Published online Nov 15, 2003. doi: 10.3748/wjg.v9.i11.2605
Revised: May 23, 2003
Accepted: June 2, 2003
Published online: November 15, 2003
AIM: To elucidate the mechanism of restenosis following balloon dilation of benign esophageal stenosis.
METHODS: A total of 49 rats with esophageal stenosis were induced in 70 rats using 5 mL of 50% sodium hydroxide solution and the double-balloon method, and an esophageal restenosis (RS) model was developed by esophageal stenosis using dilation of a percutaneous transluminal coronary angioplasty (PTCA) balloon catheter. These 49 rats were divided into two groups: rats with benign esophageal stricture caused by chemical burn only (control group, n = 21) and rats with their esophageal stricture treated with balloon catheter dilation (experimental group, n = 28). Imaging analysis and immunohistochemistry were used for both quantitative and qualitative analyses of esophageal stenosis and RS formation in the rats, respectively.
RESULTS: Cross-sectional areas and perimeters of the esophageal mucosa layer, muscle layer, and the entire esophageal layers increased significantly in the experimental group compared with the control group. Proliferating cell nuclear antigen (PCNA) was expressed on the 5th day after dilation, and was still present at 1 mo. Fibronectin (FN) was expressed on the 1st day after dilation, and was still present at 1 month.
CONCLUSION: Expression of PCNA and FN plays an important role in RS after balloon dilation of benign esophageal stenosis.
- Citation: Cheng YS, Li MH, Yang RJ, Zhang HZ, Ding ZX, Zhuang QX, Jiang ZM, Shang KZ. Restenosis following balloon dilation of benign esophageal stenosis. World J Gastroenterol 2003; 9(11): 2605-2608
- URL: https://www.wjgnet.com/1007-9327/full/v9/i11/2605.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i11.2605
Balloon catheter dilation is a common nonsurgical treatment for benign esophageal stricture. Its short-term effect is good, but its long-term effect is not so good, because esophageal restenosis is a major complication. The underlying mechanism of esophageal restenosis has not been understood yet. To study this mechanism, we established a benign esophageal stricture model and restenosis model in Sprague-Dawley (SD) rats. We performed quantitative histopathological image analysis of sections of the rat esophagus, and qualitative immunohistochemical analysis of the related indicators of proliferation and restenosis of mucous and striated muscle layers of rat esophagus dilated by a balloon catheter at different time points. This provided us with an effective experimental model for investigation of the causes of restenosis.
All protocols used for animal experiment and maintenance were approved by the Animal Ethics Committee in our university and conformed to the highest international standards of humane care.
70 male Sprague-Dawley (SD) rats weighing 305 ± 50 g were obtained from the Shanghai Experimental Animal Center (Shanghai, China). The animals were weighed on day 0 and then 1 d before sacrifice. After anesthesia with 10% ketamine (80 mg/kg) by abdominal injection, the animals were placed in a supine position and stabilized on an operating table. A 3F segmental epidural catheter was inserted into the mouth, and 10 mL of edible vinegar was injected into the stomach. The epidural catheter was then replaced by two 3F percutaneous transluminal coronary angioplasty (PTCA) balloon catheters at the mid-to-lower segment of the esophagus. The two balloons were at least 2 cm apart. These balloons were simultaneously inflated with air until they expanded to cling to the wall of the esophagus. Then 5 mL of a freshly prepared 50% sodium hydroxide solution was injected through the orifice of the balloon catheter. After three minutes the air was released from the balloon. Distilled water was injected repeatedly through the same orifice for rinsing for 1 minute. The balloon catheters were removed and the animals returned to their cages for feeding. An esophageal barium-contrast examination was performed 2 and 4 wk later to ascertain whether benign esophageal strictures had formed. We achieved 49 animal models from the 70 rats. These 49 rats were divided into two groups: rats with benign esophageal stricture caused by chemical burn only (control group, n = 21) and rats with their esophageal stricture treated with balloon catheter dilation (experimental group, n = 28).
Samples were collected at different times for immunohistochemical assay. We divided rats in both groups into seven subgroups according to the time after the dilation procedure when the samples were collected on day 1, 3, 5, 7, 14, 21 and 30. At the time of sacrifice, samples were fixed in 4% buffered formaldehyde solution.
Image analysis: Esophageal sections were stained with hematoxylin and eosin, and images were taken by a CCD camera (JVC, Osaka, Japan) and analyzed by a VIDAS imaging system (Carl Zeiss, Germany). The indicators used comprised the cross-sectional areas and perimeters of esophageal mucous layer, esophageal muscle layer, and the entire esophageal layers.
Immunohistochemical staining: ABC methods and SP methods were performed following the manufacturer's instructions using an ABC kit (Vector, USA) and an SP immunochemistry kit (Zymed Maxim, USA). The source of antibodies and their effective concentrations are listed in Table 1. The presence of platelet-derived growth factor (PDGF) and fibronectin (FN) was tested by the ABC method, and proliferating cell nuclear antigen (PCNA) was tested by the SP method.
| First antibody | Second antibody | ||||
| Goat antihuman PDGF | Promega | 1:40 | Biotinylated house antigoat IgG | Vector | 1:200 |
| Mouse antihuman FN | Life | 1:20 | Biotinylated house antimouse IgG | Vector | 1:200 |
| Mouse antihuman PCNA | Maxim | 1:20 | Biotinylated house antimouse IgG | Maxim | 1:200 |
Of the 49 model rats with benign esophageal stricture, 28 rats with esophageal restenosis were established.
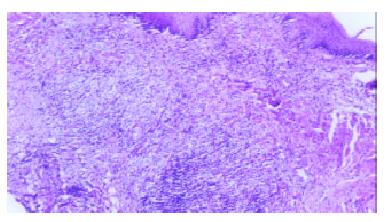
The control group showed chemical-burn lesions with an inflammatory reaction on the mucous layer of the esophagus, and comparatively slight thickening on the muscle layer of the esophagus. No broken regions were found in the muscle layer of the esophagus, and the esophageal wall was intact. Besides chemical-burn lesions, the experimental group showed mechanical damage in the mucosa of the esophagus. The muscle layer of the esophagus was thickened and broken, with accompanying inflammatory reactions (Figure 1). On the 5th day after the procedure, the broken section of the muscle layer of the esophagus became thickening, and 14 d later the degree of thickening was obvious. The changes in the cross-sectional areas and perimeters of mucosa, muscle layers, and the entire esophagus wall are listed in Table 2.
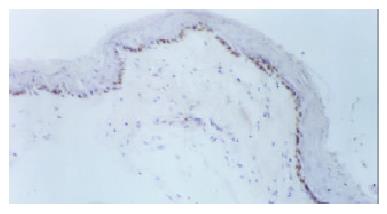
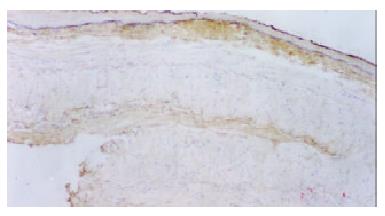
Immunohistochemical staining of benign esophageal stricture and esophageal restenosis: In the control group, basal cells of the squamous epithelium and striated-muscle cells of the esophagus exhibited no PCNA expression. Five days after the dilation procedure, PCNA expression became obvious in basal cells of the squamous epithelium, and this positive expression lasted for 30 d (Figure 2). In the control group, 3-7 d after the dilation procedure, the basal layer of the esophagus exhibited weak positive expression. Fourteen days later there was no FN expression in the basal layer. In the experimental group, on the 1st day after the procedure, the collagen fibers in submucosa and in the striated-muscle layer of esophagus were positive for FN, and this was still the case on the 14th day. After 1 mo, FN positive expression was still reasonably strong (Figure 3). PDGF was not expressed at all in striated-muscle cells from the 1st to the 30th day in both control and experimental groups.
The causes of benign esophageal stricture are numerous and complicated, and hence the models thereof are difficult to reproduce consistently. However, benign esophageal stricture eventually manifests as thickened scars and reduced luminal sizes. We used chemical burns to develop the model of benign esophageal stricture because it allowed timing to be controlled and exhibited a high rate of success. Early in the 1970s, Przymanowski et al[1] used sodium hydroxide to establish a model of benign esophageal stricture. Their method was to perform an abdominal midsection on rats, thereby exposing the lower segment of the esophagus. They used surgical thread to tightly tie the region 2-cm either side of the lower segment of the esophagus. They then inserted a stomach tube via the mouth until it reached the tied point. Sodium hydroxide solution was injected, and then rinsed out three times for 3 min with distilled water later. Then they withdrew the tube, cut the threads, and closed the abdomen. Based on their procedure, we developed a nonsurgical method to establish a model of benign esophageal stricture. Since our method did not involve surgery, it was simpler and faster. Our experimental observations demonstrated that the model was satisfactorily established. Our use of two balloon catheters made manipulation somewhat difficult. We intended to make a single catheter with two balloons, but this was found to be too difficult since the rats had a narrow esophagus that demanded fine catheters and balloons. In contrast, a double-balloon catheter with a larger caliber was easy to be constructed. Therefore, the double-balloon method was used to establish the model of benign esophageal stricture.
The technique used to establish the model of esophageal restenosis is easier. After ascertaining the stricture position by esophageal visualization, we performed balloon catheter dilation under X-ray. In this way, the esophageal restenosis model was established. In a very few cases of severe stricture, the restenosis model could not be produced due to the catheters being unable to pass through.
After the benign esophageal stricture formed, its morphology was relatively stable. It manifested as thickened muscle layers, reduced luminal sizes, and inelastic lumens. Thus it caused dysphagia. The esophageal morphology was altered by balloon catheter dilation. The esophageal mucosa exhibited not only chemical-burn lesions, but also lesions caused by mechanical damage. The thickened muscle layer of the esophagus was torn or broken, causing the areas of mucosal and muscle layers of the esophagus to increase siginficantly in the experimental group. Significant differences were also observed in the perimeters of the mucosal and muscle layers of the esophagus and in the perimeter of the entire esophagus wall. Within the same group, after the dilation procedure the areas of each layer increased rather than decreased, whilst the perimeters also increased. This indicated that dysphagia improvement was due to an enlargement of the lumen of the esophagus after dilation. Up to a certain time, these new scar tissues would further contract and cicatrize. As a result, the duct lumen was further reduced and lacked elasticity. This was one of the key factors in esophageal restenosis. This also illustrated that if there was no treatment plan after balloon dilation in benign esophageal stricture, esophageal restenosis could not be resolved[2-28].
PCNA is a type of nuclear protein equivalent to the binding protein of DNA polymerase. It coordinates the synthesis of DNA up and down strands. The quantity of PCNA is minimal in normal cells at the G0, whereas at the M stage the quantity of PCNA in transforming cells changes dramatically. The quantity of PCNA mostly declines at stage G0/G1. This quantative change coincides with DNA synthesis. Therefore, PCNA is used as an indicator to assess cell proliferation. There were a number of reports on the application of immunohistochemical methods to the study of tumor-cell proliferation[29]. In our study, we used the new method involving PCNA to investigate the basal-cell proliferation of the squamous epithelium in benign esophageal stricture by the procedure of balloon dilation. We found that there was no PCNA expression in the control group in basal cells of the squamous epithelium of the esophagus. However, in the experimental group, PCNA was expressed strongly from day 5 onwards 30 d later, PCNA expression was still positive. This consistently high proliferation of basal cells illustrated their importance in the development of esophageal restenosis.
FN was a glucoprotein with multiple functions[30]. As a noncollagenous substance in the extracellular matrix, it participates in various reactions between cells as well as between cells and the extracellular matrix, including adhesion, migration, injury, restoration, and tumor metasis. FN has two forms: A soluble dimerization in humor and a barely soluble polymerization in the extracellular matrix. After combining with its receptor through a tripeptide sequence Arg-Gug- (RGD), FN transmits cellular signals and facilitates cells' interfacing and kinetics. The study of FN expression in the lesion of benign estophageal stricture caused by balloon dilation is therefore helpful to elucidate the mechanism of proliferation and migration of cells. In the control group, we noticed that the expression of FN in the basal mucosa of the esophagus was weak, which indicates that FN expression after a chemical burn is related to the esophageal stricture. In the experimental group, soon after the procedure the squamous epithelium and striated-muscle cells expressed a large amount of FN. This reaction might be related to regulated cellular proliferation and chemotaxis. Previous studies have shown that FN has the similar function to growth factor in fibroblast cells. Even in small doses it can accelerate proliferation. An in vitro study has also shown that fibroblasts could adhere directly to the FN matrix or adhere to collagen through FN. FN can also facilitate unfolding of cells that adhere to the matrix. We also noticed that in the experimental group, FN was strongly expressed at both early and later stages after the procedure. This illustrates that FN is one of the key factors in the production of esophageal restenosis, especially at the late stage.
PDGF could stimulate the proliferation of fibroblasts in vitro[31-38]. Initially it was found in platelet granules, and afterwards its secretion was also found in normal cells and transformed cells. It exists in three biologically active isoforms: PDGF-AB, PDGF-AA, and PDGF-BB; comprising PDGF-A and PDGF-B polypeptide chains. It acts on target cells through receptors consisting of two subunits, α and β. PDGF-AB combines αα and ββ functions. In our experiment, PDGF was not expressed in striated muscle cells of the esophagus, which indicates that PDGF is not a key factor in esophageal restenosis produced by balloon dilated esophageal stricture. However, the enhanced expression of PDGF was involved in the proliferation of smooth-muscle cells. In the study of restenosis, PDGF was regarded as a strong split promoter and chemotactic factor, playing an important role in the formation of blood vessel restenosis. The full length of the esophagus in SD rats (as used in our experiments) comprised striated muscle, and hence PDGF and its function could not be shown in esophageal restenosis in these rats. Besides, in clinical settings, relatively severe chemical burns of the esophagus are usually located at the middle and lower segments of the esophagus, while the upper segment is rarely involved. The middle and lower segments of the esophagus comprise smooth muscle, while the upper segment is striated muscle. This indirectly demonstrates that PDGF expressed in smooth-muscle cells plays a greater role than that in striated-muscle cells in the formation of benign esophageal stricture and restenosis.
Edited by Zhang JZ and Wang XL
| 1. | Przymanowski Z. [Dilatational treatment of the esophageal constriction after burning in the light of experimental investigations and clinical observations (author's transl)]. Acta Biol Med (. Gdansk). 1970;15:55-116. [PubMed] |
| 2. | London RL, Trotman BW, DiMarino AJ, Oleaga JA, Freiman DB, Ring EJ, Rosato EF. Dilatation of severe esophageal strictures by an inflatable balloon catheter. Gastroenterology. 1981;80:173-175. [PubMed] |
| 3. | Chang TS, Wang W, Huang OL. One-stage reconstruction of esophageal defect by free transfer of jejunum: treatment and complications. Ann Plast Surg. 1985;15:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kochhar R, Nagi B, Mehta SK. Balloon catheter dilatation of esophageal strictures. Indian J Gastroenterol. 1988;7:97-98. [PubMed] |
| 5. | Othersen HB, Parker EF, Smith CD. The surgical management of esophageal stricture in children. A century of progress. Ann Surg. 1988;207:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Shemesh E, Czerniak A. Comparison between Savary-Gilliard and balloon dilatation of benign esophageal strictures. World J Surg. 1990;14:518-21; discussion 521-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Wang C, Wang CL, Chen CX. Four-year experience in the treatment of upper gastrointestinal strictures with balloon dilatation. Chin Med J (. Engl). 1991;104:114-118. [PubMed] |
| 8. | Strautman PR, Dorfman GS. Use of metallic stents to salvage and maintain patency in surgically created esophagocutaneous fistulas. J Vasc Interv Radiol. 1992;3:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Davies RP, Linke RJ, Davey RB. Retrograde esophageal balloon dilatation: salvage treatment of caustic-induced stricture. Cardiovasc Intervent Radiol. 1990;15:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Song HY, Han YM, Kim HN, Kim CS, Choi KC. Corrosive esophageal stricture: safety and effectiveness of balloon dilation. Radiology. 1992;184:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Chen PC. Endoscopic balloon dilation of esophageal strictures following surgical anastomoses, endoscopic variceal sclerotherapy, and corrosive ingestion. Gastrointest Endosc. 1992;38:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Broor SL, Lahoti D. Balloon dilation of corrosive esophageal strictures. Gastrointest Endosc. 1993;39:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | de Wilde I, Pieper CH, Moore SW, Hoffman B. Oesophageal stricture caused by washing powder ingestion. S Afr Med J. 1995;85:121. [PubMed] |
| 14. | Sinha KN. Foley catheter self dilatation for strictures of the upper end of oesophagus. Indian J Chest Dis Allied Sci. 1996;38:91-93. [PubMed] |
| 15. | Hwang TL, Chen MF. Surgical treatment of gastric outlet obstruction after corrosive injury--can early definitive operation be used instead of staged operation. Int Surg. 1996;81:119-121. [PubMed] |
| 16. | Panieri E, Millar AJ, Rode H, Brown RA, Cywes S. Iatrogenic esophageal perforation in children: patterns of injury, presentation, management, and outcome. J Pediatr Surg. 1996;31:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Fan S, Jiang Y, Li Z. [Intraluminal stent and balloon of intraluminal stent for prevention of esophageal stenosis due to alkali corrosive injury: experimental and clinical studies]. Zhonghua Waike Zazhi. 1996;34:170-172. [PubMed] |
| 18. | Cheng YS, Shang KZ, Zhuang QX, Li MH, Xu JR, Yang SX. Interventional therapy and cause of restenosis of esophagealbenign stricture. Huaren Xiaohua Zazhi. 1998;6:791-794. |
| 19. | Kadakia SC, Wong RK. Graded pneumatic dilation using Rigiflex achalasia dilators in patients with primary esophageal achalasia. Am J Gastroenterol. 1993;88:34-38. [PubMed] |
| 20. | Misra SP, Dwivedi M. Entrapment of guide-wire during oesophageal dilation. Trop Gastroenterol. 1997;18:117-118. [PubMed] |
| 21. | De Peppo F, Zaccara A, Dall'Oglio L, Federici di Abriola G, Ponticelli A, Marchetti P, Lucchetti MC, Rivosecchi M. Stenting for caustic strictures: esophageal replacement replaced. J Pediatr Surg. 1998;33:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Karnak I, Tanyel FC, Büyükpamukçu N, Hiçsönmez A. Esophageal perforations encountered during the dilation of caustic esophageal strictures. J Cardiovasc Surg (. Torino). 1998;39:373-377. [PubMed] |
| 23. | al-Jadaan S, Bass J. Retrograde esophageal balloon dilatation for caustic stricture in an outpatient clinic setting. Can J Surg. 1999;42:48-50. [PubMed] |
| 24. | Hunt DR, Wills VL, Weis B, Jorgensen JO, DeCarle DJ, Coo IJ. Management of esophageal perforation after pneumatic dilation for achalasia. J Gastrointest Surg. 2000;4:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Huang YC, Chen SJ, Hsu WM, Li YW, Ni YH. Balloon dilation of double strictures after corrosive esophagitis. J Pediatr Gastroenterol Nutr. 2001;32:496-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Wilsey MJ, Scheimann AO, Gilger MA. The role of upper gastrointestinal endoscopy in the diagnosis and treatment of caustic ingestion, esophageal strictures, and achalasia in children. Gastrointest Endosc Clin N Am. 2001;11:767-787, vii-viii. [PubMed] |
| 27. | Gehanno P, Guedon C. Inhibition of experimental esophageal lye strictures by penicillamine. Arch Otolaryngol. 1981;107:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Rivera EA, Maves MD. Effects of neutralizing agents on esophageal burns caused by disc batteries. Ann Otol Rhinol Laryngol. 1987;96:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Alexandrov VA, Novikov AI, Zabezhinsky MA, Stolyarov VI, Petrov AS. The stimulating effect of acetic acid, alcohol and thermal burn injury on esophagus and forestomach carcinogenesis induced by N-nitrososarcosin ethyl ester in rats. Cancer Lett. 1989;47:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Demirbilek S, Bernay F, Rizalar R, Bariş S, Gürses N. Effects of estradiol and progesterone on the synthesis of collagen in corrosive esophageal burns in rats. J Pediatr Surg. 1994;29:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Takagi K, Tashiro T, Yamamori H, Mashima Y, Nakajima N, Sunaga K. Recombinant human growth hormone and protein metabolism of burned rats and esophagectomized patients. Nutrition. 1995;11:22-26. [PubMed] |
| 32. | Yoshikawa T, Asai S, Takekawa Y, Kida A, Ishikawa K. Experimental investigation of battery-induced esophageal burn injury in rabbits. Crit Care Med. 1997;25:2039-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Bingöl-Kologlu M, Tanyel FC, Müftüoğlu S, Renda N, Cakar N, Büyükpamukçu N, Hiçsönmez A. The preventive effect of heparin on stricture formation after caustic esophageal burns. J Pediatr Surg. 1999;34:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Kaygusuz I, Celik O, Ozkaya O O, Yalçin S, Keleş E, Cetinkaya T. Effects of interferon-alpha-2b and octreotide on healing of esophageal corrosive burns. Laryngoscope. 2001;111:1999-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Wornat MJ, Ledesma EB, Sandrowitz AK, Roth MJ, Dawsey SM, Qiao YL, Chen W. Polycyclic aromatic hydrocarbons identified in soot extracts from domestic coal-burning stoves of Henan Province, China. Environ Sci Technol. 2001;35:1943-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Arzbaecher R, Jenkins JM. A review of the theoretical and experimental bases of transesophageal atrial pacing. J Electrocardiol. 2002;35 Suppl:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 314] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Demirbilek S, Aydin G, Yücesan S, Vural H, Bitiren M. Polyunsaturated phosphatidylcholine lowers collagen deposition in a rat model of corrosive esophageal burn. Eur J Pediatr Surg. 2002;12:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |