Copyright
©The Author(s) 2020.
World J Stem Cells. Mar 26, 2020; 12(3): 188-202
Published online Mar 26, 2020. doi: 10.4252/wjsc.v12.i3.188
Published online Mar 26, 2020. doi: 10.4252/wjsc.v12.i3.188
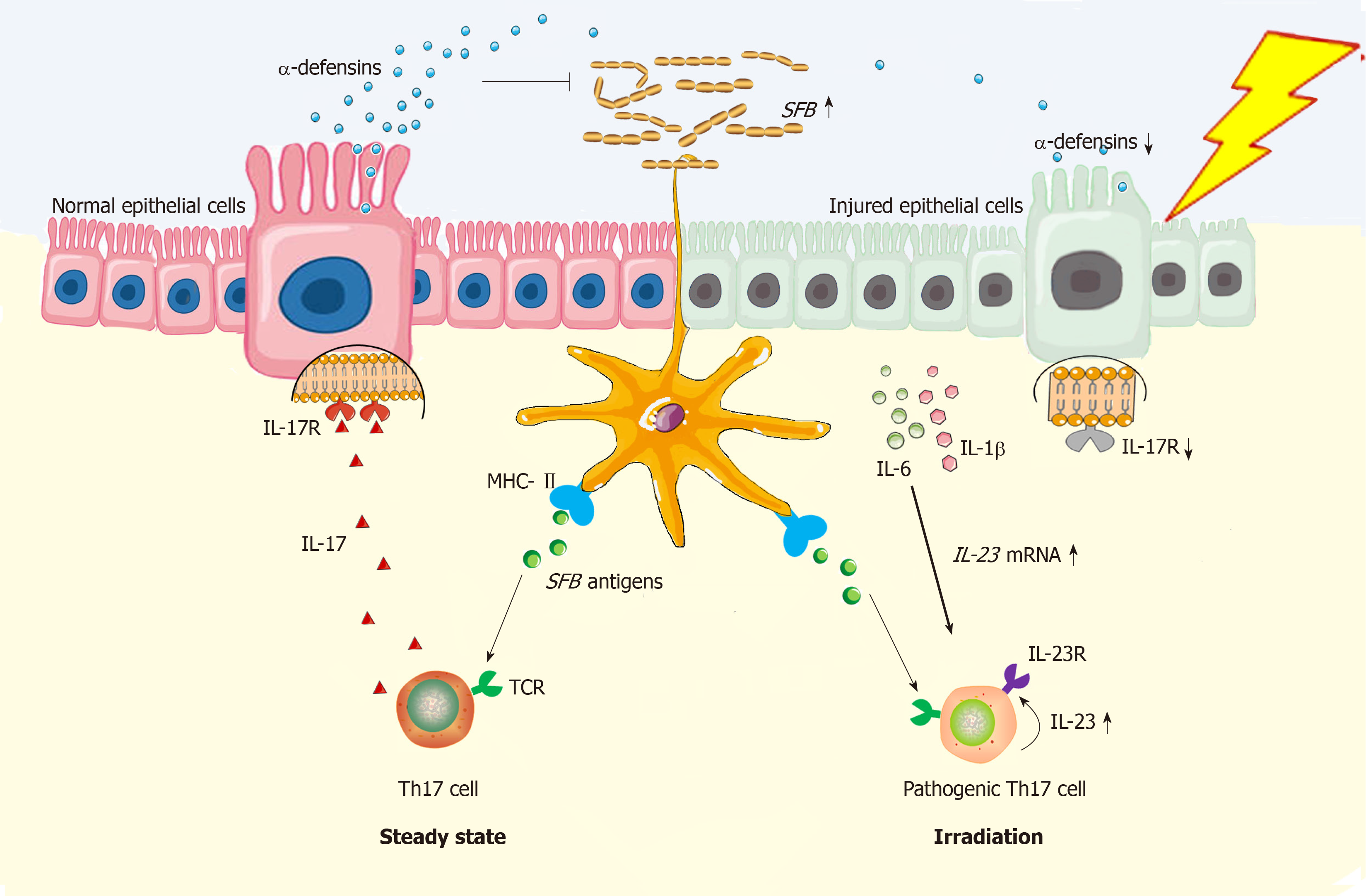
Figure 1 Schema of radiation exposure in pathogenic Th17 cell induction in gut.
In steady state, dendritic cells (DCs) are potent in Th17 induction in gut of mice because the T cell receptor recognizes the segmented filamentous bacteria (SFB) antigen presenting by DCs[28]; Meanwhile, MHC class II molecule on DCs can provide all essential signals for Th17 polarization[41]. Functionally, Th17 cells can stimulate synthesis of α-defensins by epithelial cells depending on interleukin (IL)-17/IL-17R interaction, thus protecting against SFB overgrowth in gut lumen[33]. However, under the irradiated condition, epithelial injuries will augment the local concentration of IL-1β and IL-6[31,35], which functionally upregulate expression of gene encoding IL-23[35,42]. By binding with IL-23R on Th17 cells, IL-23 is able to stimulate Th17 expansion[35]. Herein, both IL-23R/IL-22 loop and IL-23/IL-17 loop are able to increase Th17 cell-mediated immune response[26,43], thus enabling the inflammation in irradiated gut to persist. In this regard, Th17 cells are pathogenic. Besides, due to epithelial loss, low production of α-defensins will somewhat facilitate SFB overgrowth in gut lumen, thus facilitating Th17 induction as well. Collectively, Th17 cell induction will be robust in irradiated gut. DCs: Dendritic cells; SFB: Segmented filamentous bacteria; MHC-II: Major histocompatibility complex class II; TCR: T cell receptor; Th17: T helper cell 17; IL-17: Interleukin-17; IL-17R: Interleukin-17 receptor; IL-1β: Interleukin-1β; IL-6: Interleukin-6; IL-22: Interleukin-22; IL-23: Interleukin-23; IL-23R: Interleukin-23 receptor.
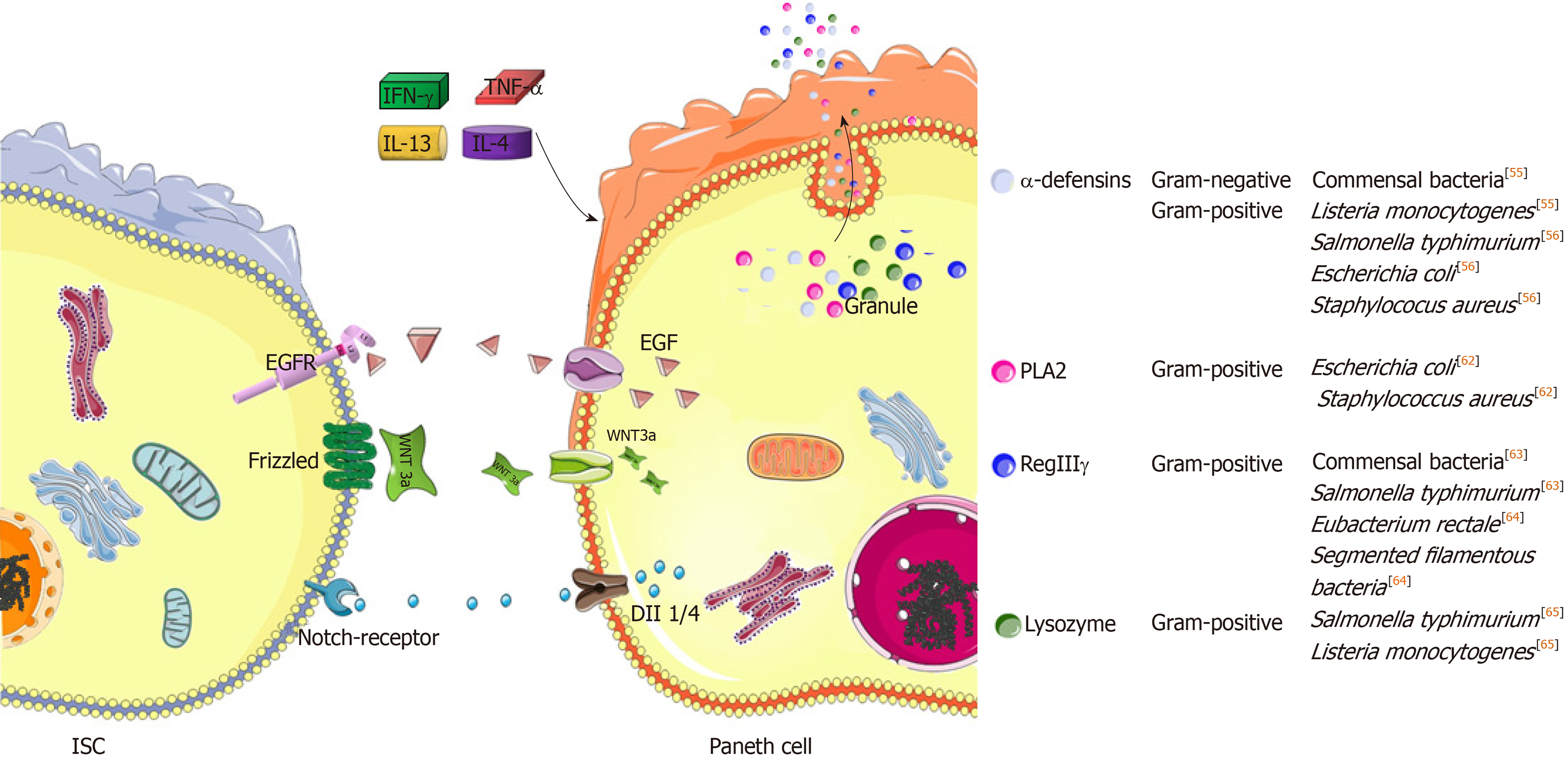
Figure 2 Specific roles of Paneth cells in maintaining epithelial homeostasis and defense.
Paneth cells are critical feeders due to their high secretion of epithermal growth factor, Wnt3a or Notch ligands to neighboring intestinal stem cells (ISCs), thus driving ISC expansion[44]. Moreover, several important peptides of antimicrobial functions are generated from Paneth cells[50]. In this situation, several inflammatory cytokines, including interferon-γ, tumor necrosis factor-α, interleukin (IL)-13 and IL-4, will elicit the degranulation of Paneth cells to antagonize luminal bacterial overgrowth. growth factor receptor; Dll1: Delta-like ligand 1; Dll4: Delta-like ligand 4; IFN-γ: Interferon-γ; TNF-α: Tumor necrosis factor-α; IL-13: Interleukin-13; IL-4: Interleukin-4; PLA2: Phospholipase A2; RegIIIγ: Regenerating islet-derived protein IIIγ.
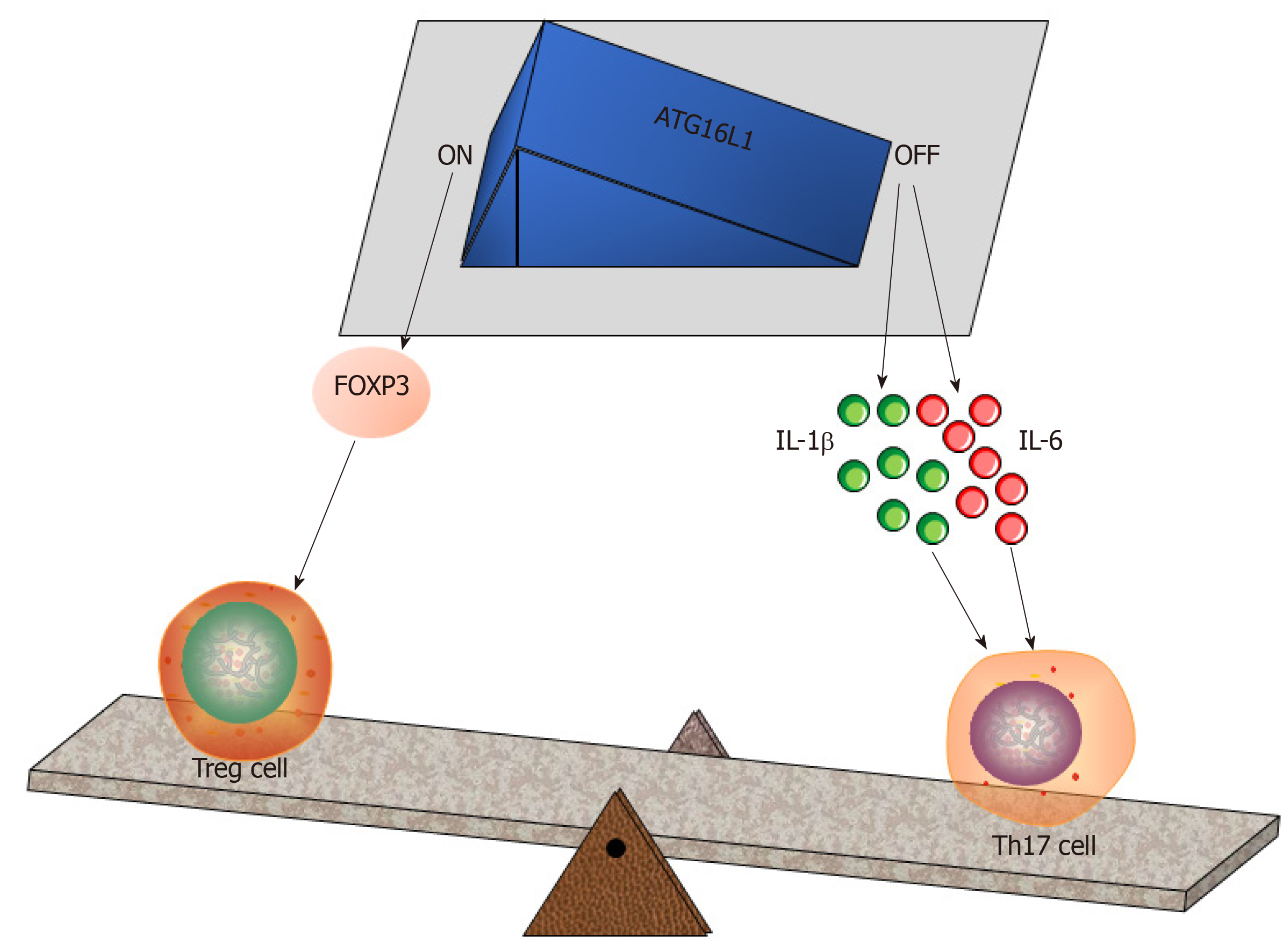
Figure 3 Autophagy-related protein 16 like protein 1 regulates the intestinal balance between Treg and Th17 cells.
The autophagy-related protein 16 like protein 1 (ATG16L1)-FOXP3 axis plays a vital role in Treg cell induction[24]. Conversely, a deficiency of ATG16L1 enables Paneth cell differentiation to be hampered, while this situation will increase interleukin (IL)-1β and IL-6 in the gut[71], thus promoting Th17 commitment. Treg: Regulatory T cell; Th17: T helper cell 17; IL-1β: Interleukin-1β; IL-6: Interleukin-6; ATG16L1: Autophagy-related protein 16 like protein 1.
- Citation: Gao YL, Shao LH, Dong LH, Chang PY. Gut commensal bacteria, Paneth cells and their relations to radiation enteropathy. World J Stem Cells 2020; 12(3): 188-202
- URL: https://www.wjgnet.com/1948-0210/full/v12/i3/188.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i3.188