Published online Nov 15, 2003. doi: 10.3748/wjg.v9.i11.2404
Revised: March 24, 2003
Accepted: June 2, 2003
Published online: November 15, 2003
AIM: To investigate the inhibition of p27kip1 gene on the growth of esophageal carcinoma cell strain (EC9706).
METHODS: Recombinant adenovirus Ad-p27kip1 was constructed and transfected into esophageal carcinoma cell EC-9706, and its effect on p27kip1 expression, the growth of esophageal carcinoma cell, DNA replication, protein synthesis, cell multiplication and apoptosis were explored by means of cell growth count, 3H-TdR, 3H-Leucine incorporation, flow cytometry, DNA fragment analysis and TUNEL.
RESULTS: Recombinant adenovirus Ad-p27kip1 was successfully constructed with a virus titer of 1.24 × 1012 pfu/mL. p27kip protein expression increased markedly after EC-9706 transfection, while incorporation quantity of 3H-TdR and 3H-Leucine decreased significantly. The growth of esophageal carcinoma cell was inhibited obviously. Testing of flow cytometry displayed a typical apoptosis peak, and DNA gel electrophoresis showed a typical apoptosis ladder. TUNEL showed the apoptosis rate of Ad-p27kip1 group and control group to be 37.3% and 1.26% (P < 0.001) respectively.
CONCLUSION: Ad-p27kip1 can inhibit the growth and multiplication of esophageal carcinoma cells and induce apoptosis. Therefore, enhanced p27kip1 expression may be a new way to treat esophageal carcinoma.
-
Citation: Wu QM, Yu JP, Tong Q, Wang XH, Xie GJ. Inhibition of adenovirus-mediated
p27kip1 gene on growth of esophageal carcinoma cell strain. World J Gastroenterol 2003; 9(11): 2404-2408 - URL: https://www.wjgnet.com/1007-9327/full/v9/i11/2404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i11.2404
As a kind of thermostable cell cycle inhibitive protein, p27 (mol. wt = 27 KD) can inhibit CDK activity and consequently regulate cell cycle negatively when combined with CyclinE-CDK2 or CyclinE-CDK4[1]. The expression level and activity of p27 protein are associated with the formation of tumor. p27 protein quantity in tumor is markedly lower than that in normal cells level and its content is also closely related to the degree of differentiation, action of molecular biology and prognosis of tumor[2-7]. In the present study, we aim to give a tentative answer to whether enhanced expression of p27 protein inhibit the growth of cancer cell
Reagent p27kip1SP reagent was obtained from Beijing Zhongshan Biology Company. Endoenzyme, Kpn I, BamH I and T4 DNA ligase were purchased from Huamei Biological Engineering Company. DMEM, RPMI1640, Lipofectamine were purchased from GIBCO/BRL Company. Low melting-temperature agarose and X-gal were obtained from Promega Company. CsCl was from Sigma Company. And high-grade neogenetic bovine serum from Hangzhou Sijiqing Biological Engineering Material Co. Ltd. p27kiplcDNA and adenovirus PCR primer were designed and synthesized by Saibaisheng Biological Company. And try Psase from Shanghai Biological Products Company. 3H-TDR and 3H-Leucine were provided by Beijing Atomic Power Research Institute. DNA-PREPTMLPR and DNA-PREPTMstain were obtained from America Beckman Coulter Company.
Plasmid, strain, adenovirus and cell line PCMV5p27kip1 was presented by Dr. Wang Gang, Urinary Surgery Research Institute of the First Hospital of Beijing Medical University. PAACCMVPLPA and PJM17 were presented by academician Wu Zhuze, No. 2 Research Institute of Military Academy of Medical Science. DH5α was presented by Dr. Peng Xu, Endocardial Department of the First Hospital of Beijing Medical University. Recombinant adenovirus was constructed by Molecular Biology Laboratory of Taihe Hospital. 293 cell was Hek cell line transferred from the gene of adenovirus E1 region, esophageal carcinoma cell strain EC9706 was presented by professor Wang Mingrong, China Academy of Medical Sciences.
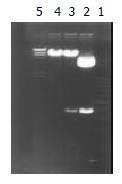
Construction of adenovirus shuttle plasmid carrying p27kip1 Double enzyme cut was performed on pCMV5 p27kip1 and pACCMVPLPA with Kpn I and BamH I respectively, and the fragments were separated at low melting-temperature agarose gel electrophoresis. The recovered p27kiplcDNA and adenovirus fragments were connected overnight with T4DNA ligase at 4 °C by directional clone, and then transferred into receptive colibacillus DH5α. From the selection of colonial amplification, a small quantity of plasmid was extracted and double cut with Kpn I and BamH I. The presence of 690 bp and 8800 bp in agarose gel after electrophoresis indicated that p27kip1cDNA had been inserted into adenovirus shuttle vector, hence successful construction of adenovirus shuttle plasmid pAd-p27kip1 carrying p27kip1.
Transfection of 293 cell by Lipofectamine-mediated pAd-p27kip1 and pJM17, and preparation of adenovirus recombinant Ad-p27kip1 (1) 293 cell was inoculated in a 9 cm plat, cultured in an incubator at 37 °C and 5% CO2, and transfected at 80% fusion; (2) DNA-lipofectamine compound was dripped in an Eppendorf tube. Plasmid pAd-p27kip1 and pJM17 were diluted in 1 mL DMEM culture fluid, and revolved for 1 s, then lipofectamine suspension was added uniformly. It was kept at room temperature for 30 min; (3) DNA-lipofectamine compound was dripped in culture flasks, and cultured in an incubator at 37 °C and 5% CO2. 0.5% agarose was added for covering (0.5% agarose contains 1 × DMEM, 10% Fcs), and cultured in an incubator with 37 °C and 5% CO2 after its coagulation, then pathological changes of 293 cell were observed.
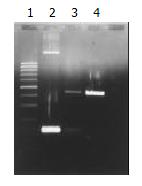
PCR appraisal of recombinant adenovirus Ad-p27kip1[8] From 293 cell undergone pathologic changes after common transfection, culture supernate 100 μL was drawn, form which cell chip was removed centrifugally, and DNA was extracted and precipitated, then PCR amplification was performed on this DNA model. Primers 1 and 2 were used for amplifying p27kip1 gene, primer 3 and primer 4 for amplifying adenovirus skeleton gene fragments. The product of PCR amplification was appraised with 0.8% agarose through electrophoresis, and held to be recombinant adenovirus Ad-p27kip1 containing human p27kip1 if gene fragments of 275 bp and 860 bp were both amplified. The sequence of primers was as follows.
Primer 1: 5’-CCTAGAGGGCAAAGTACGAGTA-3’
Primer 2: 5’-GAAGAATCGTCGGTTGCAGGTCGCT-3’
Primer 3: 5’-TCGTTTCTCAGCAAGCTGTTG-3’
Primer 4: 5’-CAATCTGAACTCAAAGCGTGG-3’
Amplification, purification and titer of recombinant adenovirus Ad-p27kip1 Recombinant adenovirus supernate 500 μL was added to 293 cell with 80% fusion, and cultured in an incubator at 37 °C and 5% CO2. Ultracentrifugation in CsCl gradient and purification were performed on the adenovirus supernate by Graham method[8]. Then purified virus fluid 100 μL, 10% SDS 20 μL, PBS 880 μL were taken to test light absorption value of OD260 and OD280 of the DNA of virus gene group, and accordingly to calculate the grannule quantity and purity of the virus. 1OD260 = 1012 pfu/mL, OD260/OD280 > 1.3 showed the purity was fairly high, virus titer pfu/mL = A260× dilution × 1012.
Transduction efficiency test of adenovirus Recombinant adenovirus Ad-LacZ was used to infect cells of esophageal carcinoma EC-9706 respectively by MOI 25, 50, 100 and 200. When X-gal was dyed, the cells dyed blue under microscope were positive cells expressed by LacZ gene, then the percentage of the cells dyed blue was calculated.
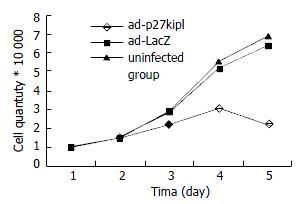
Influence of Ad-p27kip1 on growth curve of esophageal carcinoma cell 1 × 104/mL cell suspension was made of the cultured esophageal carcinoma cells (5% Fcs RPMI1640) after digestion and collection. The cells of esophageal carcinoma were inoculated in 4 pieces of culture boards with 24 holes/piece according to the quantity of 1 mL/hole, cultured for 24 h. When in 40%-50% fusion, cells were rinsed with RPMI1640, and went on culture for 24 h for synchronization. The test was divided into three groups: experimental group: Ad-p27kip1, negative control groups: Ad-LacZ, blank group: free-virus.The test groups were infected with the cells of esophageal carcinoma (RPMI1640) for 2 h with 100 MOI, during which the culture fluid was shaken every 15 min, and two hours later exchanged with 5% Fcs RPMI1640 for culture. Prior to the transfection of esophageal carcinoma cells by virus, three holes were taken as cell count to obtain the average value, afterwards daily cell count was collected for 4 d, and the changes of growth curve of esophageal carcinoma cell were recorded.
Incorporation test of 3H-TdR and 3H-leucine As described above, the experimental group and control group were cultured for 3 h with 5% Fcs RPMI1640, and then for 12 h with free-Fcs RPMI1640. Each group was added 1 μ Ci 3H-TdR or 3H-leucine, which was rinsed with PBS at the 24th, 48th and 72th hour and fixed with methyl alcohol for 10 min and absolute ethyl alcohol for 10 min. Finally, 0.1 M NaOH 200 μL was added. 200 μL of each was taken after blowing, and mixed in 5 mL scintillation liquid for overnight. On the following day, the CPM of 3H was tested, three times for each group with three wells.
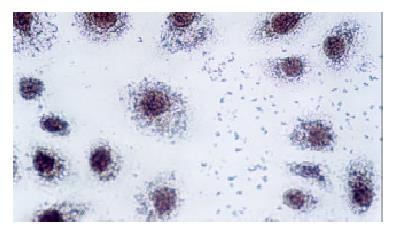
Change of p27 protein expression of cell of esophageal carcinoma p27 protein expression of the three groups was detected by immunohistochemical SP method.
FCM detection after occurrence Ad-p27kip1 effect on esophageal carcinoma cell After 48 h effect, cells from the three groups were collected, centrifugated under digestion (1000 rpm, 5 min). PBS was added for regulating the cell density to 106/mL with supernates removed. 100 μL cell suspension was put in a preparatory tube, and mixed with DNA-PREPTMLPR 200 μL. Half a minute later DNA-PREPTM stain reagent (PI staining) 2 mL was added for mixing. After staying static for half an hour, cell cycle and apoptosis were detected with FCM, and processed by SYSTEM II TM software from Coulter Company.
Apoptosis detected with DNA fragment analysis and TUENL method From the experimental group after the above-mentioned effect, genome DNA was extracted regularly. DNA fragment was analyzed by agarose gel electrophoresis. At the same time, apoptosis was detected by TUNEL method, and contrasted with that in the blank group.
Double enzyme cutting was performed on pCMV5p27kip1 and pACCMVpLpA with Kpn I and BamH I, then 690 bp fragment and 8800 bp fragment were collected respectively at low melting-temprature agarose gel electrophoresis, for the production of plasmid pAd-p27kip1 with coupled reaction. Through transformation, amplification and extraction of colibacillus, enzyme cutting appraisal were conducted to approve that pAd-p27kip1 contained p27kip1 and adenovirus carrier skeleton (Figure 1).
When contransfected with the above synthesized pAd-p27kip1 and pJM17, 293 cells underwent pathologic changes and floated as in grape clusters, which suggested that Ad-p27kip1 was produced through homologous recombinant, ultracentrifugation in CsCl gradient and purification. Spectrophotometer detection showed that the virus titer was 1.24 × 1012 pfu/mL, OD260/OD280 > 1.3, which suggested that both the virus titer and the purity were fairly high. The extracted Ad-p27kip1 adenovirus DNA underwent PCR amplification for a contrast between PCMV5p27kip1 and pACCMVpLpA, with 275 bp and 860 bp as the amplification products (Figure 2).
Cells of esophageal carcinoma were infected with Ad-LacZ by MOI 25, 50, 100, 200 respectively, then stained with X-gal 48 h later. The transfection efficiency (%) was found to be 90, 100, 100, 100 respectively. It was concluded that 100% transduction efficiency could be achieved with MOI ≥ 50, hence MOI 100 should be adopted for EC-9706 in the fellowing experiments.
Cell growth curve showed that the count of cancer cell in the experimental group declined over time, reached the bottom on the fourth day, but in Ad-Lacz group and blank group, it was just the opposite (Figure 3).
The incorporation quantity of experimental group was markedly lower than that of the control group (P < 0.001), declined over time as against the rise in Ad-LacZ group and blank group. There was no statistical difference (Table 1 and Table 2) between them.
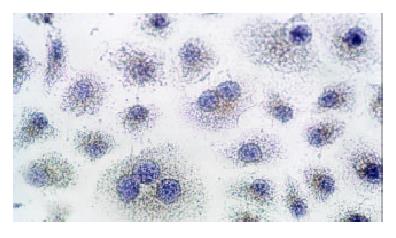
Immunocytochemical staining after virus transfection of esophageal carcinoma cells for 24 h showed that p27 expression increased clearly in experimental group, whereas there was not any change in control group (Figure 4 and Figure 5).
Forty-eight hours after Ad-p27kip1, Ad-Lacz and blank management for EC-9706 cell, FCM determination showed that apoptotic cells took on an obvious apoptosis peak prior to G0/G1 peak. The apoptosis value of Ad-p27kip1, Ad-LacZ and blank group was 32.7%, 5.72% and 0.05% respectively. Ad-p27kip1 group had the highest rate of apoptosis whereas blank group had the lowest rate.
Cell cycle from FCM is listed in Table 3. In Ad-LacZ and blank control group, G1/G0 stage ratio decreased gradually whereas S stage increased, which indicated a rapidly changing G1/G0→S procedure and active cell multiplication. However, in the experimental group, G1/G0 stage ratio was fairly high and S stage decreased. There was a significant difference from control group (P < 0.05). Cell cycle arrested G1 and inhibited cell multiplication.
After cell EC-9706 was processed by Ad-p27kip1, gel electrophoresis of genome DNA displayed a clear ladder band, but no ladder band was found in contrasting group (Figure 6).
If brownish yellow color of karyon was found with TUNEL, it showed positive apoptosis, and no color was negative. The apoptosis rate of Ad-p27kip1 group and control group was 37.3% and 1.26% respectively, and there was obvious difference χ² testing (P < 0.01).
Advances in cellular biology of tumor and molecular biology have found that the occurrence of esophageal carcinoma is a comprehensive pathologic process with multifunction, multistage and multigene variations. Activation of various oncogenes and inactivation of anti-oncogene may be the major factors for normal epithelial canceration. Genetic treatment aimed to import objective genes into gene mutation or lost cells with the use of gene engineering technology, and to have functional expression replacing the original genes in order to recover the functions and effects of original genes and correct genetic distortion or genetic loss resulted from cellular developmental disturbance and realize the treatment[9]. p53, p21, Egr-1, FHIT, VEGF, E2F-1 and hIFN-beta have been used as target genes in the treatment of esophagus carcinoma both in vivo and in vitro, and certain efficacy has been obtained[10-18]. Currently the analysis on esophagus carcinoma treatment through gene recombinant adenovirus p53 has entered the clinical stage[19], which will provide a bright future for tumor treatment.
The key to genetic treatment is the correct selection of exogenous objective genes, its import into target cells and steady expression. Koguchi[20] used adenovirus carrier to pack p27 gene to transfect astrocytes, and found its overexpression inhibited multiplication activity of astrocytes. Katner[21] used adenovirus carrier containing p27kiplcDNA to transfect human 786-0 renal carcinoma cells, and found that the cell with p27kip1 overexpression lost the growth features of tumor cells, and that CDK activity of transfected cells was inhibited obviously, and that the multiplication time was extended. Patel[22] used p27kip gene to transfect human tumor cell line AV-W9 to induce cell death.The study of p27 gene in human breast cancer cells[23], neuroblastoma cells[24], prostate carcinoma cells[25] and lymphoma[26] showed the similar results, suggesting that p27 gene could be used as an objective gene for genetic treatment of tumor.
Adenovirus carrier is of small pathogenicity and low genetic toxicity. With its wide host range, adenovirus can infect not only duplicated cells or cleavage cells, but also resting cells. Its huge package volume allows the insertion of 7.5 kb exogenic gene fragment without any active carcinogene or insertion mutation within non-integral host chromosome. The virus can reach a high titer and 100% infection rate through reproduction and purification. With its stable properties and safety for the human, it was considered as a gene conversion carrier of highly efficient expression[27] and most promising for genetic treatment[28].
Ad-p27kip1 constructed in the present study is a kind of replication of E1-deleted adenovirus vector. pJM17, the adenovirus skeleton with E1 region removed, can produce homologous recombinant with shuttle plasmids for producing infective adenovirus. Gene in E1 region is needed for adenovirus duplication, which requires that the duplication of intact adenovirus should be carried out in cell transfected by gene of E1 region. But since 293 cell is the right packaging cell for the transfection, replication of defective adenovirus has only one opportunity for infection in target cell without any duplication ability to fulfil the functions of adenovirus carrier, avoid damage of adenovirus itself to target cells and reach gene conversion.
Ad-p27kip1 containing human p27kip1 can positively connect p27kip1cDNA to CMV promoter of adenovirus. And as appraised by PCR amplification, p27 expression increased markedly after transfection of esophageal carcinoma cell. Contrasting adenovirus Ad-LacZ used in our study achieved 100% induction efficiency with MOI = 50. Ad-p27kip1 virus titer was 1.24 × 1012 pfu/mL, OD260/OD280 > 1.3, virus titer and purity were fairly high. This suggests that high expression of p27 after transfection is related to high induction efficiency and high purity, it can meet the demands of genetic treatment.
Studies demonstrated that declination of p27kip1 expression was an early event of esophageal carcinoma genesis, and also an independent prognostic factor of esophageal carcinoma[29-32].We imported human p27kip1 gene into esophageal carcinoma cell EC9706 and analyzed its biological properties through cell growth curve, 3H-TdR and 3H-Leucine incorporation experiment, FCM and DNA fragment analysis. We found that esophageal carcinoma cell displayed G1 stage block after import of the gene, and that p27 inhibited cell multiplication of esophageal carcinoma obviously, and that from G1 stage to S stage, the self-multiplication ability of the control cell decreased, and the apoptosis rate increased to 32.7%, a significant difference (P < 0.01) compared with that of control group and Ad-LacZ group. It may be concluded p27kip1 gene is an important gene for occurrence of esophageal carcinoma, decreased p27 expression may be a major factor to cell differentiation and death disturbance, while increased of p27 expression can promote cell death of esophageal carcinoma, which offers a new perspective on treating esophageal carcinoma.
Edited by Bo XN and Wang XL
| 1. | Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1442] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 2. | Harada K, Supriatno H, Sato M. Low p27Kip1 expression is associated with poor prognosis in oral squamous cell carcinoma. Anticancer Res. 2002;22:2985-2989. [PubMed] |
| 3. | Chen AJ, Meng QH, Long B, Yang GL. [Relationship between p27 expression and prognosis of colorectal carcinoma]. Ai Zheng. 2002;21:1075-1077. [PubMed] |
| 4. | Myung N, Kim MR, Chung IP, Kim H, Jang JJ. Loss of p16 and p27 is associated with progression of human gastric cancer. Cancer Lett. 2000;153:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Lacoste-Collin L, Gomez-Brouchet A, Escourrou G, Delisle MB, Levade T, Uro-Coste E. Expression of p27 (Kip1) in bladder cancers: immunohistochemical study and prognostic value in a series of 95 cases. Cancer Lett. 2002;186:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Schwerer MJ, Sailer A, Kraft K, Maier H. Cell proliferation and p27Kip1 expression in endophytic schneiderian papillomas. Laryngoscope. 2002;112:852-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Cesinaro AM, Migaldi M, Corrado S, Maiorana A. Expression of p27kip1 in basal cell carcinomas and trichoepitheliomas. Am J Dermatopathol. 2002;24:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Zhang X, Liu S, Liang C, Yang H. [Adenovirus-mediated Rb gene transfect for head and neck cancer]. Huaxi Yike Daxue Xuebao. 2001;32:194-195, 207. [PubMed] |
| 9. | Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going. J Natl Cancer Inst. 1997;89:21-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 411] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Matsubara H, Maeda T, Gunji Y, Koide Y, Asano T, Ochiai T, Sakiyama S, Tagawa M. Combinatory anti-tumor effects of electroporation-mediated chemotherapy and wild-type p53 gene transfer to human esophageal cancer cells. Int J Oncol. 2001;18:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Shimada H, Shimizu T, Ochiai T, Liu TL, Sashiyama H, Nakamura A, Matsubara H, Gunji Y, Kobayashi S, Tagawa M. Preclinical study of adenoviral p53 gene therapy for esophageal cancer. Surg Today. 2001;31:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Fujii T, Tanaka Y, Tanaka T, Matono S, Sueyoshi S, Fujita H, Shirouzu K, Kato S, Yamana H. [Experimental gene therapy using p21/WAF1 gene in esophageal squamous cell carcinoma--adenovirus infection and gene gun technology]. Gan To Kagaku Ryoho. 2001;28:1651-1654. [PubMed] |
| 13. | Wu MY, Chen MH, Liang YR, Meng GZ, Yang HX, Zhuang CX. Experimental and clinicopathologic study on the relationship between transcription factor Egr-1 and esophageal carcinoma. World J Gastroenterol. 2001;7:490-495. [PubMed] |
| 14. | Ishii H, Dumon KR, Vecchione A, Trapasso F, Mimori K, Alder H, Mori M, Sozzi G, Baffa R, Huebner K. Effect of adenoviral transduction of the fragile histidine triad gene into esophageal cancer cells. Cancer Res. 2001;61:1578-1584. [PubMed] |
| 15. | Gu ZP, Wang YJ, Li JG, Zhou YA. VEGF165 antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol. 2002;8:44-48. [PubMed] |
| 16. | Yang HL, Dong YB, Elliott MJ, Liu TJ, McMasters KM. Caspase activation and changes in Bcl-2 family member protein expression associated with E2F-1-mediated apoptosis in human esophageal cancer cells. Clin Cancer Res. 2000;6:1579-1589. [PubMed] |
| 17. | Guo WZ, Ran YL, Liu J, Yu L, Sun LX, Yang ZH. [Enhancement by hypoxia of antisense VEGF (165) gene expression in esophageal cancer cells]. Shengwu Huaxue Yu Shengwu Wuli Xuebao (. Shanghai). 2002;34:625-629. [PubMed] |
| 18. | Tsunoo H, Komura S, Ohishi N, Yajima H, Akiyama S, Kasai Y, Ito K, Nakao A, Yagi K. Effect of transfection with human interferon-beta gene entrapped in cationic multilamellar liposomes in combination with 5-fluorouracil on the growth of human esophageal cancer cells in vitro. Anticancer Res. 2002;22:1537-1543. [PubMed] |
| 19. | Shimada H, Matsubara H, Ochiai T. [Gene therapy for esophageal cancer]. Nihon Geka Gakkai Zasshi. 2002;103:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Koguchi K, Nakatsuji Y, Nakayama K, Sakoda S. Modulation of astrocyte proliferation by cyclin-dependent kinase inhibitor p27 (Kip1). Glia. 2002;37:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Katner AL, Gootam P, Hoang QB, Gnarra JR, Rayford W. A recombinant adenovirus expressing p7 (Kip1) induces cell cycle arrest and apoptosis in human 786-0 renal carcinoma cells. J Urol. 2002;168:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Patel SD, Tran AC, Ge Y, Moskalenko M, Tsui L, Banik G, Tom W, Scott M, Chen L, Van Roey M. The p53-independent tumoricidal activity of an adenoviral vector encoding a p27-p16 fusion tumor suppressor gene. Mol Ther. 2000;2:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, Lee SJ, Trepel J, Cowan K, Seth P. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997;14:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Matsuo T, Seth P, Thiele CJ. Increased expression of p27Kip1 arrests neuroblastoma cell growth. Med Pediatr Oncol. 2001;36:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Katner AL, Hoang QB, Gootam P, Jaruga E, Ma Q, Gnarra J, Rayford W. Induction of cell cycle arrest and apoptosis in human prostate carcinoma cells by a recombinant adenovirus expressing p27 (Kip1). Prostate. 2002;53:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Turturro F, Seth P. Prolonged adenovirus-mediated expression of p27 (Kip1) unveils unexpected effects of this protein on the phenotype of SUDHL-1 cells derived from t (2; 5)-anaplastic large cell lymphoma. Leuk Res. 2003;27:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 27. | Zhang WW. Development and application of adenoviral vectors for gene therapy of cancer. Cancer Gene Ther. 1999;6:113-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Tseng JF, Farnebo FA, Kisker O, Becker CM, Kuo CJ, Folkman J, Mulligan RC. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery. 2002;132:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Yasunaga M, Tabira Y, Nakano K, Iida S, Ichimaru N, Nagamoto N, Sakaguchi T. Accelerated growth signals and low tumor-infiltrating lymphocyte levels predict poor outcome in T4 esophageal squamous cell carcinoma. Ann Thorac Surg. 2000;70:1634-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Shamma A, Doki Y, Tsujinaka T, Shiozaki H, Inoue M, Yano M, Kawanishi K, Monden M. Loss of p27 (KIP1) expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Oncology. 2000;58:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Shibata H, Matsubara O, Wakiyama H, Tanaka S. The role of cyclin-dependent kinase inhibitor p27 in squamous cell carcinoma of the esophagus. Pathol Res Pract. 2001;197:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Tanière P, Martel-Planche G, Saurin JC, Lombard-Bohas C, Berger F, Scoazec JY, Hainaut P. TP53 mutations, amplification of P63 and expression of cell cycle proteins in squamous cell carcinoma of the oesophagus from a low incidence area in Western Europe. Br J Cancer. 2001;85:721-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |