Published online Jul 15, 2003. doi: 10.3748/wjg.v9.i7.1625
Revised: January 14, 2003
Accepted: February 8, 2003
Published online: July 15, 2003
AIM: As the conventional combined liver-small bowel transplantation is complicated with many postoperative complications, the aim of this study was to describe a modified technique for the combined liver-small bowel transplantation with preservation of the duodenum, partial head of pancreas and hepatic biliary system in pigs.
METHODS: Composite liver/small bowel allotransplantations were undertaken in 30 long-white pigs. The graft included liver, about 3 to 4 m proximal jejunum, duodenum and partial pancreatic head. Vessels reconstructions included subhepatic vena cava-vena cava anastomosis, aorta-aorta anastomosis and portal-splenic vein anastomosis.
RESULTS: Without immunosuppressive treatment, the median survival time of the animals was 6 d (2 to 12 d), and about 76.9% (20/26) of the animals survived for more than 4 d after operation.
CONCLUSION: The modified technique is feasible and safe for the composite liver/small bowel transplantation with duodenum and pancreas preserved in pigs. And also this technique can simplify the operation and decrease possible postoperative complications.
- Citation: Yin ZY, Ni XD, Jiang F, Li N, Li YS, Li JS. Modified technique for combined liver-small bowel transplantation in pigs. World J Gastroenterol 2003; 9(7): 1625-1628
- URL: https://www.wjgnet.com/1007-9327/full/v9/i7/1625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i7.1625
As a result of total parenteral nutrition (TPN) induced end-stage liver disease, 60% to 70% of recipients of intestinal transplant procedures require simultaneous liver allografts[1,2]. Although many clinical liver/small bowel transplantations (LSBT) were reported from different medical centers[3-5], it remains an experimental procedure[6]. Compared to the widely used rat LSBT model[7,8], large animal models such as LSBT in pigs were rarely reported.
As a conventional composite liver-small bowel graft requires a loop of defunctionalized (Roux) allograft small bowel for biliary drainage[9], its posttransplant biliary complications include anastomotic leaks and obstruction in 12% of the cases, with significantly associated morbidity and mortality in clinical reports[1]. In the present study, we modified the technique for LSBT by preserving the duodenum, partial head of pancreas and hepatic biliary system, and also we modified the conventional vena cava and the portal drainage anastomotic methods. Experience with this technique for the LSBT in pigs has not been described previously.
Donor preparation 60 long-white pigs weighing 20-40 Kg with random sex were undertaken 30 LSBTs. The weight of the donor was generally lower than that of the corresponding recipient. No immunosuppressive treatment was given in the group.
Preoperative treatment The animals were not allowed to eat for 24 h and drink for 4 h before operation respectively. The gut decontamination was attempted in all donors with an oral antibiotic preparation 3 d before surgery.
After anesthesia with 25 mg/kg of intravenous pentobarbital sodium, the animal was intubated and mechanically ventilated with a mixture of oxygen, nitrous oxide and isoflurane. In addition, the standard intravenous antibiotic prophylaxis was instituted with cefotaxime at the time of surgery.
Initial exposure and isolation of the abdominal organs The procurement varied in details but followed the standard techniques for human multiorgan retrieval[10-12]. Briefly, the donor operation was performed through a midline laparotomy. Cares should be taken not to damage the urethra of male pigs when opening the abdomen. The liver was mobilized by dividing its suspensory attachments. The right gastric and right gastroepiploic vessels were divided, and the pylorus was transected, which allowed the stomach to be reflected craniad. The proximal 3 to 4 m of jejunum (the total porcine small bowel is about 15 m) together with the liver was procured as the graft. After the redundant small bowel was dissected, its supply vessels were ligated. Then the intestinal tract was transected at the beginning of the descending colon, and then the redundant small bowel and the colon were removed from the operative field. Thus the duodenum, proximal jejunum and the aorta could be well exposed. The left gastric artery was ligated at the celiac axis.
