Published online Feb 15, 2003. doi: 10.3748/wjg.v9.i2.309
Revised: September 4, 2002
Accepted: November 4, 2002
Published online: February 15, 2003
AIM: Helicobacter pylori can be diagnosed by invasive or non-invasive tests but to obtain bacteria for culture and antibiotic susceptibility testing, an upper GI endoscopy is often required. The string test may be a minimally-invasive alternative method of obtaining H. pylori samples. This study evaluates the sensitivity and specificity of the string test in the diagnosis of H. pylori in comparison with endoscopic means of diagnosis.
METHODS: This was a prospective open comparative study of patients with dyspepsia with endoscopy-based tests as gold standard (defined as a positive CLO test and antral histology). Fasting patients swallowed the encapsulated-string (Entero-test Hp), which was withdrawn after 1 h. The gastric juice from the string was plated onto H. pylori-selective media for culture. Helicobacter pylori was identified by typical colony morphology, gram stain and biochemical test results.
RESULTS: Thirty dyspeptic patients were recruited of whom 21 (70%) were positive for H. pylori according to the gold standard. The sensitivity, specificity, positive predictive value, and negative predictive value for the string test were 38%, 100%, 100% and 41% respectively, and for endoscopic biopsies 81%, 100%, 100%, 69% respectively (P = 0.004). Logistic regression showed that only abundant growth density from endoscopic biopsy cultures to be a predictor of a positive string test (P = 0.018).
CONCLUSION: The string test is an alternative method to endoscopy in obtaining H. pylori but has a low sensitivity compared to endoscopic biopsies.
-
Citation: Leong RW, Lee CC, Ling TK, Leung WK, Sung JJ. Evaluation of the string test for the detection of
Helicobacter pylori . World J Gastroenterol 2003; 9(2): 309-311 - URL: https://www.wjgnet.com/1007-9327/full/v9/i2/309.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i2.309
The diagnosis of H. pylori can be made by invasive or non-invasive tests[1]. While non-invasive methods offer distinct advantages in terms of cost, ease-of-effort and comfort to patients, the retrieval of H. pylori for microscopy, culture, DNA analysis, cytotoxin profiling, strain identification and antibiotic susceptibility testing often require an endoscopy with gastric mucosal biopsies. With a rising incidence in antibiotic resistance, failed eradication therapy now occurs in up to 20% of all patients[2,3]. In this situation, obtaining H. pylori for antibiotic susceptibility testing may be necessary, with the goal of choosing an appropriate antibiotic regimen that will result in likely successful eradication of resistant organisms[3,4]. In this regard, there is a growing need to develop a cost effective and minimally-invasive means of obtaining the bacteria for culture and antibiotic susceptibility testing[4,5].
The string test consists of an encapsulated string that is swallowed and then later withdrawn orally along with gastric juice that may harbor H. pylori. It may be a method by which H. pylori can be obtained with minimal invasiveness. Studies have shown string test sensitivity as high as 78% to 97%[4,6]. However these results are achieved in highly specialized centers with a dedicated team and expertise in the string test technique. The same high sensitivity may not be reproduced in a standard microbiology laboratory with less experience with this procedure. Other studies used PCR on the string test to improve the sensitivity through the detection of H. pylori-specific DNA[7-10]. However this method does not obtain live H. pylori for culture and antibiotic susceptibility testing. We therefore evaluated the utility of the string test compared with endoscopy-based methods and more specifically the sensitivity and specificity of culture from the string compared with culture from endoscopic biopsies.
Adult patients, 18-80 years of age, with dyspepsia were prospectively recruited from the Endoscopy Department of the Prince of Wales Hospital, prior to oesophagogastroduodenoscopy (OGD). Patients were excluded if they had previous H. pylori eradication therapy, recent use of antibiotics, histamine receptor antagonists or proton pump inhibitors within the 4 wk prior to OGD, a previous endoscopy or diagnostic test for H. pylori, predominant reflux symptoms, or previous gastric surgery or gastric malignancy. Signed informed consent was obtained from every patient. The study was in accordance with the declaration of Helsinki.
