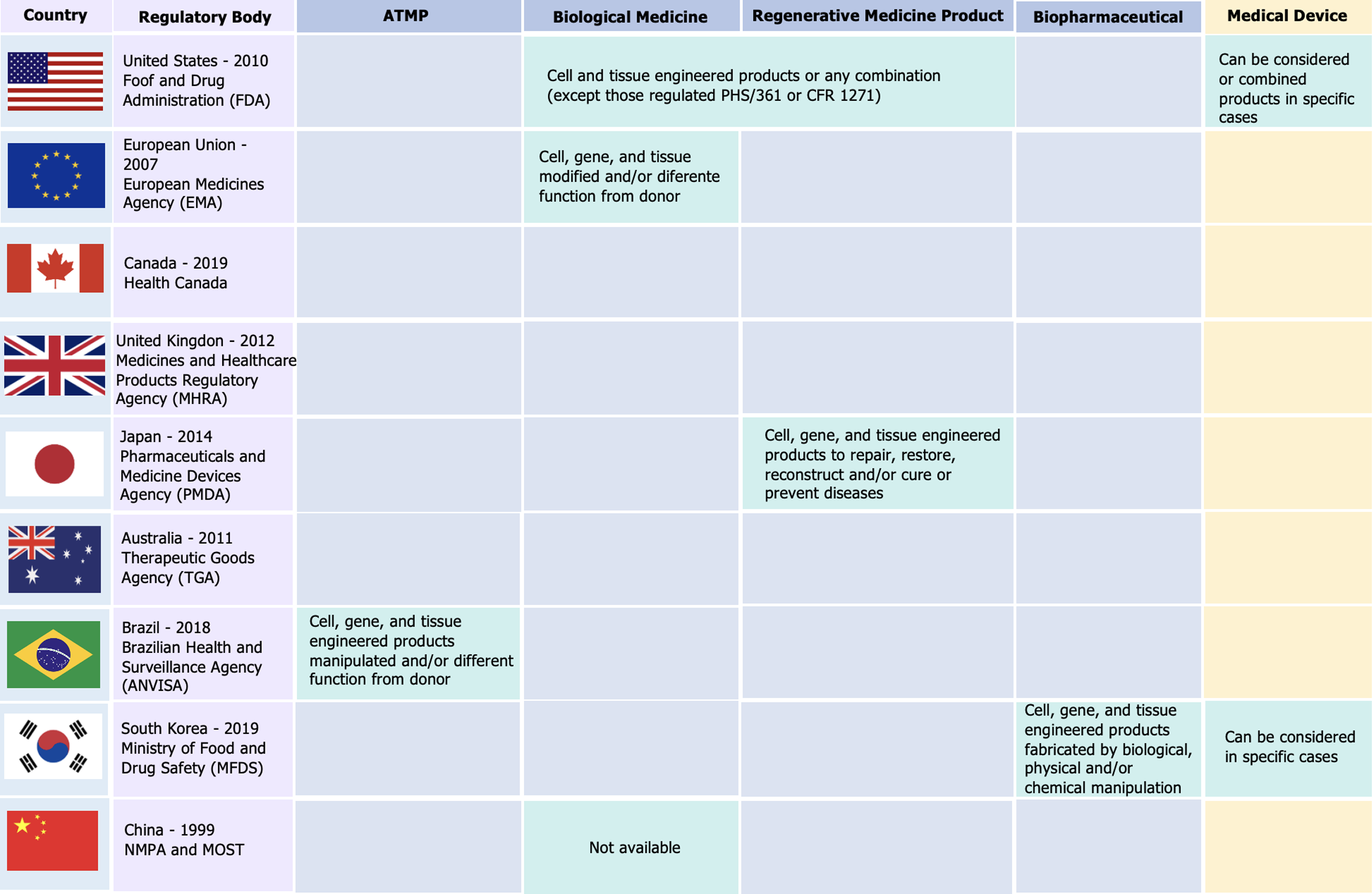
Figure 3 Regulatory landscape for advanced therapeutic products worldwide.
ATMP: Advanced therapeutic product; MOST: National Health Commission of the People’s Republic of China; NMPA: State Administration for Market Regulation.
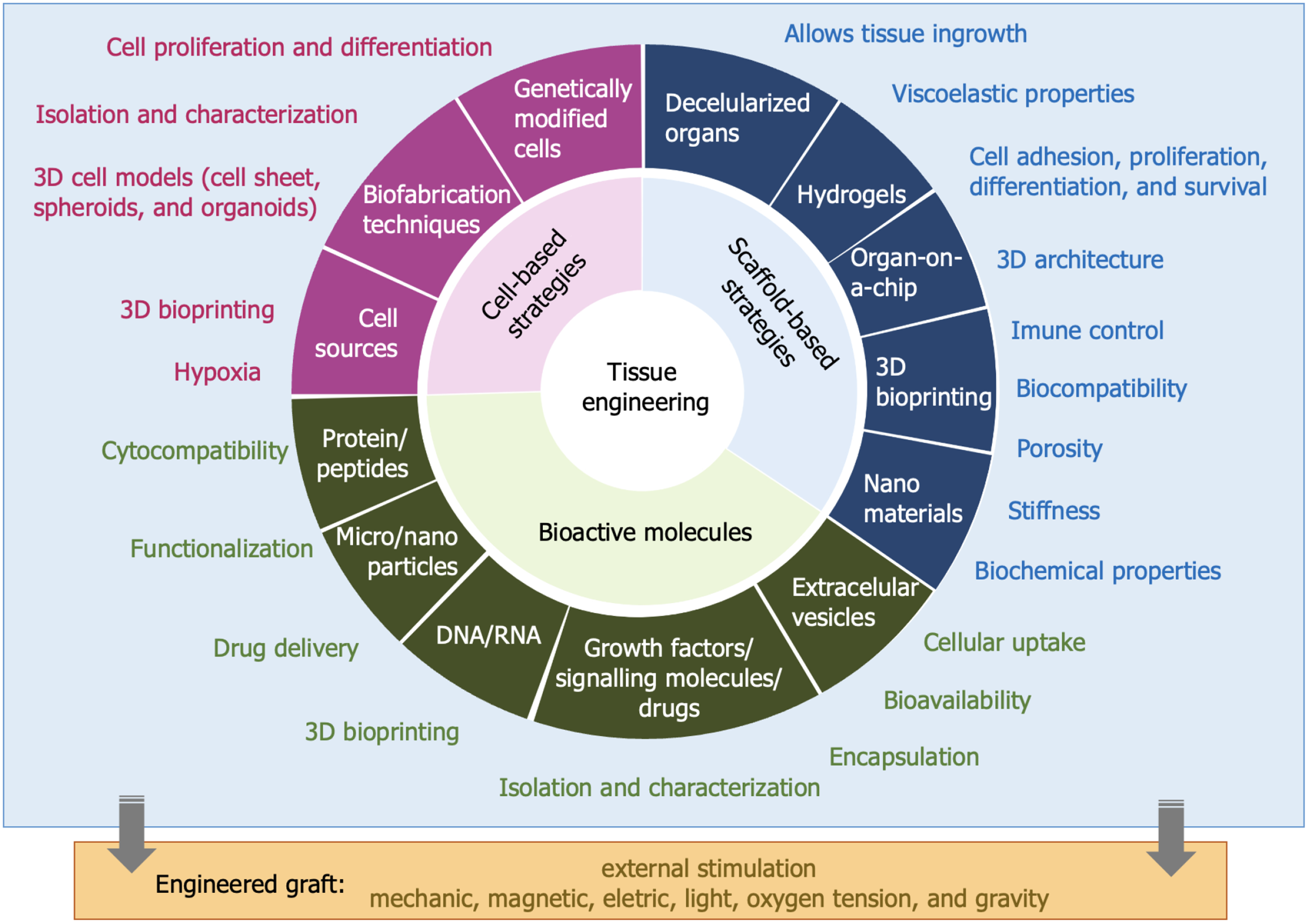
Figure 4 Tissue engineering basics.
Three essential components required for tissue regeneration are: (1) Living cells that can proliferate and differentiate to form new tissue; (2) Scaffolds that provide structural support for cell attachment and tissue formation; and (3) Bioactive molecules that promote cell proliferation, differentiation, and tissue development. 3D: Three-dimensional.
- Citation: Granjeiro JM, Borchio PGM, Ribeiro IPB, Paiva KBS. Bioengineering breakthroughs: The impact of stem cell models on advanced therapy medicinal product development. World J Stem Cells 2024; 16(10): 860-872
- URL: https://www.wjgnet.com/1948-0210/full/v16/i10/860.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i10.860