Dissection of the vessels The suprahepatic vena cava was firstly dissected and encircled. An extensive Kocher maneuver allowed visualization of the inferior vena cava and its branch. The subhepatic vena cava was dissected and encircled. The left and right renal veins were ligated respectively.
With division of the left retroperitoneal artery, the superior mesenteric and celiac arteries were identified by extending dissection of the aorta. The right and left renal arteries were then isolated and ligated. The subrenal aorta was isolated and encircled distally for the eventual insertion of an infusion cannula. The abdominal aorta was also encircled above the celiac axis for later crossclamping when cold fluid was infused through the distal aortic cannula[13]. It should be mentioned that dissection of the celiac trunk would always open the diaphragm and result in pneumothorax.
The splenic vein was then freed and prepared for portal perfusion cannulation after division of the splenic artery. The splenic vein should be well protected when dividing the splenic artery[14].
In situ cooling and removal of the organs Unlike the clinical transplantation, it was imperative to collect the donor's blood for the recipient operation. Before the infusion with cold solution, the donor's blood was collected from the iliac artery.After completion of the preliminary dissections and collection of the donor's blood, the liver and small bowel connected by the portal vein and the aortic segment were lavaged in situ with UW solution. Briefly, the donor was fully heparinized and the previously encircled proximal aorta was crossclamped, and the distal donor aorta was cannulated with infusion of cold UW solution. For the simultaneous portal venous infusion, a venous cannula was placed into the splenic vein and infused with the UW solution. The intrapericardial inferior vena cava and the subhepatic vena cava were transected to decompress the infused solution as in other reports[15,16]. The amount of infusion was variable (between 50 mL/Kg and 100 mL/Kg), guided by blanching the organs and estimated by palpation of the degree of cooling. If the intestine did not feel cold after limited perfusion, there was no reason for concern, providing it was blanched, further surface cooling after immersion in cold fluid was rapid as the intestine is a hollow organ[11]. It is important to avoid both venous hypertension and overperfusion of the intestine and pancreas, as overperfusion might result in duodenopancreatic and small bowel edema[13,17]. Some solution was injected into the gallbladder to lavage the bile tract.
After infusion, the graft containing liver, hepatic hilus, pancreatic-duodeno complex, spleen together with the splenic vein, and small bowel was achieved with preservation of a segment of aorta containing the superior mesenteric artery and celiac trunk in continuity. The intestine was entrapped by stapling its two ends and carried with the specimen throughout the preservation. Thus the graft was en bloc removed and stored in UW solution at 0 to 4 °C.
Back table procedure Back table procedure was performed in the cold UW solution. It included suturing the orifice of suprahepatic vena cava and the proximal end of the aorta. The spleen was removed and the splenic vein was well preserved. The body and tail of the pancreas along the portal vein were isolated and transected, leaving partial pancreatic head attached to the allograft duodenum. This preserved the superior and inferior pancreatic duodenal arcades. The stump of the pancreas was stapled and then oversewn with a running suture using 4-0 polypropylene. The gallbladder was removed and a catheter was placed into the cystic duct stump for the early decompression and study of the donor biliary system during the early postoperative period.
After anesthesia, a monitor was placed on the recipient. Two venous catheters were inserted for transfusion and central venous pressure monitoring. The arterial blood pressure was monitored through a thigh artery catheter.
Intestinal resection When the abdomen was opened, the small bowel and its mesentery were dissected. Most of the intestine was removed for an artificial short bowel with only about 30 cm left at each end.
Vascular preparation The subrenal aorta was exposed and encircled 2 cm under the renal artery. Small arterial and lymphatic vessels along the aorta were ligated to avoid later bleeding or lymphorrhea. Subhepatic vena cava was also dissected and encircled. The place just above the left renal vein was routinely used for the donor out-flow anastomosis. The hilar of the recipient liver was dissected. The common bile duct and the liver artery were transected while the portal vein was crossclamped with a bulldog after transection.
Hepatectomy The resection of the recipient liver was another major step. The total vascular exclusive technique for hepatectomy in human being was not suitable to the pig, so the liver was removed lobe by lobe. Since the retrohepatic vena cava in the pig was passing through the liver with numerous small hepatic veins draining to this segment besides the major hepatic veins, it was hard to be skeletonized. In order to avoid massive bleeding when dissecting the retrohepatic vena cava, a small part of the liver around the retrohepatic vena cava was always saved and oversewn.
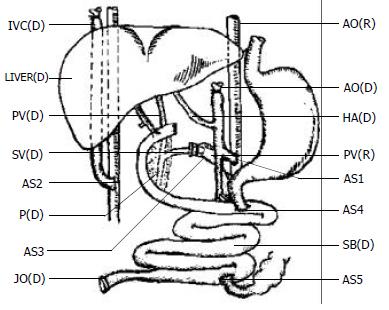
Graft implantation and revascularization The transplantation methods varied in details but followed the principles as described previously[11]. The typical reconstruction is shown in Figure 1.
The graft was placed in an orthotopic position. The arterial inflow was created via an end-to-side anastomosis of the graft aorta to the subrenal native aorta with a running polypropylene suture. Donor and recipient vena cava were anastomosed end to side. The venous outflow was a modification of the piggyback fashion with the end of the graft subhepatic vena cava anastomosed to the side of the native subhepatic vena cava.
Before reperfusion, unclamping and perfusion of the allograft liver were achieved after a lavage of 300 to 500 mL donor blood or Ringer's solution through the splenic vein.
Anastomosis of the donor splenic vein to the recipient portal vein By using the graft splenic venous stump, a branch point left could be clamped separately and anastomosed to the recipient portal vein to allow outflow of the retained recipient viscera (stomach, pancreas, and spleen as well as the remaining native intestine). Since the splenic vein was smaller than the recipient portal vein, the cuff technique was always used for this anastomosis.
Gastrointestinal reconstruction The proximal duodenum was closed. The intestinal continuity was established proximally by end-to-side recipient-to-donor jejuno-jejunal anastomosis. The end of the recipient's distal ileum was anastomosed to the side of the donor distal jejunum including a donor distal intestinal vent for the early decompression and surveillance endoscopies as described in some clinical reports[18,19]. The bile was drained with a catheter in the donor's cystic tract.
Postoperative management After operation, the animal was returned to the monitor room, where hemodynamic monitoring and mechanical ventilation were performed as needed 24 h after operation. Due to the high rate of inflammatory complications, broad-spectrum antibacterial prophylaxis was administered once for 5 d. Lactated Ringer’s solution and parenteral nutrition were given daily until the animal was able to eat and drink.
The appearance of the allograft ostomy and the amount of ostomy output were useful clinical signs of graft dysfunction. Ostomy losses up to 100 cc/kg per day were acceptable and could be compensated by supplementary intravenous fluids.
After reperfusion, the liver was soft and pink with prompt bile production, evidenced through the cystic duct catheter. If the liver was harder than normal, the outflow of the liver might be obstructed and the vena cava anastomosis was required to be checked. The small bowel would be perfused well, with good mesenteric arterial inflow and venous outflow. The peristalsis and intraluminal mucous production were evident within 15 min after reperfusion.
Animals died suddenly after reperfusion were ruled out from the statistic series. 4 recipients died because of post reperfusion syndrome and operative techniques. The other 26 LSBT pigs had a median survival time of 6 d (from 2 to 12 d). The surgical records are shown in Table 1, and the values were expressed as median (range).
| Parameters | Values |
| Weight of the donor (Kg) | 22.5 (19-25) |
| Weight of the recipient (Kg) | 25 (22-38) |
| Length of the graft small bowel (m) | 3.4 (2.8-4.2) |
| Weight of the graft liver (g) | 760 (620-960) |
| Collection of the donor blood (ml) | 800 (400-1200) |
| Cooling solution (ml) | 1400 (1200-2000) |
| Donor operative time (hr) | 3.3 (2.8-3.6) |
| Back table time (hr) | 0.8 (0.5-1.1) |
| Preservation time (hr) | 3.8 (3.0-4.5) |
| Total cold ischemia time (hr) | 5.6 (3.8-6.6) |
| Total operative time (hr) | 8.2 (7.0-11.4) |
| Postoperative survival time (day) | 6 (2-12) |
| Survival rate (more than 4 d) | 76.9%(20/26) |
During the first three days, the intestinal graft stoma appeared healthy, and the mucosa was pink, moist, and well vascularized. No intestinal edema was found in most cases with stomal output averaging 500 mL/d and characterized by bile-stained stool. The high stomal output would decrease with time.
All liver grafts functioned immediately after serum bilirubin and transaminase levels peaked on the first postoperative day and fell rapidly thereafter.
Neither the duodenal allografts experienced signs of ischemia or stump leakage, nor experienced any biliary complication. Abdominal drains were monitored serially for amylase and lipase. Chemical pancreatitis was observed during the early postoperative period with lipase-rich fluid drainage. The biopsies of the dead animals indicated mild pancreatitis in the remained pancreas.
Histopathologic studies of the grafts showed no significant preservation injury. None of the biopsies obtained in the first postoperative week had histological evidence of submucosal bacterial invasion. The frequent cause of death was postoperative rejection convinced by the graft biopsies when the animal was dead.
Specialties of porcine anatomy and LSBT model Firstly, the porcine liver is divided into 4 relatively independent lobes. There are obvious borderlines between lobes. This is the reason why we can remove the liver lobe by lobe when total vascular exclusive technique can not be used in hepatectomy. Secondly, the porcine retrohepatic vena cava is passing through the liver parenchyma with numerous small hepatic veins outflow to this segment besides the major hepatic veins. It is dangerous to remove the liver parenchyma when dissecting the retrohepatic vena cava. This is the reason why the classic piggyback liver transplantation is not suitable to the pig. The vena cava anastomosis was modified by replacing the major hepatic vein (or suprahepatic vena cava) anastomosis in classical piggyback transplantation with the subhepatic vena cava anastomosis (Figure 1). This modification has at least three advantages in porcine LSBT: (1). It is safe to remove the liver, for the whole recipient liver is not moved to expose the retrohepatic vena cava. (2). The subhepatic vena cava anastomosis can be easily performed. (3). The subhepatic vena cava anastomosis can adjust a flexible anastomotic interval to make the aorta-aorta and the portal-splenic vein anastomosis easier.
The third difference of porcine anatomy is that the interval between the celiac axis and superior mesenteric artery is longer than that of human being. It is about 2 to 2.5 cm in general, so the Carrel patch with celiac axis and superior mesenteric artery in human LSBT is not suitable to the pig. The long segment of aorta with celiac axis and superior mesenteric artery was used in porcine LSBT. (Figure 1).
Feasibility and safety of the porcine LSBT with pancreatic head and duodenum In clinical practice, Abu-Elmagd suggested that LSBT with pancreatic head and duodenum had some advantages including avoidance of biliary complications and simplification of the operative procedure [20]. These possible advantages might exist in the animal LSBT.
The LSBT transplant procedure is a much more arduous surgical endeavor. The technique retaining the duodenum and the head of pancreas would simplify the back table preparation and avoid risks associated with dissection of the donor hepatic hilus.
Retrieval for composite grafts using the standard technique involves an obligatory reconstruction of the biliary system with a defunctionalized loop of proximal allograft jejunum[3]. In this porcine LSBT model, no biliary reconstruction is required to eliminate the source of complications such as bile leakage, bile tract stricture or even the death of recipient. Liver transplantation related biliary complication rate is about 12%, which would result in about 19% of death of them[21,22]. The LSBT with partial pancreas and duodenum would remarkably decrease such complications.
Without donor or recipient bowel for Roux-en-Y biliary reconstruction would enhance the potential benefits of any intestinal segment in freeing the pig for TPN, as it is directly kept in continuity with the alimentary tract.
The advantage of the composite technique is to maintain the hepatic hilus. The use of liver artery and superior mesenteric artery with a large arterial conduit would minimize the risk of hepatic artery thrombosis compared to isolated graft[18,23].
In the experiments, we found that the graft with duodenum and partial pancreas was also convenient to be implanted as compared to the standard LSBT. Only three vascular anastomoses were required, including aorta-aorta anastomosis, vena cava-vena cava anastomosis and portal-splenic vein anastomosis. The liver artery anastomosis and biliary reconstruction are not necessary when this method is used. Since the aorta and vena cava are end-to-side anastomosed with the recipient's aorta and vena cava partially excluded during the anastomosis. This method would avoid not only hemodynamic damages, but also possible kidney injuries, postoperative thrombus and lower limb ischemia.
Inclusion of the duodenum and pancreatic head to maintain continuity of the biliary system was associated with early postoperative allograft pancreatitis, and no significant morbidity was reported[17]. This complication was also found in our study. It could be detected by measuring pancreatic enzymes in peritoneal fluid from abdominal drains and serum pancreatic enzymes[20]. Early diagnosis and aggressive surgical management of the native pancreatitis have eliminated the need for repeated transplantation[18]. At present, the following possible ways are considered to protect the allografted pancreas from postoperative pancreatitis. (1). To limit the cold solution and the pressure of perfusion[13]. (2). To procure the pancreas entirely with the graft and avoid over-dissection of the tissue and vessels around the duodenum and pancreas[24]. (3). To ligate the pancreatic tract and suture the pancreatic interface definitely. (4). To use somatostatin after operation.
Some reports suggested that LSBT with duodenum and pancreas head preserved would neither increase the possibility of rejection nor require more immunosuppressive treatment than that of the standard LSBT without pancreas and duodenum[18,23]. The presence of allograft pancreas in the multivisceral allograft was not an important risk factor for mortality, and the incidence of rejection of the pancreas was only 12% in some report[18]. So the technique with preservation of the pancreas is safe in composite LSBT.
In summary, the modified technique is feasible and safe for composite liver/small bowel transplantation with duodenum and pancreas preserved. This technique can simplify the operation procedure and decrease possible postoperative complications. The immunosuppressive treatment in this large animal LSBT model needs to be further studied.
Edited by Yuan HT, Zhu LH and Wang XL
| 1. | Reyes J, Bueno J, Kocoshis S, Green M, Abu-Elmagd K, Furukawa H, Barksdale EM, Strom S, Fung JJ, Todo S. Current status of intestinal transplantation in children. J Pediatr Surg. 1998;33:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Grant D. Intestinal transplantation: 1997 report of the international registry. Intestinal Transplant Registry. Transplantation. 1999;67:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 241] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Grant D, Wall W, Mimeault R, Zhong R, Ghent C, Garcia B, Stiller C, Duff J. Successful small-bowel/liver transplantation. Lancet. 1990;335:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 305] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Todo S, Reyes J, Furukawa H, Abu-Elmagd K, Lee RG, Tzakis A, Rao AS, Starzl TE. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270-80; discussion 280-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 231] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Goulet O, Jan D, Sarnacki S, Brousse N, Colomb V, Salomon R, Cuenod B, Piloquet H, Ricour C, Revillon Y. Isolated and combined liver-small bowel transplantation in Paris: 1987-1995. Transplant Proc. 1996;28:2750. [PubMed] |
| 6. | Muiesan P, Dhawan A, Novelli M, Mieli-Vergani G, Rela M, Heaton ND. Isolated liver transplant and sequential small bowel transplantation for intestinal failure and related liver disease in children. Transplantation. 2000;69:2323-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Zhong R, He G, Sakai Y, Zhang Z, Garcia B, Li XC, Jevnikar A, Grant D. The effect of donor-recipient strain combination on rejection and graft-versus-host disease after small bowel/liver transplantation in the rat. Transplantation. 1993;56:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Li XC, Zhong R, He G, Sakai Y, Garcia B, Jevnikar A, Grant D. Host immune suppression after small bowel/liver transplantation in rats. Transpl Int. 1994;7:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Furukawa H, Kaubu-Elmagd K, Reyes JL. Technical aspects of intestinal transplantation In: Braverman MH, Tawas RL, editors. Surgical Technology International II, San Francisco CA. TF Laszlo 1994: 165-170. . |
| 10. | Starzl TE, Todo S, Tzakis A, Alessiani M, Casavilla A, Abu-Elmagd K, Fung JJ. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335-344. [PubMed] |
| 11. | Casavilla A, Selby R, Abu-Elmagd K, Tzakis A, Todo S, Reyes J, Fung J, Starzl TE. Logistics and technique for combined hepatic-intestinal retrieval. Ann Surg. 1992;216:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Williams JW, Sankary HN, Foster PF. Technique for splanchnic transplantation. J Pediatr Surg. 1991;26:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Abu-Elmagd K, Fung J, Bueno J, Martin D, Madariaga JR, Mazariegos G, Bond G, Molmenti E, Corry RJ, Starzl TE. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann Surg. 2000;232:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | de Ville de Goyet J, Mitchell A, Mayer AD, Beath SV, McKiernan PJ, Kelly DA, Mirza D, Buckles JA. En block combined reduced-liver and small bowel transplants: from large donors to small children. Transplantation. 2000;69:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Starzl TE, Hakala TR, Shaw BW, Hardesty RL, Rosenthal TJ, Griffith BP, Iwatsuki S, Bahnson HT. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223-230. [PubMed] |
| 16. | Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343-348. [PubMed] |
| 17. | Casavilla A, Selby R, Abu-Elmagd K, Tzakis A, Todo S, Starzl TE. Donor selection and surgical technique for en bloc liver-small bowel graft procurement. Transplant Proc. 1993;25:2622-2623. [PubMed] |
| 18. | Reyes J, Fishbein T, Bueno J, Mazariegos G, Abu-Elmagd K. Reduced-size orthotopic composite liver-intestinal allograft. Transplantation. 1998;66:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Todo S, Tzakis AG, Abu-Elmagd K, Reyes J, Fung JJ, Casavilla A, Nakamura K, Yagihashi A, Jain A, Murase N. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation. 1992;53:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Abu-Elmagd K, Reyes J, Todo S, Rao A, Lee R, Irish W, Furukawa H, Bueno J, McMichael J, Fawzy AT. Clinical intestinal transplantation: new perspectives and immunologic considerations. J Am Coll Surg. 1998;186:512-525; discussion 525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Lopez RR, Benner Kg, Ivancev K, Keeffe EB, Deveney CW, Pinson CW. Management of biliary complications after liver transplantation. Am J Surg. 1992;163:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 356] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Bueno J, Abu-Elmagd K, Mazariegos G, Madariaga J, Fung J, Reyes J. Composite liver--small bowel allografts with preservation of donor duodenum and hepatic biliary system in children. J Pediatr Surg. 2000;35:291-295; discussion 291-295;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Kato T, Romero R, Verzaro R, Misiakos E, Khan FA, Pinna AD, Nery JR, Ruiz P, Tzakis AG. Inclusion of entire pancreas in the composite liver and intestinal graft in pediatric intestinal transplantation. Pediatr Transplant. 1999;3:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |