Copyright
©The Author(s) 2020.
World J Stem Cells. Feb 26, 2020; 12(2): 123-138
Published online Feb 26, 2020. doi: 10.4252/wjsc.v12.i2.123
Published online Feb 26, 2020. doi: 10.4252/wjsc.v12.i2.123
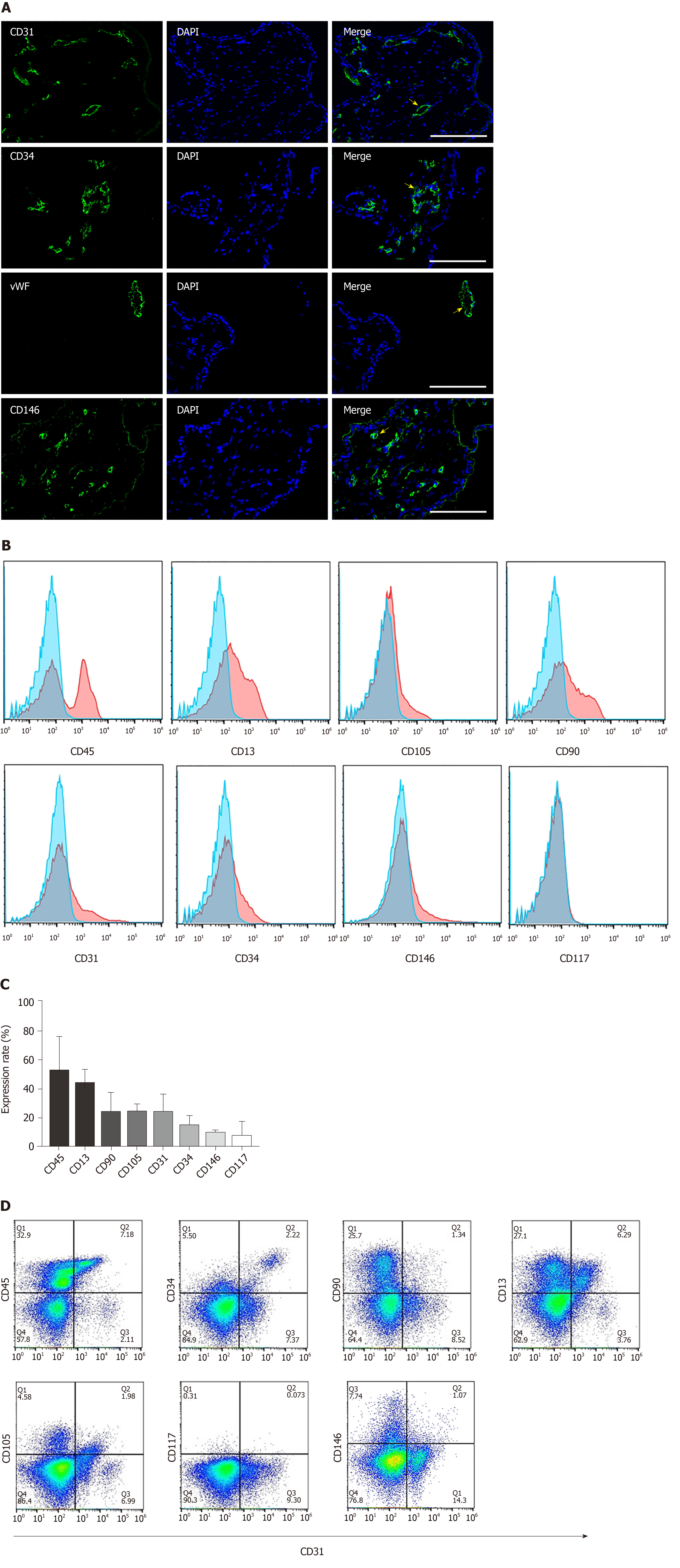
Figure 1 Characterization and cellular composition of the human early gestation placenta.
Cellular composition of the human early gestation placenta chorionic villi was characterized. A: Immunofluorescence staining of CD31, CD34, von Willebrand factor and CD146 showed positive cells, depicted with arrows, located in chorionic villi and organized into blood vessel structures; B, C: Flow cytometry analysis of the surface markers of single cells derived by enzymatic dissociation of the placental chorionic villi; each marker’s expression is quantitively presented (data were expressed as mean ± SD, n = 3); D: Flow cytometry analysis of the co-expression of typical endothelial cell marker CD31 and other surface markers. Scale bar = 100 µm.
Figure 2 Isolation procedure of endothelial colony-forming cells from placental chorionic villi.
A: Depiction of the isolation of chorionic villus endothelial colony-forming cells from chorionic-villus cells by enzymatic dissociation of chorionic villus tissue, followed by CD31 magnetic bead sorting and subsequent manual clonal isolation and expansion; B: Morphology of the colonies formed by CD31+-sorted cells. CD31 was expressed only by colonies that had a cobblestone-like morphology and not by ones that displayed spindle shaped morphology. Two representative colonies are shown for each morphology; C: Immunofluorescence staining of isolated colonies for expression of CD31 and CD144. Scale bar = 100 µm.
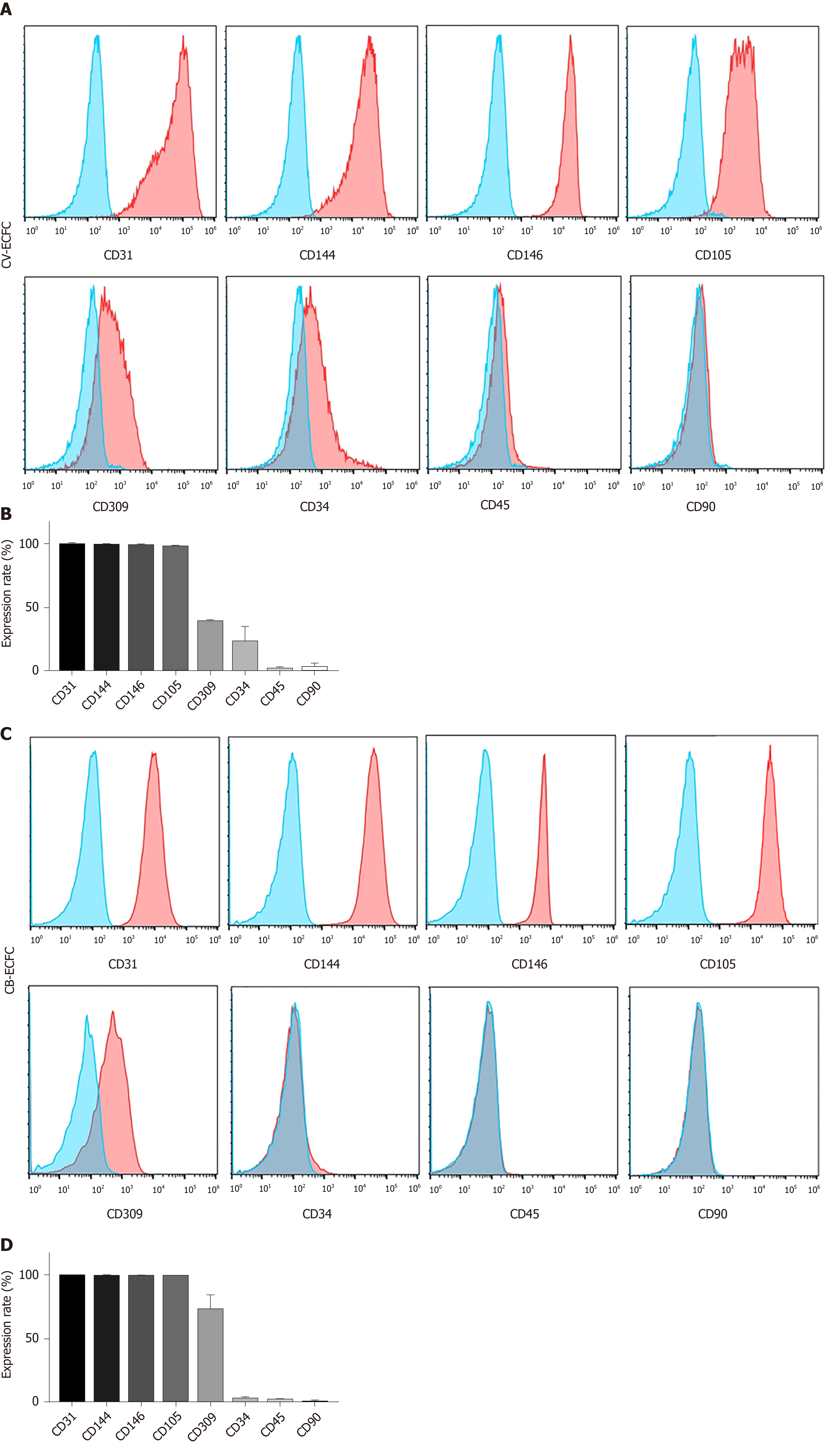
Figure 3 Chorionic villi-derived endothelial colony-forming cells express typical endothelial surface markers, similar to cord blood-derived endothelial colony-forming cells.
A: Flow cytometry immunophenotypic analyses demonstrate that chorionic villus endothelial colony-forming cells were positive for the endothelial markers CD31, CD144, CD146, CD105, CD309, low expression of CD34, and were negative for the hematopoietic and MSC markers CD45 and CD90, respectively; B: Quantification of the markers; C and D: Flow cytometry immunophenotypic analysis of the surface marker of cord blood-derived endothelial colony-forming cells. Data are expressed as mean ± SD, n = 3.
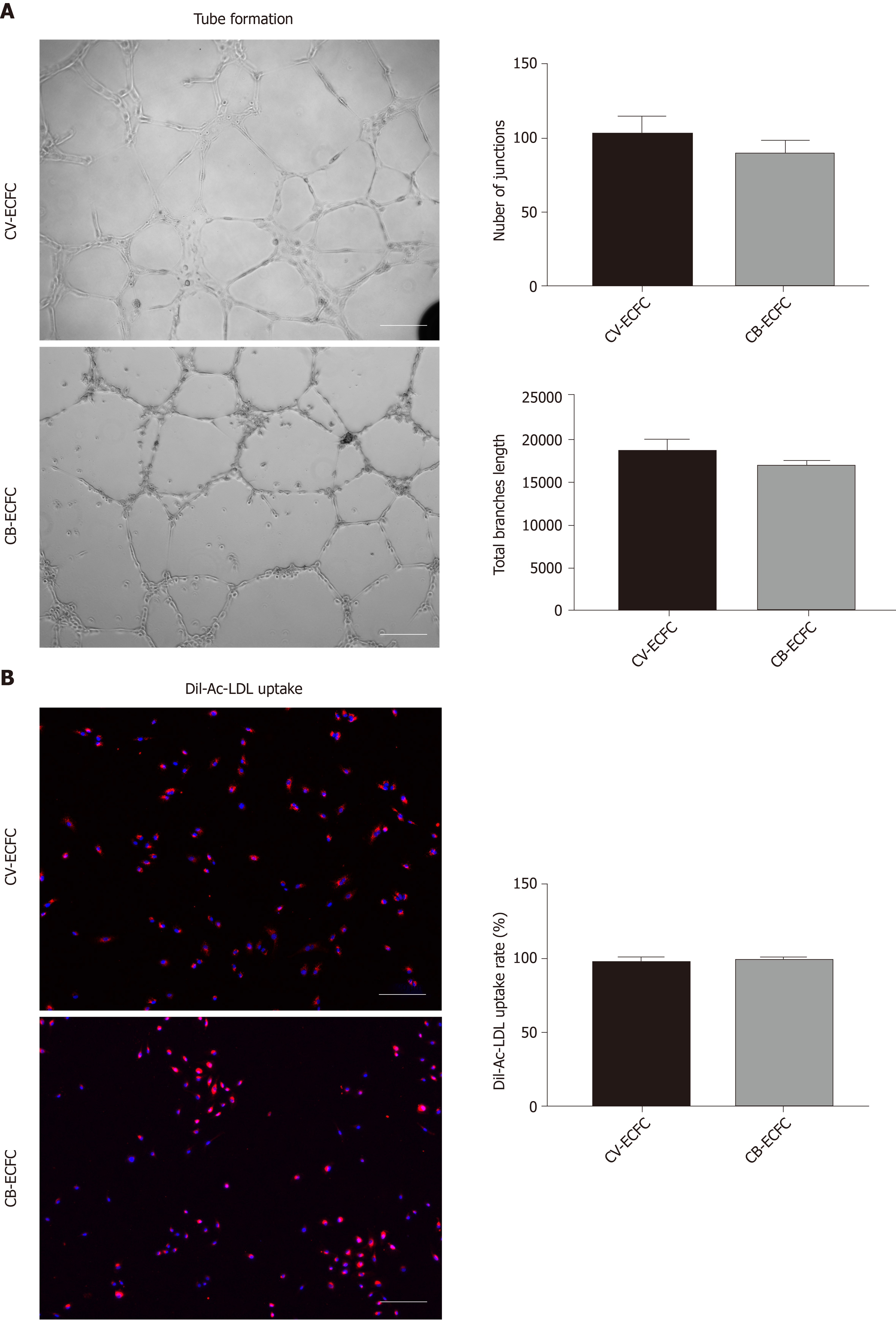
Figure 4 Chorionic villus endothelial colony-forming cells have endothelial functions.
A: Representative phase-contrast images of chorionic villus endothelial colony-forming cells (CV-ECFCs), cord blood-derived ECFCs (CB-ECFCs) tube formation assay, and quantification of junction numbers and total branch lengths. There is no difference between CV-ECFCs and CB-ECFCs; B: Representative images for Dil-Ac-LDL uptake by CV-ECFCs and CB-ECFCs. There is no difference in their respective uptake rates. Data are expressed as mean ± SD, n = 3, Scale bar = 100 µm.
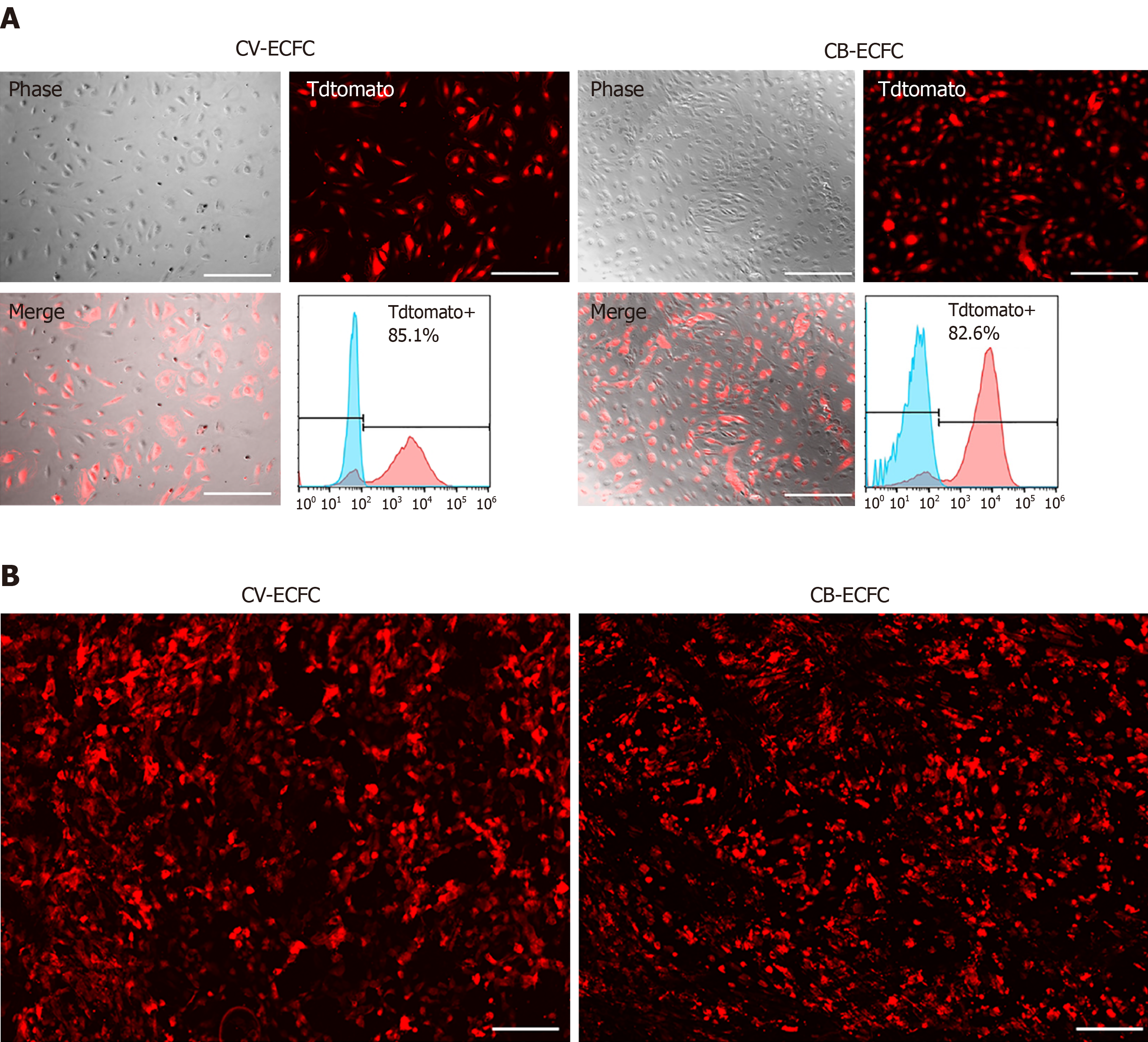
Figure 5 Chorionic villus endothelial colony-forming cells can be transduced by lentiviral vector and are compatible with a small intestinal submucosa scaffold.
A: Representative images of chorionic villus endothelial colony-forming cells (CV-ECFCs) and cord blood-derived ECFCs (CB-ECFCs) transduced with a pCCLc-MNDU3-LUC-PGK-Tomato-WPRE lentiviral vector. Flow cytometric analysis showed a transduction rate of 85.1% and 82.8%, respectively; B: tdTomato lentiviral vector-transduced CV-ECFCs (left panel) and CB-ECFCs (right panel) seeded onto a small intestinal submucosa extracellular matrix scaffold, showing adherence. Scale bar = 100 µm.
- Citation: Gao K, He S, Kumar P, Farmer D, Zhou J, Wang A. Clonal isolation of endothelial colony-forming cells from early gestation chorionic villi of human placenta for fetal tissue regeneration. World J Stem Cells 2020; 12(2): 123-138
- URL: https://www.wjgnet.com/1948-0210/full/v12/i2/123.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i2.123