The string test protocol was based on that described by Samuels et al[6]. The patients had fasted overnight prior to presentation. A 7 mm diameter gelatin capsule, which contained a 90 cm-long nylon string with a weighted end, was used for the string test (Enterotest HP, HDC Corporation, San Jose, CA, USA). A 20 cm-long portion of the string was pulled out from the capsule, and this proximal end was tapped to the patients' cheek to prevent the whole string from being swallowed. The capsule was then swallowed with up to 200 mL of water. The capsule dissolved within the gastric lumen releasing the string to absorb the gastric mucus. After an hour the string was retrieved orally. The proximal 30 cm of the string was cut using sterile scissors and discarded. The distal gastric acid-impregnated string was flushed with 5 mL of normal saline to reduce contamination by naso- and oropharyngeal organisms, then placed into a sterile specimen bottle containing 3 mL of brain-heart-infusion broth (BHIB) and immediately sent for processing. Gastric juice was squeezed from the string and the juice and approximately 5 microliter aliquot was plated onto each of two different H. pylori-selective media-Skirrows agar (containing vancomycin 10 mg/L, trimethorprim 5 mg/L, polymixin B 2500 IU/L) and Wilkins-Chalgren agar with Dent supplement (vancomycin 10 mg/L, trimethoprim 5 mg/L, cefsulodin 5 mg/L, amphotericin B 5 mg/L). The remaining BHIB/gastric juice was centrifuged at 14000 rpm for 5 min. The supernatant was discarded and the concentrated pellet was resuspended in 200 microliter of BHIB and plated onto another set of the same 2 selective media. Therefore, every patient's string sample was inoculated onto 4 culture plates. The plates were incubated in micro-aerophilic conditions, (37 °C, 10% carbon dioxide) and 95%-100% humidity. On day 3-4, suspected H. pylori colonies were identified and replated onto horse-blood agar and cultured for up to 7 more days. Formal identification was made by typical colony morphology, Gram stain, and positive biochemical test results (oxidase, catalase, and urease tests). Contaminants were gram stained but not formally identified.
Patients underwent OGD and had an antral biopsy taken for rapid urease test (CLO test, Tri-Med, Va, USA), and 2 antral and 2 body biopsies for histopathology, and 2 antral biopsies for culture. The gold standard for the presence of H. pylori was defined by a positive CLO test and antral histopathology. Growth of H. pylori from antral biopsies was graded as scant, moderate or abundant growth corresponding with the H. pylori colonies found in 1, 2 or = 3 quadrants of the culture plate respectively. Antral specimens were examined with hematoxylin-eosin stain and Giemsa stain and examined by a pathologist unaware of the patient's H. pylori status. Helicobacter pylori seen on antral histology was graded according to the Sydney system as normal (no H. pylori), mild (focal bacteria), moderate (bacteria in several areas), or marked (abundance of bacteria in most glands)[11].
The Chi square test was used for comparison of categorical data and the Fisher's exact test was used when an expected variable was below 5%. The McNemar test was used to detect the symmetry of the differences between the string test and other tests. A P value of < 0.05 was considered significant.
Thirty-three patients were approached to participate in this study. One patient refused participation and 2 patients attempted to but could not swallow the string capsule due to gagging and dry retching. The remaining 30 patients (14 males and 16 females with mean age of 52 years, age range 19-77 years) had both the string test and the OGD. There were no complications from the string test or from the OGD, apart from minor transient discomfort or gagging on swallowing the string test and during withdrawal of the string.
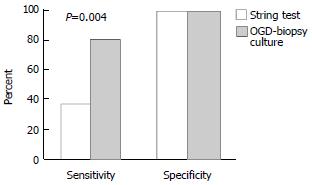
Twenty-one out of 30 (70%) patients had H. pylori infection based on the gold standard definition of a positive CLO test and antral histopathology. The string test detected H. pylori in only 8 patients and was negative in 22 patients. The sensitivity, specificity, positive predictive value and negative predictive value for the string test were 38%, 100%, 100% and 41% respectively. In comparison, the endoscopic biopsy culture method yielded a positive result in 17 patients with a sensitivity, specificity, positive predictive value and negative predictive value of 81%, 100%, 100% and 69%, respectively. (P = 0.004). Figure 1 illustrates this difference. The McNemar test showed that the string test result was significantly different to the gold standard (P < 0.001), endoscopic biopsy culture (P = 0.004), CLO test (P < 0.001) and histopathology (P < 0.001). The comparison between the string-test and with the OGD-based tests is presented in Table 1 and Table 2.
| Helicobacter pylori status | CLO test | Histology | Antral biopsy culture | String test culture | Number of patients (n = 30) |
| + | + | + | + | + | 8* |
| + | + | + | + | - | 9 |
| + | + | + | - | - | 4 |
| - | - | + | - | - | 1 |
| - | - | - | - | - | 8 |
| Sensitivity % | Sensitivity % | PPV % | NPV % | Accuracy % | |
| CLO test | 100 | 100 | 100 | 100 | 100 |
| Antral histology | 100 | 95 | 89 | 100 | 97 |
| Antral biopsy culture | 81 | 100 | 100 | 69 | 77 |
| String test culture | 38 | 100 | 100 | 41 | 57 |
The string test was more likely to be positive when there was also abundant growth of H. pylori from endoscopic biopsies, and less likely to be positive for moderate, scant or no growth (string test positive in 80% and 16% respectively, P = 0.011, Table 3). However, the string test result was not statistically associated with the density of H. pylori organisms on antral histopathology (string test positive in 50% of cases of marked density and 18% of moderate or lower density, P = 0.158, Table 4). Logistic regression showed that abundant growth from endoscopic biopsy cultures to be the only significant predictor of a positive string test (OR: 23.1, 95% CI: 1.71-310, P = 0.018).
| Cultures of endoscopic biopsies and growth rate of Helicobacter pylori | ||||
| Abundant | Moderate | Scant | No growth | |
| String test negative (n = 22) | 1 | 2 | 6 | 13 |
| String test positive (n = 8) | 4 | 1 | 3 | 0 |
| Total | 5 | 3 | 8 | 14 |
| Antral histology and density of Helicobacter pylori | ||||
| Marked | Moderate | Mild | None present | |
| String test negative (n = 22) | 4 | 8 | 2 | 8 |
| String test positive (n = 8) | 4 | 2 | 2 | 0 |
| Total | 8 | 10 | 4 | 8 |
Overall on OGD, 18 (60%) patients had endoscopic findings (5 patients with peptic ulcer disease and 13 patients with gastroduodenitis). The string test was positive in 4 out of 5 (80%) patients with peptic ulcers, 3/13 (23%) of patients with gastroduodenitis, and 1/12 (8%) of patients with no pathology (P = 0.009).
The concentrated Skirrow medium provided the best result for the string test and grew H. pylori in all 8 patients who had a positive string test result. In contrast the Wilkins-Chalgren medium was positive in only 2 of these patients. Contamination of the plates with naso- and oropharyngeal organisms was the main reason for the low string test sensitivity, and occurred in 37/60 Wilkins-Chalgren plates, and 18/60 of the Skirrow plates. The contaminants were predominantly gram positive cocci and gram negative rods. A few plates had fungal contaminants. The plates with the concentrated BHIB were more likely to grow H. pylori, but also tended to have more contaminants. We attempted to reduce the load of oropharyngeal commensals by having the patients gargle their throats with water just prior to string extraction. However this did not improve the overall sensitivity of detecting H. pylori (data not presented). Contamination of the batch BHIB was excluded by the use of a fresh BHIB sample as a negative control. There was no growth of contaminants from this negative control. Also no contamination was reported from the antral biopsy cultures, which were also transported in BHIB.
The string test demonstrated only 38% sensitivity in detecting H. pylori at our center contrasting previous results[4,6]. In contrast, direct endoscopic mucosal biopsy cultures yielded a significantly higher sensitivity of 81%. This low sensitivity of the string test could be attributable to either overgrowth of contaminants, or failure to collect sufficient H. pylori onto the string, or death of the organisms during handling.
To prevent oropharyngeal and nasopharyngeal organisms from overgrowing the culture plates require the use of H. pylori-selective media. We only used 2 types of H. pylori selective media (total of 4 plates per patient) whereas 3 types were used in Samuel's study[6]. Samuel's study showed that there was no clear superiority of any one selective medium over another and that different strains of H. pylori may unpredictably preferentially grow in a certain medium and not the others. Therefore the use of all 3 media may improve the pick up rate[6]. However, other experts have advocated on the use of only 2 types of selective plates for identifying H. pylori[12].
Helicobacter pylori may have been successfully collected on the string but plate contaminants or improper handling may have resulted in the negative culture. PCR can be performed to detect H. pylori-specific DNA sequences and this may increase the positive results. Previous studies have shown the high sensitivity of PCR-based string test[7-10]. In one study the string test sensitivity increased from 37% with culture to 93% when PCR was utilized[8]. However PCR testing is not a practical alternative in many centers and this will add to the overall cost of diagnosis. Also the main purpose of H. pylori culture in adults is to determine antibiotic susceptibility and this information is not determined by PCR.
The string test reported by Samuels et al[6] with very high sensitivity rate of 97% requires intensive laboratory methods, is labor-intensive, and requires the use of many culture plates. A vacuum hood was used when plating the selective media and this might reduce airborne contamination. String tests were processed immediately without refrigeration and this may improve the viability of the H. pylori. Six plates, instead of 4, were used and this may have also improved the yield of culture. Therefore, while the string test is an alternative method to an OGD for obtaining samples of H. pylori, the low sensitivity coupled with the demanding methodology may mean that it is not a realistic or possible option in many laboratories at present. Modifications of the methodology are required.
In conclusion, the string test is a minimally-invasive test to obtain H. pylori. As with other H. pylori culture techniques it has 100% specificity but in contrast to previous studies, a low sensitivity was demonstrated. Despite the use of H. pylori-selective media, overgrowth of commensal organisms remains the major obstacle with this technique.
RWL Leong is partially supported by the Athelstan and Amy Saw Overseas Medical Research Fellowship from the University of Western Australia
Edited by Xia HHX
| 1. | Vaira D, Holton J, Menegatti M, Ricci C, Gatta L, Geminiani A, Miglioli M. Review article: invasive and non-invasive tests for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14 Suppl 3:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Gisbert JP, Pajares JM. Helicobacter pylori therapy: first-line options and rescue regimen. Dig Dis. 2001;19:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Guslandi M. Review article: Alternative antibacterial agents for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2001;15:1543-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Perez-Trallero E, Montes M, Alcorta M, Zubillaga P, Telleria E. Non-endoscopic method to obtain Helicobacter pylori for culture. Lancet. 1995;345:622-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Perez-Trallero E, Montes M, Larrañaga M, Arenas JI. How long for the routine Helicobacter pylori antimicrobial susceptibility testing The usefulness of the string test to obtain Helicobacter for culture. Am J Gastroenterol. 1999;94:3075-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Samuels AL, Windsor HM, Ho GY, Goodwin LD, Marshall BJ. Culture of Helicobacter pylori from a gastric string may be an alternative to endoscopic biopsy. J Clin Microbiol. 2000;38:2438-2439. [PubMed] |
| 7. | Ferguson DA, Jiang C, Chi DS, Laffan JJ, Li C, Thomas E. Evaluation of two string tests for obtaining gastric juice for culture, nested-PCR detection, and combined single- and double-stranded conformational polymorphism discrimination of Helicobacter pylori. Dig Dis Sci. 1999;44:2056-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Parejo R, Garcia-Arata I, de Rafael L, Canton R, Olivares F, Boixeda D, Lorente Minarro M, Camarero H, Escobar y C. Usefulness of the enterotest method for the diagnosis of Helicobacter pylori infection in children. Gut. 1998;43:A74. |
| 9. | Roth DE, Velapatiño B, Gilman RH, Su WW, Berg DE, Cabrera L, Garcia E. A comparison of a string test-PCR assay and a stool antigen immunoassay (HpSA) for Helicobacter pylori screening in Peru. Trans R Soc Trop Med Hyg. 2001;95:398-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Domínguez-Bello MG, Cienfuentes C, Romero R, García P, Gómez I, Mago V, Reyes N, Gueneau de Novoa P. PCR detection of Helicobacter pylori in string-absorbed gastric juice. FEMS Microbiol Lett. 2001;198:15-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3550] [Article Influence: 122.4] [Reference Citation Analysis (3)] |
| 12. | Pérez-Trallero E, Montes M. String test for Helicobacter pylori. J Clin Microbiol. 2000;38:4303. [PubMed] |